- 1College of Physical Education, Hunan Normal University, Changsha, China
- 2College of Optical and Electronic Technology, China Jiliang University, Hangzhou, China
- 3Independent Researcher, Windermere, FL, United States
Background: Early exercise interventions targeting lower limb muscles are critical for enhancing motor development in children with cerebral palsy (CP). While both resistance training, which enhances muscular strength and endurance, and power training, which targets explosive force production and movement velocity, fall under the umbrella of strength training, this focused review synthesizes current evidence on muscle hypertrophy resulting from these two modalities in children with CP.
Methods: The Web of Science Core Collection, Scopus, and Embase were searched through March 2025. Eligible studies were randomized controlled trials assessing muscle fascicle length or proxy indicators of muscle fiber diameter following resistance or power training in children with CP. A random-effects meta-analysis was performed to calculate Cohen's d comparing strength training with regular physiotherapy.
Findings: Eight studies met the inclusion criteria and were systematically reviewed, with five included in the meta-analysis. These five studies reported outcomes from 80 participants in the strength training group and 73 participants in the traditional physiotherapy group. All participants were ambulatory children classified with low to mild levels on the Gross Motor Function Classification System. Resistance training significantly increased muscle fiber diameter (d = 0.82, 95% CI = 0.54–1.09), whereas power training did not (d = 0.35, 95% CI = −0.29 to 0.99). Neither training modality produced a significant increase in muscle fascicle length (resistance training: d = 0.19, 95% CI = −0.17 to 0.56; power training: d = 0.37, 95% CI = −0.27 to 1.01). Notably, one study comparing power and resistance training demonstrated a highly significant improvement in muscle fascicle length (d = 1.20, 95% CI = 0.13–2.27), which may be attributed to the high-velocity, high-load nature of concentric power training.
Interpretation: Current evidence favors resistance training to increase muscle fiber diameter in ambulatory children with CP. As individuals progress, maximal loads and repetitions should be progressively increased and complemented with explosive power training to further enhance muscle fascicle length and lower limb function. The optimal protocol for children with high levels of functional disability remains to be established.
1 Introduction
Cerebral palsy (CP) is a neurological disorder resulting from non-progressive brain damage or developmental defects during fetal or infant brain development, affecting approximately 1.5–3 per 1,000 live births globally (1). Preterm infants are at increased risk for cerebral white matter injury due to genetic factors, nutritional deficiencies, infections, or placental damage (2). These factors can trigger hypoxic-ischemic events, leading to CP via injuries to the cortex, subcortical regions, or basal ganglia. Additionally, birth-related ischemia, hypoxia, postnatal encephalitis, and cerebral hemorrhage may contribute to CP development. Clinically, CP is classified by manifestations such as spasticity, dyskinesia, ataxia, and abnormal tone, with spastic CP being the most common in children. Damage to neuromotor components disrupts skeletal muscle function (3), resulting in central motor deficits and postural abnormalities. Children aged 2–5 years with spastic CP exhibit approximately 22% lower muscle volume and markedly reduced lower-limb muscle cross-sectional area compared to their typically developing peers (4). Likewise, shortening of the rectus femoris—one of several affected muscles—reduces strength, contraction velocity, and range of motion, thereby impairing daily activities (5). Furthermore, skeletal muscles in CP frequently stiffen during activity, leading to weakness, imbalance, and reduced endurance, which hinders motor control and increases the risk of deformities. Ultimately, musculoskeletal malformations and muscle contractures significantly reduce quality of life, with persistent spasms often resulting in lifelong motor disabilities.
The absence of fixed deformities and high behavioral plasticity in young children with CP enables timely interventions to support muscular and motor development. Current treatments primarily involve long-term rehabilitation, such as gait training (6). Among traditional and emerging approaches, resistance training has gained interest as a non-invasive and cost-effective method to counter disease-related declines in muscle mass and function (7). Since weak lower limb muscles contribute to impaired gait, resistance training holds potential for improvement. Two systematic reviews support its benefits in enhancing strength, balance, and mobility in children with CP (8, 9). However, few studies have examined the impact of strength training on muscle morphology, with only one systematic review available that addresses overall strength training (10).
An important nuance in modern strength training lies in pairing exercise type with load and velocity to target strength-endurance, strength-speed, or speed-strength (11). Traditional resistance programs begin with a strength-endurance phase—refining technique and fortifying connective tissues to prepare novices for heavier loads—then progress to a strength-speed phase that maximizes force production and muscle hypertrophy via high loads. Advanced protocols add a speed-strength (power training) phase, using moderate loads with high-velocity exercises to maximize acceleration, movement velocity, and power output. In able-bodied trained children, power training yields superior gains in muscle mass (12), strength, power (13), and mobility (14) compared to traditional high-load resistance training, leading some researchers to advocate it as a replacement—rather than a complement—to resistance training for high-performance athletics (14). Restricted fiber-diameter growth in children with CP impairs both strength development and muscle power (7). This raises the question of whether power training—which involves rapid eccentric or concentric movements—offers advantages for muscle hypertrophy and function compared to resistance training. However, evidence of its effects in children with CP remains limited. van Vulpen et al. (15) examined power training targeting the plantar flexors using functionally loaded, multi-joint movements that emphasized ankle push-off, high movement velocity to simulate daily activities, and progressive loading at 50%–70% of maximum unloaded speed. Their assessments of isometric and dynamic muscle functions, along with muscle tone, provided preliminary evidence that moderate power output can enhance real-life functional capacities in children with CP. It should be kept in mind that, disuse-related muscle limitations in children with CP may restrict their ability to perform high-load—typically >80% of one-repetition maximum (1RM)—resistance training, while functional limitations may also impede rapid force exertion required for power training. Consequently, comparing these two strength training modalities is of significant interest. To our knowledge, no dedicated meta-analysis has yet addressed this specific micro aspect.
Therefore, the purpose of this systematic review was to summarize current evidence on the effects of resistance and power training on lower limb muscle morphology in children with CP. The findings are expected to foster academic dialogue and guide the optimization of muscle hypertrophy through evidence-based strength training protocols in clinical practice.
