- 1Department of Public Health, J.N. Medical College, KLE Academy of Higher Education & Research, Belagavi, India
- 2Department of Paediatrics, J.N. Medical College, KLE Academy of Higher Education & Research, Belagavi, India
- 3Department of Epidemiology and Biostatistics, KLE Academy of Higher Education & Research, Belagavi, India
- 4Food and Micro-Nutrient Analysis Laboratory, KLE Academy of Higher Education & Research, Belagavi, India
Introduction: Rapid urbanization in low- and middle-income countries has led to the expansion of slums, where children face a heightened risk of undernutrition. This study aimed to determine the prevalence of undernutrition and its determinants among children residing in the urban slums of Belagavi, Karnataka.
Methods: The anthropometric measurements, clinical signs, demographic information, and dietary history of children aged 9–36 months from urban slums were assessed. The chi-square test, bivariate analysis, and multivariable logistic regression were used to identify the risk factors at the child, maternal, and household levels for undernutrition.
Results: The prevalence of stunting, wasting, and underweight among children aged 9–36 months was 44%, 11%, and 25%, respectively. Common predictors of stunting and underweight included low birth weight, short maternal stature, lack of maternal exposure to print media, and maternal consumption of iron–folic acid during pregnancy. A lack of maternal exposure to print media was also associated with wasting. In addition, stunting was linked to male sex and low maternal education, while underweight was associated with children from non-Hindu and non-Muslim religious backgrounds, and maternal lack of autonomy or control over household finances. Wasting, however, was associated with the 24–36 months age group and maternal gestational diabetes.
Conclusion: A high level of undernutrition was observed in the urban slums of Belagavi, with the prevalence of stunting exceeding the national and state averages. Undernutrition was linked to maternal, child, and household factors, including low birth weight, maternal stature, education, and autonomy.
Introduction
Malnutrition continues to threaten children's ability to thrive and survive, representing one of the greatest social challenges globally (1, 2). The World Health Assembly and Sustainable Development Goals target ending all forms of malnutrition by 2030, yet the progress remains uneven (3, 4). Undernutrition is particularly alarming in urban India, where 30.1% of children under 5 years are stunted, 18.5% are wasted, and 27.3% are underweight (5). Malnourished children face the highest risk of mortality and morbidity, along with some far-reaching consequences, affecting not only individuals but also societal and economic outcomes (6, 7).
In India, the extent of malnutrition is disproportionately high in urban slums, where children are more vulnerable than their counterparts in non-slum urban areas (8, 9). Low- and middle-income countries (LMICs) have experienced a significant growth in urbanization over the past few decades, leading to the development of slums characterized by overcrowding, unhygienic living conditions, and inadequate access to healthcare (9). Migrants to urban slums are particularly at risk, as they transition from a food-secure environment to precarious urban settings. Specific factors that contribute to poor nutritional status in children include a lack of immunization, early weaning, and dependence on street food, exacerbating undernutrition in this population and highlighting the urgent need for nutrition surveillance in these areas (9, 10).
Despite national efforts to address malnutrition, data from urban slums remains limited. Interventions related to malnutrition have historically focused on rural areas, leaving the unique vulnerabilities of urban slums unexplored. Karnataka, one of India's rapidly urbanizing states, exhibits substantial variation in health outcomes, but the nutritional status and its determinants among children in urban slums remain poorly understood. This study aims to assess the nutritional status and determinants of undernutrition among children aged 9–36 months in the urban slums of Belagavi, Karnataka.
Methods
Study context
The analysis presented in this study of the prevalence and determinants is from the data of the parent randomized controlled trial (RCT) conducted in the urban slums of Belagavi (CTRI Reg no: CTRI/2022/06/043002). The protocol for the RCT was approved by the Ethical Committee (Human) for Ph.D. Research, KAHER, Belagavi. Permission was obtained from the District Health Office, Taluka Health Office, and medical officers of the respective slum areas of Belagavi to conduct the study. Informed written consent was obtained from all the mothers as per the guidelines given by the Indian Council of Medical Research (ICMR), India.
