- 1Department of Ultrasonic Medicine, West China Second University Hospital of Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Tibet Autonomous Region Women’s and Children’s Hospital, West China Second University Hospital of Sichuan University, Lhasa, China
- 4Xizang Region Child Development Clinical Medical Research Center, Xizang, China
Congenital ventricular aneurysm is a rare malformation affecting all cardiac chambers, and is most frequently observed in the left ventricle. Congenital ventricular aneurysm is characterized by localized bulges in the ventricular wall, typically distinct from the normal myocardium, and it shows akinesia or dyskinesia. Using multimodality imaging, we diagnosed a case of ventricular septal aneurysm complicated by biventricular myocardial non-compaction in a child. After excluding various potential causes, we considered a congenital developmental abnormality as the most likely pathogenic factor.
1 Introduction
A left ventricular (LV) aneurysm typically occurs in middle-aged and elderly patients with coronary heart disease. It is characterized by ventricular expansion in the infarcted region, accompanied by myocardial thinning, necrosis, and subsequent replacement with fibrous tissue (1). The thinning of the ventricular wall in affected areas leads to impaired or paradoxical heart contraction (1). This condition most frequently arises as a complication of acute myocardial infarction, though multiple other etiological factors have been identified. These include traumatic injury, hypertrophic cardiomyopathy, infectious processes such as tuberculosis and endocarditis, inflammatory manifestations of connective tissue disorders, coronary vascular pathologies (notably Kawasaki disease), and congenital coronary artery origin anomalies (1). In contrast to these acquired forms, congenital ventricular aneurysm represents an exceptionally rare clinical entity, with an estimated prevalence of approximately 5/1,000,000 individuals, showing no sex predilection, and its etiology remains unclear (2).
Ventricular non-compaction cardiomyopathy is characterized by an abundance of prominent ventricular trabeculae and deep intertrabecular recesses. The pathogenesis of this condition is thought to be associated with disturbances in endocardial morphogenesis, leading to the stagnation of normal cardiac development (3).
We reported a case of LV congenital septal aneurysm complicated by biventricular non-compaction, as shown by multimodal imaging, in a boy. To the best of our knowledge, this finding has not been previously been reported.
2 Case description
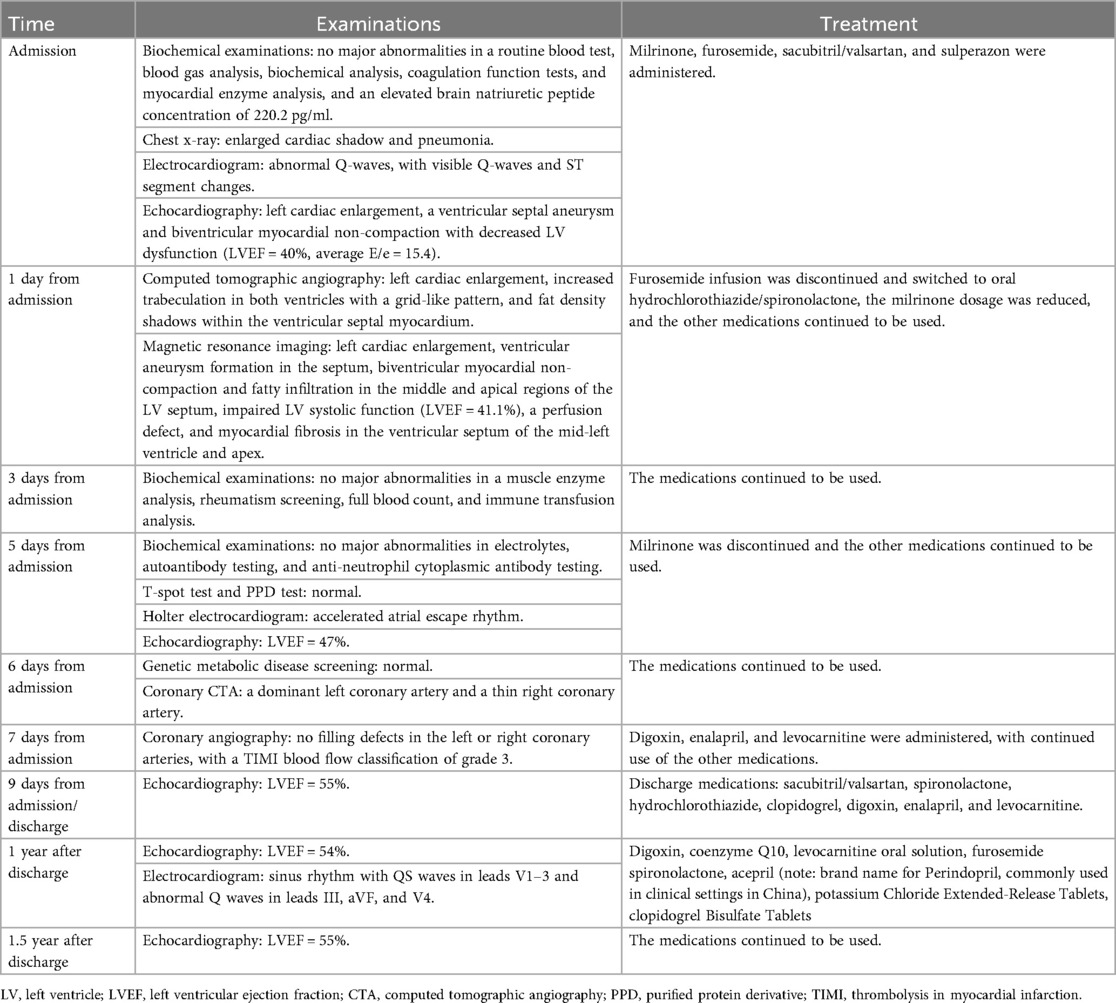
