- 1Department of Pharmacology and Toxicology, Radboud Institute of Health Sciences, Radboud University Medical Center, Nijmegen, Netherlands
- 2Department of Genetics, Section Metabolic Diagnostics, University Medical Center Utrecht, Utrecht, Netherlands
- 3Department of Pediatric Cardiology, Erasmus MC – Sophia, Rotterdam, Netherlands
- 4Institute of Clinical Pharmacy and Pharmacotherapy, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany
- 5Department of Clinical Pharmacology, University Children’s Hospital, Belgrade, Serbia
- 6Department of Pharmacology, Clinical Pharmacology and Toxicology, School of Medicine, University of Belgrade, Belgrade, Serbia
- 7Department of Paediatrics and Adolescent Medicine, Medical University of Vienna, Vienna, Austria
- 8Intensive Care and Department of Pediatric Surgery, Erasmus MC Sophia Children’s Hospital, Rotterdam, Netherlands
Introduction: Enalapril is an angiotensin-converting enzyme (ACE) inhibitor (ACEi) which is widely used in the management of (paediatric) hypertension and heart failure (HF). There is a significant interindividual variability in the patient's response to enalapril that is not completely understood. Therefore, we aimed to examine the potential of metabolic profiling for stratifying paediatric patients with HF due to congenital heart disease (CHD) in terms of treatment response to enalapril. Additionally, we investigated metabolic profiles in CHD patients and healthy controls.
Methods: CHD patients aged 0–6 years of age who previously participated in a multi-centre and multinational pharmacokinetic safety bridging study of enalapril were included. Patients were defined as responder when aldosterone levels decreased after a single administration of enalapril. Non-responders were those with an increase in their aldosterone levels. We applied an untargeted mass spectrometry-based metabolomics approach on serum. By using both supervised and unsupervised learning algorithms, we compared metabolic profiles between responders and non-responders as well as between patients and age and sex matched healthy controls.
Results: In total, 63 patients were included with a median age of 132 (IQR 54–211) days and 46 controls [97 (63–160) days]. 41 of 63 patients responded to enalapril therapy. Their baseline characteristics were similar to non-responders (n = 22). A total of 1,820 unique features were identified. Responders were distinguished from non-responders using a supervised learning algorithm based on 94 features (p = 0.05). Furthermore, metabolic profiles could distinguish between patients and controls based on an unsupervised learning algorithm which revealed 278 relevant features (p = 0.001).
Conclusions: These are the first data to demonstrate a clear metabolic signature in children with CHD using ACEi. We identified metabolites whose concentrations were both associated with ACEi response and HF. This indicates more severe HF in patients with more profound treatment response. Our results will therefore allow further studies aiming at disentangling variability in ACEi treatment response.
Introduction
Enalapril is an angiotensin-converting enzyme (ACE) inhibitor (ACEi) which is widely used in the management of (paediatric) hypertension and heart failure (HF). It exhibits its effect after hepatic metabolism to its active metabolite: enalaprilat. Enalaprilat inhibits ACE, leading to a decrease in formation of angiotensin II (ATII) and a diminished aldosterone secretion. This results in downregulation of the Renin Angiotensin Aldosterone System (RAAS), thereby decreasing blood pressure (BP) and reducing the cardiac afterload (1), ultimately improving left ventricle function (2). In adults, there is significant interindividual variability in the patient's response to ACEi based on both physiological (e.g., BP) and biochemical (e.g., RAAS markers) parameters, and 50% of hypertensive patients still fail to achieve target BPs (3). This variability in treatment response is not completely understood, even when taking factors potentially influencing drug disposition like disease (4–7), food intake (8) and race (9) into account. As the moderate effectiveness of ACEi therapy and unexplained variability in therapy remained over the past decade, innovative approaches were pursued in order to better understand treatment response and ultimately improve effectiveness.
Metabolomics is increasingly used to understand variation to treatment response (10). This is the analysis of small molecules within a biologic specimen of which the concentration is a reflection of all the biochemical processes in one individual. Because it accounts for many sources of variation, including those in the genome, transcriptome and proteome, it aids in understanding disease and response to therapy (3, 11). In the adult population, genotype dependent differences in the metabolomic response to ACEi intake were investigated and several dipeptides were considered as potential functional markers of ACE activity in adult patients, regardless of indication (11). Also, metabolites could predict a poor response to lisinopril—which is also an ACEi—in the treatment of hypertension (10) or were related to ACE-activity (11). Thus, discovered metabolites offer leads in predicting treatment response and individualize treatment in adults.
Paediatric HF, however, differs from adult HF with regards to aetiology, clinical manifestation, comorbidities and prevalence (12). Also, there is an increased activity of the RAAS during infancy and childhood (up to four years of age) (13). Therefore, adult findings cannot simply be extrapolated to the paediatric population. To the best of our knowledge, no metabolomic studies have been conducted in paediatric patients treated with ACEi.
In a multicentre, multinational Phase II/III prospective, open-label pharmacokinetic bridging study (LENA), the pharmacokinetics (PK), pharmacodynamics (PD) and safety of enalapril were investigated in children with HF due to congenital heart disease (CHD) (14). Results showed interindividual variability in response to treatment (15). As adult metabolomic studies have helped in disentangling treatment response to ACEi, we aimed to examine the potential of metabolic profiling for stratifying paediatric patients with CHD in terms of treatment response, ultimately aiming at individualizing treatment. We additionally investigated metabolic profiles in healthy paediatric controls and compared those to profiles patients with CHD-related HF to better understand this condition. A small number of paediatric studies investigated metabolic profiles in variety of diseases and comparing patient profiles to healthy controls has led to a better understanding of a wide variety of diseases. This could offer the opportunity to identify diagnostic or therapeutic biomarkers.
Methods
Patient characteristics
This exploratory metabolomic study was part of a multicentre, multinational Phase II/III prospective, open-label, pharmacokinetic bridging study in patients with CHD (LENA) (14). In short, study patients were treated with newly developed orodispersible minitablets (ODMT) of enalapril and systematically assessed for PK and pharmacodynamics (PD), as well as clinical parameters. For this metabolomic study, only CHD patients aged 0–6 years of age whose parents specifically consented for this sub study could be included.
