- 1Department of Public Health, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia
- 2Department of Public Health, Bichena Primary Hospital, Bichena, Amhara, Ethiopia
- 3Department of Nursing, Bichena Primary Hospital, Bichena, Amhara, Ethiopia
- 4Department of Environmental Health, Injibara University, Injibara, Amhara, Ethiopia
- 5Department of Midwifery, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia
Background: Despite progress in reducing neonatal mortality rates in Ethiopia, the country still has a high neonatal mortality rate compared with the global average. Primary hospitals are critical in delivering basic neonatal care, particularly in rural areas. However, data on neonatal mortality and contributing factors in these settings are scarce. This study aimed to determine the survival status and predictors of neonatal mortality among neonates admitted to Bichena Primary Hospital, Northwest Ethiopia.
Methods: A retrospective cohort study was conducted among 638 neonates admitted to the Bichena Primary Hospital neonatal intensive care unit from January 1, 2021, to April 30, 2023. Neonates were selected via a consecutive sampling method. Data were collected from medical records using a pretested checklist. A Kaplan–Meier survival curve was used to estimate the neonatal survival time, and a Cox proportional hazard regression model was used to identify independent predictors of neonatal mortality.
Results: Of the 638 neonates followed, 21.5% died during the study period. The overall incidence rate of death was 66.69 per 1,000 neonate days. Hypothermia, birth injury, perinatal asphyxia, preterm birth, maternal history of abortion, low birth weight, and neonatal hypoglycemia were independent predictors of neonatal mortality.
Conclusion and recommendation: The study found a high rate of neonatal mortality, exceeding rates reported in other regions of Ethiopia. Most predictors were preventable and treatable. Therefore, early identification of obstetric complications, immediate interventions and postnatal care are crucial to reduce neonatal mortality and enhance overall neonatal outcomes.
1 Introduction
1.1 Background
Neonatal mortality refers to the death of a live-born baby within the first 28 days (1) and measures the quality of maternal and child healthcare (2). Neonatal deaths account for 47% of the global under-five mortality (3). Among these deaths, 36% occur during the first 24 h (2, 4), 37% occur between days 1 and 7, and 27% occur between days 7 and 27 (2). In low- and middle-income countries, neonatal deaths account for 53.1% of all under5 deaths (5). From 1990 to 2019, neonatal deaths worldwide decreased from 5 million to 2.4 million (3), resulting in a decline in the mortality rate from 37 deaths per 1,000 live births in 1990 to 18 deaths per 1,000 live births in 2021 (6).
In East Africa, the Neonatal mortality rate (NMR)increased from 33.6 per 1,000 live births in 2017 (7) to 51.32 per 1,000 live births in 2021 (8). NMRs also vary across countries: Kenya reported a rate of 19.6 per 1,000 live births in 2018 (9), Eritrea reported a rate of 16.5 (10), and Ethiopia reported 26.84 per 1,000 live births (11). In Ethiopia, neonatal mortality is a major public health issue (12). The Amhara region has the highest rate of 46 per 1,000 live births, which is higher than that reported in the other areas (12). There is also a geographical clustering of neonatal deaths in Ethiopia, with Amhara being one of the most affected areas (7, 13), emphasizing the need to address NMRs in this region.
Neonatal mortality in Ethiopia improved from 39 deaths per 1,000 live births in 2005–29 deaths in 2016 (14) but slightly increased to 33 deaths in 2019 (12), which is higher than the global average of 18 deaths per 1,000 live births (4). This suggests inadequate progress toward achieving Sustainable Development Goals (SDG) 3.3, which aims to reduce NMRs to less than 12 by 2030. The identified risk factors were home birth, rural residency, gestational age, sepsis, perinatal asphyxia (PNA), longer birth intervals, and respiratory distress (7, 15). Additionally, mothers of advanced age and primipara mothers are at increased risk of neonatal mortality (11).
Numerous efforts are underway to improve neonatal survival. The SDGs aim to reduce neonatal mortality to 12 per 1,000 live births by 2030 (4). Additionally, the Every Newborn Action Plan (ENAP) focuses on preventing stillbirths and newborn deaths (16). Ethiopia has committed to integrating life-saving measures into the national strategy outlined in the Health Sector Transform Plan (HSTP), to achieve an NMR of 10 per 1,000 live births by 2020 (17).
Despite efforts, neonatal mortality remains high in Ethiopia, with a current rate of 33 deaths per 1,000 live births (14). To reduce NMRs, it is crucial to identify and address the underlying causes of neonatal deaths starting from primary hospitals, which are the first point of care for newborns (18), and provide basic neonatal care (19). Therefore, this study aimed to investigate the survival status and predictors of mortality among neonates admitted to Bichena Primary Hospital in Northwest Ethiopia. These findings support the goals of the Ethiopian Hospital Alliance for Quality (EHAQ) program by generating new evidence to support evidence-based care in neonatal care (20).
2 Methods and materials
2.1 Study area and period
The study took place at the Bichena Primary Hospital neonatal intensive care unit located in Bichena Administrative Town in the Amhara Region, Northwest Ethiopia. It is approximately 273 km from Addis Ababa, the capital of Ethiopia, and 225 km from Bahir Dar, the capital of the Amhara Regional State. The hospital has been operational since April 2015, providing healthcare services to a population exceeding 450,000. In addition to providing various inpatient and outpatient services, including a neonatal intensive care unit, the hospital provides to the areas surrounding Enemay Woreda and Debay Telatgen.
The neonatal intensive care unit of Bichena Primary Hospital has a maximum of 9 beds, with a monthly average of 35 neonates being admitted, yielding, on average, 400–500 annual admissions of neonates. During the study period from January 1, 2021, to April 2023, the NICU was equipped with four room heaters, one infant radiant warmer, one fan radiant warmer, one incubator, four oxygen concentrators, and oxygen cylinders. However, Bubble CPAP was not available.
2.2 Study design
A retrospective cohort study was conducted among neonates admitted to the NICU (Neonatal Intensive Care Unit) at Bichena Primary Hospital, Northwest Ethiopia.
2.3 Populations
2.3.1 Source population
The source population comprised all neonates admitted to the NICU at Bichena Primary Hospital, North West Ethiopia, during the study period.
2.3.2 Study population
The study population comprised neonates admitted to the NICU at Bichena Primary Hospital, Northwest Ethiopia, between January 1, 2021, and April 2023, who met the inclusion criteria.
2.4 Eligibility criteria
2.4.1 Inclusion criteria
All admitted neonates’ medical records with complete information during the study period (January 1, 2021–April 30, 2023) in the NICU at Bichena Primary Hospital were included.
2.4.2 Exclusion criteria
Neonates with incomplete records of major variables (e.g., date of admission, date of discharge/death, treatment outcomes), lost records, revisits, or neonates who were initially admitted but were immediately referred to specialized health facilities for further management were excluded from the study.
2.5 Sample size determination and sampling procedure
2.5.1 Sample size determination
The sample size is estimated via the double population proportion formula in Epi Info version 7. This is done by considering different significant variables from the previous study and by considering the following assumption: the level of significance is 5%, the power is 80%, P1 is the proportion of neonatal mortality among the exposed group, and P2 is the proportion outcome among the not exposed group. The ratio of the population exposed to not exposed neonates was 1:1. The anticipated mortality rate among neonates who were delivered via assisted methods (P1, exposed) was 12.2%, whereas that for those delivered without assistance (P2, unexposed) was 21.2% (21); 10% was added for potential incompleteness. Hence, the final sample size was determined to be 638 neonates admitted to the NICU at Bichena Primary Hospital.
2.5.2 Sampling technique and procedures
A consecutive sampling method was employed to select study participants, involving the sequential selection of participants using their medical record numbers until the desired sample size was achieved.