2 Methods
2.1 Search strategy
Two researchers (B.L. and Y.Z.) conducted an electronic search of the Web of Science Core Collection, Scopus, and Embase from inception to March 2025. The search combined the keywords “children” and “cerebral palsy” with either “exercise,” “training,” or “rehabilitation,” and further paired these with “muscle fiber diameter,” “muscle volume,” “muscle thickness,” “muscle size,” “cross-sectional area,” “muscle fiber length,” or “muscle fascicle length.”
Inclusion criteria comprised randomized controlled trials of resistance or power training interventions targeting lower-limb muscle morphology in CP participants aged 6–17 years (ambulatory or non-ambulatory) who had undergone no surgery in the preceding 12 months. Only full-length, English-language research articles were considered, while reviews, conference papers, and protocols were excluded. Two researchers (B.L. and X.Z.) independently screened the records and resolved disagreements by consensus.
2.2 Data extraction
Two researchers (B.L. and Y.Z.) extracted data using a standard sheet available on Figshare (doi: 10.6084/m9.figshare.28642094.v1). Extracted data included participant demographics, exercise characteristics, and outcomes of interest. Additionally, risk-of-bias information was recorded on a separate datasheet using ROB 2 tool (16).
Outcome measures included muscle fascicle length and proxy indicators of muscle fiber diameter—specifically muscle volume, thickness, or cross-sectional area. Typically, either the means with standard deviations or the mean change with paired p-values were extracted. Data reported as medians and interquartile ranges were converted to means and standard deviations using McGrath et al.'s quantile estimation method (metamedian, version 1.2.1) (17).
2.3 Data synthesis
Data were analyzed with the R package meta (version 8.0-2). Owing to heterogeneity among participants and interventions, we applied a random-effects meta-analysis and synthesized pooled effects as Cohen's d (18), despite the small sample sizes of the constituent studies. An effect was considered significant when its 95% confidence interval (CI) excluded zero. Heterogeneity was assessed using the I2 statistic. No subgroup analysis was conducted due to the inclusion of only one power training study. When a study reported multiple muscle morphology metrics, each outcome was treated as independent. To mitigate potential effect size inflation from multiple measurements within a single study, a sensitivity analysis using an inverse-variance weighted common effect size was conducted. Contour-enhanced funnel plots were used to visualize potential publication bias. Of note, two systematically reviewed studies were excluded from the meta-analysis because of control-arm inconsistencies (see “Results” for details); nonetheless, their control-adjusted effect sizes are discussed with the principal findings.
3 Results
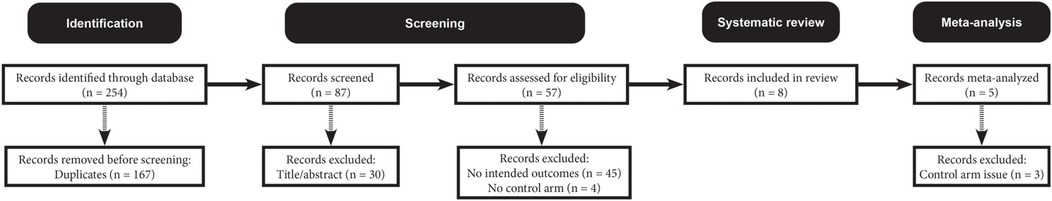
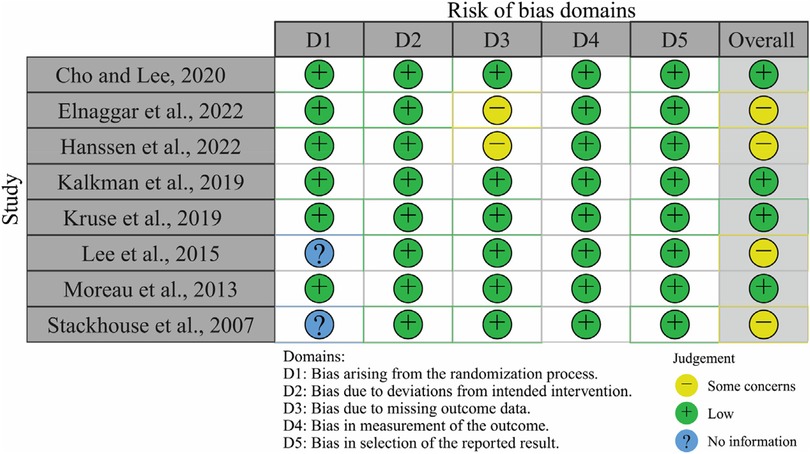
Figure 1 outlines the literature screening process. The search initially identified 254 unique records, which were subsequently narrowed down to eight eligible studies (19–26). Figure 2 visualizes the risk-of-bias assessment. Lee et al. (24) and Stackhouse et al. (26) did not specify their randomization methods, although this omission may not critically affect result validity. Elnaggar et al. (20) and Hanssen et al. (21) used partial imputation for missing data without detailing the affected data, raising concerns about outcome precision. Consequently, these studies present some issues in data presentation. Overall, the eight included studies qualify as valid randomized controlled trials, justifying their inclusion in the systematic review and meta-analysis.