Data collection time frame and eligibility screening for the RCT
The data for the current analysis were collected from March to July 2023. After obtaining a list of slums from the Taluka Health Office, it was determined that the slums in Belagavi fell under six primary health centers (PHCs). Permission to conduct the study was then secured from the respective medical officers of these PHCs. Data collected from all eligible and ineligible children aged 9–36 months at the screening time for the registered RCT were included in the present analysis.
Sample size estimation
The sample size of the randomized controlled trial was n = 360, calculated using a 95% confidence interval and 90% power, considering an attrition of 1.05 and design effect of 1.2. The following formula was used:
The previous randomized controlled trial showed a mean difference in height of the participants pre- and post-intervention of 14.7 ± 2.1 and 13.8 ± 1.7 cm, in the experimental and control groups, respectively (11, 12).
Data collection and nutritional assessment methods for RCT screening
Data were collected by a trained researcher at the time of the screening of the children for the randomized controlled trial. Mothers/caregivers were interviewed to collect data on correlates such as child, maternal, and household-level characteristics.
Anthropometric data were collected using standardized procedures. For the final measurement, an average of triplicate measurements was utilized. Weight was measured using an electronic scale with a digital screen to the nearest 0.01 kg (child and parent). Height was measured using an infantometer and a stadiometer (child and parents) to the nearest 0.1 cm. A non-flexible tape was used to measure mid-upper arm circumference and head and chest circumference to the nearest 0.1 cm.
Children were screened for signs of nutritional deficiencies. A child was considered to have signs of anemia if they presented with any one of the following: pale skin, pale eyes, pale tongue, or pale or spoon-shaped nails. Signs of a vitamin A deficiency included whether the child exhibited Bitot's spot, dry eyes, or scarring. A vitamin D deficiency was indicated by knock-knees, bowed legs, a widening of the wrist, Harrison's sulcus, or frontal bossing.
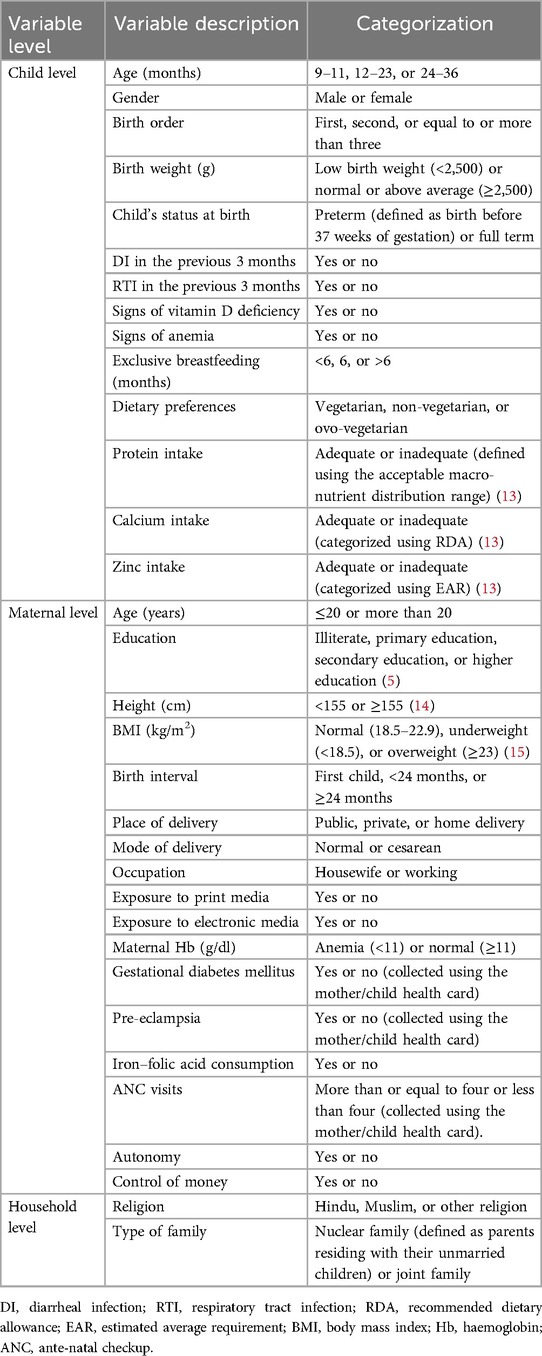
Variable description
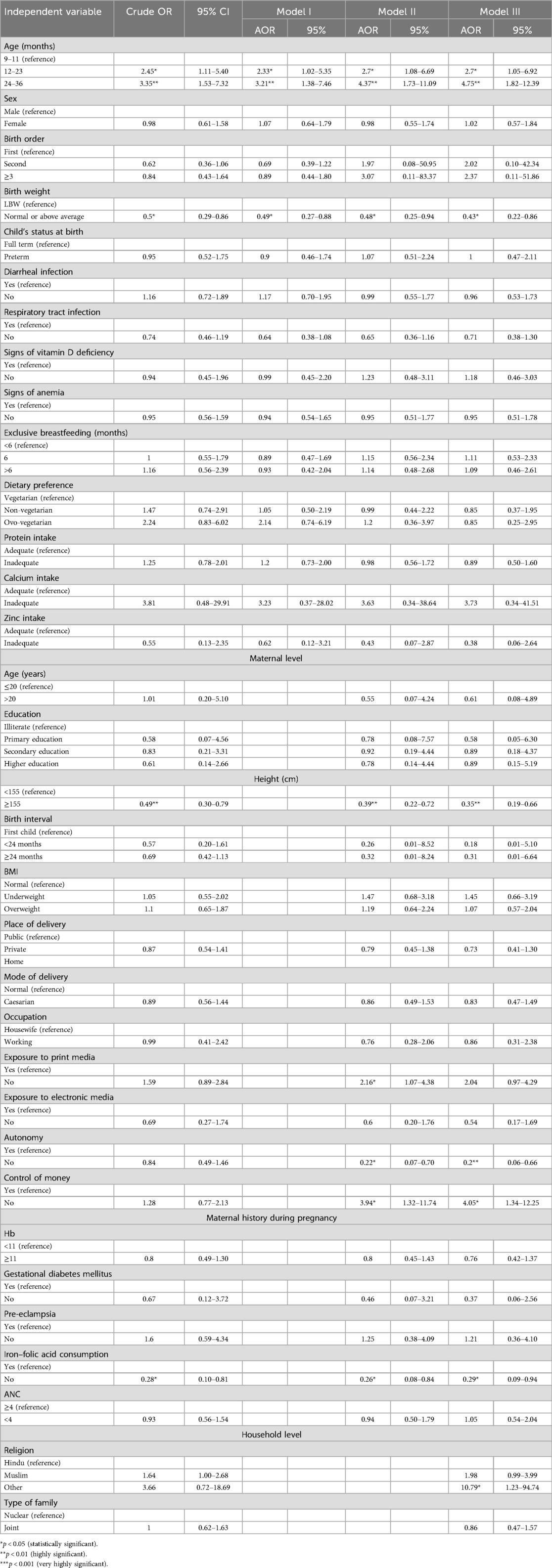
Table 1 describes the independent variables considered for this study.
The outcome variables included the prevalence of stunting, wasting, and underweight among children. Stunting, wasting, and underweight were defined as height-for-age Z score (HAZ), weight-for-height Z score (WHZ), and weight-for-age Z score (WAZ), respectively, more than 2 standard deviations (SD) below the median of the WHO growth standards (16). These outcomes were assessed using the WHO Anthro software (17).