2.6 Study variables
The dependent variable for this study was the time to death of the neonate. The independent variables were sociodemographic factors, including maternal age, place of residence, sex of the neonate, and age of the neonate; maternal and obstetric factors, gravidity, parity, Antenatal care (ANC), mode of delivery, place of delivery, duration of labor, time of initiation of breastfeeding, amniotic fluid status, premature rupture of membrane (PROM), antepartum hemorrhage, eclampsia, type of pregnancy, history of abortion, stillbirth, history of fetal death, anemia, hepatitis, Syphilis and HIV/AIDS; and neonatal factors, including date of admission, weight, Apgar (Appearance, Pulse, Grimace, Activity, Respiration) score, and gestational age. Neonatal medical conditions such as sepsis, perinatal asphyxia (PNA), respiratory distress syndrome (RDS), sepsis, hypoglycemia, and temperature at admission.
2.7 Operational definitions
Neonatal anemia was defined as a hemoglobin level of less than 13 gr/dl or a hematocrit of less than 39% (22).
Birth asphyxia was diagnosed when the newborn had at least one of the following signs: not breathing or gasping, <30 breaths per minute, or an APGAR score <7, neonatal neurologic sequelae (seizures, coma, and hypotonic), or multiple organ involvement (kidney, lungs, liver, heart, and intestines) (23).
Censored: a neonate who was alive at the end of the study or lost to follow-up, including discharged to home, discharged against medical advice, or transferred to other health institutions without knowing the outcome (24).
Event: death of a neonate after NICU admission as evidenced by physician confirmation.
Follow-up time-From the time of admission until either an event or censorship occurs.
Hepatitis: Diagnosed as positive if the hepatitis antigen test is positive during pregnancy or during neonate admission if not tested previously.
Low birth weight: defined as a birth weight admission weight of less than 2,500 grams.
Preterm: refers to births occurring before 37 completed weeks of gestation (25).
Survival status: outcome of the neonate; either death or censored.
Survival time: Measures the follow-up time from admission to the NICU to the occurrence of an event or censoring (26).
Syphilis: Diagnosed as positive if the VDRL test is positive during pregnancy or during neonate admission if not tested previously.
Time origin: date of admission.
Time scale: days from the admission of a neonate to the occurrence of an outcome.
2.8 Data collection tools and procedures
The data extraction tool was adapted from the NICU registration book (27), which has been used by other studies previously (26, 28–30). The questionnaire was prepared in the English language. Four BSC nurses currently working in the NICU of Bichena Primary Hospital were involved as data collectors, and two MSC nurses who are hospital staff were supervisors. Survival time was obtained by considering the time between the date of admission and the date of discharge (the date of death or censoring).
2.9 Data quality control
A pretest was performed on 5% (32) of the sample size in the hospital before the actual data were collected. The necessary modifications and corrections were made to ensure its validity. Before starting the data collection, one-day training was given on the objectives of the study, the contents of the checklist, and issues related to the confidentiality of the neonates’ profiles. The principal investigator and supervisors spot-checked and reviewed the completed checklist to ensure completeness and were examined through a random selection of cards by the principal investigator.
2.10 Data processing and analysis
The data were cleaned, coded, and edited to ensure completeness and consistency. The cleaned data were entered into Epi-Data version 3.1 and exported to STATA 14.0 for analysis.
Descriptive statistics, such as frequencies with percentages and means with standard deviations, were used to describe the study population. Tables and figures were also used for data presentation. A variance inflation factor (VIF) >10 and tolerance (T) <0.1 were considered suggestive of the existence of multicollinearity. We found that none of the variables in our model had a VIF greater than 10 or a T less than 0.1, indicating that multicollinearity was not a concern.
A Kaplan–Meier curve was used to estimate the survival status of neonates admitted to the NICU at different times. The log-rank test was used to compare the survival experience of categorical predictor variables.
The assumptions of proportional hazards were verified via statistical tests and graphical tests. The log minus log plots were assessed to confirm whether they were approximately parallel to each other over time, indicating that the proportional hazards assumption was met. The p values of all variables and the overall global test results in the final multivariable Cox regression model were assessed, with values greater than 0.05 indicating that the proportional hazard assumption was fulfilled. Schoenfeld residual proportional hazard test was used to confirm the independence between the residuals and time. A slope of zero between the residuals and time indicated a good model fit. The Cox-Snell residuals test was also used to evaluate the model's goodness of fit.
Factors associated with outcome variable with a p-value less than 0.25 in bivariate Cox regression were analyzed via multivariate Cox regression. The adjusted hazard ratios (AHR) with 95% confidence intervals (CIs) from the multivariate Cox regression were used as a measure of association, and variables with a p-value <0.05 were considered significant predictors.
3 Results
3.1 Sociodemographic characteristics of the study participants
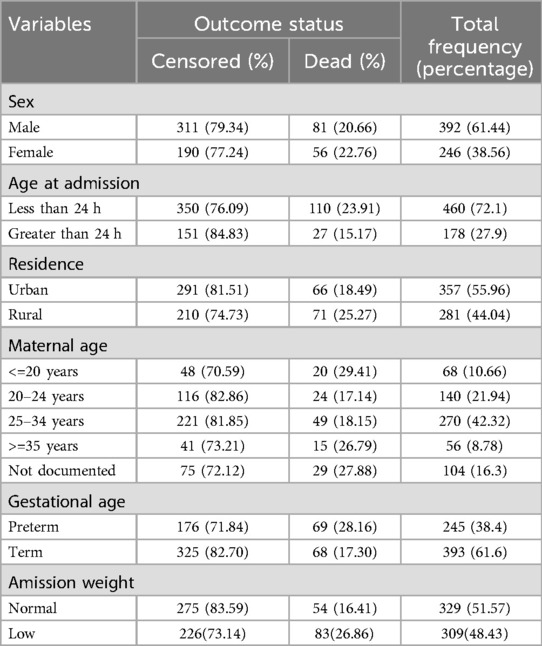
A total of 638 neonates were followed during the study period. Among the participants, the majority of neonates in the study were male (61.44%), while the percentage of deaths was slightly greater in females (22.76%) than in males (20.66%). Furthermore, a notable 72.1% of neonates were admitted within 24 h of birth. Concerning residence, approximately half (55.96%) of the neonates were from urban areas. Additionally, the majority of mothers (42.32%) were in the age range of 25–34 years. Finally, 61.6% of the newborns were born at term, whereas 38.4% were born preterm (Table 1).
3.2 Maternal and obstetric characteristics of mothers
Among the 638 neonates in the study, the majority of mothers (94.51%) received ANC, whereas 5.49% did not. The majority of neonates were born spontaneously (66.77%), followed by cesarean section (12.7%), instrumental delivery (17.71%), and assisted breech delivery (2.82%). The place of delivery varied, with 63.48% of neonates born at the same facility, 29.31% from other facilities, and 7.21% born at home. Regarding the history of abortion, 97.18% of the neonates were born to mothers with no history of abortion. The majority of neonates (88.71%) were born without any obstetric complications, although 11.29% experienced complications (Table 2).
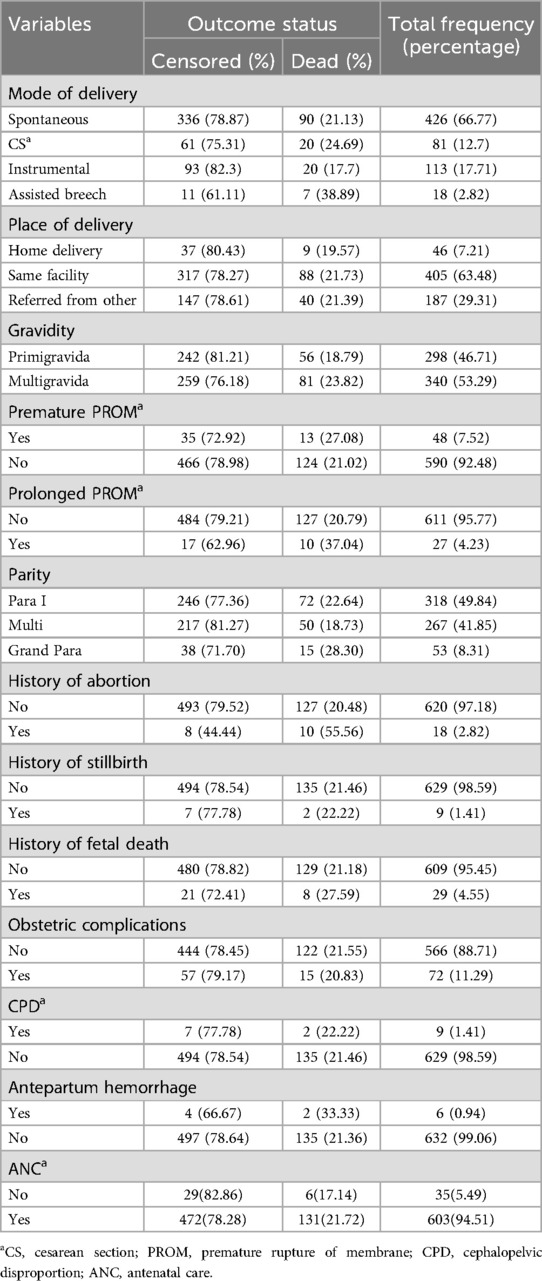
Table 2. Maternal and obstetric characteristics of mothers whose neonates were admitted in the study.
