- 1Department of Pediatrics and Public Health, Università Degli Studi di Torino, Turin, Italy
- 2Immunorheumatology Unit, Ospedale Infantile Regina Margherita, Città Della Salute e Della Scienza, Turin, Italy
- 3Postgraduate School of Pediatrics, Università Degli Studi di Torino, Turin, Italy
- 4Division of Pediatric Cardiology, Ospedale Infantile Regina Margherita, Città Della Salute e Della Scienza, Turin, Italy
Acute myocarditis (AM) is an inflammation of the myocardium with a rapid onset of typically <1 month. The use of anakinra (ANK) for treating inflammatory AM in adults has been recently described; however, while some reports are promising, its efficacy remains debated. Here, we present a case of severe AM with concomitant systemic symptoms [fever, elevated C-reactive protein (CRP)] in a pediatric patient who was successfully treated with high-dose ANK. A literature review of similar published cases is also presented. A 14-year-old boy was admitted for AM with concomitant pericarditis. At disease onset, the patient presented with high fever and elevated CRP (163 mg/L) and troponin I (14,816 ng/L). Treatment with ibuprofen (30 mg/kg/day), intravenous immunoglobulin (80 g in 24 h), and colchicine (0.5 mg per day) were initiated without benefit and with further worsening of contractile function [Ejection Fraction (EF) 26%]. Consequently, inotropic support and intravenous methylprednisolone were started, leading to a partial improvement of EF (45%). Due to the inability to reduce inotropic support, a rescue treatment with ANK (7 mg/kg/day) in continuous intravenous infusion was started, resulting in progressive improvement and normalization of left ventricular systolic function. Our literature review identified five case reports of pediatric AM successfully treated with ANK. Most cases presented elevated inflammatory markers (ferritin and CRP) and/or concomitant pericarditis. We conclude that ANK, especially at high doses, may be useful for treating severe pediatric AM, particularly when associated with severe inflammation and/or pericarditis.
1 Introduction
Acute myocarditis (AM) is an inflammation of the myocardium with an onset of typically <1 month caused by abnormal immunoreactivity, drug or toxic substance exposure, or infection (1). Its clinical spectrum is variable, ranging from minor illness to high-risk cardiac conditions, including severe heart failure, refractory arrhythmias, cardiogenic shock, and sudden cardiac death (2, 3). AM treatment is mostly supportive, and evidence supporting the role of immunosuppression as a treatment strategy is limited (4).
The past ten years have seen a growing body of evidence, both in murine models (5) and in humans (6), showing an activation of the NOD-like receptor protein 3 (NLRP3) inflammasome during AM, ultimately leading to increased interleukin (IL)-1 levels in affected patients. These observations provided the rationale for using IL-1 inhibitors, especially anakinra (ANK), for treating AM. ANK is a recombinant human IL-1 receptor antagonist that neutralizes the biological activity of IL-1α and IL-1β by competitive binding to the IL-1 receptor type I (IL-1RI) (7, 8).
The use of ANK in treating inflammatory AM has primarily been described in adults; however, while some reports are promising, its efficacy remains debated (9). In the pediatric setting, ANK has primarily been used to treat Multisystem Inflammatory Syndrome in Children (MIS-C), a severe autoinflammatory condition following SARS-CoV-2 infection in children. MIS-C is characterized by persistent fever, mucocutaneous signs resembling Kawasaki disease, and heart failure (10). Published reports on ANK in pediatric patients with AM but without MIS-C are sparse.
Here, we report the case of a 14-year-old patient with severe AM and pericarditis who responded to IL-1 inhibition with ANK. We also discuss this case in light of a literature review of currently available evidence on the use of ANK in children with AM.
2 Case report
A 14-year-old boy (weight 57 kg) with sudden onset of thoracic and interscapular pain and fever was admitted to the local emergency room. The patient was previously healthy, played sports at a competitive level, and had no family history of heart disease.
Vital signs on admission were essentially normal, and physical examination found a hemodynamically stable patient with rhythmic cardiac activity and no pathological heart murmurs. Electrocardiogram (ECG) was pathological, showing sinus rhythm with ascending S-T elevation in V4–V6, DI, DII, and AVF. Blood tests showed neutrophil leukocytosis (WBC count 21,160; neutrophils 83%), increased inflammatory response markers [C-reactive protein (CRP) 163 mg/L], and elevation in cardiac enzyme levels (high-sensitivity troponin I 14,816 ng/L); NT-proBNP was 3,140 ng/L (normal <450 ng/L) (Day 0; Figure 1).
