- University Children’s Hospital Regensburg (KUNO-Clinics), University of Regensburg, Regensburg, Germany
Multisystem inflammatory syndrome in children (MIS-C) is a hyperinflammatory disease that occurs after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report a 9-month-old male infant with two episodes of hyperinflammatory disease, each involving the coronary arteries, within a short period of time during the SARS-CoV-2 pandemic. Although both episodes met the official criteria for MIS-C, this case illustrates the difficulty in distinguishing MIS-C from its main differential diagnosis, Kawasaki disease (KD). Recurrence of postviral hyperinflammatory disease is rare. Compared with KD, the recurrence of MIS-C is even rarer, but clinicians should be aware of this possibility. Our case also emphasizes the need to follow up these patients closely and to detect sequelae regularly, especially cardiovascular sequelae, at an early stage.
Introduction
A new rare hyperinflammatory disease, now known as multisystem inflammatory syndrome in children (MIS-C), emerged shortly after the beginning of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (1). Its main characteristics are fever, elevated inflammatory parameters, and multiple organ dysfunction, in particular cardiac dysfunction and severe shock, and hyperinflammation is considered the presumed cause. MIS-C shares similarities with Kawasaki disease (KD), but epidemiological, clinical, and immunological differences may help to distinguish MIS-C from KD (2, 3). The clinical course of children affected by MIS-C tends to be more severe and may be potentially life-threatening, requiring a rapid diagnosis and prompt treatment (4). Complete recovery without sequelae is the usual course even with severe initial presentations (5). We describe a male infant who had two episodes of hyperinflammatory disease, each involving the coronary arteries, within a short period of time during the SARS-CoV-2 pandemic and discuss the difficulty in distinguishing MIS-C from KD.
Case report
First episode
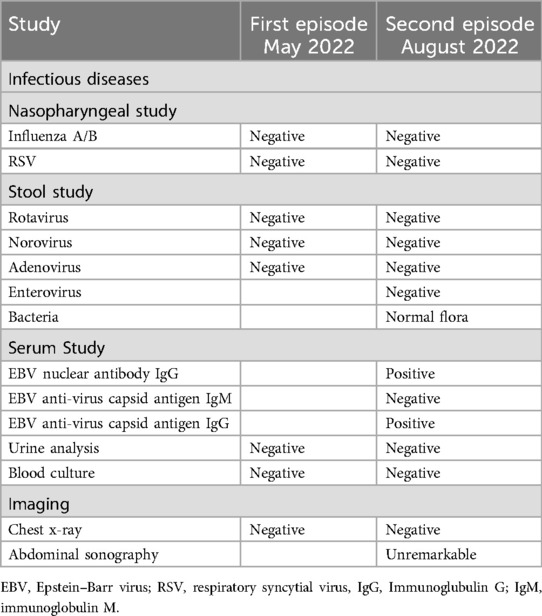
A previously healthy 9-month-old male infant, up to date with routine immunizations (without the SARS-CoV-2 vaccine, as it was not yet licensed for this age group), presented to our emergency department with a fever of up to 39.8°C for 8 days, accompanied by rhinorrhea. Prior to this presentation, oral antibiotic therapy with amoxicillin/clavulanic acid had been initiated by the general pediatrician, which was given for 3 days without clinical improvement. Diarrhea and vomiting had also been present for 3 days. On presentation, the infant appeared ill with low-grade fever, tachycardia, and normal room air oxygen saturation, otherwise, there were no further indicators on physical examination to explain the persistent fever. The absence of symptoms consistent with KD was notable, including, but not limited to, extremity changes, skin rash, conjunctivitis, oral