Among inclusion criteria were a diagnosis of HF due to CHD, need for afterload reduction by drug therapy and a bodyweight greater than 2.5 kg. Patients needed to be naive to ACEi or already on ACEi but willing to switch to enalapril ODMTs. Patients were excluded when using dual ACEi therapy, renin inhibitors, ATII antagonists and non-steroidal anti-inflammatory drugs. Patients with a BP below the fifth percentile for age were also excluded. The full list of in- and exclusion criteria was listed in the study protocol (14). A minimum of 60 patients needed to be included with at least 37 patients below the age of 12 months in order to obtain adequate paediatric PK data and to accurately describe the dose-exposure in this population. For this metabolomic study, control samples were derived from sex and age matched healthy children, of which blood samples were obtained before any small medical procedure or surgery.
Ethical approval
For the initial LENA study (including metabolomics substudy), the medical ethics review board of the responsible centers approved the protocol. This included the Univerzitetska Dečja Klinika in Belgrade, Serbia, the Medizinische Universität in Wien, Austria, the Göttsegen Gyorgy Orszagos Kardiologiai Intezet HPHC in Budapest, Hungary, the Erasmus Medical Center in Rotterdam, the Netherlands, the Wilhelmina Kinderziekenhuis of the University Medical Centre in Utrecht, The Netherlands as well as the Great Ormond Street Hospital for Children, NHS Trust GOSH in London, United Kingdom. For the collection of healthy control samples, the Erasmus Medical Center medical ethics review board approved the protocol. Written informed consent was obtained from all subjects' families before enrolment.
Study procedures
Enalapril was administered orally as ODMTs and intake at the initial dose visit took place at the clinic. Patients naive to ACEi therapy were uptitrated according to a defined dose titration scheme (Supplementary Table S1) and patients who were already on enalapril remained on the same dose when switched to the enalapril ODMTs. Clinical parameters assessed at the initial dose visit included the Ross score for accurate grading of the presence and severity of paediatric HF (range: 0–12) (16), shortening fraction (SF) based on echocardiography and non-invasive mean arterial pressure (BP). Blood was withdrawn for RAAS activity [including renin, angiotensin I (ATI), aldosterone & plasma renin activity (PRA)] before administration and 4 h after administration of enalapril. Also, a full PK curve was collected with sampling points at 1, 2, 4, 6 & 12 h after administration. For metabolomic analysis, blood was withdrawn before enalapril administration.
Laboratory analysis
PD and PK markers, including renin, PRA, ATI, enalapril and enalaprilat concentrations were measured as previously described (17). In short, enalapril and enalaprilat concentrations were determined using high-performance liquid chromatography. The enalapril and enalaprilat pharmacokinetics were characterized by the maximum serum concentration (Cmax) as well as area under the curve (AUC) from zero to infinity. This AUC was calculated by the trapezoidal rule with infinity extrapolation (17). Renin was determined using a chemiluminescent immunoassay based on monoclonal antibodies. PRA and ATI were determined in a 125I radioimmunoassay using ATI antibodies and circulating immunoreactive ATI. Aldosterone was measured by a previously validated immunoassay. This assay was validated in the aldosterone calibration range between 43 and 958 pg/ml. The mean difference between the original and repeat of the incurred LENA sample reanalysis was –4% (18).
Metabolic sample preparation and profiling
Metabolites were analysed in serum by, a semi-quantitative direct-infusion high-resolution mass spectrometry-based metabolomics method in combination with a nano-electrospray ionization source, as described previously (19). Briefly, metabolites were extracted from 7.5 µl of serum by adding 140 µl methanol with stable isotope labeled internal standards (acylcarnitines and amino acids). This solution was centrifuged for five minutes at 17,000 g and the supernatant diluted with 45 µl 0.3% formic acid. This was filtered using a methanol preconditioned 96 well filter plate (Acro prep, 0.2 m GHP, NTRL, 1 ml well; Pall Corporation, Ann Arbor, MI, USA) and a vacuum manifold. The sample filtrate was collected in a 96 well plate (Advion, Ithaca, NY, USA). Samples (13 µl) were injected in triplicate into the Q-Exactive high-resolution mass spectrometer using a TriVersa NanoMate system (Advion, Ithaca, NY, USA) controlled by Chipsoft software (version 8.3.3, Advion). The Q-Exactive high-resolution mass spectrometer was operated in positive and negative ion mode in a single run, with automatic polarity switching. There were two time segments of 1.5 min with a total run time of 3.0 min. Scan range was 70–600 mass to charge ratio (m/z), resolution was 140,000 at m/z = 200. Data acquisition was performed using Xcalibur software (Thermo Scientific™, Waltham, MA, USA). Using MSConvert15 (ProteoWizard Software Foundation, Palo Alto, CA, USA), raw data files containing scanning time, m/z and peak intensity were converted to mzXML format. Data processing and peak calling was done using an in-house developed pipeline (https://github.com/UMCUGenetics/DIMS). Detected mass peaks were annotated by matching the m/z of the mass peak with a range of five parts per million to metabolite masses present in the Human Metabolome Database (HMDB) (19). Metabolite annotations without adduct ions in negative or positive mode ([M - H]–,[M + H]+), or with the single adduct ions [M + Na]+, [M + K]+, and [M + Cl]− were selected For each sample, the intensities of these five mass peaks were summed, resulting in one (summed) mass peak intensity per metabolite annotation: ∼6,600 summed mass peaks in total. Next, exogenous and drug metabolite annotations were excluded resulting in ∼3,900 summed mass peaks in total, corresponding to ∼1,900 metabolite (isobaric compounds) annotations, since mass peaks can account for several isomers. For each mass peak per patient sample, the deviation from the intensities in control samples was indicated by a Z-score, calculated by: Z-score=(intensity patient sample—mean intensity control samples)/standard deviation intensity control samples.