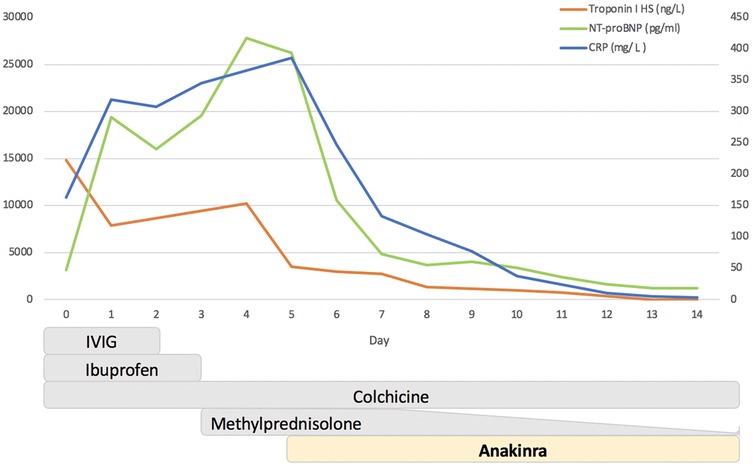
Figure 1. Timeline of changes in C-reactive protein (CRP), troponin I, and NT-proBNP after therapeutic interventions. The Y-axis on the left refers to troponin I (ng/L) and NT-proBNP (pg/ml), while the Y-axis on the right refers to CRP (mg/L). IVIG, intravenous immunoglobulin.
Echocardiogram showed hypokinesis of the posterolateral wall and mild-to-moderate impaired global left ventricular contraction [estimated B-mode ejection fraction (EF) 45%–48%]. Mild mitral insufficiency was present. Right ventricular function appeared normal. Mild posterior pericardial effusion of 5–6 mm was present. Blood cultures assessing the presence of pathogens commonly associated with myocarditis (CMV, EBV, Enterovirus, Parvovirus, Adenovirus, HSV1-2, Coxsackie, SARS-CoV-2) were negative. Myocardial biopsy was not performed.
In the setting of myopericarditis of unknown cause, treatment with ibuprofen (30 mg/kg per day), intravenous immunoglobulin (IVIG) (80 g in 24 h), and colchicine (0.5 mg per day) was started. On Day 2, the patient's clinical condition deteriorated with the resumption of fever, hypotension, lung rales, contraction of diuresis, hepatomegaly, and worsening of contractile function (EF 40%). The patient was transferred to the ICU and placed under CPAP; treatment with furosemide and inotropes (adrenaline, milrinone, and levosimendan) was started.
Further worsening of the patient's clinical condition was observed on Day 3, with a severe reduction of contractile function (EF 26%) and signs of low cardiac output (diuresis contraction, diffuse ST elevation on ECG, and increased serum lactate up to 12 mmol/L). Augmentation of inotropic support and intubation were necessary. Immunomodulatory therapy was potentiated with bolus corticosteroid treatment (methylprednisolone 600 mg/day). Ibuprofen was withdrawn, while colchicine was maintained.
On Day 5, the patient's condition was stationary, with echocardiographic improvement in contractile function (EF 45%). However, a clinical picture of heart failure persisted with the inability to wean the patient from inotropic therapy and mechanical ventilation. Blood tests demonstrated the persistence of a systemic inflammatory response (CRP 386 mg/L) and myocardial damage (troponin I 2,710 ng/L); NT-proBNP was 26,265 ng/L (Figure 1).
Despite the hyperinflammatory state (ferritin 667 ng/ml), no clinical or laboratory criteria suggestive of Still's disease or Macrophage Activation Syndrome were present. Considering the inflammatory markers, the persistence of pericardial effusion, and the inability to decrease inotrope support, a rescue treatment with ANK 7 mg/kg/day in continuous intravenous infusion was started on Day 5 (Figure 1).
A progressive improvement on a clinical level was subsequently observed, alongside a reduction in inflammatory markers and normalization of left ventricle systolic function. The patient was extubated after 3 days (Day 8), and inotropic treatment was discontinued after 9 days (Day 14). Treatment with ANK was reduced at 100 mg/day and switched to subcutaneous administration on Day 10 and discontinued on Day 15. Steroid therapy was progressively tapered without relapse (Figure 1).