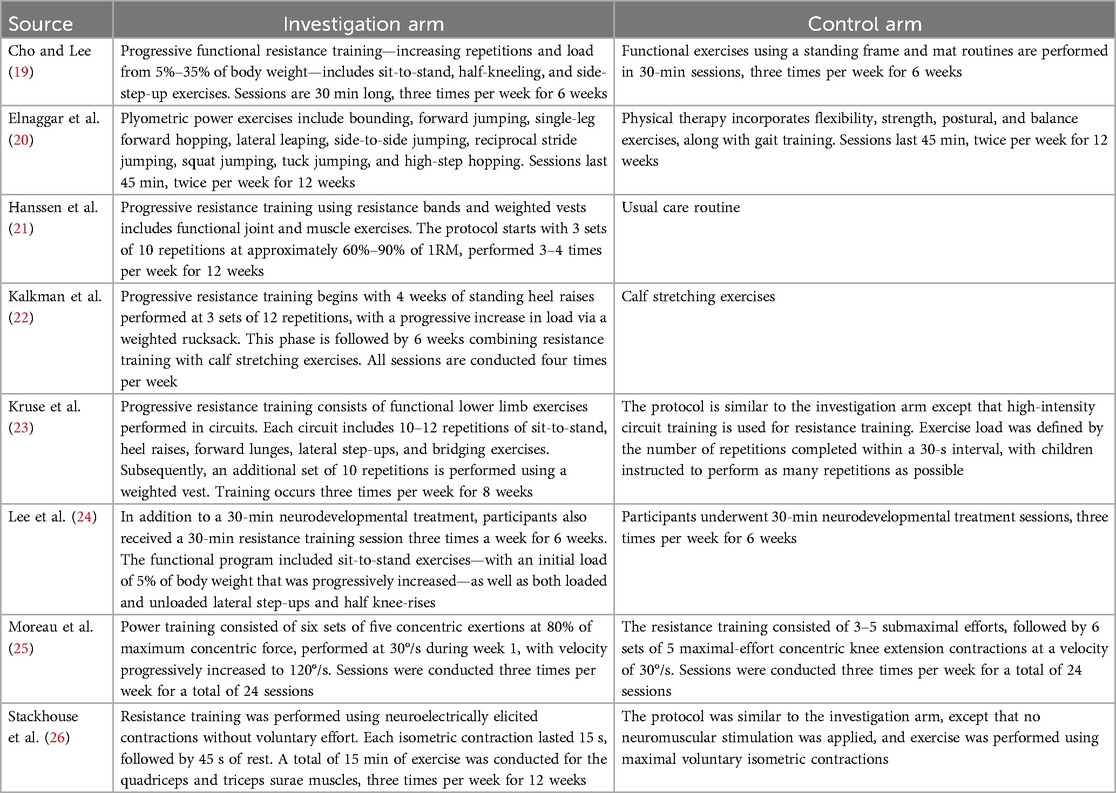
Table 1 presents the training characteristics of all reviewed studies. Kruse et al. (23) and Moreau et al. (25) featured a control arm categorized as power training, and Stackhouse et al. (26) employed a typical resistance training control, while the remaining studies used regular physiotherapy as control. Although a network meta-analysis would be ideal, the limited evidence warranted a traditional meta-analysis comparing resistance or power training to regular physiotherapy. Consequently, five (19–22, 24) of the eight studies were included in the meta-analysis, with only Elnaggar et al. (20) categorized as power training.
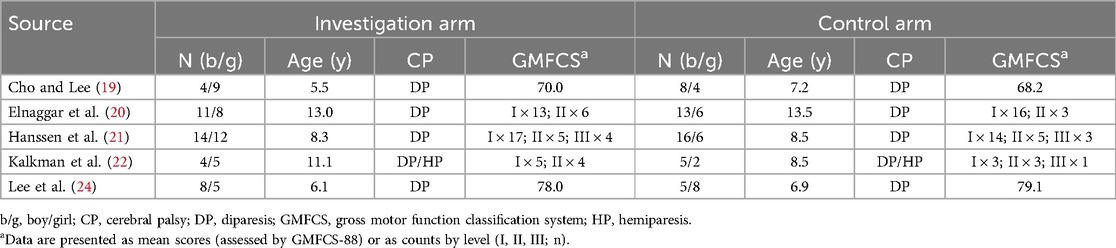
Table 2 summarizes the participant demographics of meta-analyzed studies. The intervention arm included 80 participants (51.3% boys), while the control arm comprised 73 participants (64.4% boys). All but one study recruited children with diparesic CP. Notably, all studies enrolled ambulatory children with lower gross motor function classification system (GMFCS) levels (I–III), indicating relatively adequate baseline mobility for participating in exercise training.
Figure 3 shows the effects of resistance and power training on muscle fiber diameter, as measured by ultrasonography in all studies. Resistance training yielded a significant large effect size, while the single power training study leaves it unclear whether plyometric exercises effectively increase muscle thickness. Sensitivity analysis confirms the robustness of the results to the data synthesis method. Although the main results indicate low heterogeneity, the sensitivity analysis reveals heterogeneity—an expected outcome given participant and intervention variations. Figure 4 shows the effect of resistance and power training on muscle fascicle length. Neither type of training yielded significant improvement over regular physiotherapy.
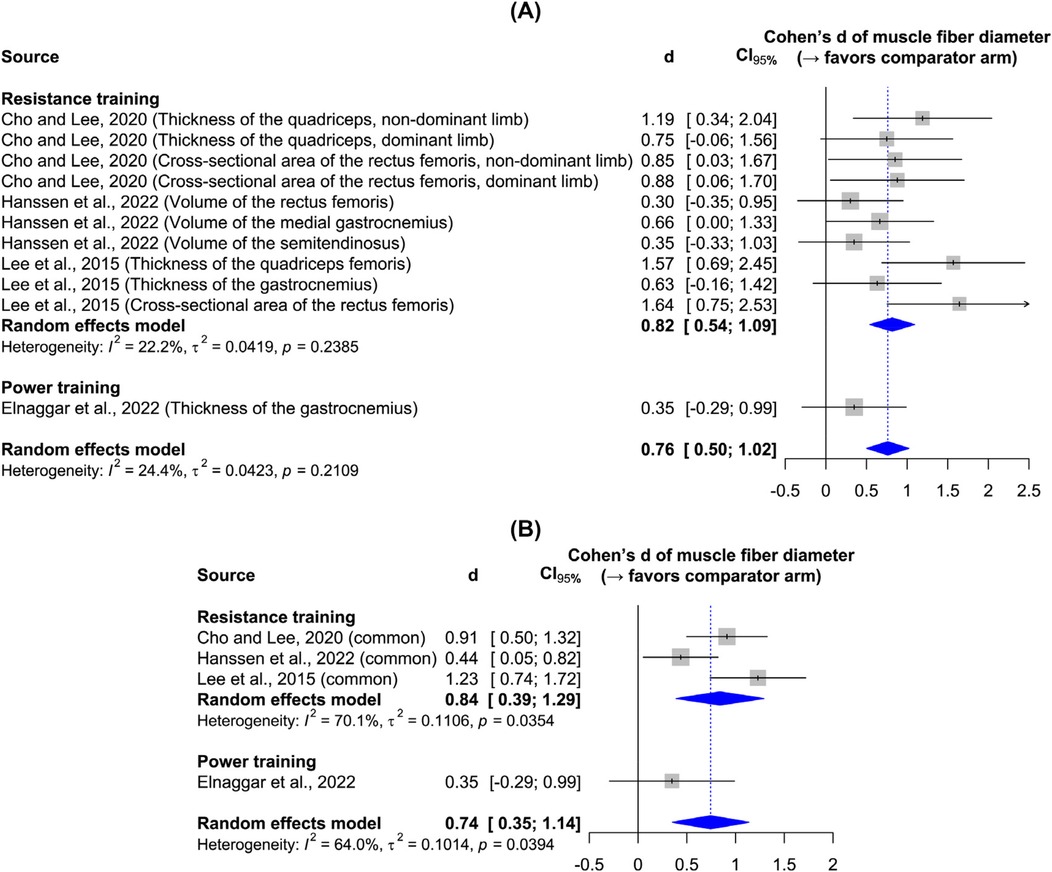
Figure 3. Forest plots: (A) main results of the effect on muscle fiber diameter; (B) sensitivity analysis.