Data analysis
Patients were categorized as responder or non-responder based on the difference between their pre and post-dose aldosterone levels. Understanding the difference in aldosterone response is of value as this is the final and direct effector of the RAAS, and, independent of BP, its levels are associated with clinical outcomes in children (20). As inhibition of the RAAS by enalapril would normally lead to decreased aldosterone levels, patients were defined as responders when their aldosterone levels decreased after administration of enalapril. The difference in BP after enalapril administration was not used to categorise patients as accurate BP measurements in infants is prone to error and therefore less reliable. Differences between groups were assessed using a Mann–Whitney U-test. For continuous variables, data were expressed as median values with interquartile ranges (IQR) when not normally distributed. For comparison between PK and PD markers prior to and after administration, a Wilcoxon signed rank test was used. To display categoric variables, numbers and percentages were used. Statistical analysis took place using SPSS version 25.0.
MetaboAnalyst was used to analyse metabolic features. The majority of metabolites are isobaric compounds and do not have unique nominal masses. With direct infusion mass spectrometry, these isobaric compounds cannot be separated and a feature can therefore represent one or multiple metabolites. Both principal component analysis (PCA) (unsupervised learning algorithm) and partial least-squares discriminant analysis (PLS-DA) (supervised learning algorithm) were employed to visualize and interpret differences, aiming to distinguish responders from non-responders and patients from healthy controls. For feature selection, e.g., selection of features that differed significantly between two groups, a two sample T-test on raw data was applied. Data were not filtered as the number of features did not exceed 5,000 and all features were included in the analysis. We did not correct for multiple testing To achieve clearer results, data ware scaled using auto scaling (mean-centred and divided by the standard deviation of each variable). Assuming equal group variance as many biological measurements have a typical pattern of dispersion with a normal distribution, two-sample T-tests were used to determine metabolites of interest to differentiate between groups. For all identified important features, the relation to cardiovascular diseases for the identified metabolite, but also for its isobaric compounds were investigated (21). Last, to potentially link unknown metabolic features, a clustering analysis was conducted to reveal up- or downregulated pathways between groups.
Data sharing statement
Our raw data were published at MetaboLights, which is a database for Metabolomics experiments and derived information. (Link will follow after acceptation).
Results
Patient characteristics
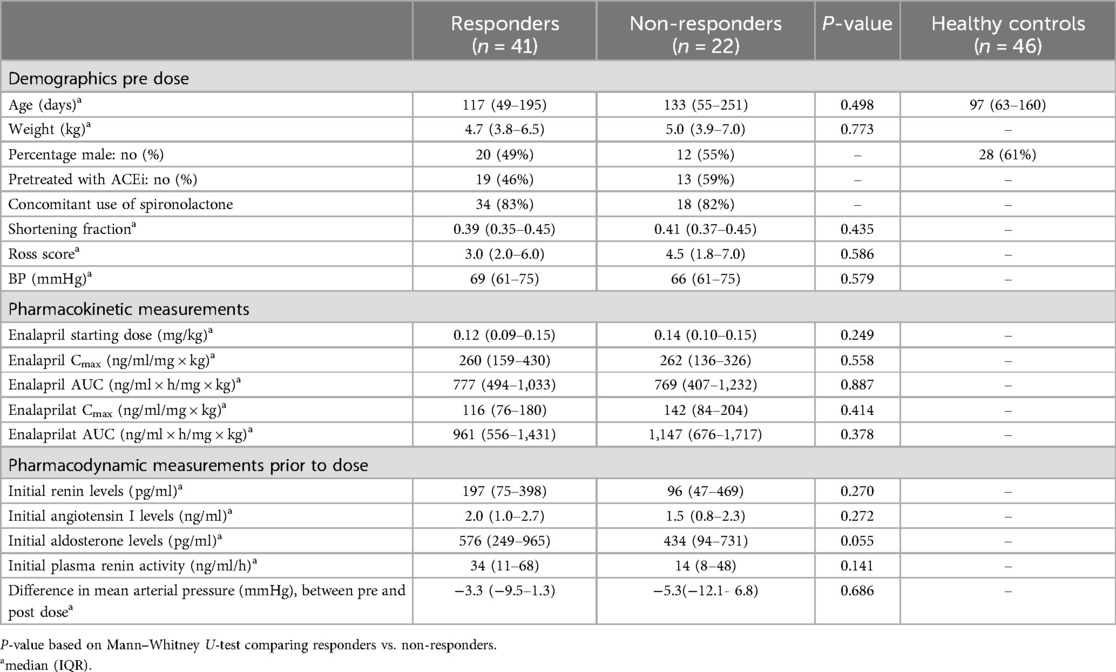
In total, 67 patients were included in the LENA study and all gave consent for participation in this metabolomic study. Of these 67 patients, almost half (n = 31) were diagnosed with an AVSD (46%), 21 with a VSD (31%), three with mitral valve insufficiency (5%), eleven patients had a variety of diagnosis including a complete AV-canal defect, a double outlet right ventricle, hypoplastic left heart syndrome, patent arterial duct and transposition of the great arteries. Of one patient, the diagnosis was not registered and could not be recalled. Serum samples were collected, and for 63 of these patients, aldosterone (before and after administration of enalapril) and metabolite levels (before administration only) were available. Based on differences between aldosterone levels prior to and after enalapril administration, responders (n = 41) and non-responders (n = 22) were defined. Median change in aldosterone levels was−144.3 (IQR −353.9 to −60.1 pg/ml) for responders, whereas non-responders had a median change of 61.2 (28.4–210.4) pg/ml. Both patient groups had similar baseline characteristics prior to enalapril therapy (Table 1). Ross scores of both groups indicate mild HF, while SFs are within normal paediatric ranges (between 29% and 46%, lower percentages indicating worse LV function). There was an equal decrease in BP after enalapril administration, four hours post dose, in responders (median −3.3, IQR −9.5 to 1.3 mmHg) and non-responders (median, −5.3, IQR −12.1 to 6.8 mmHg) (Wilcoxon signed rank, p = 0.005 and p = 0.042, respectively). Although statistically not significant, median initial aldosterone levels were higher in responders [576 (IQR 249–965) pg/ml] compared to non-responders [434 (IQR 94–731)] (p = 0.055).
Control samples were collected from 46 children with a median age of 97 (IQR 63–160) days, this was equal to the median age of our patients [132 (IQR 54–211) days] (Mann–Whitney U, p = 0.306).