Cardiac magnetic resonance imaging (MRI) performed 1 month after onset (Figures 2A,B) showed a non-dilated left ventricle with mildly reduced systolic function [EF 52% [normal range 56%–76%], EDIV 66 ml/m2 [normal range 56–104 ml/m2], EDSI 31.5 ml/m2 [normal range 16–40 ml/m2], and SV 57 ml] and no involvement of right ventricle. Edema and late gadolinium enhancement (LGE) were present at the mid-lateral, infero-lateral wall, and mid-apical anterior wall with intramyocardial and subepicardial patterns (see Figures 2A,B). At 40 days after onset, the patient was stable and asymptomatic. CRP, troponin, and NT-proBNP levels had normalized. Sinus rhythm on ECG was observed; negative T waves persisted in DI, aVL, and V4–V6. Echocardiogram showed normalized global and segmental systolic function and no pericardial effusion. The patient was subsequently discharged with ongoing outpatient cardiological and immunological follow-up.

Figure 2. Cardiac magnetic resonance imaging performed 1 month after acute myocarditis onset, two-chamber view: T2 weighted imaging (A) enables visualization of myocardial edema in the anterior wall concordant with regional late gadolinium enhancement (B) with an epi and mid-myocardial distribution.
No myocarditis flare was observed during follow-up. After 6 months off therapy, the patient experienced a mild flare of pericarditis but without myocardial involvement. The patient had no fever or signs suggestive of systemic JIA (no rash, no arthritis). Blood tests showed mild elevation of CRP (15 mg/L), with normal ferritin and ESR, and negative autoantibodies. A screening for recent infections (coxasachiae, EBV, adenovirus) excluded a post-infectious pericarditis. ANK 100 mg/day was re-started, leading to rapid improvement. Now, 18 months after re-starting ANK, the patient is in complete remission while receiving a low dose of Anakinra (100 mg two days/week).
3 Literature review
3.1 Search strategy
Our literature review adhered to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) 2020 guidelines (11). A systematic literature search was conducted in PubMed on August 5th, 2024, without language restriction using predefined MESH terms “AND” and “OR.” The following search terms were used: [“myocardic” (All Fields) OR “myocarditis” (MeSH Terms) OR “myocarditis” (All Fields) OR “myocarditides” (All Fields)] AND [“interleukin 1 receptor antagonist protein” (MeSH Terms) OR “interleukin 1 receptor antagonist protein” (All Fields) OR “ANK” (All Fields)].
3.2 Eligibility criteria
Studies were included if they fulfilled the following criteria: patients (age <18 years) with a confirmed diagnosis of AM via cardiac MRI or myocardial biopsy; use of ANK to treat myocarditis during the acute phase. Case reports, case series, and prospective and retrospective studies were included. Studies were excluded if they reported on MIS-C or other COVID-19-related cardiovascular complications, involved animal testing, or were review articles.
3.3 Data extraction
Titles and abstracts were initially assessed for inclusion by two reviewers (Fr Li and Fe Fo), with discrepancies arbitrated by the senior author (Fr Li). Full-text screening of relevant articles was conducted independently by Fr Li and Fe Fo to decide which satisfied the inclusion criteria.
Data extracted from the selected studies and combined in a shared Excel spreadsheet included demographic data (age and gender), study design, publication year, patient comorbidities, symptoms, length of hospitalization, duration of management, treatment used, and patient outcomes.
3.4 Results
Our search string identified 78 studies, of which five were deemed relevant according to the inclusion criteria. Most were case reports (12–15). Only one of ten patients in the retrospective case series (16) was diagnosed with AM and included here. Patient characteristics, study source, and clinical histories of the five patients plus our patient are summarized in Table 1.