3.3 Maternal medical diagnosis
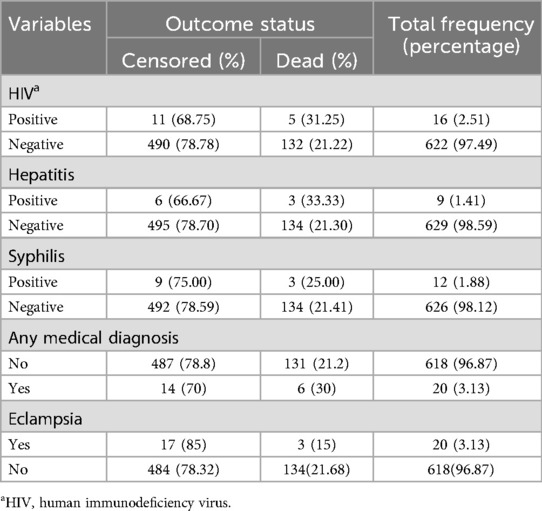
In the present study, among mothers of neonates, 2.51% were HIV positive, whereas 97.49% were not exposed. Hepatitis was present in a small fraction (1.41%) of mothers. Eclampsia was diagnosed in 3.13% of the mothers of the neonates (Table 3).
3.4 Neonatal medical diagnosis
Among the admitted neonates, the majority (86.05%) were hypothermic during admission. Neonates admitted with suspected neonatal sepsis accounted for 67.55% of the neonates. Almost three-fourths (74.29%) of the neonates were asphyxiated, and 18.03% of them received resuscitation. Birth injury or trauma was observed in 18.97% of the neonates (Table 4).
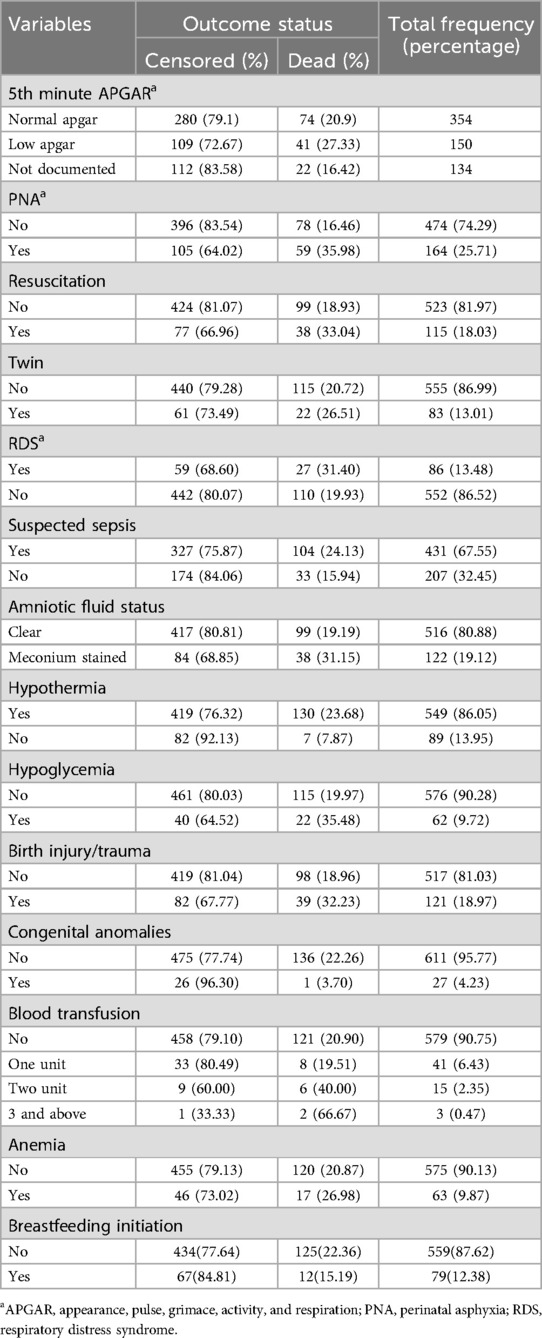
Table 4. Neonatal medical-related factors among neonates admitted to the NICU of Bichena primary hospital Northwest Ethiopia, January 1st, 2021–April 30, 2023.
3.5 Causes of neonatal death
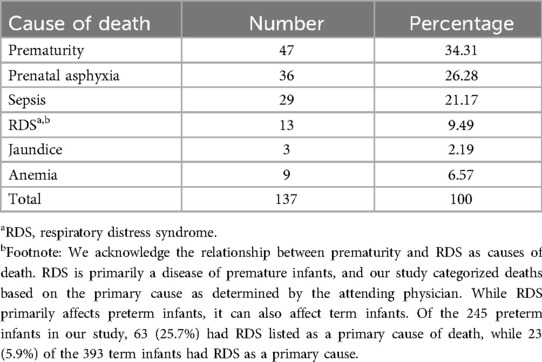
As shown in the table below, prematurity (34.31%) was the leading cause of neonatal death, followed by birth asphyxia, which accounted for 26.28% of neonatal deaths, whereas anemia was the least common cause of neonatal death (Table 5).
3.6 Survival status of the neonates and incidence rate of neonatal mortality
The study included 638 neonates, resulting in 2,046 neonate days. The study period was from January 1, 2021, to April 20, 2023, with a follow-up period of 1–21 days. The neonates’ discharge statuses were as follows: 37.1% (237 neonates) recovered, 21.5% (137 neonates) died, 34.0% (217 neonates) were transferred, and 7.4% (47 neonates) left against medical advice. The main reasons for transfer were prematurity and low birth weight (<1,500 g) requiring specialized NICU care, uncontrolled seizures in asphyxiated neonates, severe jaundice needing intensive phototherapy, respiratory distress (especially RDS) requiring CPAP, and severe sepsis. The total incidence rate of neonatal mortality at the NICU of Bichena Primary Hospital was 66.96 (CI 56.64, 79.17) per 1,000 neonate days.
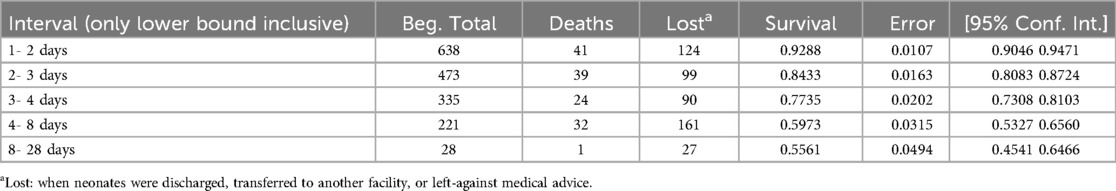
The estimated cumulative survival probability during the follow-up period was 92.88% (95% CI: 90.46%–94.71%) on the first day, 84.33% (95% CI: 80.83%–87.24%) at the end of the second day, 77.35% (95% CI: 73.08%–81.03%) at the end of the third day, 59.73% (95% CI: 53.27%–65.60%) from days four to seven, and 55.61% (95%: 45.41%–64.66%) from days eight to 28 (Table 6).