Metabolomic analysis
Responders vs. non-responders
First, the metabolic profiles were analysed in patients only, and a total of 1,820 unique features were identified. Unsupervised PCA did not display two distinct clusters of individuals. Using PLS-DA, responders and non-responders differed significantly (Figure 1). A two-sample T-test, however, revealed 94 significantly different features (p-value threshold of 0.05) between responders and non-responders of which the 15 most important ones (sorted by highest t-value) are visualised in Figure 1C (full list of identified features with corresponding isobaric compounds listed in Supplementary Table S1, sorted by t-value).
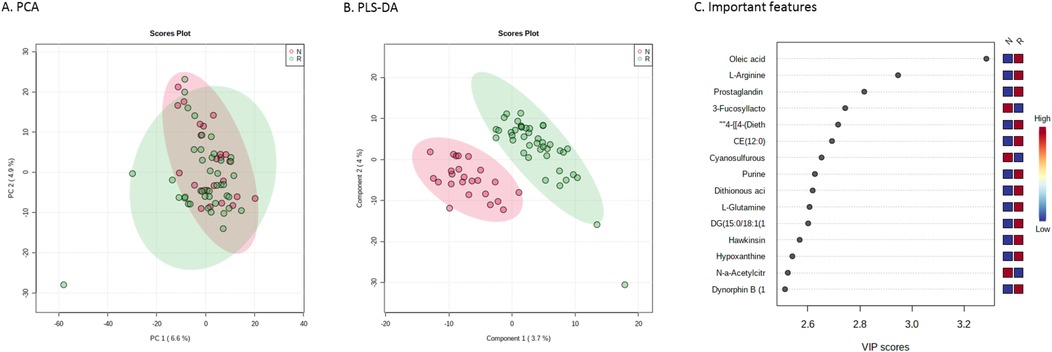
Figure 1. Metabolic profiles of responders and non-responders to enalapril therapy. (A) Principal component analysis score plot and (B) partial least-squares discriminant analysis serving as visualization for distinguishing responders (in green) and not-responders (in red) to enalapril therapy. (C) Important features plot, indicating the 15 most important metabolites that were relevant for distinguishing responders from non-responders. PC, principal component; VIP, variable importance in projection.
Healthy controls vs. CHD patients
Next, metabolic profiles in patients were compared to healthy controls. Here, PCA revealed two distinct clusters of individuals (Figure 2). Using again a two-sample T-test, this revealed 278 unique features (p-value threshold of 0.001), listing the most distinctive features in Figure 2 (full list of features with corresponding isobaric compounds in Supplementary Table S2).
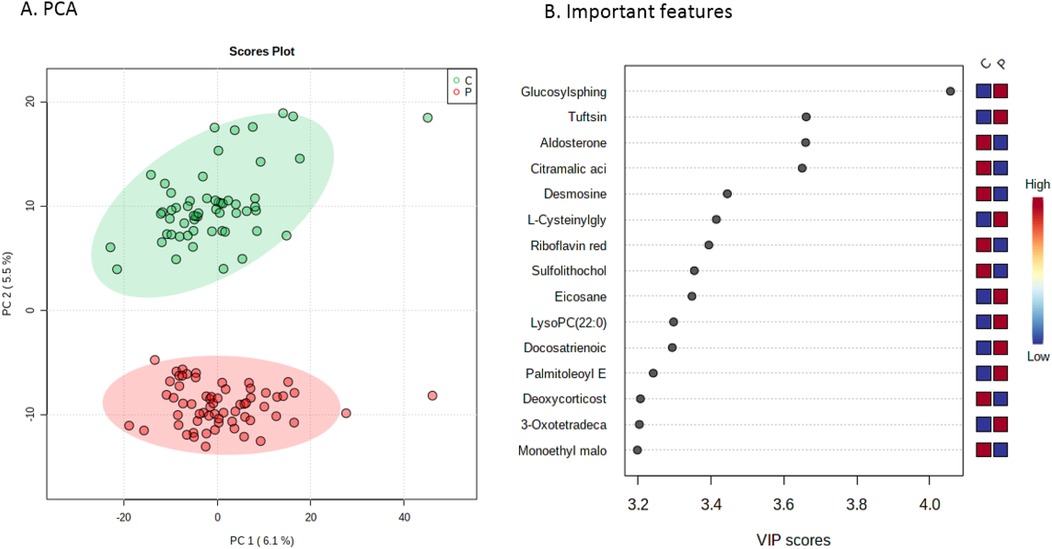
Figure 2. Metabolic profiles of CHD patients vs. controls. (A) Principal component analysis score plot serving as visualization for distinguishing patients (in red) from healthy controls (in green). (B) Important features plot, indicating the 15 most important metabolites that were relevant for distinguishing patients from controls. PC, principal component; VIP, variable importance in projection.
Last, clustering analysis did not reveal (groups of) pathways that were up- or downregulated and could explain differences between groups.
Discussion
This metabolomic study in paediatric patients with CHD-related HF was designed to identify metabolites that distinguish patients who respond to enalapril therapy based on their aldosterone levels from patients who do not respond. Our untargeted metabolomic approach identified a distinct metabolic signature for patients responding to enalapril therapy. As a secondary objective, the difference in metabolic profiles patients and healthy age-matched controls was assessed to better understand the pathophysiology of CHD-related HF. Our analysis demonstrated a clear difference between patients and healthy controls based on their metabolic profiles.