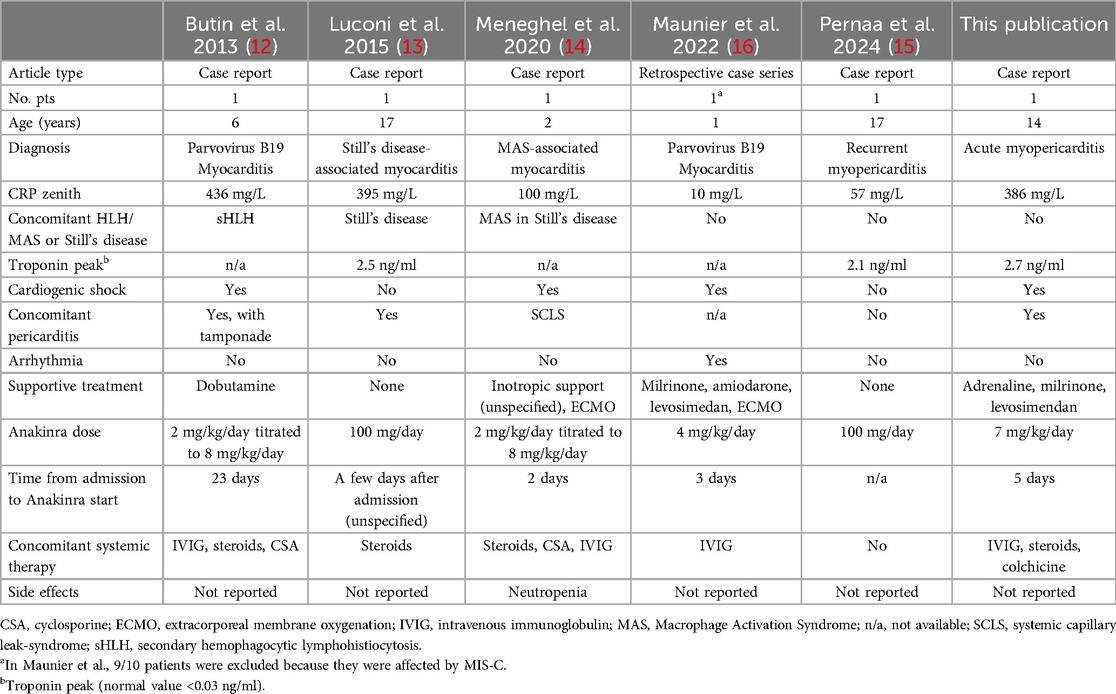
Table 1. Published case reports of acute myocarditis treated with anakinra, including this case report.
Patient ages ranged from 1 to 17 years. Two patients were diagnosed with myopericarditis and four with myocarditis. Four patients required inotropic support, two of whom also required extracorporeal membrane oxygenation (ECMO). ANK dosage was reported as 100 mg/day (2 studies) or between 2 mg/kg/day and 8 mg/kg/day (4 studies). All five patients responded to ANK, and neutropenia was the only adverse event reported (14) (Table 1).
4 Discussion
We report the case of a pediatric patient with AM associated with hyperinflammation who responded to IL-1 inhibition with ANK. ANK has been successfully used to treat myocarditis in adults (17–20); however, the recent ARAMIS trial failed to demonstrate its efficacy in a cohort of adult patients with AM (9). In the ARAMIS trial, 120 patients with myocarditis diagnosed by MRI and elevated cardiac enzymes were randomized to receive either 100 mg/day of ANK or a placebo. Overall, the incidence of severe adverse events was low in both groups and was equally distributed. However, the study lacked sufficient statistical power to detect differences in a cohort of patients who generally had very mild myocarditis without systemic symptoms; only 10% of the enrolled patients had an EF of <50%. Furthermore, the dose of ANK administered, at <2 mg/kg/day, was low (9).
In the pediatric population, the use of ANK in myocarditis is reported far less frequently, with only 5 cases published to date. Therefore, this is the sixth case report of ANK in a pediatric patient with AM. Notably, most patients had severe myocarditis, with 67% requiring inotropic support and 33% needing ECMO. Despite their overall severity, all patients responded to ANK, with significant improvements in both systemic symptoms and cardiac function (12–16).
Our patient experienced a rapid onset of severe myocarditis, accompanied by significantly elevated inflammatory markers. Notably, three of the previously published cases treated with ANK also had myocarditis in the context of concomitant hemophagocytic lymphohistiocytosis/Macrophage Activation Syndrome (HLH/MAS) or Still's disease (12–14). Myocarditis is rare at the presentation of Still disease and MAS with an estimated incidence <5% in both diseases (14, 21, 22). The efficacy of ANK in treating MAS has been demonstrated in various studies and is commonly used in everyday practice (23). Interestingly, our patient did not meet the criteria for MAS but exhibited only a persistent high fever with high RCP. This suggests that ANK may be beneficial not only for myocarditis secondary to systemic illnesses such as MAS and Still's disease but also for AM with concomitant prominent systemic inflammation. According to published studies, only 27% of patients with AM have significantly elevated inflammatory markers, such as CRP (24). ANK is likely beneficial in cases of AM associated with systemic inflammation because it acts at multiple levels. First, like its role in MAS, ANK antagonizes IL-1, a key cytokine involved in the inflammatory process (7). Additionally, ANK modulates blood pressure and improves overall circulatory performance (25). Secondly, ANK may exert a direct effect on the myocardium, counteracting the increase of IL-1 due to persistent inflammasome activation in cardiomyocytes (6, 7).