3.7 Kaplan–Meier and log-rank test results for several predictor variables
The Kaplan–Meier survival estimate revealed that the survival probability of neonates was high on the first day after admission. However, this probability decreased over time. According to the graph, nearly all newborn deaths occurred within the first week of hospitalization. The total neonatal survival rate was 61.65%, with a standard error of 0.0416 (95% CI 0.52, 0.69) during the follow-up period (Figure 1).
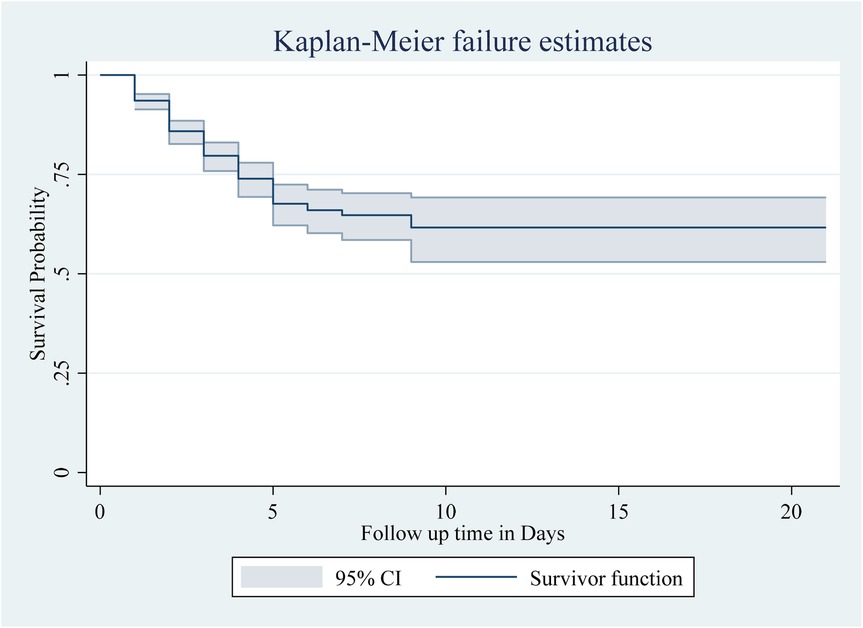
Figure 1. The overall Kaplan–Meier survival curve with a 95% confidence interval showing the survival time of neonates in the study.
Among the numerous predictor variables, log-rank tests revealed statistically significant differences in newborn survival probabilities. A lower birth weight (log-rank test, χ2 = 15.07, p < 0.001; Figure 2), respiratory distress (log-rank test, χ2 = 21.77, p < 0.001; Figure 3), preterm gestational age (log-rank test, χ2 = 24.61, p < 0.001; Figure 4), hypothermia (log-rank test, χ2 = 11.70, p < 0.001; Figure 5), and a history of abortion in a previous pregnancy (log-rank test, χ2 = 19.25, p < 0.001; Figure 6) were associated with a significantly lower probability of survival.
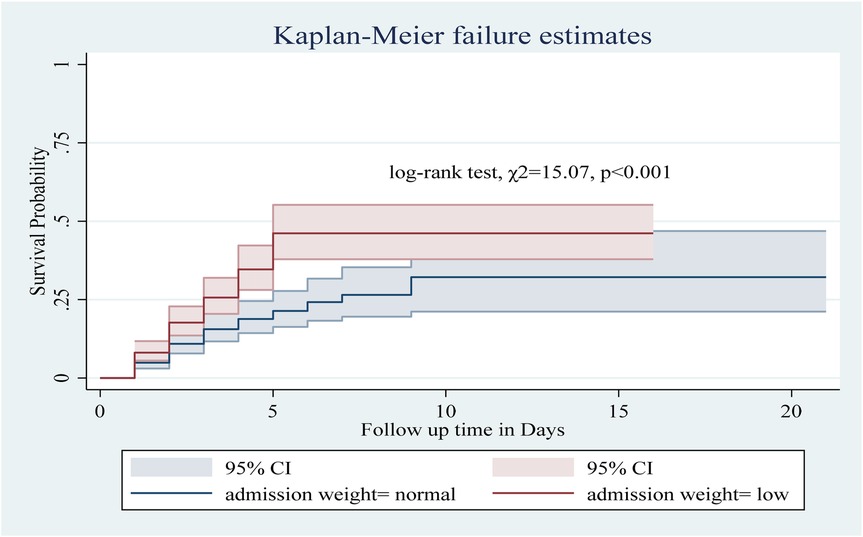
Figure 2. Kaplan–Meier curve showing the survival probability of neonates admitted to the NICU based on admission weight.
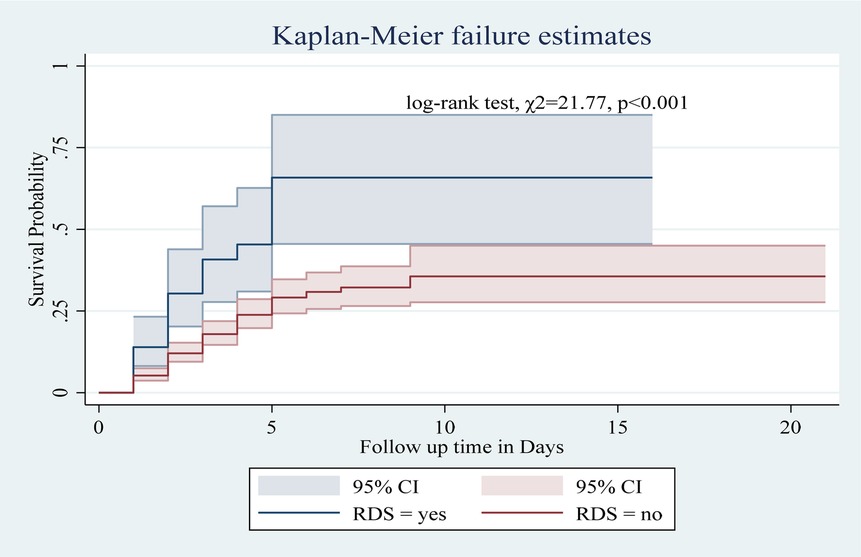
Figure 3. Kaplan–Meier curve showing the survival probability of neonates admitted to the NICU based on RDS.
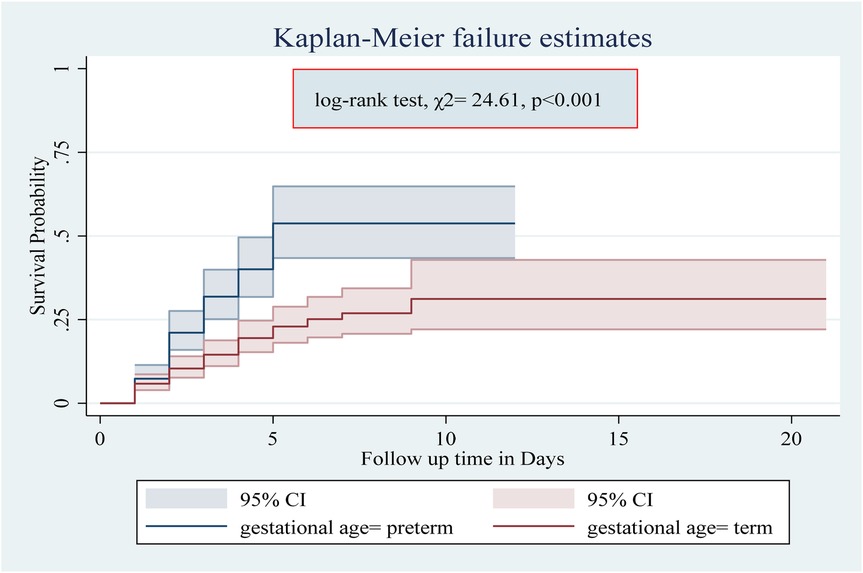
Figure 4. Kaplan–Meier curve showing the survival probability of neonates admitted to the NICU based on gestational age.