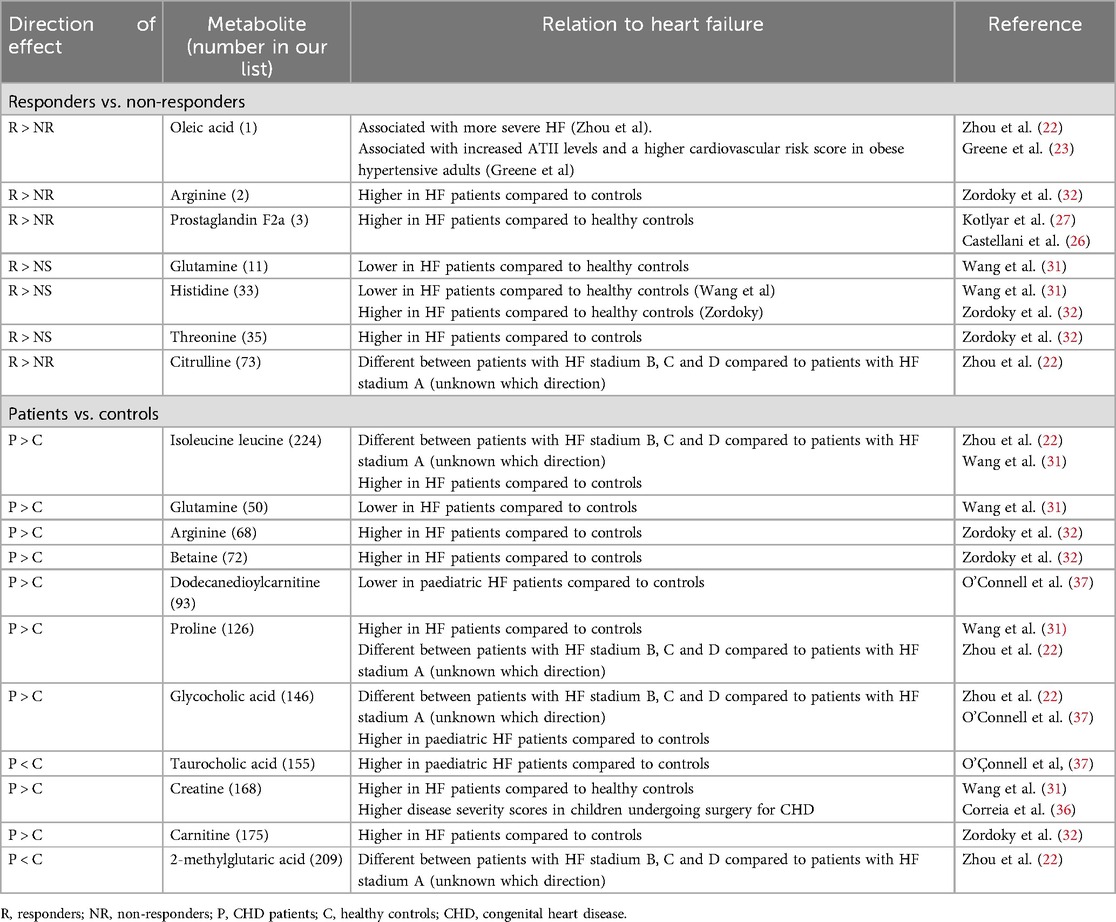
Responders vs. non-responders some of the metabolites associated with response to enalapril therapy have been previously associated with more severe cardiovascular diseases in other cohorts (Table 2). This includes oleic acid, arginine, prostaglandin F2a and threonine, which were all higher in responders vs. non-responders. Oleic acid has been associated with more severe HF (22) as well as with increased ATII levels in obese hypertensive adults (23). Also, an interaction between oleic acid and ATII led to lower cell viability and an increased number of vascular small muscle cells (24). As higher oleic and ATII levels are both present in patients with a higher cardiovascular risk score, Greene et al. hypothesized that oleic acid and ATII might interact to accelerate vascular disease in obese hypertensive patients. Furthermore, arginine and prostaglandin 2a have been associated with (more severe) HF. There are higher serum concentrations of arginine and reduced myocardial nitrogen oxide production (25), as well as increased prostaglandin F2a and threonine levels in patients with HF (26, 27). Also, although not statistically significant, PD markers of the RAAS were higher in our responders compared to non-responders (p = 0.055–0.272) before enalapril administration, suggesting increased RAAS activation in these patients. Because RAAS is activated in high-risk cardiovascular patients, together with the above mentioned associations for metabolites, this might indicate that patients responding to enalapril therapy may have been more severely ill. The definition of responders based on a single aberrant level of aldosterone following a dose of ACE inhibition could constitute a limitation of our study design. After a significant period of heart failure therapy, alternate pathways of aldosterone formation might occur, this is known as the aldosterone escape theory (REF). Yet, we feel that in our population in which the majority of patient were not on heart failure treatment for a long period of time, aldosterone is still the main identifier for treatment response.
Even though Ross scores and SFs were equal between responders and non-responders, these classifications have limitations. The use of the Ross score remains still need to be validated as a surrogate clinical endpoint in large number of patients, impairing its current value for scoring HF severity in children (28). Also, SF determination relies on normal left ventricle shape, which may be altered in the presence of congenital heart defects (29). Thus, less pronounced differences in disease severity may very well exist between groups, even though Ross scores and SFs indicated mild heart failure only. However, not all metabolites support this finding. Glutamine, which was higher in responders, promoted cardiovascular health by exerting antioxidant and anti-inflammatory effects as well as by optimizing nitric oxide synthesis in adults (30). In accordance, in adult HF patients, glutamine levels were lower (31). For histidine and citrulline, available data are conflicting, suggesting both a negative and positive relationship with HF (22, 31, 32). None of the other features distinguishing responders from -non-responders was associated with cardiovascular disease or RAAS.
Patients vs. controls
Interestingly, although unsupervised learning clearly separated the CHD patients and controls, none of the top 15 identified metabolites had a known association with cardiovascular disease except for aldosterone, which was lower in patients. Because 50% of all patients were pretreated with ACEi before the start of enalapril therapy, this can explain the lower aldosterone levels in patients vs. controls. All other important features (top 15) were not previously linked to disease, except for glucosylsphingosine, which was significantly lower in patients. This is a well-known marker for Gaucher disease, a rare genetic disorder in which sphingolipids accumulate in cells, leading to immune dysregulation and skeletal disease (33). Yet, why glucosylsphingosine levels are higher in our paediatric patients, needs investigation.
Although the top 15 features do not have a known association with cardiovascular disease, other features further down the list were previously associated with (more severe) HF. Multiple metabolomic studies in adult HF patients (22, 31, 32) revealed a great number of dysregulated metabolites that could be potential biomarkers. Many of the reported metabolites that were associated with (more severe) HF, were also higher in our patients than controls (isoleucine, arginine, betaine, proline, creatine & carnitine (Table 2). For instance, creatine will be elevated in case of muscle damage and was higher in patients vs. controls. Whether this can be attributed to cardiac muscle damage is unknown. Again, for glutamine, which was higher in our patients, levels were lower in HF patients compared to healthy controls (31). Although glutamine levels are significantly lower in children compared to adults (34), this cannot explain the observed differences.