Statistical analysis
Categorical data were reported as frequencies and percentages, while continuous variables were summarized using means and SD. The chi-square test was used to assess the significant differences between undernutrition and explanatory variables or covariates. In addition, simple or crude logistic regression was used to examine the association between each covariate and the outcome variable. Eventually, multiple or multivariable logistic regression was used to examine the independent effect of each covariate on child malnutrition. We applied three models for the analysis: Model 1 was adjusted for individual child-level characteristics, Model 2 accounted for both child and maternal-level characteristics, and Model 3 incorporated adjustments for child, maternal, and household-level characteristics. Further, the equation for the multivariable logistic regression model is expressed as
where π is the probability of the occurrence of the event (stunting, wasting, or underweight), βi is the regression coefficient associated with the reference group, and Xi is the explanatory variables. Stata 16 software was used for all the statistical analyses.
Results
A total of 420 children living in the urban slums of Belagavi were screened for the main study, with complete data available for 362 children in the present analysis.
Descriptive analysis
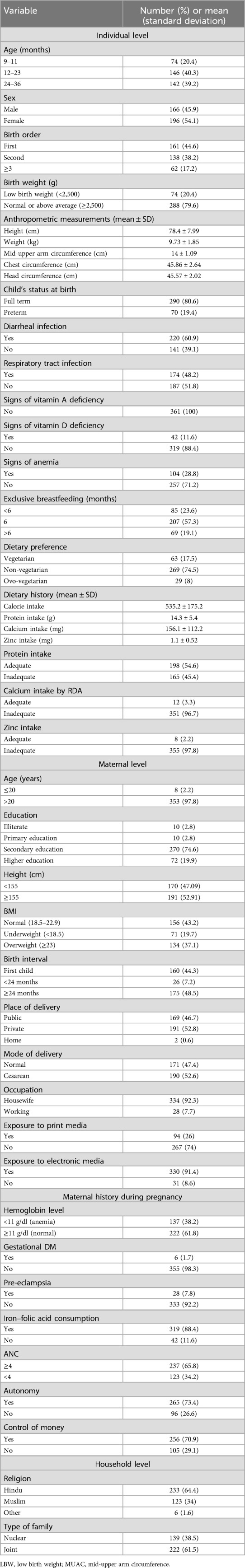
As shown in Figure 1, the prevalence among children aged 9–36 months was 44.2% for stunting, 11.05% for wasting, and 25.14% for underweight. Descriptive statistics of the child, maternal, and household-level characteristics are presented in Table 2. The majority of the children were aged 12–36 months (79.5%) and more than half were female (54.1%). Most of the children were first in birth order (44.6%), had normal or above-average birth weight (79.6%), and were full term (80.6%). Diarrhea and respiratory tract infection (RTI) were reported at least once in the previous 3 months in 60.9% and 48.2% of the individuals, respectively. No signs of vitamin A deficiency were observed, while 11.6% showed signs of a vitamin D deficiency and 28.8% exhibited signs of anemia. Nearly all the children had an inadequate intake of calcium (96.7%) and zinc (97.8%) in their diet. The majority of mothers had completed their secondary education (74.6%), were housewives (92.3%), had exposure to electronic media (91.4%), and 74% had no exposure to print media. Most households belonged to the Hindu religion (64.4%) and reported living in a joint family (61.5%).
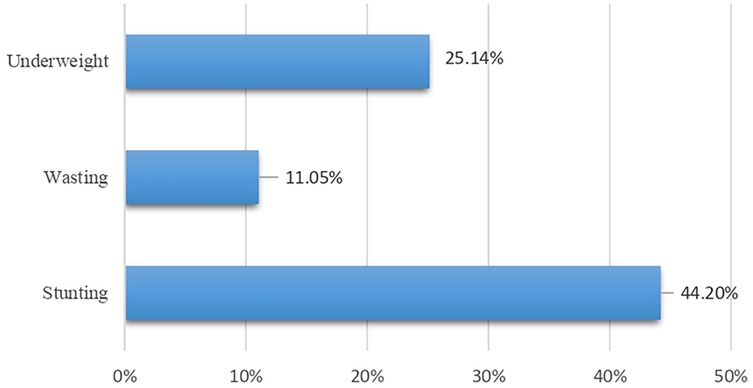
Figure 1. Prevalence of undernutrition in the urban slums of Belagavi among children aged 9–36 months.