A 5-year-old boy presented with a history of cough and two fever episodes within 3 weeks. Upon admission, his vital signs were stable (T: 36.5 ℃, HR 98 bpm, RR 20 bpm). A physical examination showed the absence of wheezing, with notable cardiothoracic findings. These findings included precordial prominence, cardiomegaly on percussion (documented as 0.5-cm lateral displacement of the apical impulse from the left midclavicular line at the 5th intercostal space), and a regular cardiac rhythm without audible murmurs. His personal history (including prenatal examinations) and familial medical histories were noncontributory. A chest x-ray showed an enlarged cardiac shadow and pneumonia. Electrocardiographic findings included abnormal Q-waves, with visible Q-waves in leads II, III, aVF, and V2–V4. Additionally, ST segment changes were observed (ST segment elevation of 0.1–0.2 mV in leads V1–V4) (Figure 1A), along with QTc prolongation, left atrial heterogeneity, and double-compartment hypertrophy. Echocardiography indicated left cardiac enlargement [Left atrial volume = 28.3 ml (Z score = 4.0), LVend-diastolic diameter = 42 mm (Z score = 5.6)], a ventricular septal aneurysm in the basal and intermediate segments measuring 30 mm × 13.8 mm × 19.0 mm (Figure 2), and biventricular myocardial non-compaction with decreased LV dysfunction [ejection fraction (EF) = 40%, average E/e' = 15.4]. Computed tomographic angiography was systematically conducted for an accurate morphological assessment of the ventricular aneurysm (including topographic localization, volumetric measurement, and perianeurysmal anatomy). This technique showed left cardiac enlargement, increased trabeculation in both ventricles with a grid-like pattern, and fat density shadows within the ventricular septal myocardium (Figure 3), and a ventricular septal aneurysm. A Holter electrocardiogram showed an accelerated atrial escape rhythm (Figure 1B). Magnetic resonance imaging showed left cardiac enlargement [Left atrial volume = 34.82 ml (Z score = 4.9), LV end-diastolic volume = 76.41 ml (Z score = 4.1)], local aneurysm formation in the ventricular septum, biventricular myocardial non-compaction, and fatty infiltration in the middle and apical regions of the LV septum (Figure 4). LV systolic function was impaired (LV ejection fraction = 41.1%) and the right ventricular ejection fraction was normal (55.4%). First-pass perfusion imaging showed a perfusion defect in the ventricular septum of the mid-left ventricle and apex. Delayed enhancement imaging indicated myocardial fibrosis in the above area, which was likely secondary to myocardial ischemia.