Our patient also exhibited moderately elevated ferritin levels, indicative of a systemic hyperinflammatory state. This finding is observed in other cases included in our review, and it is reasonable to infer that increased levels of ferritin, along with other inflammatory markers, confirm the presence of a hyperinflammatory condition. This supports the rationale for using IL-1 antagonists in this context (6).
Another interesting factor observed in our case and two previously reported cases is the concomitant presence of pericarditis (13, 15). While data in pediatrics are scarce, in adults, the estimated incidence of elevated troponin in patients with pericarditis is 28.8%; nevertheless, most of these patients have normal left ventricular function. The incidence of cardiac insufficiency secondary to myocarditis in patients with pericarditis is around 5% (peri-myocarditis). Patients with peri-myocarditis have a good long-term prognosis if they survive the acute phase of the disease. Recently, one adult patient with peri-myocarditis was successfully treated with ANK, confirming that it may be a promising therapeutic option even for older patients (26, 27). The efficacy of ANK in treating idiopathic pericarditis has been confirmed in numerous retrospective case series (28, 29). In our case, ANK may have had a positive effect on pericardial effusion, ultimately improving cardiac contraction. Given its proven effects on systemic inflammation and its role in treating pericarditis, it is possible that ANK could be particularly effective in a subgroup of patients with AM associated with pericarditis and/or systemic inflammation.
In this report, ANK was administered at a high dose (i.e., 7 mg/kg/day) via intravenous infusion. It is worth noting that in two previously published cases, the treating physicians initially started with a lower dose (2 mg/kg/day) but quickly increased it due to cardiac deterioration (12, 14). These observations suggest that ANK could be initiated at a high dose in cases of AM and that a lack of response to a lower dose does not necessarily indicate treatment failure. Interestingly, data suggest a higher dose of ANK may be more effective in treating cardiac dysfunction in MIS-C, especially in severe cases (30).
Neither our patient nor the previously published cases of AM treated with ANK experienced severe adverse events, with only one case of transient neutropenia reported (14). Of note, according to previous experience with ANK in MIS-C patients, the drug seems safe even at high doses, with increased ALT being the most frequent side effect, which usually disappears after dose adjustment (30).
This study has some limitations. First, in this case, as in others, ANK was administered after IVIG and steroids. Second, there is significant heterogeneity in how treatment response was assessed in previous reports, making it impossible to quantify the drug response in more detail.
5 Conclusion
In summary, we report the case of a patient experiencing life-threatening AM associated with hyperinflammation, who showed significant cardiological and systemic improvement following treatment with high-dose ANK. A systematic literature review highlighted five other pediatric cases of AM treated with ANK. The results appear promising in this limited series, particularly in patients with AM associated with severe inflammation and/or pericarditis. Further studies are needed to evaluate the safety and efficacy of IL-1 inhibitors in this context and to define the optimal dose and timing of ANK treatment in pediatric myocarditis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