changes, or cervical lymphadenopathy. Further diagnostics based on the current medical history and current clinical symptoms are shown in Table 1, which were unremarkable. Due to the persistent fever, non-response to antibiotics, and gastrointestinal symptoms, the presence of a hyperinflammation syndrome was suspected. The SARS-CoV-2 anti-spike antibodies were slightly positive (12.5 U/ml, normal range 0–0.79 U/ml), whereas the anti-nucleocapsid antibodies were negative, yet no prior history of infection or exposure to an infected individual was documented. His echocardiogram showed normal biventricular function but small aneurysms of the left main coronary artery (2.6 mm, Z-score +2.6) and left anterior descending coronary artery (2.2 mm, Z-score +2.8) (6). The following day, fever persisted (day 9) and there was a further increase in D-Dimers and N-terminal pro B-type natriuretic peptide (NT-proBNP). Based on these findings, the abnormal laboratory findings (Table 2), and the revised MIS-C definition by the Centers for Disease Control and Prevention (CDC) (7), MIS-C was considered the most likely differential diagnosis in the context of the global SARS-CoV-2 pandemic. In light of this, according to the Bavarian State Office for Health and Food Safety (LGL), 109,284 confirmed new SARS-CoV-2 infections were reported in Bavaria in the presumed week of infection (week 16 of the calendar year). The predominant type was Omicron BA.2 and the highest number of confirmed new infections was in week 12, with 309,345 infections (8). However, based on the findings, there was a possibility that incomplete KD (IKD) may also be a differential diagnosis (Table 3). According to the current guidelines, immunomodulatory therapy with intravenous immunoglobulins (2 g/kg) and prednisolone (2 mg/kg divided into three single doses) and anti-inflammatory therapy with acetylsalicylic acid (40 mg/kg divided into four single doses) were started (9). There was a good response to therapy with rapid normalization of body temperature, improvement of general condition, inflammatory markers, and resolved coronary artery aneurysms. The patient was discharged on the fifth day after admission with acetylsalicylic acid (5 mg/kg once a day) for antiplatelet effect. At the regular follow-up visits after 3 and 6 weeks, he presented in a good clinical condition with normal cardiac findings. No signs of skin peeling were observed. The steroid therapy was tapered for 20 days and the acetylsalicylic acid was stopped after 6 weeks. Importantly, between the first and second follow-up visits, the patient had a polymerase chain reaction (PCR)-proven SARS-CoV-2 infection with mild symptoms of an upper respiratory tract infection (Figure 1).
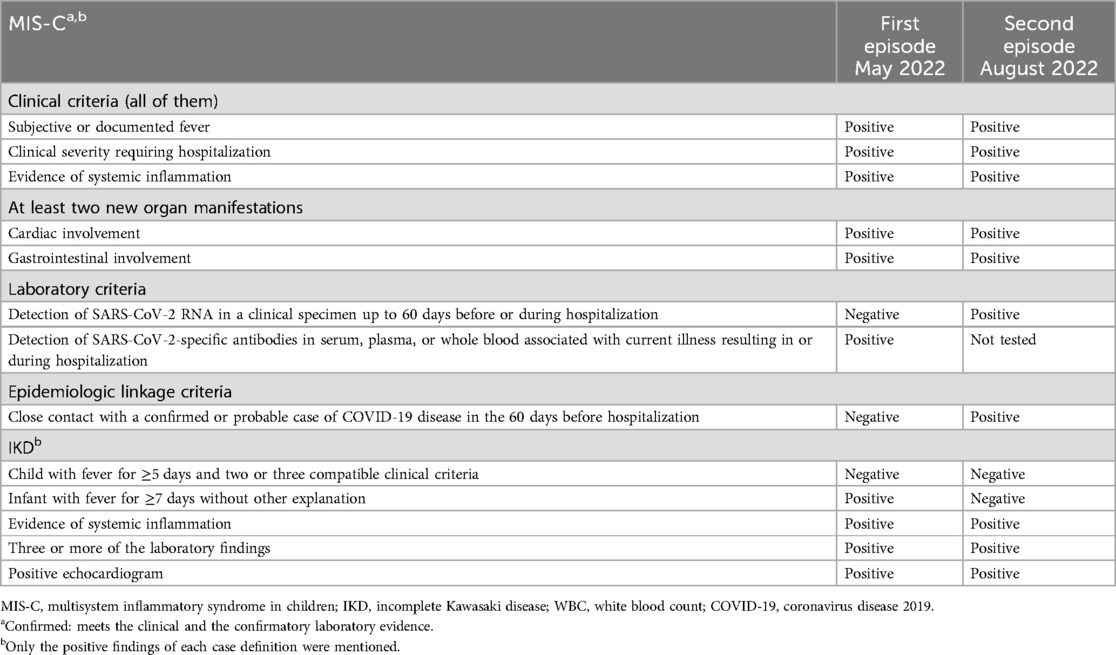
Table 3. Comparison of the adapted multisystem inflammatory syndrome in children case definition (7) and adapted incomplete Kawasaki disease case definition (17) and the findings in the patient.