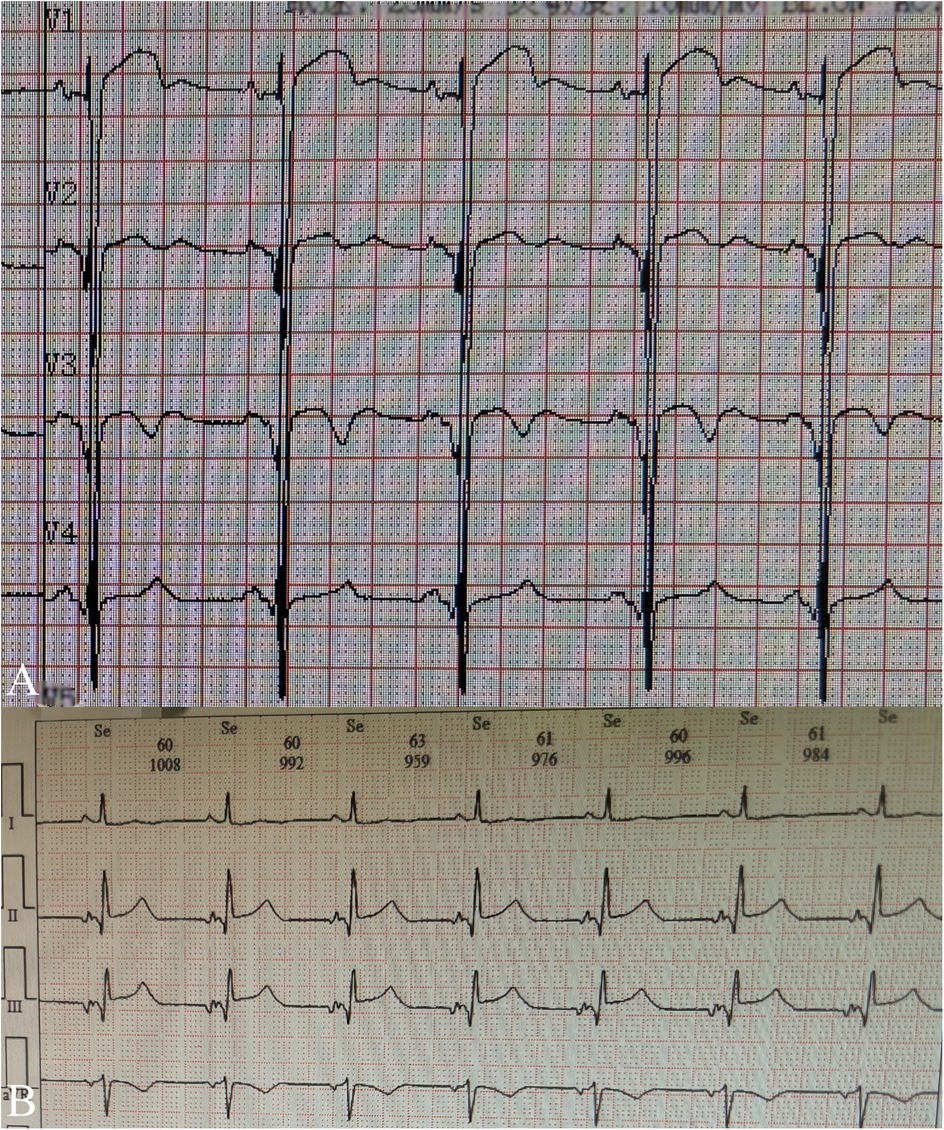
Figure 1. Electrocardiogram fingdings during hospitalization: (A) routine electrocardiogram shows abnormal Q-waves, with visible Q-waves in leads V2–V4 and ST segment elevation of 0.1–0.2 mV in leads V1–V4; (B) Excerpt from holter electrocardiogram monitoring reveals accelerated atrial ectopic rhythm.