To the best of our knowledge, we are the first to report metabolomic profiles in paediatric patients on ACEi therapy (35).. Three metabolomic studies were conducted in children with HF. Correia et al. investigated metabolic profiles of 28 children undergoing surgery for CHD (36). These had a median Risk Adjustment for Congenital Heart Surgery (RACHS) score of 2 (IQR2-3), with 6 being the highest and 1 the lowest score possible. Patients were slightly older than in our cohort (median age 6.6 months, IQR 4.0–18.9 months), and more severely ill. Directly after surgery, patients had multiple organ failure [median Pediatric Logistic Organ Dysfunction II (PELODII) score of 11 (IQR 11–20.75)]. Out of 15 predefined metabolites sampled before surgery, eight metabolites (3-d-hydroxybutyrate, acetone, acetoacetate, citrate, lactate, creatine, creatinine, and alanine) were associated with postoperative PELODII and RACHS scores. Of these metabolites, only creatine (no. 168) and creatinine (no.305) differentiated our patients from controls.
Additionally, out of 521 predefined metabolites, 44 metabolites distinguished 26 patients with single ventricle disease aged 2–19 years of age from controls (37). These included acylcarnitines, amino acids and bile acids. Three of these metabolites were also significantly different between patients and healthy controls in our cohort: dodecanedioylcarnitine (no. 93) and two bile acids; taurocholic acid (no. 155) and glycocholic acid (no. 146). Interestingly, while glycocholic and taurocholic acid levels were higher and dodecanedioylcarnitine levels lower in patients compared to controls, we observed the opposite.
Last, in infants undergoing cardiopulmonary bypass surgery, pre and postoperatively, 165 serum metabolites were measured, aiming to discriminate between survivors and non-survivors, as well as to predict length of stay at the intensive care unit (38). These patients had a median Aristotle score of 10.0 (3.0–19.5), indicating medium procedure-adjusted complexity (score ranges between 1.5 and 25). Multiple pathways demonstrated changes between groups, yet, none of their top 15 metabolites matched with our results. Concluding, when looking at these three paediatric studies, there is only little overlap between identified important features. This could be due to differences in age, diagnosis or disease severity as these factors are well known influencers of the metabolome (39, 40).
Although metabolomic studies in children on ACEi are not available, the effect of ACEi on the metabolome was investigated in adults. In a population-based metabolomics study, 517 metabolites in 1,361 individuals were investigated and findings were replicated in another cohort based on 1,964 individuals (11). Patients on ACEi were selected regardless of ACEi indication. There were differences in the concentrations of five dipeptides and three ratios of di- and oligopeptides between ACEi users and non-users. Two of these dipeptides (aspartylphenylalanine & phenylalanylserine) even showed significant associations with BP response and thus qualified as read outs of ACE-activity. However, these metabolites were not considered important features in our cohort, neither for distinguishing responders from non-responders nor for discriminating between patients and controls. Also 2-oxoglutarate, which predicted lisinopril response in adult hypertensive patients (10), was not included in our lists of important features. Possibly, this could be explained by the demographic differences and different ACEi indications (hypertension vs. HF).
While our study provides new insights in the metabolic perturbations in children with CHD using enalapril, there are some limitations. First, the sample size is relatively small with 63 patients and 46 controls. Due to the complexity of metabolomic studies, there are currently no standard methods for sample size determination. Nevertheless, when compared to existing cohorts investigating the metabolome in the paediatric population, we believe, our study included sufficient patients. Besides, it has the advantage of a narrow age range of the included patients. Also, conducting a larger study including a similar population is challenging as the prevalence of CHD patients using ACEi is relatively low and high quality sampling and analysis for RAAS markers is difficult in a clinical setting (12). As blood for metabolomic analysis in controls was withdrawn after these children were sedated for a minor surgical procedure, this could be considered another limitation. Even though controls were healthy and age-matched, there could be a distorting effect of the sedation they received. Most frequently, anaesthesia was induced with sevoflurane or propofol/fentanyl, of which the effect on the systemic metabolome is still uncertain. In 500 adult surgical patients with high end-tidal sevoflurane concentrations, levels of L-glutamine, pyroglutamic acid, sphinganine and L-selenocysteine were elevated compared to patients with low concentrations (41). In our population, however, concentrations of these metabolites in controls were not elevated, making a sedation-induced effect on the metabolome unlikely. Last, as we used an untargeted metabolomic approach, we could not make the distinction between isobaric compounds. For instance, aldosterone could be replaced by metabolites having a similar m/z ratio that include cortisone or 19-Oic-deoxycorticosterone. Although we cannot identify the exact metabolite that has a distinctive value, we assessed the possible association of all isobaric compounds with cardiovascular disease. This did not lead to different findings and conclusions.
Concluding, the extensive, untargeted metabolomic analysis described here in children with CHD is novel and will allow further studies aiming at disentangling variability in treatment response. Although our initial aim was to distinguish responders from non-responders, we believe, our results indicate that, based on metabolite levels, children with more severe HF exhibit a more profound decrease in their aldosterone levels to enalapril therapy than children with less severe HF. Also, there were very clear differences in metabolic profile between patients and controls, which may help to better understand the pathobiology of paediatric CHD related HF and to develop predictive biomarkers. Ultimately, these metabolites could be valuable in terms of monitoring disease progression.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
NS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. IH: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. JJ: Methodology, Software, Supervision, Writing – original draft, Writing – review & editing. MD: Supervision, Writing – original draft, Writing – review & editing. SL: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. CM: Supervision, Writing – original draft, Writing – review & editing. SD: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research leading to these results has received funding from the EU's Seventh Framework Programme (FP7/2007-2013) under grant agreement n°602295 (LENA).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1530063/full#supplementary-material
Supplementary Table S1 | Important features for distinguishing responders from non-responders.
Supplemetary Table S2 | Important features for distinguishing patients from controls.
Abbreviations
ACE, angiotensin-converting enzyme; ACEi, angiotensin-converting enzyme inhibitor; ATI, angiotensin I; ATII, angiotensin II; AUC, area under the curve; BP, blood pressure; CHD, congenital heart disease; Cmax, maximum serum concentration; HF, heart failure; IQR, interquartile range; LV, left ventricle; BP, mean arterial pressure; ODMT, orodispersible minitablet; PCA, principal component analysis; PD, pharmacodynamics; PELOD, pediatric logistic organ dysfunction; PK, pharmacokinetics; PLS-DA, partial least-squares discriminant analysis; PRA, plasma renin activity; RAAS, renin angiotensin aldosterone system; VSMC, vascular small muscle cells.