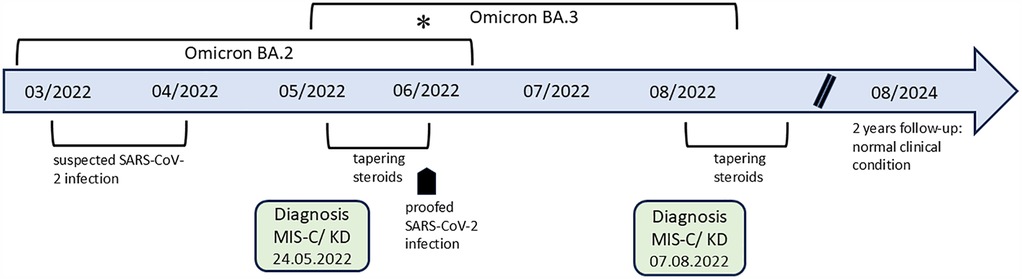
Figure 1. The timeline shows the course of both episodes. There was a change of SARS-CoV-2 Omicron subtype in Bavaria, with a predominance of variant BA.3 (supposed second infection) compared to variant BA.2 (supposed first infection), indicated with an asterisk.
Second episode
The patient presented again to our Emergency Department 3 months after being discharged from the hospital with fever and decreased general condition. The fever had persisted for 4 days and was resistant to oral antibiotic therapy with amoxicillin/clavulanic acid administered for 2 days. As already mentioned, the patient had a PCR-proven SARS-CoV-2 infection with mild symptoms 7 weeks before. On presentation, the infant was ill with fever, tachycardia, and diarrhea. The clinical examination revealed a reddened ear on the right side and a reddened throat, otherwise, no other indicators could be found. Comparable to the first episode, the absence of symptoms consistent with KD was again notable. Further diagnostics based on the current medical history and current clinical symptoms are shown in Table 1, which were unremarkable, with the exception of a positive Epstein–Barr virus (EBV) serology. The initial echocardiography was normal and the antibiotic therapy was continued. The patient showed no clinical improvement, but inflammatory markers and NT-proBNP increased (Table 2). Serial echocardiography again revealed a renewed small aneurysm of the left main coronary artery (2.7 mm, Z-score +2.6) (6). Thus, a second episode of hyperinflammatory disease was again considered the most likely diagnosis (Table 3). At the time of confirmed infection, 7 weeks before the second episode, in week 25 of the calendar year, 79,051 new infections were reported in Bavaria. In comparison to the first episode, the predominant variant had changed to Omicron BA.3 and the highest number of new infections was in week 29, with 130,676 infections (8). An immunomodulatory therapy and platelet aggregation inhibition with the same regimen as in the first episode was started and he showed a prompt clinical improvement to therapy. No signs of skin peeling were observed. Until now, follow-up care has included a period of more than 2 years after diagnosis of the second episode with normal clinical and echocardiographic findings (most recently left main coronary artery 2.7 mm, Z-score +1.6 and left anterior descending coronary artery 1.8 mm, Z-score +0.51) (Figure 1) (6).