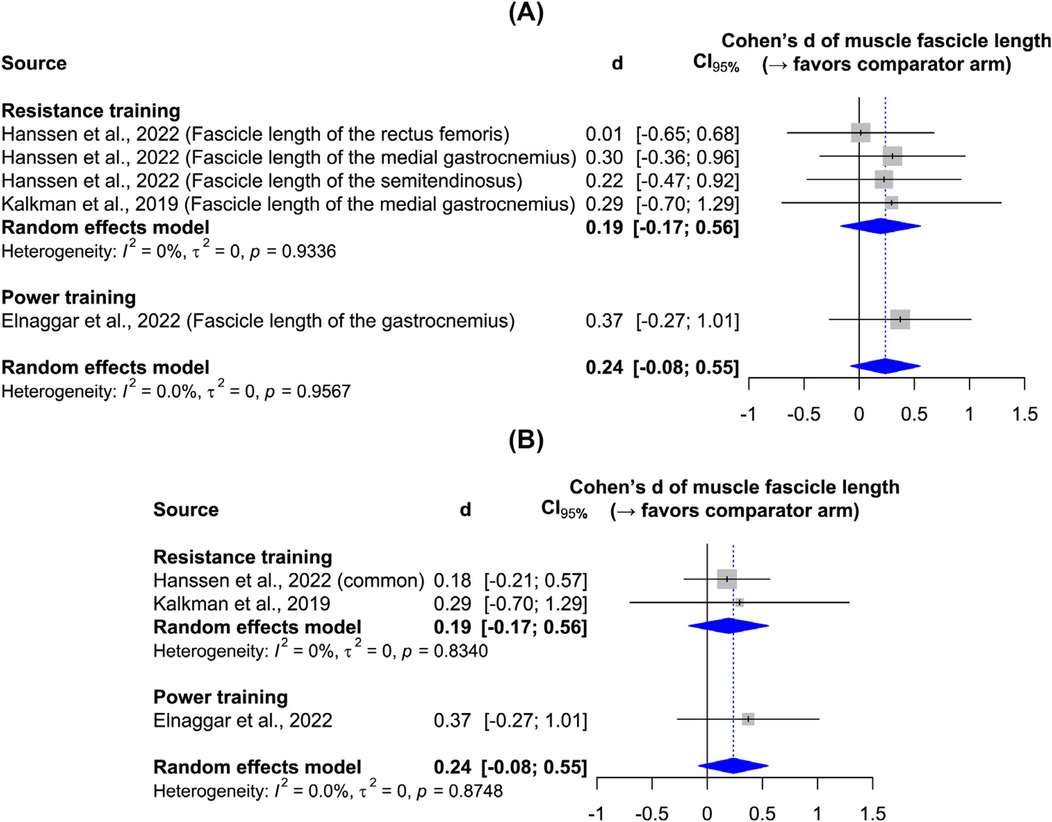
Figure 4. Forest plots: (A) main results of the effect on muscle fascicle length; (B) sensitivity analysis.
Figure 5 illustrates the risk of bias from potential missing publications. For muscle fiber diameter, results span all significance levels, suggesting no obvious publication bias. In contrast, only studies reporting non-significant improvements in muscle fascicle length have been published. Although the evidence is limited, these findings suggest that without innovative approaches, short-term (i.e., less than half a year) resistance or power training may have no effect on muscle fascicle length.