References
1. Mori Y, Nakazawa M, Tomimatsu H, Momma K. Long-term effect of angiotensin-converting enzyme inhibitor in volume overloaded heart during growth: a controlled pilot study. J Am Coll Cardiol. (2000) 36(1):270–5. doi: 10.1016/S0735-1097(00)00673-2
2. Konstam MA, Kronenberg MW, Rousseau MF, Udelson JE, Melin J, Stewart D, et al. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (studies of left ventricular dysfunction) investigators. Circulation. (1993) 88(5 Pt 1):2277–83. doi: 10.1161/01.CIR.88.5.2277
3. Flaten HK, Monte AA. The pharmacogenomic and metabolomic predictors of ACE inhibitor and angiotensin II receptor blocker effectiveness and safety. Cardiovasc Drugs Ther. (2017) 31(4):471–82. doi: 10.1007/s10557-017-6733-2
4. Todd PA, Goa KL. Enalapril. A reappraisal of its pharmacology and therapeutic use in hypertension. Drugs. (1992) 43(3):346–81. doi: 10.2165/00003495-199243030-00005
5. Dickstein K, Till AE, Aarsland T, Tjelta K, Abrahamsen AM, Kristianson K, et al. The pharmacokinetics of enalapril in hospitalized patients with congestive heart failure. Br J Clin Pharmacol. (1987) 23(4):403–10. doi: 10.1111/j.1365-2125.1987.tb03069.x
6. Schwartz JB, Taylor A, Abernethy D, O'Meara M, Farmer J, Young J, et al. Pharmacokinetics and pharmacodynamics of enalapril in patients with congestive heart failure and patients with hypertension. J Cardiovasc Pharmacol. (1985) 7(4):767–76. doi: 10.1097/00005344-198507000-00023
7. Kelly JG, Doyle G, Donohue J, Laher M, Vandenburg MJ, Currie WJ, et al. Pharmacokinetics of enalapril in normal subjects and patients with renal impairment. Br J Clin Pharmacol. (1986) 21(1):63–9. doi: 10.1111/j.1365-2125.1986.tb02823.x
8. Moffett BS, DiSanto AR, Espinosa O, Hou J, Colabuono P. Bioequivalence of enalapril oral solution for treatment of pediatric hypertension and enalapril tablets. Clin Pharmacol Drug Dev. (2014) 3(6):493–8. doi: 10.1002/cpdd.129
9. Weir MR, Chrysant SG, McCarron DA, Canossa-Terris M, Cohen JD, Gunter PA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. (1998) 31(5):1088–96. doi: 10.1161/01.HYP.31.5.1088
10. Sonn BJ, Saben JL, McWilliams G, Shelton SK, Flaten HK, D'Alessandro A, et al. Predicting response to lisinopril in treating hypertension: a pilot study. Metabolomics. (2019) 15(10):133. doi: 10.1007/s11306-019-1601-7
11. Altmaier E, Menni C, Heier M, Meisinger C, Thorand B, Quell J, et al. The pharmacogenetic footprint of ACE inhibition: a population-based metabolomics study. PLoS One. (2016) 11(4):e0153163. doi: 10.1371/journal.pone.0153163
12. Smeets NJL, Schreuder MF, Dalinghaus M, Male C, Lagler FB, Walsh J, et al. Pharmacology of enalapril in children: a review. Drug Discov Today. (2020) 25(11).
13. Fiselier T, Monnens L, van Munster P, Jansen M, Peer P, Lijnen P. The renin-angiotensin-aldosterone system in infancy and childhood in basal conditions and after stimulation. Eur J Pediatr. (1984) 143(1):18–24. doi: 10.1007/BF00442742
14. Bajcetic M, de Wildt SN, Dalinghaus M, Breitkreutz J, Klingmann I, Lagler FB, et al. Orodispersible minitablets of enalapril for use in children with heart failure (LENA): rationale and protocol for a multicentre pharmacokinetic bridging study and follow-up safety study. Contemp Clin Trials Commun. (2019) 15:100393. doi: 10.1016/j.conctc.2019.100393
15. Laeer S, Cawello W, Burckhardt BB, Ablonczy L, Bajcetic M, Breur JMPJ, et al. Enalapril and enalaprilat pharmacokinetics in children with heart failure due to dilated cardiomyopathy and congestive heart failure after administration of an orodispersible enalapril minitablet (LENA-studies). Pharmaceutics. (2022) 14(6). doi: 10.3390/pharmaceutics14061163
16. Ross RD. The ross classification for heart failure in children after 25 years: a review and an age-stratified revision. Pediatr Cardiol. (2012) 33(8):1295–300. doi: 10.1007/s00246-012-0306-8
17. Burckhardt BB, Tins J, Ramusovic S, Läer S. Tailored assays for pharmacokinetic and pharmacodynamic investigations of aliskiren and enalapril in children: an application in serum, urine, and Saliva. J Pediatr Pharmacol Ther. (2015) 20(6):431–52.26766933
18. Makowski N, Ciplea AM, Ali M, Burdman I, Bartel A, Burckhardt BB, et al. A comprehensive quality control system suitable for academic research: application in a pediatric study. Bioanalysis. (2020) 12(5):319–33. doi: 10.4155/bio-2019-0242
19. Haijes HA, Willemsen M, Van der Ham M, Gerrits J, Pras-Raves ML, Prinsen HCMT, et al. Direct infusion based metabolomics identifies metabolic disease in Patients’ dried blood spots and plasma. Metabolites. (2019) 9(1). doi: 10.3390/metabo9010012
20. Liu Y, Lin Y, Zhang M-M, Li X-H, Liu Y-Y, Zhao J, et al. The relationship of plasma renin, angiotensin, and aldosterone levels to blood pressure variability and target organ damage in children with essential hypertension. BMC Cardiovasc Disord. (2020) 20(1):296. doi: 10.1186/s12872-020-01579-x
21. Opialla T, Kempa S, Pietzke M. Towards a more reliable identification of isomeric metabolites using pattern guided retention validation. Metabolites. (2020) 10(11). doi: 10.3390/metabo10110457