Discussion
We report a case of recurrent hyperinflammation in an infant and highlight the challenge of distinguishing between MIS-C and KD/IKD. Recurrences of KD are rare. A comparison of data from the United States and Japan showed a recurrence rate of 1.7% in the United States, rising to 3.5% in Asians and Pacific Islanders (10). Thus far, there have been few reports on the worsening of symptoms in patients with suspected MIS-C after initial clinical improvement due to immunomodulatory therapy and both cases can be characterized as rebounds (11, 12). Another study described patients who had undergone MIS-C who did not develop MIS-C again after SARS-CoV-2 reinfection (13). Importantly, one case report presented a 9-year-old boy with two distinct illnesses that occurred within a few months, which were both rated as MIS-C, and therefore, a recurrence of the disease was considered (14). This case highlights, on the one hand, the different possible phenotypes of MIS-C and, on the other hand, the possibility that a subset of children may have a predisposition for repeat MIS-C. Another reason for recurrence, or perhaps in parallel, could be the different virulence of the SARS-CoV-2 subtypes triggering MIS-C.
It has been proposed by several studies that specific risk factors may play a role in the development of MIS-C. These include epidemiological factors, such as age and gender; pre-existing conditions, such as asthma and obesity; and congenital immune deficiencies (15, 16).
Meeting the criteria of the MIS-C definition by the CDC may be challenging, especially if not all typical clinical signs of a disease are present or when the clinical pictures are similar, as in KD/IKD and MIS-C. This represents a particular challenge during infancy when persistent fever is the sole clinical finding or when there are subtle or fleeting clinical signs in addition to fever. Therefore, initiating adequate therapy may be delayed (17). These circumstances put the patient at risk of disease progression or sequelae. The CDC requires detection or contact with SARS-CoV-2 as one crucial point in their diagnostic criteria for MIS-C (7). In the initial episode, antibodies against the spike protein were detected at a low level, while those against the nucleocapsid were negative. It is important to consider the following when evaluating this finding. The patient was not breastfed and the mother had neither a proven SARS-CoV-2 infection nor a SARS-CoV-2 vaccination before or during pregnancy. Therefore, it appears conceivable that the positive serology against a previous SARS-CoV-2 infection was not transmitted transplacentally, with uncertainty regarding a possible maternal sub-clinical SARS-CoV-2 infection. Furthermore, we interpreted the positive EBV serology found during the second episode as transferred antibodies due to the immunoglobulins that had been administered 3 months earlier. It is possible that maternal antibodies could be a factor, however, it should be noted that the mother did not appear to have experienced an EBV infection, at least from a clinical perspective. Although we have no proof of this, it would be unusual for maternal antibodies to persist until a child reaches the age of 12 months. One would actually expect that the antibodies would be higher as MIS-C develops within 2–6 weeks after contact or active infection with SARS-CoV-2. However, in a matched analysis of convalescent subjects, anti-nucleocapsid IgG levels fell significantly over time, with 8% of subjects being seronegative at 5 weeks, 34% at 6 months, and 48% at 12 months (18). In addition, in a study conducted in regions of Germany with a relatively high incidence of SARS-CoV-2 infections, a few patients who tested positive by PCR showed no seroconversion (19). Therefore, the absence of antibodies against the nucleocapsid antigen and even the low level of antibodies against the spike protein may not exclude a recent infection with SARS-CoV-2.
Symptoms of hyperinflammation reappeared in the patient more than 6 weeks (44 days) after the end of steroid therapy for the first episode. In between, he was in a normal clinical condition, including normal echocardiographic findings during two follow-up visits, but experienced a symptomatic infection with SARS-CoV-2, proven by PCR, 7 weeks before the second episode. The CDC definition and criteria led us to suspect a possible second episode of MIS-C, but, similar to the first episode, incomplete Kawasaki disease may also be a possibility (Table 3). SARS-CoV-2 antibodies were not determined because of the previously proven SARS-CoV-2 infection. In addition, it should be noted that the detection of SARS-CoV-2 antibodies would generally become less important as population immunity increases due to ongoing exposure or vaccination. This makes seropositivity less specific as a diagnostic marker for MIS-C (20). The reason for the possible SARS-CoV-2 reinfection within a few months may be due to the following. In the time period between the possible first asymptomatic and the proven second symptomatic infections, there was a change in the Omicron subtype as mentioned above (Figure 1). The Omicron variants contained many new mutations in the spike protein, which caused a rapid spread around the world and large outbreaks among children and adolescents (21). Furthermore, the second infection occurred during the period when the patient was still receiving systemic steroid therapy in a tapering form. Steroids may influence the dysregulated immune response in a positive way but may exert inhibitory effects on the immune defense (22). In addition, it needs to be considered that infants generally have a different and less effective humoral immune response than older children following natural infections. This may result from the inability of bone marrow at this age to establish a pool of long-lived plasma cells (23). All these reasons make the body more susceptible and can contribute to possible reinfection.