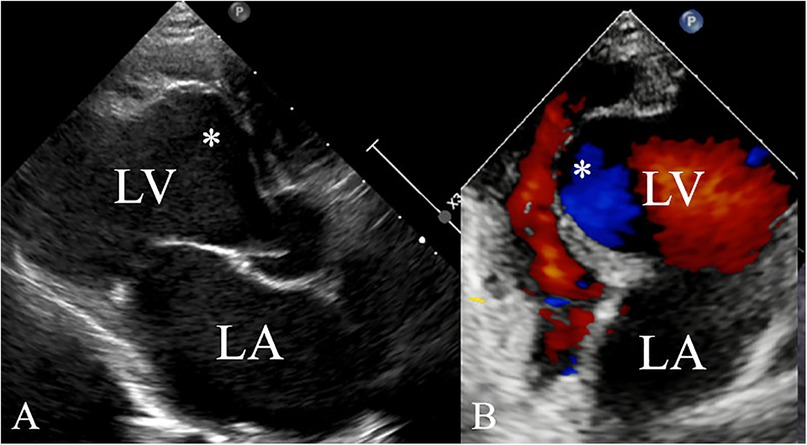
Figure 2. Ventricular aneurysm (*) detected by echocardiography (A) of the left ventricle in the long-axis view and (B) the apical four-chamber view.
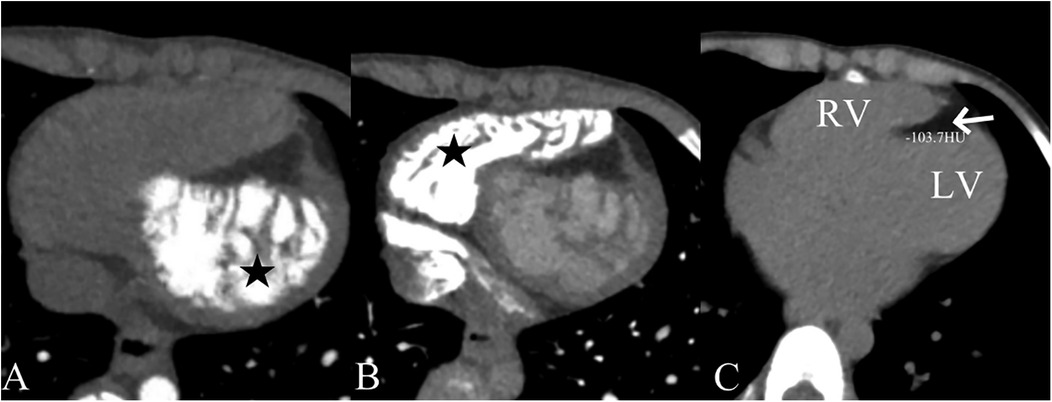
Figure 3. Computed tomographic angiography findings showing left (A) and right (B) ventricular myocardial non-compaction (★) and fat density shadows within the ventricular septal myocardium (arrow). The computed tomographic value was −103.7 HU (C).