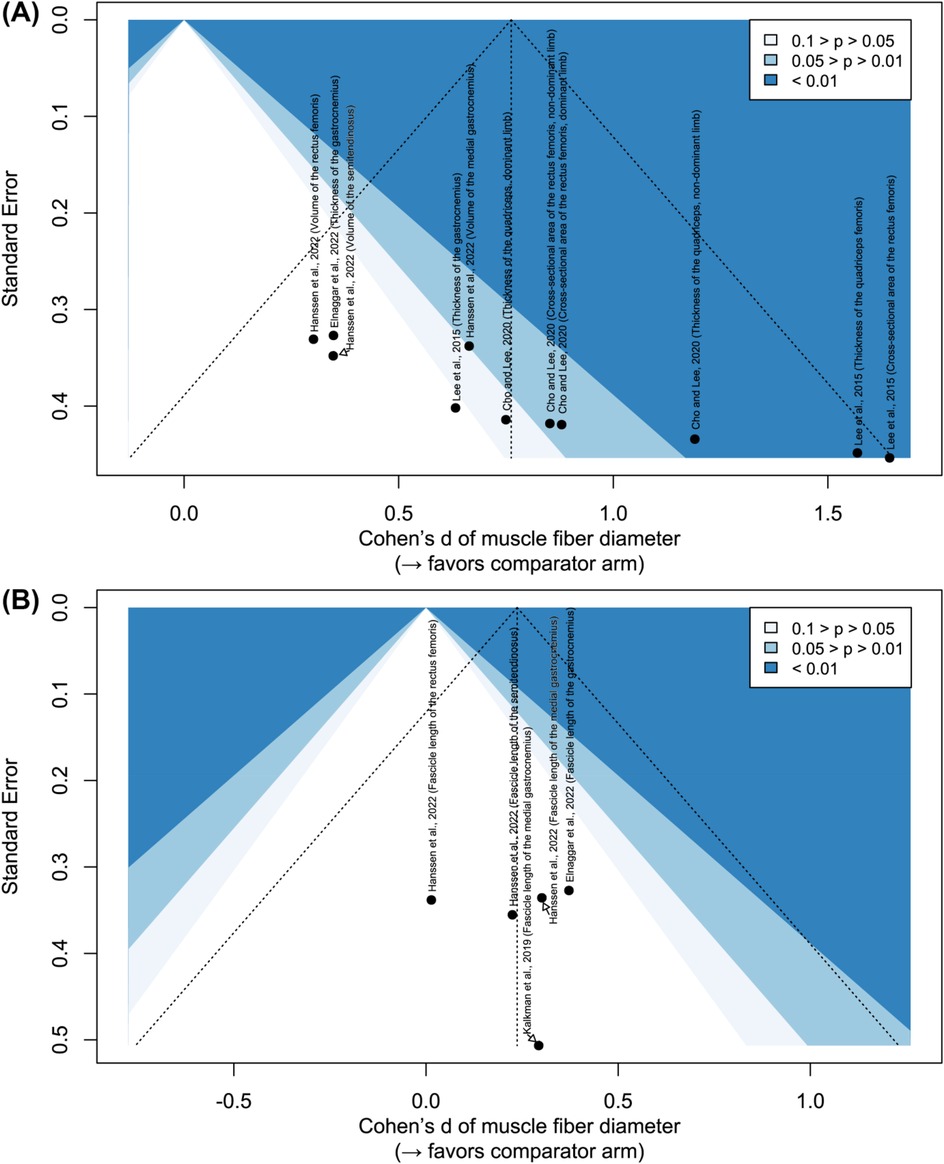
Figure 5. Contour-enhanced funnel plots: (A) effect on muscle fiber diameter; (B) effect on muscle fascicle length.
Three reviewed studies not included in the meta-analysis are presented for additional insights. In Kruse et al. (23), resistance training significantly improved vastus lateralis thickness but did not affect medial gastrocnemius thickness or fascicle length, while power training showed no differences in these measures. In Moreau et al. (25), resistance training produced a greater increase in the cross-sectional area of the rectus femoris than power training (d = 0.70, 95% CI: −0.32 to 1.72, with power training as control), whereas power training significantly enhanced rectus femoris fascicle length compared to resistance training (d = 1.20, 95% CI: 0.13–2.27, with resistance training as control). In Stackhouse et al. (26), adding neuromuscular stimulation to resistance training further improved the cross-sectional area of the quadriceps (d = 1.00, 95% CI: −0.31 to 2.32, with resistance training as control).
4 Discussion
An earlier meta-analysis (6) found that traditional gait training improves gait speed but not strength training in children with CP. Two subsequent meta-analyses (8, 9) provided updated evidence on the positive effects of strength training on mobility. The new contribution of the present review is its focused analysis of strength training modalities aimed at optimizing muscle morphology in children with CP, offering updated insights for guiding clinical practice during their developmental years.
Despite our effort to synthesize all evidence on the effectiveness of strength training, only ambulatory children with CP who demonstrated adequate cognitive function were recruited in the reviewed studies. This presents a significant limitation to the generalizability of the findings to children with severe motor impairments. While the overall prevalence of CP remains relatively stable (1), the severity, classified by GMFCS levels, varies across regions. In Australia, individuals with severe motor impairments (GMFCS levels IV and V) account for 29.3% of affected persons (27), similar to the 22.4% reported in Sweden (28). In contrast, in developing countries such as India, the proportion rises to 55.3% (29). Notwithstanding early evidence that strength training improves functional mobility in children with GMFCS IV (30, 31), its effects on muscle morphology remain unstudied, leaving a critical research gap for non-ambulatory children. This discrepancy underscores that the current findings and interpretations largely apply to children capable of following exercise instructions.
Although evidence on power training is limited (20, 25), current data strongly favor resistance training for increasing muscle fiber diameter. This is expected, as muscle hypertrophy during strength training is driven by loading effect—higher loads are more effective than submaximal loads in untrained individuals (32). Moreau et al. (25) compared fixed-speed, maximal loading with high-velocity, high-repetition submaximal loading, and their findings support the meta-analysis results. Moreover, performing more repetitions at lower loads until volitional failure leads to greater discomfort and fatigue without additional strength benefits (33, 34), which is less desirable for untrained children. Thus, current evidence favors using resistance training to enhance muscle fiber diameter in children with CP.
Despite limited evidence of muscle hypertrophy from power training, it is important to note that power and strength contribute to distinct aspects of lower extremity neuromuscular function. For instance, dynamic steady-state balance is strongly correlated with maximal strength in children (35), whereas gait speed is closely linked to peak power in children with CP (36). From a developmental standpoint, muscle strength precedes power, and together they achieve a full spectrum of neuromuscular capacity. It may be logical to prioritize resistance training for muscle hypertrophy, then integrate dynamic power training to enhance functional capacity in children with CP; however, further evidence is needed to confirm this sequencing.