We admit that, in both episodes, IKD has to be considered as the most likely differential diagnosis (Table 3). This is supported by the values of some laboratory parameters, such as lymphocytes and thrombocytes, which are considered important differentiators between MIS-C and KD/IKD, and the age of our patient, which is in contrast to most reported patients (1, 2). A reliable distinction between MIS-C and KD/IKD would be of great importance as the diagnoses have different risks of cardiovascular involvement and clinical decompensation. In light of this, there is ongoing focus on leveraging various tools, such as biomarkers, to enhance this differentiation. For example, an analysis was conducted by the working group of the International Kawasaki Disease Registry (IKDR) of a substantial and varied cohort of patients suffering from KD and MIS-C, with the objective of identifying disparities in cardiac biomarkers. It was demonstrated that higher NT-proBNP (≥1,500 ng/L) and troponin I (≥20 ng/L) values are indicative of MIS-C compared to KD with reasonable specificity and may be clinically useful in differentiation (24). In our case, this would be applicable in the context of the second episode. Echocardiography represents a further point at which these two diseases can differ with regard to the cardiovascular system. Coronary artery involvement is more prevalent in patients with KD/IKD, while heart valve involvement, left ventricular systolic dysfunction, and pericardial effusion are more prevalent in MIS-C (25). Another possible way to differentiate between MIS-C and KD was demonstrated using circulating endothelial cells, a marker for inflammatory vessel wall damage. The amount of circulating endothelial cells was significantly higher in KD compared to MIS-C. One possible explanation for this could be that the changes in the vessel walls in MIS-C are secondary to systemic inflammation, possibly due to a cytokine storm, and not necrotizing arteritis as observed in KD (26). In addition, it has been shown that the determination of immune profiles can facilitate the differentiation between these two diseases, but, similar to the determination of circulating endothelial cells, this was not possible in our institution at the time of presentation (27). However, no specific laboratory marker exists for either MIS-C or KD and MIS-C has been identified in infants who appear to have a less critical clinical course than older children (28, 29). Therefore, we argue that after a thorough workup for alternative diagnoses (Table 1); a failure to improve on antibiotics; coronary artery aneurysms, which are very rarely described in other circumstances; and rapid improvement after immunomodulatory treatment, a diagnosis of recurrence of hyperinflammation can be made in the absence of clear evidence of MIS-C or IKD (30, Table 3).
Conclusion
This case report aims to raise awareness of the potential for recurrence of hyperinflammation. It also highlights the fact that the current diagnostic criteria for MIS-C may not accurately identify all affected children adequately and the primary challenge in this clinical case was to make an accurate diagnosis. This is especially the case in infants, in whom the clinical course and the course of laboratory parameters may differ from older children. Further, it should be highlighted that despite two episodes of a hyperinflammatory disease within a short period of time, there was a good response to an immunomodulatory therapy with rapid clinical improvement and normalization of the affected coronary arteries. There is still uncertainty about the long-term course of hyperinflammation that occurred during the SARS-CoV-2 pandemic. Therefore, to enhance our knowledge in depth, it is necessary that these patients are followed up closely and regularly to detect sequelae, especially cardiovascular sequelae, at an early stage.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