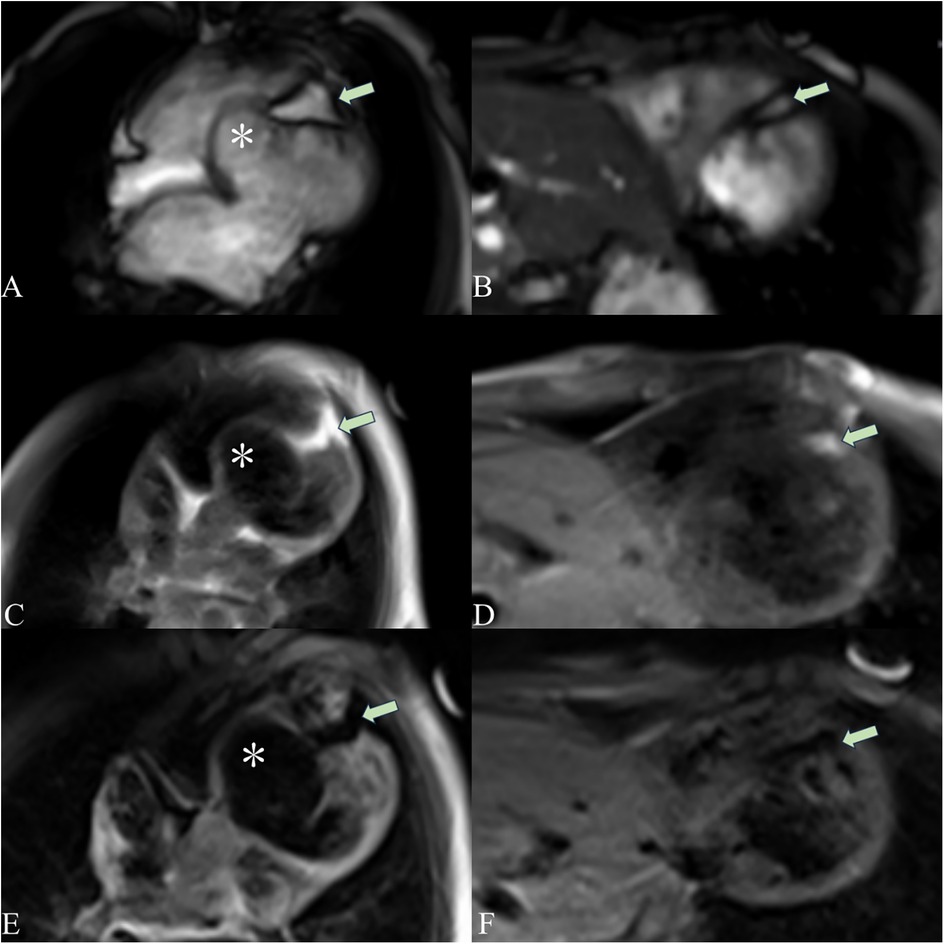
Figure 4. Cardiac magnetic resonance imaging showing a ventricular aneurysm (*) and fatty infiltration. The cardiac magnetic resonance four-chamber view cine sequence (A), T1-weighted image (C), and T2 fat-suppression sequence (E) show a ventricular aneurysm (*) and fatty signals (arrows) in the middle and apical regions of the LV septum. The short-axis cine sequence (B), T1-weighted image (D), and T2 fat-suppression sequence (F) demonstrate fatty signals (arrows) in the middle and apical regions of the LV septum. On T1-weighted imaging, fat appears as a high-signal intensity area. The T2 fat-suppression sequence suppresses fat signals.
The patient showed an elevated brain natriuretic peptide concentration of 220.2 pg/ml. Coronary computed tomographic angiography was conducted because of the boy's myocardial ischemia, despite an echocardiogram indicating a normal coronary origin and proximal segment. This imaging technique showed a dominant left coronary artery and a thin right coronary artery. Subsequently, coronary angiography was carried out to exclude coronary artery disease. The angiography examination showed no filling defects in the left or right coronary arteries, with a thrombolysis in myocardial infarction blood flow classification of grade 3. Comprehensive evaluations, including a blood routine test, blood gas analysis, biochemical analysis, coagulation function tests, myocardial enzyme analysis, muscle enzyme analysis, rheumatism screening, full blood count, immune transfusion analysis, electrolytes, autoantibody testing, anti-neutrophil cytoplasmic antibody testing, genetic metabolic disease screening, a T-spot test, and a purified protein derivative test, showed no major abnormalities. These findings ruled out endocarditis, tuberculosis, connective tissue disease, and other inflammatory causes. Whole-exome sequencing of the proband identified multiple phenotype-associated genetic variants, with subsequent parental segregation analysis by Sanger sequencing. However, a comprehensive pathogenicity assessment in accordance with the ACMG/AMP guidelines showed that the classification of these variants was variants of uncertain significance. Notably, a comprehensive genomic analysis showed no detectable rare pathogenic variants associated with the boy's clinical presentation. Consequently, the cardiac condition in this case was attributed to congenital factors. The parents opted for conservative management, and based on the patient's examination outcomes and evolving condition, the medication regimen was sequentially adjusted from milrinone, furosemide, sulperazon, and sacubitril/valsartan to sacubitril/valsartan, spironolactone, hydrochlorothiazide, clopidogrel, digoxin, enalapril, and levocarnitine. This treatment resulted in a stable current condition with an improved LVEF (55%) at discharge. The patient has been followed up for one and a half years, continues to receive conservative treatment, and remains in stable condition (Table 1).