While current evidence does not show a clear effect of resistance or power training on muscle fascicle length (CIs crossing zero), it would be premature to conclude that strength training yields minimal adaptations. In fact, Moreau et al. (25)—not included in the meta-analysis—demonstrated a very large effect size for power training compared to resistance training. A key difference is that Moreau and colleagues employed concentric training at 80% of maximum force, whereas other studies (20–22) used lower loads. For healthy, trained individuals, increasing muscle fascicle length requires accentuated eccentric-load strength training (37, 38). Overall, the literature indicates that higher loads, preferably utilizing power training principles (either concentric or eccentric), are necessary to enhance muscle fascicle length. Notably, most studies employed lower loads with body weight or weighted vests, which, while effective for hypertrophy, may not sufficiently target fascicle length. Ideally, strength-training programs for children with CP should progressively incorporate higher loads — 70%–85% of 1 RM for resistance training and 60%–80% of 1 RM for power training (7)—and clinicians should always fine-tune these loads to each child's GMFCS level and individual needs.
We suggest three future research directions to complement muscle mass and strength development protocols. First, joint intervention approaches warrant mention. Muscle strength—and by extension, function—in children with CP is influenced by spasticity (39). While it remains debated whether underdeveloped lower limb muscles directly contribute to spasticity, early reduction of spasticity appears to facilitate improvements in motor function. To address muscle spasticity in CP, treatments such as Botulinum neurotoxin type A (BoNT-A), selective dorsal rhizotomy, and extracorporeal shockwave therapy are available (40). For example, BoNT-A reduces spasticity and rigidity, improving lower limb movement and muscle tone (41). In CP, BoNT-A injections inhibit acetylcholine release at neuromuscular junctions, block nerve-muscle signal conduction, reduce spasms, and enhance joint mobility (42). Williams et al. (43) combined BoNT-A injections with functional resistance training in children with CP, resulting in significant muscle hypertrophy and functional gains. Thus, physiotherapists may consider combining invasive treatments with non-invasive strength training to optimize muscle adaptations in children with CP. Second, we echo the recent calls by Modlesky and Matias for precision nutrition interventions (44). Nutritional supplements are effective in enhancing muscle growth and function in various populations (45), including CP (46, 47). Therefore, integrating strength training with nutritional supplementation warrants further investigation. Third, children with CP are often born into families with low socioeconomic status (48, 49). Given their socioeconomic limitations, long-term participation in center-based rehabilitation is often impractical for these families. Future research should explore telehealth approaches for strength training protocols, which would involve training parents as personal trainers and conducting periodic follow-ups to monitor progress.
Finally, this review does not address the impact of different strength training modalities on functional motor capacity. However, it is clear that muscle morphology underpins proper motor function, with muscle strength developing before power, which in turn precedes overall motor capacity. Therefore, the primary finding remains that resistance training targeting muscle hypertrophy should be prioritized, with complementary power training following initial adaptations.
In conclusion, this focused review highlights the distinct effects of resistance and power training on muscle adaptations in ambulatory children with CP. Practical barriers, such as participant recruitment and long-term trial compliance, have impeded data collection and precluded consensus on optimal strength-training protocols. Nonetheless, current evidence favors initial resistance training to induce muscle fiber hypertrophy. If strength training principles established in able-bodied populations apply here, loads should be progressively raised to submaximal intensities (∼80% of 1 RM) and paired with power training to further promote fascicle lengthening and mobility; these protocols should be tested in long-term trials (6–12 months). In the meantime, the efficacy of strength training—and its optimal protocols—for children with CP who have severe cognitive and mobility impairments (GMFCS IV and V) remains largely unexamined. Future research should prioritize this cohort and assess whether integrating strength training, invasive interventions, and tailored nutrition yields synergistic benefits.
Author contributions
BL: Conceptualization, Writing – original draft. JY: Writing – review & editing. YF: Writing – original draft. YX: Writing – review & editing. XZ: Writing – review & editing. YZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by Hunan Provincial Education Department Scientific Research Key Project “Academic Research Methods and Norms in Sports” (Grant No.: 23A0093; Grant holder: Prof. Fan Yunxiang).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patel DR, Neelakantan M, Pandher K, Merrick J. Cerebral palsy in children: a clinical overview. Transl Pediatr. (2020) 9:S125–35. doi: 10.21037/tp.2020.01.01
2. Korzeniewski SJ, Slaughter J, Lenski M, Haak P, Paneth N. The complex aetiology of cerebral palsy. Nat Rev Neurol. (2018) 14(9):528–43. doi: 10.1038/s41582-018-0043-6
3. Howard JJ, Herzog W. Skeletal muscle in cerebral palsy: from belly to myofibril. Front Neurol. (2021) 12:620852. doi: 10.3389/fneur.2021.620852
4. Barber L, Hastings-Ison T, Baker R, Barrett R, Lichtwark G. Medial gastrocnemius muscle volume and fascicle length in children aged 2 to 5 years with cerebral palsy. Dev Med Child Neurol. (2011) 53(6):543–8. doi: 10.1111/j.1469-8749.2011.03913.x