M-JD: Conceptualization, Investigation, Writing – original draft. HM: Writing – review & editing. MK: Writing – review & editing. MM: Writing – review & editing. SG: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank our patient and his family for allowing us to present this case. We also thank Thomas Luginger and Dr Alexander Kiefer for their help in preparing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
2. Sharma C, Ganigara M, Galeotti C, Burns J, Berganza FM, Hayes DA, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat Rev Rheumatol. (2021) 17:731–48. doi: 10.1038/s41584-021-00709-9
3. Rivas MN, Arditi M. Kawasaki disease: pathophysiology and insights from mouse model. Nat Rev Rheumatol. (2020) 16:391–405. doi: 10.1038/s41584-020-0426-0
4. Bichali S, Bonnet M, Lampin ME, Baudelet JB, Reumaux H, Domanski O, et al. Impact of time to diagnosis on the occurrence of cardiogenic shock in MIS-C post-COVID-19 infection. World J Pediatr. (2023) 19:595–604. doi: 10.1007/s12519-022-00681-8
5. Lee S, Erdem G, Yasuhara J. Multisystem inflammatory syndrome in children associated with COVID-19: from pathophysiology to clinical management and outcomes. Minerva Pediatr (Torino). (2024) 76:268–80. doi: 10.23736/S2724-5276.23.07205-1
6. Dallaire F, Dahdah N. New equations and a critical appraisal of coronary artery Z scores in healthy children. J Am Soc Echocardiogr. (2011) 24:60–74. doi: 10.1016/j.echo.2010.10.004
7. Melgar M, Lee EH, Miller AD, Lim S, Brown CM, Yousaf AR, et al. Council of state and territorial epidemiologists/CDC surveillance case definition for multi- system inflammatory syndrome in children associated with SARS-CoV-2 infection—United States. MMWR Recomm Rep. (2022) 71:1–14. doi: 10.15585/mmwr.rr7104a1
8. Available at: www.lglg.bayern.de (Accessed April, 2025).
9. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19. Arthritis Rheumatol. (2022) 74:e1–20. doi: 10.1002/art.42062
10. Maddox RA, Holman RC, Uehara R, Callinan LS, Guest JL, Schonberger LB, et al. Recurrent Kawasaki disease: USA and Japan. Pediatr Int. (2015) 57:1116–20. doi: 10.1111/ped.12733
11. Pawar RS, Tarkasband VA, Patil RK, Naik AV. Second episode of multisystem inflammatory syndrome in children: relapse, rebound, or recurrence? Pediatr Infect Dis J. (2021) 40:e452. doi: 10.1097/INF.0000000000003249
12. Brisca G, Olcese C, Derchi ME, Trocchio G, Caorsi R, Moscatelli A, et al. Efficacy of anakinra on rebound of multisystem inflammatory syndrome. Pediatr Int. (2022) 64:e15337. doi: 10.1111/ped.15337
13. Buddingh EP, Vossen ACTM, Lamb HJ, van der Palen RLF, Brinkman DMC, et al. Reinfection with severe acute respiratory syndrome coronavirus 2 without recurrence of multisystem inflammatory syndrome in children. Pediatr Infect Dis J. (2021) 40:e491–2. doi: 10.1097/INF.0000000000003280
14. Hancock WC, Green AM, Creel C, Moyen S, Collins KP, Pishko SD, et al. Two distinct illnesses consistent with MIS-C in a pediatric patient. Pediatrics. (2022) 149:e2021053123. doi: 10.1542/peds.2021-053123
15. Rhedin S, Lundholm C, Horne A, Smew AI, Osvald EC, Haddadi A, et al. Risk factors for multisystem inflammatory syndrome in children—a population-based cohort study of over 2 million children. Lancet Reg Health Eur. (2022) 19:100443. doi: 10.1016/j.Ianepe.2022.100443
16. Bellos E, Santillo D, Vantourout P, Jackson HR, Duret A, Hearn H, et al. Heterozygous BTNL8 variants in individuals with multisystem inflammatory syndrome in children (MIS-C). J Exp Med. (2024) 221:e20240699. doi: 10.1084/jem.20240699
17. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease; a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