3 Discussion
Congenital ventricular aneurysm is rare and hypothesized to be associated with prenatal viral infections, coronary artery embolism due to placental sinus abnormalities or stenosis, and focal ventricular wall weakening resulting from intrinsic embryonic developmental anomalies (4). There is limited literature on ventricular aneurysms in children. Some reports have indicated that these aneurysms are predominantly located in the apex rather than the septum (5). Non-compaction of the myocardium is characterized by excessive trabeculation and predominantly involves the left ventricle. In some cases, the right ventricle may also be affected—either concurrently with the left ventricle or as an isolated manifestation. This condition can occur in isolation or coexist with other cardiomyopathies. With advances in imaging techniques, its detected incidence in the pediatric population has been increasing. The etiology is heterogeneous, encompassing both genetic and acquired forms (6, 7).
Only four cases (8–11) of ventricular aneurysm coexisting with myocardial non-compaction have been reported, with three cases in adults and one case in a child (11). The pediatric case involved a newborn with a congenital apex LV aneurysm and LV non-compaction, who presented with treatment-resistant heart failure and passed away at 5 months of age. Notably, ventricular septal aneurysms complicated by biventricular myocardial non-compaction have not previously been reported. In this case, the young patient presented with no notable personal or familial medical history. Following a chest x-ray indicating an enlarged cardiac shadow, subsequent examinations showed the presence of ventricular septal aneurysms complicated by biventricular non-compaction. Despite evidence of myocardial infarction and decreased cardiac function upon imaging assessment, the child remained asymptomatic. Coronary artery lesions and genetic abnormalities were ruled out, with no prior history of myocarditis or related trauma. Computed tomography and magnetic resonance imaging indicated fatty infiltration in the midventricular septum and apex. Consequently, we speculate that these concurrent lesions were due to a congenital developmental abnormality. We also speculate that the process of pathogenesis is relatively slow myocardial development of the corresponding segment of the ventricular septum owing to fatty infiltration of the ventricular septum, while the muscular ventricular septal segment continues to grow, resulting in local aneurysmal bulging of the ventricular septum to the right ventricle (12). Myocardial ischemia ensues because of damage to the microcirculation of the nondense and dense layers (13), as well as disruption of the cardiac spiral structure by the ventricular aneurysm over time (14). Consequently, this cascade of events may perpetuate a vicious cycle, ultimately culminating in myocardial infarction and scar formation. However, a limitation of this hypothesis is that it has not been confirmed by histopathology. We recommend that the patient be followed up regularly for a long time because he may be at risk for progressive cardiac deterioration and even death.
4 Conclusion
We report a case of LV septal aneurysm complicated by biventricular non-compaction, as shown by multimodal imaging, in a child. To the best of our knowledge, this condition has not previously been reported. Our case illustrates the feasibility of conservative treatment for such pediatric patients, though long-term follow-up remains necessary.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of West China Second University Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Writing – original draft. JC: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by National Key Research and Development Program of China under No. 2023YFC2705701, Science and Technology Program of Xizang Autonomous Region under No. XZ202401ZY0013.