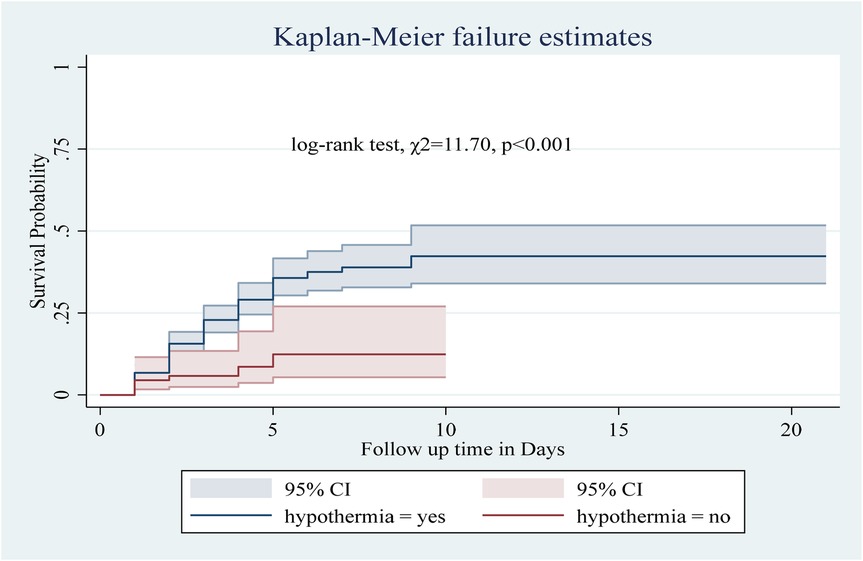
Figure 5. Kaplan–Meier curve showing the survival probability of neonates admitted to the NICU based on hypothermia.
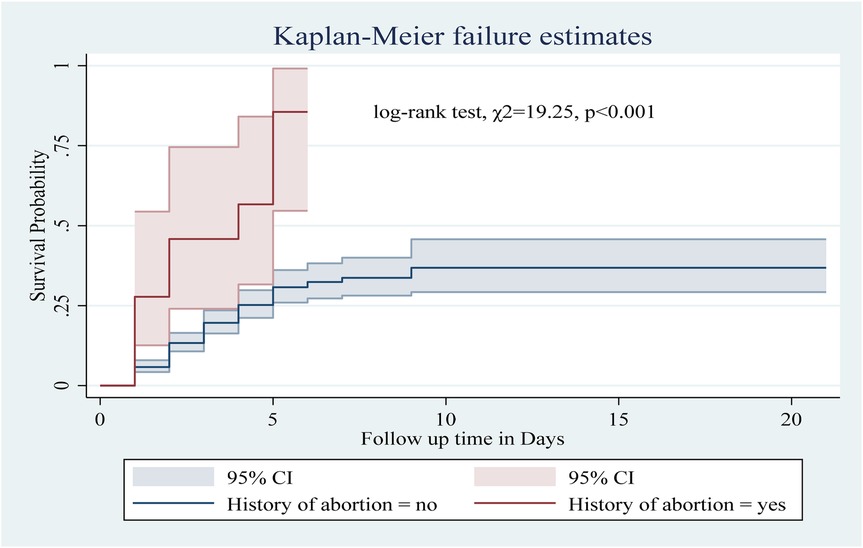
Figure 6. Kaplan–Meier curve showing the survival probability of neonates admitted to the NICU based on history of abortion.
3.8 Predictors of neonatal mortality among neonates admitted to the NICU
In this study, bivariate Cox regression analysis was initially conducted with several variables, including hypothermia, birth injury/trauma, perinatal asphyxia, gestational age, history of abortion, birth weight, gravidity, premature ROM, prolonged ROM, suspected sepsis, amniotic fluid status, hepatitis exposure, residence, antepartum hemorrhage, and hypoglycemia. Variables showing a significance level of p < 0.25 in the bivariate analysis were further considered for the multivariable Cox regression model. The backward elimination method was employed for variable selection, with biological plausibility and insights from previous studies guiding the process. The final adjusted hazard ratios (AHRs) with 95% confidence intervals (CIs) were determined, and variables with a p-value < 0.05 were identified as significant predictors of neonatal mortality.
In this study, neonates with hypothermia had a 2.59-fold greater risk of death than those without hypothermia (AHR: 2.59, 95% CI: 1.17–5.70). Similarly, the presence of birth injury/trauma and perinatal asphyxia increased the risk of death by 1.82 (AHR: 1.82, 95% CI: 1.2, 2.76) and 1.65 times (AHR: 1.65, 95% CI: 1.11–2.45), respectively. Preterm neonates were at a 2.29-fold greater risk of death than term neonates (AHR: 2.29, 95% CI: 1.55, 3.38). Neonates from mothers with a history of abortion had a 2.13 times greater risk of death than those from mothers without a history of abortion (AHR: 2.13, 95% CI: 1.06, 4.25). Low birth weight increased the risk of death by 1.6 times (AHR: 1.6, 95% CI: 1.09, 2.34). Finally, neonates with hypoglycemia had a 1.71-fold greater risk of death than those without hypoglycemia (AHR: 1.71, 95% CI: 1.06 2.74) (Table 7).
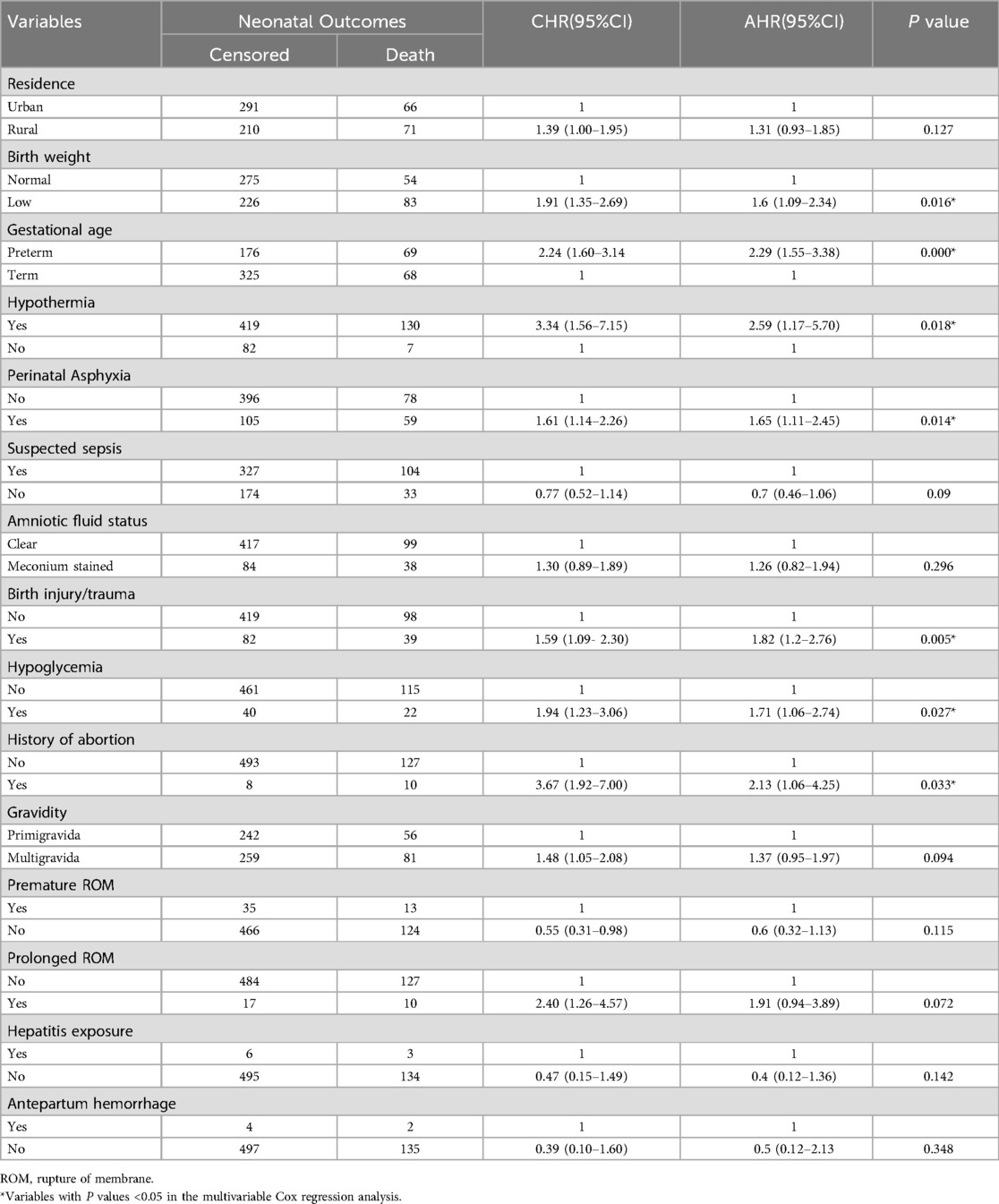
Table 7. Bivariate and multivariate Cox regression analyses of predictive factors of neonatal mortality in the NICU.