18. Amjadi MF, Adyniec RR, Gupta S, Bashar SJ, Mergaert AM, Braun KM, et al. Anti-membrane antibodies persist at least one year and discriminate between past coronavirus disease 2019 infection and vaccination. J Infect Dis. (2022) 226:1897–902. doi: 10.1093/infdis/jiac263
19. Laub O, Leipold G, Toncheva AA, Peterhoff D, Einhauser S, Neckermann P, et al. Symptoms, SARS-CoV-2 antibodies, and neutralization capacity in a cross sectional-population of German children. Front Pediatr. (2021) 9:678937. doi: 10.3389/fped.2021.678937
20. McCrindle BW, Manlhiot C. SARS-CoV-2-related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? JAMA. (2020) 324:246–8. doi: 10.1001/jama.2020.10370
21. Torjesen I. COVID-19: omicron variant is linked to steep rise in hospital admissions of very young children. Br Med J. (2022) 376:o110. doi: 10.1136/bmj.o110
22. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. (2017) 17:233–47. doi: 10.1038/nri.2017.1
23. Semmes EC, Chen JL, Goswami R, Burt TD, Permar SR, Fouda GG, et al. Understanding early-life adaptive immunity to guide interventions for pediatric health. Front Immunol. (2021) 11:595297. doi: 10.3389/fimmu.2020.595297
24. Walton M, Raghuveer G, Harahsheh A, Portman MA, Lee S, Khoury M, et al. Cardiac biomarkers aid in differentiation of Kawasaki disease from multisystem inflammatory syndrome in children associated with COVID-19. Pediatr Cardiol. (2025) 46:116–26. doi: 10.1007/s00246-023-03338-z
25. Alkan F, Bircan O, Bal A, Bayturan S, Zengin N, Coskun S. Comparison of early characteristics of multisystemic inflammatory syndrome and Kawasaki disease in children and the course of Kawasaki disease in the pandemic. BMC Pediatr. (2024) 24:485. doi: 10.1186/s12887-024-04966-x
26. Fabi M, Petrovic B, Andreozzi L, Corinaldesi E, Filice E, Biagi C, et al. Circulating endothelial cells: a new possible marker of endothelial damage in Kawasaki disease, multisystem inflammatory syndrome in children and acute SARS-CoV-2 infection. Int J Mol Sci. (2022) 23:10106. doi: 10.3390/ijms231710106
27. Moreews M, Le Gouge K, Khaldi-Plassart S, Pescarmona R, Mathieu AL, Malcus C, et al. Polyclonal expansion of TCR vbeta 21.3+ CD4+ and CD8+ T cells is a hallmark of multisystem inflammatory syndrome in children. Sci Immunol. (2021) 6:eabh1516. doi: 10.1126/sciimmunol.abh1516
28. Hufnagel M, Armann J, Jakob A, Doenhardt M, Diffloth N, Hospach A, et al. A comparison of pediatric inflammatory multisystem syndrome temporarily-associated with SARS-CoV-2 and Kawasaki disease. Sci Rep. (2023) 13:1173. doi: 10.1038/s41598-022-26832-5
29. Godfred-Cato S, Tsang CA, Giovanni J, Abrams J, Oster ME, Lee EH, et al. Multisystem inflammatory syndrome in infants <12 months of age, United States, May 2020–January 2021. Pediatr Infect Dis J. (2021) 40:601–5. doi: 10.1097/INF.0000000000003149
Keywords: hyperinflammation, infant, recurrence, multisystem, inflammatory, case report
Citation: Dechant M-J, Michel H, Kabesch M, Melter M and Gerling S (2025) Case Report: Two episodes of hyperinflammation in an infant consistent with multisystem inflammatory syndrome in children: recurrence or rather two different diseases?. Front. Pediatr. 13:1559244. doi: 10.3389/fped.2025.1559244
Received: 12 January 2025; Accepted: 13 May 2025;
Published: 5 June 2025.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Alenka Gagro, Children's Hospital Zagreb, CroatiaKate Webb, University of Cape Town, South Africa
Arthur Chang, University of Nebraska Medical Center, United States
Copyright: © 2025 Dechant, Michel, Kabesch, Melter and Gerling. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Markus-Johann Dechant, bWFya3VzLmRlY2hhbnRAYmFybWhlcnppZ2UtcmVnZW5zYnVyZy5kZQ==
 Markus-Johann Dechant
Markus-Johann Dechant Holger Michel
Holger Michel Michael Kabesch
Michael Kabesch Michael Melter
Michael Melter Stephan Gerling
Stephan Gerling