5. Moreau NG, Teefey SA, Damiano DL. In vivo muscle architecture and size of the rectus femoris and vastus lateralis in children and adolescents with cerebral palsy. Dev Med Child Neurol. (2009) 51(10):800–6. doi: 10.1111/j.1469-8749.2009.03307.x
6. Moreau NG, Bodkin AW, Bjornson K, Hobbs A, Soileau M, Lahasky K. Effectiveness of rehabilitation interventions to improve gait speed in children with cerebral palsy. Systematic review and meta-analysis. Phys Ther. (2016) 96(12):1938–54. doi: 10.2522/ptj.20150401
7. Moreau NG, Lieber RL. Effects of voluntary exercise on muscle structure and function in cerebral palsy. Dev Med Child Neurol. (2022) 64(6):700–8. doi: 10.1111/dmcn.15173
8. Merino-Andrés J, García de Mateos-López A, Damiano DL, Sánchez-Sierra A. Effect of muscle strength training in children and adolescents with spastic cerebral palsy: a systematic review and meta-analysis. Clin Rehabil. (2022) 36(1):4–14. doi: 10.1177/02692155211040199
9. Shilesh K, Karthikbabu S, Rao PT. The impact of functional strength training on muscle strength and mobility in children with spastic cerebral palsy—a systematic review and meta-analysis. Dev Neurorehabil. (2023) 26(4):262–77. doi: 10.1080/17518423.2023.2218905
10. Gillett JG, Boyd RN, Carty CP, Barber LA. The impact of strength training on skeletal muscle morphology and architecture in children and adolescents with spastic cerebral palsy: a systematic review. Res Dev Disabil. (2016) 56:183–96. doi: 10.1016/j.ridd.2016.06.003
11. Comfort P, Haff GG, Suchomel TJ, Soriano MA, Pierce KC, Hornsby WG, et al. National strength and conditioning association position statement on weightlifting for sports performance. J Strength Cond Res. (2023) 37(6):1163–90. doi: 10.1519/jsc.0000000000004476
12. Fernandez Ortega JA, los Reyes YGD, Garavito Peña FR. Effects of strength training based on velocity versus traditional training on muscle mass, neuromuscular activation, and indicators of maximal power and strength in girls soccer players. Apunts Sports Med. (2020) 55(206):53–61. doi: 10.1016/j.apunsm.2020.03.002
13. Xiao W, Bai X, Geok SK, Yu D, Zhang Y. Effects of a 12-week functional training program on the strength and power of Chinese adolescent tennis players. Children. (2023) 10(4):635. doi: 10.3390/children10040635
14. Xiao W, Bai X, Soh KG, Zhang Y. Effects of functional training on tennis-specific physical fitness and functional movement screen in junior tennis players. PLoS One. (2024) 19(9):e0310620. doi: 10.1371/journal.pone.0310620
15. van Vulpen LF, de Groot S, Rameckers E, Becher JG, Dallmeijer AJ. Improved walking capacity and muscle strength after functional power-training in young children with cerebral palsy. Neurorehabil Neural Repair. (2017) 31(9):827–41. doi: 10.1177/1545968317723750
16. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
17. McGrath S, Zhao X, Ozturk O, Katzenschlager S, Steele R, Benedetti A. Metamedian: an R package for meta-analyzing studies reporting medians. Res Syn Meth. (2024) 15(2):332–46. doi: 10.1002/jrsm.1686
18. Lin L, Aloe AM. Evaluation of various estimators for standardized mean difference in meta-analysis. Stat Med. (2021) 40(2):403–26. doi: 10.1002/sim.8781
19. Cho H-J, Lee B-H. Effect of functional progressive resistance exercise on lower extremity structure, muscle tone, dynamic balance and functional ability in children with spastic cerebral palsy. Children. (2020) 7(8):85. doi: 10.3390/children7080085
20. Elnaggar RK, Alghamdi MS, Alenazi AM, Alghadier M, Mahmoud MZ, Elsayed AEA, et al. Mechanical and morphological changes of the plantar flexor musculotendinous unit in children with unilateral cerebral palsy following 12 weeks of plyometric exercise: a randomized controlled trial. Children. (2022) 9(11):1604. doi: 10.3390/children9111604
21. Hanssen B, Peeters N, De Beukelaer N, Vannerom A, Peeters L, Molenaers G, et al. Progressive resistance training for children with cerebral palsy: a randomized controlled trial evaluating the effects on muscle strength and morphology. Front Physiol. (2022) 13:911162. doi: 10.3389/fphys.2022.911162
22. Kalkman BM, Holmes G, Bar-On L, Maganaris CN, Barton GJ, Bass A, et al. Resistance training combined with stretching increases tendon stiffness and is more effective than stretching alone in children with cerebral palsy: a randomized controlled trial. Front Pediatr. (2019) 7:333. doi: 10.3389/fped.2019.00333
23. Kruse A, Schranz C, Svehlik M, Tilp M. The effect of functional home-based strength training programs on the mechano-morphological properties of the plantar flexor muscle-tendon unit in children with spastic cerebral palsy. Pediatr Exerc Sci. (2019) 31(1):67–76. doi: 10.1123/pes.2018-0106
24. Lee M, Ko Y, Shin MMS, Lee W. The effects of progressive functional training on lower limb muscle architecture and motor function in children with spastic cerebral palsy. J Phys Ther Sci. (2015) 27(5):1581–4. doi: 10.1589/jpts.27.1581
25. Moreau NG, Holthaus K, Marlow N. Differential adaptations of muscle architecture to high-velocity versus traditional strength training in cerebral palsy. Neurorehabil Neural Repair. (2013) 27(4):325–34. doi: 10.1177/1545968312469834
26. Stackhouse SK, Binder-Macleod SA, Stackhouse CA, McCarthy JJ, Prosser LA, Lee SCK. Neuromuscular electrical stimulation versus volitional isometric strength training in children with spastic diplegic cerebral palsy: a preliminary study. Neurorehabil Neural Repair. (2007) 21(6):475–85. doi: 10.1177/1545968306298932
27. Reid SM, Carlin JB, Reddihough DS. Using the gross motor function classification system to describe patterns of motor severity in cerebral palsy. Dev Med Child Neurol. (2011) 53(11):1007–12. doi: 10.1111/j.1469-8749.2011.04044.x
28. Westbom L, Hagglund G, Nordmark E. Cerebral palsy in a total population of 4–11 year olds in Southern Sweden. Prevalence and distribution according to different CP classification systems. BMC Pediatr. (2007) 7(1):41. doi: 10.1186/1471-2431-7-41