Acknowledgments
The authors are grateful to Yamei Zhou and Lei Hu who contributed to the images accompanying this article. We thank Ellen Knapp, PhD, from Liwen Bianji (Edanz) (https://www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Antunes MJ, Antunes PE. Left-ventricular aneurysms: from disease to repair. Expert Rev Cardiovasc Ther. (2005) 3(2):285–94. doi: 10.1586/14779072.3.2.285
2. Sharma JR, Oforl-Amanfo G, Marboe C, Quaegebeur J. Congenital left ventricular aneurysm with pericardial effusion: prenatal diagnosis, surgical management and follow-up. Pediatr Cardiol. (2002) 23(4):458–61. doi: 10.1007/s00246-002-1528-y
3. Mirza H, Mohan G, Khan W, Alkhatib A, Kaur I, Asif M, et al. A review of left ventricular non-compaction cardiomyopathy (LVNC). J Community Hosp Intern Med Perspect. (2022) 12(6):51–63. doi: 10.55729/2000-9666.1120
4. Ohlow MA, Secknus MA, Geller JC, von Korn H, Lauer B. Prevalence and outcome of congenital left ventricular aneurysms and diverticula in an adult population. Cardiology. (2009) 112(4):287–93. doi: 10.1159/000159122
5. Ohlow MA, von Korn H, Lauer B. Characteristics and outcome of congenital left ventricular aneurysm and diverticulum: analysis of 809 cases published since 1816. Int J Cardiol. (2015) 185:34–45. doi: 10.1016/j.ijcard.2015.03.050
6. Srivastava S, Yavari M, Al-Abcha A, Banga S, Abela G. Ventricular non-compaction review. Heart Fail Rev. (2022) 27(4):1063–76. doi: 10.1007/s10741-021-10128-3
7. Rath A, Weintraub R. Overview of cardiomyopathies in childhood. Front Pediatr. (2021) 9:708732. doi: 10.3389/fped.2021.708732
8. Ahn JH, Koh JS, Park JR, Park MJ, Min JH, Cho SY, et al. Isolated left ventricular noncompaction with a congenital aneurysm presenting with recurrent embolism. J Cardiovasc Ultrasound. (2012) 20(2):103–7. doi: 10.4250/jcu.2012.20.2.103
9. Daprati A, Sassi CG, Garatti A, Saitto G, Menicanti L. Congenital left ventricular aneurysm with myocardial noncompaction pattern. Asian Cardiovasc Thorac Ann. (2020) 28(8):504–6. doi: 10.1177/0218492320949833
10. Shan S, He X, He L, Wang M, Liu C. Coexistence of congenital left ventricular aneurysm and prominent left ventricular trabeculation in a patient with LDB3 mutation: a case report. J Med Case Rep. (2017) 11(1):229. doi: 10.1186/s13256-017-1405-1
11. Ootani K, Shimada J, Kitagawa Y, Konno Y, Miura F, Takahashi T, et al. Congenital left ventricular aneurysm coexisting with left ventricular non-compaction in a newborn. Pediatr Int. (2014) 56(5):e72–4. doi: 10.1111/ped.12415
12. Fernandez-Valls M, Srichai MB, Stillman AE, White RD. Isolated left ventricular apical hypoplasia: a new congenital anomaly described with cardiac tomography. Heart. (2004) 90(5):552–5. doi: 10.1136/hrt.2003.010637
13. Stöllberger C, Finsterer J. Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr. (2004) 17(1):91–100. doi: 10.1016/S0894-7317(03)00514-5
Keywords: congenital ventricular aneurysm, left ventricle, multimodality imaging, biventricular myocardial non-compaction, child
Citation: Zhou J and Chen J (2025) Case Report: Multimodality imaging of a congenital ventricular septal aneurysm complicated by biventricular myocardial non-compaction in a child. Front. Pediatr. 13:1559102. doi: 10.3389/fped.2025.1559102
Received: 11 January 2025; Accepted: 17 September 2025;
Published: 1 October 2025.
Edited by:
Liqun Sun, Zhejiang University, ChinaReviewed by:
Michael Yeong, The University of Queensland, AustraliaCecilia Lazea, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Yusuf Shieba, South Valley University, Egypt
Copyright: © 2025 Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiao Chen, Y2hlbmppYW9Ac2N1LmVkdS5jbg==
 Jie Zhou
Jie Zhou Jiao Chen
Jiao Chen