4 Discussion
The NMR at the NICU of Bichena Primary Hospital was 66.96 per 1,000 neonate days, significantly higher than rates reported in other regions of Ethiopia. For instance, Bombe Primary Hospital in southern Ethiopia reported a rate of 20.8 per 1,000 neonatal days (31), and Arba Minch General Hospital reported 31.6 per 1,000 neonate days of follow-up (28). Further, a study in Gurage Zone Public Hospitals reported 36.9 per 1,000 neonate days (32), and a tertiary hospital in Addis Ababa Ethiopia also reported 19.2 deaths per 1,000 live births (29). Such discrepancies can be attributed to differences in healthcare practices, hospital levels, sample size, and the demographic characteristics of admitted neonates. For example, Bombe Primary Hospital admitted a higher proportion of low-risk neonates, which may partly explain their lower NMR (31).
The NMR observed in our study was also much higher than international neonatal mortality rates, particularly in high-income and middle-income countries. In Australia, the NMR is approximately 2.3 per 1,000 live births, which is far lower than the rate observed in our study. Similarly, in middle-income countries, neonatal mortality remains significantly lower, with India reporting approximately 22.73 per 1,000 live births and South Africa around 11 per 1,000 live births. While our study reported NMR per 1,000 neonate days, these international figures, measured per 1,000 live births, still highlight major disparities in neonatal care (33).
Additionally, the study revealed a cumulative incidence rate of 215 per 1,000 newborns admitted to Bichena Primary Hospital, indicating that 21.5% of admitted newborns died during the follow-up period. This is twice the neonatal death rate at Bombe Primary Hospital, 10.6% (31) but comparable to rates at Arba Minch General Hospital (20.8%) (28), Debre Markos Hospital (21.3%) (34), and Gurage Zone Public Hospitals (22.7%) (32).
In this study, we identified several risk factors contributing to neonatal mortality. Being preterm increases the risk of death by 2.29 times compared with term neonates. This finding is supported by studies conducted in various locations, including Addis Ababa, Ethiopia (29), the Hadiya Zone (35), Jimma (36), and Debre Tabor (37). It is well known that preterm neonates are not fully prepared to live outside the uterus, as their bodies are not fully mature (29). Given the high risk of mortality and morbidity associated with preterm birth, it is better to transfer preterm neonates to Level II or Level III hospitals as soon as possible after stabilization for specialized care. Ensuring CPAP availability in primary hospitals is also crucial for improving respiratory support and reducing mortality in preterm neonates. Therefore, policymakers should prioritize CPAP provision and implement policies to facilitate prompt referrals to higher-level facilities, ultimately enhancing neonatal survival and long-term health outcomes.
Additionally, low-birth-weight neonates were at a 1.6-fold greater risk of death. This is in agreement with studies from Bombe (31), the Hadiya Zone (35), the Amhara regional state (38), Addis Ababa (39), and Asmara (10). Policymakers should prioritize establishing and maintaining a Level II or III NICU in hospitals that care for neonates, particularly in regions with high rates of preterm birth and low-birth-weight neonates. This will enable healthcare providers to provide specialized care and treatment to neonates who require it, and improve their chances of survival and long-term health outcomes. Additionally, hospitals should be equipped with the minimum set of equipment and supplies available to provide the most possible care for newborns, as per international standards and guidelines.
Similarly, this study revealed that perinatal asphyxia increased the risk of death by 1.65 times. This finding agrees with studies conducted in Hosanna (30), at a tertiary hospital, Addis Ababa (29), and Wolaita Sodo University Hospital in Ethiopia (40). A meta-analysis revealed that asphyxia increases the risk of neonatal death (41). Perinatal asphyxia leads to oxygen deprivation, which causes hypoxia, hypercarbia, and acidosis, potentially damaging vital organs and resulting in death (32). The unchanged death rate related to perinatal asphyxia may be attributed to delayed or inadequate care during labor and neonatal resuscitation (35). Consequently, pregnant women need to seek prompt medical care for any delivery complications. Clinicians should focus on providing timely, quality care, in early detection and management of perinatal asphyxia. Policymakers should prioritize improving maternal healthcare access by ensuring obstetricians are available during labor.
Furthermore, Neonates with hypothermia had a 2.59-fold greater risk of death than did those without hypothermia. This finding is consistent with studies conducted in Bombe primary hospitals (31), Wollega University referral hospitals (42), specialized hospitals in the Amhara region, and (38) the Hadiya Zone (35) from Ethiopia. A study from Malawi Hospital also reported that all neonates admitted with hypothermia died (43), indicating its significant role in mortality. This finding highlights the importance of keeping newborns warm and comfortable, especially in the first 24 h after birth. Clinicians should be alert about preventing neonatal hypothermia by using warm blankets and maintaining a cozy environment. Policymakers should ensure hospitals have access to warm blankets and heating devices to keep newborns safe and comfortable.
Moreover, this study revealed that birth injury/trauma increased the risk of death by 1.82 times. This finding is supported by studies conducted in Ethiopia (44). This might be related to cranial injuries in neonates, which can result in bleeding, hypovolemic shock, and various complications. These conditions deteriorate the health status of the neonate, resulting in neonatal death. This finding highlights the importance of careful monitoring and management of neonates with birth trauma, as these injuries may lead to adverse outcomes. Policymakers are recommended to improve access to quality maternal healthcare by training providers in emergency obstetric care and developing standards to minimize birth injuries.
Furthermore, hypoglycemia increases the risk of death by 1.71 times compared with those without this condition. A study conducted in Ethiopia supports the present study (38). The possible reason may be a delay in the diagnosis of hypoglycemia, as undiagnosed and untreated hypoglycemia can cause brain damage and potentially result in death (25). This finding emphasizes the importance of early diagnosis and treatment of hypoglycemia in neonates. Parents should be aware of the signs and symptoms, such as shakiness, irritability, and lethargy, and seek medical attention if they suspect hypoglycemia. For sick neonates unable to breastfeed, alternative feeding methods such as cup or tube feeding should be considered, particularly during transport, to help maintain glucose levels. Clinicians also should monitor blood glucose levels and provide supplements as needed.
Finally, neonates from mothers with a history of abortion had a 2.13-fold greater risk of death than those from mothers without a history of abortion. This finding aligns with those of previous studies in Ethiopia and abroad (45–48). Further research is needed to explore the biological mechanism of different types of abortion-related to subsequent pregnancy (49). This finding emphasizes the importance of pregnant women disclosing previous abortions and reproductive health issues to their healthcare providers. Clinicians should be aware of abortion history as a risk factor and take steps to identify and manage underlying health issues. Policymakers should improve access to quality reproductive healthcare through education and counseling on reproductive health and family planning.
This study has several strengths, including a large sample size of 638 neonates, a cohort study design, and standardized data collection by trained NICU nurses. The study accounted for seasonal variation over two years and provides valuable insights for future prospective cohort studies in primary hospitals. However, the study also has some limitations. The retrospective design did not address potential predictors such as socioeconomic status, quality of care, or the service provider. Additionally, the study was conducted in a single health institution and had potential loss to follow-up. Moreover, the absence of CPAP in the NICU may have impacted neonatal outcomes. Addressing these limitations and focusing on identified risk factors can help future research improve neonatal care and outcomes.