29. Brien M, Krishna D, Ponnusamy R, Cameron C, Moineddin R, Coutinho F. Motor development trajectories of children with cerebral palsy in a community-based early intervention program in rural south India. Res Dev Disabil. (2024) 154:104829. doi: 10.1016/j.ridd.2024.104829
30. dos Santos AN, Neves da Costa CS, Baldessar GMT, Rocha NACF. Functional strength training in child with cerebral palsy GMFCS IV: case report. Dev Neurorehabil. (2013) 16(5):308–14. doi: 10.3109/17518423.2012.731085
31. Smati S, Annie P-L, Mathilde C, Martin L, Ballaz L. Effect of power training on locomotion capacities in children with cerebral palsy with GMFCS level III–IV. Disabil Rehabil. (2023) 45(14):2329–35. doi: 10.1080/09638288.2022.2090623
32. Lopez P, Radaelli R, Taaffe DR, Newton RU, Galvão DA, Trajano GS, et al. Resistance training load effects on muscle hypertrophy and strength gain: systematic review and network meta-analysis. Med Sci Sports Exerc. (2021) 53(6):1206–16. doi: 10.1249/mss.0000000000002585
33. Fisher JP, Steele J. Heavier and lighter load resistance training to momentary failure produce similar increases in strength with differing degrees of discomfort. Muscle Nerve. (2017) 56(4):797–803. doi: 10.1002/mus.25537
34. dos Santos WDN, Vieira CA, Bottaro M, Nunes VA, Ramirez-Campillo R, Steele J, et al. Resistance training performed to failure or not to failure results in similar total volume, but with different fatigue and discomfort levels. J Strength Cond Res. (2021) 35(5):1372–9. doi: 10.1519/jsc.0000000000002915
35. Muehlbauer T, Gollhofer A, Granacher U. Associations between measures of balance and lower-extremity muscle strength/power in healthy individuals across the lifespan: a systematic review and meta-analysis. Sports Med. (2015) 45(12):1671–92. doi: 10.1007/s40279-015-0390-z
36. Pontiff ME, Batra A, Li L, Moreau NG. Muscle power is associated with higher levels of walking capacity and self-reported gait performance and physical activity in individuals with cerebral palsy. Front Physiol. (2025) 15:1488905. doi: 10.3389/fphys.2024.1488905
37. Walker S, Trezise J, Haff GG, Newton RU, Häkkinen K, Blazevich AJ. Increased fascicle length but not patellar tendon stiffness after accentuated eccentric-load strength training in already-trained men. Eur J Appl Physiol. (2020) 120(11):2371–82. doi: 10.1007/s00421-020-04462-x
38. Duclay J, Martin A, Duclay A, Cometti G, Pousson M. Behavior of fascicles and the myotendinous junction of human medial gastrocnemius following eccentric strength training. Muscle Nerve. (2009) 39(6):819–27. doi: 10.1002/mus.21297
39. Damiano DL, Quinlivan J, Owen BF, Shaffrey M, Abel MF. Spasticity versus strength in cerebral palsy: relationships among involuntary resistance, voluntary torque, and motor function. Eur J Neurol. (2001) 8(s5):40–9. doi: 10.1046/j.1468-1331.2001.00037.x
40. Kudva A, Abraham ME, Gold J, Patel NA, Gendreau JL, Herschman Y, et al. Intrathecal baclofen, selective dorsal rhizotomy, and extracorporeal shockwave therapy for the treatment of spasticity in cerebral palsy: a systematic review. Neurosurg Rev. (2021) 44(6):3209–28. doi: 10.1007/s10143-021-01550-0
41. Olver J, Esquenazi A, Fung VSC, Singer BJ, Ward AB. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: international consensus statement. Eur J Neurol. (2010) 17(s2):57–73. doi: 10.1111/j.1468-1331.2010.03128.x
42. Çağlar Okur S, Uğur M, Şenel K. Effects of botulinum toxin A injection on ambulation capacity in patients with cerebral palsy. Dev Neurorehabil. (2019) 22(4):288–91. doi: 10.1080/17518423.2018.1502832
43. Williams SA, Elliott C, Valentine J, Gubbay A, Shipman P, Reid S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: the impact on muscle morphology and strength. Disabil Rehabil. (2013) 35(7):596–605. doi: 10.3109/09638288.2012.711898
44. Modlesky CM, Matias AB. Muscle in children with cerebral palsy: current evidence, knowledge gaps, and emerging research opportunities. Pediatr Res. (2024). doi: 10.1038/s41390-024-03422-x
45. Antonio J, Candow DG, Forbes SC, Gualano B, Jagim AR, Kreider RB, et al. Common questions and misconceptions about creatine supplementation: what does the scientific evidence really show? J Int Soc Sports Nutr. (2021) 18(1):13. doi: 10.1186/s12970-021-00412-w
46. Theis N, Brown MA, Wood P, Waldron M. Leucine supplementation increases muscle strength and volume, reduces inflammation, and affects wellbeing in adults and adolescents with cerebral palsy. J Nutr. (2021) 151(1):59–64. doi: 10.1093/jn/nxaa006
47. Zhao Y, He L, Peng T, Liu L, Zhou H, Xu Y, et al. Nutritional status and function after high-calorie formula vs. Chinese food intervention in undernourished children with cerebral palsy. Front Nutr. (2022) 9:960763. doi: 10.3389/fnut.2022.960763
48. Delobel-Ayoub M, Ehlinger V, Klapouszczak D, Duffaut C, Arnaud C, Sentenac M. Prevalence and characteristics of children with cerebral palsy according to socioeconomic status of areas of residence in a French department. PLoS One. (2022) 17(5):e0268108. doi: 10.1371/journal.pone.0268108
Keywords: exercise modalities, fascicle length, muscle hypertrophy, muscle mass, muscle volume, plyometric training, strength training
Citation: Liu B, You J, Fan Y, Xia Y, Zhang X and Zhang Y (2025) Resistance or power training to enhance lower limb muscle morphology in ambulatory children with cerebral palsy? A focused systematic review with meta-analysis. Front. Pediatr. 13:1546156. doi: 10.3389/fped.2025.1546156
Received: 17 December 2024; Accepted: 23 May 2025;
Published: 24 June 2025.
Edited by:
Elisabet Rodby-Bousquet, Lund University, SwedenReviewed by:
Kelly Greve, Cincinnati Children's Hospital Medical Center, United StatesEvgenia Manousaki, Karolinska Institutet (KI), Sweden
Copyright: © 2025 Liu, You, Fan, Xia, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jizhi You, MzU5Mjc1NTMyQHFxLmNvbQ==; Yunxiang Fan, MTEyNzFAaHVubnUuZWR1LmNu; Xiang Zhang, emhhbmd4NTgxQGh1bm51LmVkdS5jbg==; Yang Zhang, eXpoYW5nNjhAY3JpbXNvbi51YS5lZHU=
 Bo Liu
Bo Liu Jizhi You2*
Jizhi You2* Yang Zhang
Yang Zhang