FL: Conceptualization, Formal analysis, Methodology, Writing – original draft. FFo: Conceptualization, Formal analysis, Methodology, Writing – original draft. FFe: Data curation, Writing – original draft. CC: Data curation, Writing – review & editing. CR: Data curation, Writing – review & editing. DM: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank Melanie Gatt, PhD, for editorial assistance provided on behalf of Health Publishing & Services srl. This assistance was funded by SOBI in compliance with Good Publication Practice (GPP3) guidelines.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ammirati E, Veronese G, Bottiroli M, Wang DW, Cipriani M, Garascia A, et al. Update on acute myocarditis. Trends Cardiovasc Med. (2021) 31(6):370–9. doi: 10.1016/j.tcm.2020.05.008
2. Law YM, Lal AK, Chen S, Čiháková D, Cooper LT Jr, Deshpande S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. (2021) 144(6):e149. doi: 10.1161/CIR.0000000000001011
3. Law YM, Lal AK, Chen S, Cihakova D, Cooper LT Jr, Deshpande S, et al. Diagnosis and management of myocarditis in children: a scientific statement from the American Heart Association. Circulation. (2021) 144(6):e123–e35. doi: 10.1161/CIR.0000000000001001
4. Ferone E, Segev A, Tempo E, Gentile P, Elsanhoury A, Baggio C, et al. Current treatment and immunomodulation strategies in acute myocarditis. J Cardiovasc Pharmacol. (2024) 83(5):364–76. doi: 10.1097/FJC.0000000000001542
5. Wang Y, Gao B, Xiong S. Involvement of Nlrp3 inflammasome in Cvb3-induced viral myocarditis. Am J Physiol Heart Circ Physiol. (2014) 307(10):H1438–47. doi: 10.1152/ajpheart.00441.2014
6. Toldo S, Kannan H, Bussani R, Anzini M, Sonnino C, Sinagra G, et al. Formation of the inflammasome in acute myocarditis. Int J Cardiol. (2014) 171(3):e119–21. doi: 10.1016/j.ijcard.2013.12.137
7. Cavalli G, Colafrancesco S, Emmi G, Imazio M, Lopalco G, Maggio MC, et al. Interleukin 1alpha: a comprehensive review on the role of il-1alpha in the pathogenesis and treatment of autoimmune and inflammatory diseases. Autoimmun Rev. (2021) 20(3):102763. doi: 10.1016/j.autrev.2021.102763
8. Thomson RJ, Singh A, Knight DS, Buckley J, Lamb LE, Captur G. Anakinra treats fulminant myocarditis from neisseria meningitidis septicaemia and haemophagocytic lymphohistiocytosis: a case report. Eur Heart J Case Rep. (2021) 5(6):ytab201. doi: 10.1093/ehjcr/ytab201
9. Morrow DA, Verbrugge FH. In-perspective: the aramis double-blind randomized placebo-controlled trial of anakinra for the treatment of acute myocarditis. Eur Heart J Acute Cardiovasc Care. (2023) 12(9):627–8. doi: 10.1093/ehjacc/zuad102
10. Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, et al. Sars-Cov-2-induced kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. (2020) 146(2):e20201711. doi: 10.1542/peds.2020-1711
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Butin M, Mekki Y, Phan A, Billaud G, Di Filippo S, Javouhey E, et al. Successful immunotherapy in life-threatening parvovirus B19 infection in a child. Pediatr Infect Dis J. (2013) 32(7):789–92. doi: 10.1097/INF.0b013e31828df4d1
13. Luconi N, Risse J, Busato T, Galland J, Mandry D, Voilliot D, et al. Myocarditis in a young man with adult onset still’s disease successfully treated with il-1 blocker. Int J Cardiol. (2015) 189:220–2. doi: 10.1016/j.ijcard.2015.04.071
14. Meneghel A, Martini G, Amigoni A, Pettenazzo A, Padalino M, Zulian F. Case report: life-threatening macrophage activation syndrome with fulminant myocarditis successfully rescued by high dose intravenous anakinra. Front Pediatr. (2020) 8:635080. doi: 10.3389/fped.2020.635080
15. Pernaa N, Vakkuri A, Arvonen M, Kuismin O, Santaniemi W, Glumoff V, et al. Germline Havcr2/tim-3 checkpoint inhibitor receptor deficiency in recurrent autoinflammatory myocarditis. J Clin Immunol. (2024) 44(3):81. doi: 10.1007/s10875-024-01685-x
16. Maunier L, Charbel R, Lambert V, Tissieres P, Group Cs. Anakinra in pediatric acute fulminant myocarditis. Ann Intensive Care. (2022) 12(1):80. doi: 10.1186/s13613-022-01054-0
17. Cavalli G, Foppoli M, Cabrini L, Dinarello CA, Tresoldi M, Dagna L. Interleukin-1 receptor blockade rescues myocarditis-associated end-stage heart failure. Front Immunol. (2017) 8:131. doi: 10.3389/fimmu.2017.00131