5 Conclusion
In this study, we reported a high rate of neonatal mortality, exceeding rates reported in other regions of Ethiopia. Hypothermia, birth injury, perinatal asphyxia, preterm birth, maternal history of abortion, low birth weight, and neonatal hypoglycemia were independent predictors of neonatal mortality. As our findings revealed, most predictors were preventable and treatable. Therefore, special attention should be given to the early identification of obstetric complications, immediate interventions, and postnatal care to reduce the risk of neonatal mortality and enhance overall neonatal outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Amhara Region Research Ethics Review Board (NoH/R/T/T/D/07/54). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study involved a retrospective review of medical records, which posed minimal risk to participants and did not involve direct contact with them. A letter was submitted to the hospital administrative body of Bichena Primary Hospital to obtain permission and cooperation for data collection. Before starting data collection, written and signed consent was obtained from the head of the hospital. The confidentiality of patient information was ensured, and data were used solely for the study.
Author contributions
AE: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DB: Supervision, Visualization, Writing – original draft, Writing – review & editing. AN: Supervision, Visualization, Writing – original draft, Writing – review & editing. AY: Supervision, Visualization, Writing – original draft, Writing – review & editing. TS: Supervision, Visualization, Writing – original draft, Writing – review & editing. BE: Supervision, Visualization, Writing – original draft, Writing – review & editing. KB: Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was financially supported for data collection by Bichena Primary Hospital; however, it had no role in any part of the study. The content is solely the responsibility of the authors.
Acknowledgments
We would like to thank Bichena primary hospital staff members, data collectors, and supervisors for their cooperation in the data collection process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chan GJ, Goddard FG, Hunegnaw BM, Mohammed Y, Hunegnaw M, Haneuse S, et al. Estimates of stillbirths, neonatal mortality, and medically vulnerable live births in Amhara, Ethiopia. JAMA Network Open. (2022) 5(6):e2218534. doi: 10.1001/jamanetworkopen.2022.18534
2. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. (2014) 384(9938):189–205. doi: 10.1016/S0140-6736(14)60496-7
3. World Health Organization. Newborns: Improving Survival and Well-being. Geneva: World Health Organization (2020).
4. IGME U. Levels & Trends in Child Mortality: Estimates: Report 2018. New York: Who/Unicef/World Bank/Un (2018). p. 1–48.
5. Li Z, Karlsson O, Kim R, Subramanian S. Distribution of under-5 deaths in the neonatal, postneonatal, and childhood periods: a multicountry analysis in 64 low-and middle-income countries. Int J Equity Health. (2021) 20:1–11. doi: 10.1186/s12939-020-01327-9
6. Estimation UI-aGfCM. Levels & Trends in Child Mortality: Report 2021. New York: United Nations Children’s Fund (UNICEF) (2021).
7. Grady SC, Frake AN, Zhang Q, Bene M, Jordan DR, Vertalka J, et al. Neonatal mortality in east Africa and West Africa: a geographic analysis of district-level demographic and health survey data. Geospat Health. (2017) 12(1):5–7. doi: 10.4081/gh.2017.501
8. Tesema GA, Teshale AB, Tessema ZT. Incidence and predictors of under-five mortality in east Africa using multilevel weibull regression modeling. Arch Public Health. (2021) 79(1):1–13. doi: 10.1186/s13690-021-00727-9
9. Masaba BB, Mmusi-Phetoe RM. Neonatal survival in Sub-Sahara: a review of Kenya and South Africa. J Multidiscip Healthc. (2020) 13:709–16. doi: 10.2147/JMDH.S260058
10. Andegiorgish AK, Andemariam M, Temesghen S, Ogbai L, Ogbe Z, Zeng L. Neonatal mortality and associated factors in the specialized neonatal care unit Asmara, Eritrea. BMC public Health. (2020) 20(1):1–9. doi: 10.1186/s12889-019-8118-x
11. Shiferaw K, Mengistie B, Gobena T, Dheresa M, Seme A. Neonatal mortality rate and its determinants: a community–based panel study in Ethiopia. Front Pediatr. (2022) 10:3–6. doi: 10.3389/fped.2022.875652
12. Ethiopian Public Health Institute(EPHI) [Ethiopia], ICF. Ethiopia Mini Demographic and Health Survey 2019: Final Report. Rockville, Maryland, USA: EPHI and ICF (2021).
13. Gudayu TW. Epidemiology of neonatal mortality: a spatial and multilevel analysis of the 2019 mini-Ethiopian demographic and health survey data. BMC Pediatr. (2023) 23(1):1–14. doi: 10.1186/s12887-023-03838-0
14. Ethiopian Public Health Institute (EPHI), ICF. Ethiopia mini Demographic and Health Survey 2019: Key Indicators. Rockville, Maryland, USA: EPHI and ICF (2019).
15. Aynalem YA, Shiferaw WS, Akalu TY, Dargie A, Assefa HK, Habtewold TD. The magnitude of neonatal mortality and its predictors in Ethiopia: a systematic review and meta-analysis. Int J Pediatr. (2021) 2021:1–10. doi: 10.1155/2021/7478108
16. WHO U. Every Newborn: An Action Plan to end Preventable Deaths. Geneva: World Health Organization (2014). p. 2017–18.
17. Federal Ministry of Health. National Strategy for Newborn and Child Survival in Ethiopia, 2015/16–2019/20. Addis Ababa, Ethiopia: FMoH (2015).
19. Federal Ministry of Health of Ethiopia. Neonatal Intensive Care Unit (Nicu) Training Participants’ Manual. Addis Ababa: Federal Ministry of Health (2021).
20. Federal Ministry of Health. Ethiopian Hospital Alliance for Quality 4th Cycle Evidence Based Care (EBC) Project Document and Change Package Clinical Service Directorate. Addis Ababa: Federal Ministry of Health (2021). p. 1–5.
21. Woday Tadesse A, Mekuria Negussie Y, Aychiluhm SB. Neonatal mortality and its associated factors among neonates admitted at public hospitals, pastoral region, Ethiopia: a health facility based study. PLoS One. (2021) 16(3):e0242481. doi: 10.1371/journal.pone.0242481
22. Aslamzai M, Danish Y, Hakimi T, Jawadi B. Evaluation of the factors associated with anemia in neonates admitted to the neonatal unit of Maiwand teaching hospital: a cross-sectional study. Global Pediatrics. (2024) 8:100164. doi: 10.1016/j.gpeds.2024.100164
23. Desalew A, Sintayehu Y, Teferi N, Amare F, Geda B, Worku T, et al. Cause and predictors of neonatal mortality among neonates admitted to neonatal intensive care units of public hospitals in eastern Ethiopia: a facility-based prospective follow-up study. BMC Pediatr. (2020) 20(1):1–11. doi: 10.1186/s12887-020-02051-7
24. Asmare Y, Mekonen H. Survival status and Predictor of Mortality among Premature Neonate Admitted to Neonatal Intensive Care Unit from 2013 to 2017 in Tikur Anbesa Specialized Hospital, Addis Ababa, Ethiopia, 2018. Nursing Addis Ababa: Addis Ababa University (2018). p. 70.
25. Rosa-Mangeret F, Benski A-C, Golaz A, Zala PZ, Kyokan M, Wagner N, et al. 2.5 million annual deaths—are neonates in low-and middle-income countries too small to be seen? A bottom-up overview on neonatal morbi-mortality. Trop Med Infect Dis. (2022) 7(5):64. doi: 10.3390/tropicalmed7050064
26. Woelile TA, Kibret GT, Workie HM, Amare AT, Tigabu A, Aynalem YA, et al. Survival status and predictors of mortality among low-birth-weight neonates admitted to the neonatal intensive care unit at felege hiwot comprehensive specialized hospital, Bahir Dar, Ethiopia, 2020. Pediatric Health Med Ther. (2021) 12:451–66. doi: 10.2147/PHMT.S323526
27. Ministry of Health, Ethiopia. Hospital/Clinic Neonatal Intensive Care Unit (NICU) Register. Addis Ababa: Ministry of Health (2022).