22. Zhou J, Chen X, Chen W, Zhong L, Cui M. Comprehensive plasma metabolomic and lipidomic analyses reveal potential biomarkers for heart failure. Mol Cell Biochem. (2021) 476(9):3449–60. doi: 10.1007/s11010-021-04159-5
23. Greene EL, Lu G, Zhang D, Egan BM. Signaling events mediating the additive effects of oleic acid and angiotensin II on vascular smooth muscle cell migration. Hypertension. (2001) 37(2):308–12. doi: 10.1161/01.HYP.37.2.308
24. Lu G, Meier KE, Jaffa AA, Rosenzweig SA, Egan BM. Oleic acid and angiotensin II induce a synergistic mitogenic response in vascular smooth muscle cells. Hypertension. (1998) 31(4):978–85. doi: 10.1161/01.HYP.31.4.978
25. Kaye DM, Parnell MM, Ahlers BA. Reduced myocardial and systemic L-arginine uptake in heart failure. Circ Res. (2002) 91(12):1198–203. doi: 10.1161/01.RES.0000047506.52381.90
26. Castellani S, Paladini B, Paniccia R, Di Serio C, Vallotti B, Ungar A, et al. Increased renal formation of thromboxane A2 and prostaglandin F2 alpha in heart failure. Am Heart J. (1997) 133(1):94–100. doi: 10.1016/S0002-8703(97)70253-9
27. Kotlyar E, Vita JA, Winter MR, Awtry EH, Siwik DA, Keaney JF Jr, et al. The relationship between aldosterone, oxidative stress, and inflammation in chronic, stable human heart failure. J Card Fail. (2006) 12(2):122–7. doi: 10.1016/j.cardfail.2005.08.005
28. Hsu DT, Pearson GD. Heart failure in children: part I: history, etiology, and pathophysiology. Circ Heart Fail. (2009) 2(1):63–70. doi: 10.1161/CIRCHEARTFAILURE.108.820217
29. Tissot C, Singh Y, Sekarski N. Echocardiographic evaluation of ventricular function-for the neonatologist and pediatric intensivist. Front Pediatr. (2018) 6:79. doi: 10.3389/fped.2018.00079
30. Durante W. The emerging role of l-glutamine in cardiovascular health and disease. Nutrients. (2019) 11(9). doi: 10.3390/nu11092092
31. Wang J, Li Z, Chen J, Zhao H, Luo L, Chen C, et al. Metabolomic identification of diagnostic plasma biomarkers in humans with chronic heart failure. Mol Biosyst. (2013) 9(11):2618–26. doi: 10.1039/c3mb70227h
32. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS One. (2015) 10(5):e0124844. doi: 10.1371/journal.pone.0124844
33. Murugesan V, Chuang W-L, Liu J, Lischuk A, Kacena K, Lin H, et al. Glucosylsphingosine is a key biomarker of Gaucher disease. Am J Hematol. (2016) 91(11):1082–9. doi: 10.1002/ajh.24491
34. Hammarqvist F, Angsten G, Meurling S, Andersson K, Wernerman J. Age-related changes of muscle and plasma amino acids in healthy children. Amino Acids. (2010) 39(2):359–66. doi: 10.1007/s00726-009-0446-1
35. Fridman MD, Mital S. Perspective on precision medicine in paediatric heart failure. Clin Sci (Lond). (2017) 131(6):439–48. doi: 10.1042/CS20160414
36. Correia GDS, Ng KW, Wijeyesekera A, Gala-Peralta S, Williams R, MacCarthy-Morrogh S, et al. Metabolic profiling of children undergoing surgery for congenital heart disease. Crit Care Med. (2015) 43(7):1467–76. doi: 10.1097/CCM.0000000000000982
37. O'Connell TM, Logsdon DL, Mitscher G, Payne RM. Metabolic profiles identify circulating biomarkers associated with heart failure in young single ventricle patients. Metabolomics. (2021) 17(10):95. doi: 10.1007/s11306-021-01846-8
38. Davidson JA, Pfeifer Z, Frank B, Tong S, Urban TT, Wischmeyer PA. Metabolomic fingerprinting of infants undergoing cardiopulmonary bypass: changes in metabolic pathways and association with mortality and cardiac intensive care unit length of stay. J Am Heart Assoc. (2018) 7(24):e010711. doi: 10.1161/JAHA.118.010711
39. Gu H, Pan Z, Xi B, Hainline BE, Shanaiah N, Asiago V, et al. 1H NMR metabolomics study of age profiling in children. NMR Biomed. (2009) 22(8):826–33. doi: 10.1002/nbm.1395
40. Barba I, Andrés M, Garcia-Dorado D. Metabolomics and heart diseases: from basic to clinical approach. Curr Med Chem. (2019) 26(1):46–59. doi: 10.2174/0929867324666171006151408
Keywords: heart failure, enalapril, pharmacokinetics, children, ACE inhibitor
Citation: Smeets NJL, van Hoek IN, Jans JJM, Dalinghaus M, Laer S, Bajcetic M, Male C and de Wildt SN (2025) Untargeted metabolic analysis in serum samples reveals metabolic signature in children with congenital heart failure on enalapril therapy. Front. Pediatr. 13:1530063. doi: 10.3389/fped.2025.1530063
Received: 18 November 2024; Accepted: 2 April 2025;
Published: 28 April 2025.
Edited by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeReviewed by:
Paul Kantor, University of Southern California, United StatesSomanshu Banerjee, University of California, Los Angeles, United States
Copyright: © 2025 Smeets, van Hoek, Jans, Dalinghaus, Laer, Bajcetic, Male and de Wildt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. N. de Wildt, Uy5kZXdpbGR0QGVyYXNtdXNtYy5ubA==
 N. J. L. Smeets
N. J. L. Smeets I. N. van Hoek2
I. N. van Hoek2 J. J. M. Jans
J. J. M. Jans M. Dalinghaus
M. Dalinghaus S. Laer
S. Laer M. Bajcetic
M. Bajcetic S. N. de Wildt
S. N. de Wildt