18. Cavalli G, Pappalardo F, Mangieri A, Dinarello CA, Dagna L, Tresoldi M. Treating life-threatening myocarditis by blocking interleukin-1. Crit Care Med. (2016) 44(8):e751–4. doi: 10.1097/CCM.0000000000001654
19. De Luca G, Campochiaro C, Dinarello CA, Dagna L, Cavalli G. Treatment of dilated cardiomyopathy with interleukin-1 inhibition. Ann Intern Med. (2018) 169(11):819–20. doi: 10.7326/L18-0315
20. Parisi F, Paglionico A, Varriano V, Ferraccioli G, Gremese E. Refractory adult-onset still disease complicated by macrophage activation syndrome and acute myocarditis: a case report treated with high doses (8 mg/kg/D) of anakinra. Medicine (Baltimore). (2017) 96(24):e6656. doi: 10.1097/MD.0000000000006656
21. Ciancia S, Cappella M, De Fanti A, Iughetti L. Perimyocarditis as first sign of systemic onset juvenile idiopathic arthritis treated successfully with anakinra: a case-based review. Acta Biomed. (2020) 91(4). doi: 10.23750/abm.v91i4.9093
22. Svantesson H, Björkhem G, Elborgh R. Cardiac involvement in juvenile rheumatoid arthritis. A follow-up study. Acta Paediatr Scand. (1983) 72(3):345–50. doi: 10.1111/j.1651-2227.1983.tb09726.x
23. Baldo F, Erkens RGA, Mizuta M, Rogani G, Lucioni F, Bracaglia C, et al. Current treatment in macrophage activation syndrome worldwide: a systematic literature review to inform the metaphor project. Rheumatology (Oxford). (2025) 64(1):32–44. doi: 10.1093/rheumatology/keae391
24. Durani Y, Egan M, Baffa J, Selbst SM, Nager AL. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. (2009) 27(8):942–7. doi: 10.1016/j.ajem.2008.07.032
25. Urwyler SA, Ebrahimi F, Burkard T, Schuetz P, Poglitsch M, Mueller B, et al. Il (interleukin)-1 receptor antagonist increases ang (angiotensin [1–7]) and decreases blood pressure in obese individuals. Hypertension. (2020) 75(6):1455–63. doi: 10.1161/HYPERTENSIONAHA.119.13982
26. Imazio M, Brucato A, Barbieri A, Ferroni F, Maestroni S, Ligabue G, et al. Good prognosis for pericarditis with and without myocardial involvement: results from a multicenter, prospective cohort study. Circulation. (2013) 128(1):42–9. doi: 10.1161/CIRCULATIONAHA.113.001531
27. Panejko A, Ołpińska B, Łoboz-Rudnicka M, Gruszecka K, Jaroch J. Life-saving treatment with anakinra in severe relapse of still disease with perimyocarditis and aseptic pneumonia. Pol Arch Intern Med. (2024) 134(6):16725. doi: 10.20452/pamw.16725
28. Caorsi R, Insalaco A, Bovis F, Martini G, Cattalini M, Chinali M, et al. Pediatric recurrent pericarditis: appropriateness of the standard of care and response to il-1 blockade. J Pediatr. (2023) 256:18–26.e8. doi: 10.1016/j.jpeds.2022.11.034
29. Finetti M, Insalaco A, Cantarini L, Meini A, Breda L, Alessio M, et al. Long-term efficacy of interleukin-1 receptor antagonist (anakinra) in corticosteroid-dependent and colchicine-resistant recurrent pericarditis. J Pediatr. (2014) 164(6):1425–31.e1. doi: 10.1016/j.jpeds.2014.01.065
Keywords: acute myocarditis, anakinra, case report, myopericarditis, pericarditis, severe inflammation
Citation: Licciardi F, Fornari F, Ferroni F, Covizzi C, Riggi C and Montin D (2025) Treatment of pediatric severe acute myopericarditis with anakinra: a case report and literature review. Front. Pediatr. 13:1544126. doi: 10.3389/fped.2025.1544126
Received: 12 December 2024; Accepted: 12 March 2025;
Published: 23 April 2025.
Edited by:
Giovanni Filocamo, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, ItalyReviewed by:
Lampros Fotis, National and Kapodistrian University of Athens, GreeceLucia Viola Camilla Mauri, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
Martina Rossano, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
Copyright: © 2025 Licciardi, Fornari, Ferroni, Covizzi, Riggi and Montin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Federico Fornari, ZmVkZXJpY28uZm9ybmFyaUBlZHUudW5pdG8uaXQ=
 Francesco Licciardi
Francesco Licciardi Federico Fornari
Federico Fornari Francesca Ferroni4
Francesca Ferroni4 Carlotta Covizzi
Carlotta Covizzi Davide Montin
Davide Montin