28. Dessu S, Kote M, Gebremeskel F, Girum T. Predictors of neonatal mortality among neonates who admitted in neonatal intensive care unit at Arba Minch general hospital. Ethiop J Health Dev. (2019) 33(1):46–52.
29. Menalu MM, Gebremichael B, Desta KW, Kebede WM, Tarekegn FN, Mulu GB, et al. Time to death and its predictors among neonates who were admitted to the neonatal intensive care unit at tertiary hospital, Addis Ababa, Ethiopia: retrospective follow up study. Front Pediatr. (2022) 10:913583. doi: 10.3389/fped.2022.913583
30. Demisse AG, Alemu F, Gizaw MA, Tigabu Z. Patterns of admission and factors associated with neonatal mortality among neonates admitted to the neonatal intensive care unit of university of Gondar hospital, northwest Ethiopia. Pediatric Health Med Ther. (2017) 8:57. doi: 10.2147/PHMT.S130309
31. Berhanu B, Oljira L, Demana M, Negash B, Mamo Ayana G, Beshir Raru T, et al. Survival and predictors of mortality among neonates admitted to neonatal intensive care unit at bombe primary hospital, southern Ethiopia: institution-based retrospective cohort study. Pediatric Health Med Ther. (2021) 12:239–49. doi: 10.2147/PHMT.S303158
32. Chekole B, Terefe TF, Tenaw SG, Zewudie BT, GebreEyesus FA, Kassaw A, et al. Survival status, length of stay, and predictors of mortality among neonates admitted in the neonatal intensive care unit of Gurage zone public hospitals. SAGE Open Nursing. (2023) 9:23779608231187480. doi: 10.1177/23779608231187480
33. World Health Organization. Neonatal Mortality Rate (per 1,000 Live Births). Geneva: World Health Organization (2024). Available online at: https://data.who.int/indicators/i/E3CAF2B/A4C49D3?form=MG0AV3
34. Alebel A, Wagnew F, Petrucka P, Tesema C, Moges NA, Ketema DB, et al. Neonatal mortality in the neonatal intensive care unit of Debre Markos referral hospital, northwest Ethiopia: a prospective cohort study. BMC Pediatr. (2020) 20(1):1–11. doi: 10.1186/s12887-020-1963-z
35. Moges S, Dejene Ermias Mekango A, Astatkie A. Mortality and its predictors among neonates admitted to a neonatal intensive care unit in Hadiya zone, southern Ethiopia: a retrospective cohort study. J Pediatr Neonatol. (2021) 2(1):1012.
36. Seid SS, Ibro SA, Ahmed AA, Olani Akuma A, Reta EY, Haso TK, et al. Causes and factors associated with neonatal mortality in neonatal intensive care unit (NICU) of Jimma university medical center, Jimma, south west Ethiopia. Pediatric Health Med Ther. (2019) 10:39–48. doi: 10.2147/PHMT.S197280
37. Sk D, Munye T, Munye B. Prevalence of Neonatal Mortality and Its Associated Factor among Neonates who Admitted in Neonatal Intensive Care Unit At Debre Tabor General Hospital, South Gondar, Amhara, Ethiopia. Brussels: Longdom Publishing (2021).
38. Yeshaneh A, Tadele B, Dessalew B, Alemayehu M, Wolde A, Adane A, et al. Incidence and predictors of mortality among neonates referred to comprehensive and specialized hospitals in amhara regional state, north Ethiopia: a prospective follow-up study. Ital J Pediatr. (2021) 47(1):1–11. doi: 10.1186/s13052-021-01139-9
39. Zelalem Ayichew M, Derseh Gezie L, Gelagay AA, Anmut Bitew D. Neonatal mortality and associated factors among neonates admitted to neonatal intensive care unit of Gandhi memorial hospital in Addis Ababa, Ethiopia, 2019. BMC Pediatr. (2022) 22(1):1–9. doi: 10.1186/s12887-022-03339-6
40. Orsido TT, Asseffa NA, Berheto TM. Predictors of neonatal mortality in neonatal intensive care unit at referral hospital in southern Ethiopia: a retrospective cohort study. BMC Pregnancy Childbirth. (2019) 19(1):1–9. doi: 10.1186/s12884-019-2227-5
41. Pratiwi SR, Prasetya H, Murti B. The effect of asphyxia on neonatal death: a meta-analysis. J Matern Child Health. (2020) 5(4):413–21. doi: 10.26911/thejmch.2020.05.04.08
42. Tolossa T, Wakuma B, Mengist B, Fetensa G, Mulisa D, Ayala D, et al. Survival status and predictors of neonatal mortality among neonates admitted to neonatal intensive care unit (NICU) of Wollega university referral hospital (WURH) and nekemte specialized hospital, western Ethiopia: a prospective cohort study. PLoS One. (2022) 17(7):e0268744. doi: 10.1371/journal.pone.0268744
43. Phoya F, Langton J, Dube Q, Iroh Tam P-Y. Association of neonatal hypothermia with morbidity and mortality in a tertiary hospital in Malawi. J Trop Pediatr. (2020) 66(5):470–8. doi: 10.1093/tropej/fmz086
44. Tesfay N, Tariku R, Zenebe A, Dejene Z, Woldeyohannes F. Cause and risk factors of early neonatal death in Ethiopia. PLoS One. (2022) 17(9):e0275475. doi: 10.1371/journal.pone.0275475
45. Davidz IKL, Joewono HT, Nugroho HSW. The multifactorial causes of neonatal mortality: a literature review. Indian J Forensic Med Toxicol. (2022) 16(1):647.
46. Valadbeigi T, Dara N, Tabatabaee H, Tajalli S, Etemad K, Hosseini A, et al. Maternal risk factors of neonatal mortality in Iran: a case-control study. Int J Pediatr. (2020) 8(2):10865–74. doi: 10.22038/ijp.2019.44502.3683
47. Eshete A, Tadesse H, Tesfaye T, Abiy S. Magnitude and risk of neonatal death in neonatal intensive care unit at referral hospital in Godeo zone: a prospective cohort study. Pan Afr Med J. (2021) 38(1):201. doi: 10.11604/pamj.2021.38.201.20650
48. Girma D, Dejene H, Adugna L. Predictors of neonatal mortality in Ethiopia: a comprehensive review of follow-up studies. Int J Pediatr. (2022) 2022:3. doi: 10.1155/2022/1491912
Keywords: survival status, predictors, neonate, primary hospitals, Ethiopia
Citation: Enawgaw AS, Belay D, Nigate A, Yeshiwas AG, Shumet T, Endalew B and Bishaw KA (2025) Survival status and predictors of mortality among neonates admitted to neonatal intensive care unit at Bichena Primary Hospital, Northwest Ethiopia. A retrospective cohort study. Front. Pediatr. 13:1529089. doi: 10.3389/fped.2025.1529089
Received: 20 November 2024; Accepted: 19 March 2025;
Published: 9 April 2025.
Edited by:
Zubaida Farouk, Bayero University Kano, NigeriaReviewed by:
Scott Guthrie, Vanderbilt University Medical Center, United StatesMaria Livia Ognean, Lucian Blaga University of Sibiu, Romania
Iretiola Fajolu, University of Lagos, Nigeria
Copyright: © 2025 Enawgaw, Belay, Nigate, Yeshiwas, Shumet, Endalew and Bishaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anley Shiferaw Enawgaw, YW5sZXlzaGlmZXJhdzlAZ21haWwuY29t; YW5sZXlfc2hpZmVyYXdAZG11LmVkdS5ldA==
 Anley Shiferaw Enawgaw
Anley Shiferaw Enawgaw Debas Belay2
Debas Belay2 Almaw Genet Yeshiwas
Almaw Genet Yeshiwas Bekalu Endalew
Bekalu Endalew Keralem Anteneh Bishaw
Keralem Anteneh Bishaw