Table 3 presents the results of the chi-square tests examining the association between various child, maternal, and household characteristics with different forms of undernutrition (stunting, wasting, and underweight). Stunting was significantly associated with low birth weight, low maternal education, and short maternal height. Wasting was significantly associated with child age. Characteristics including child age, low birth weight, short maternal height, and iron–folic acid (IFA) consumption were significantly associated with underweight.
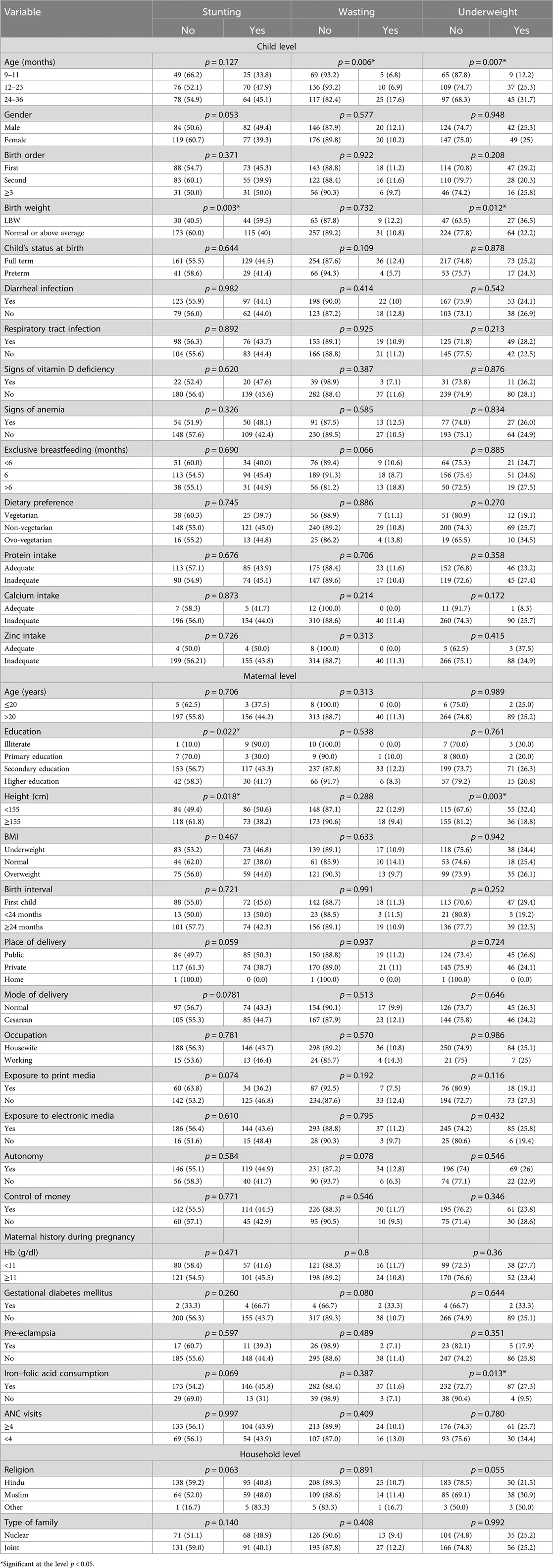
Table 3. Chi-square test results of the association between child, maternal, and household characteristics and undernutrition.
Association between child, maternal, and household characteristics and undernutrition: crude and multivariable analyses
Stunting
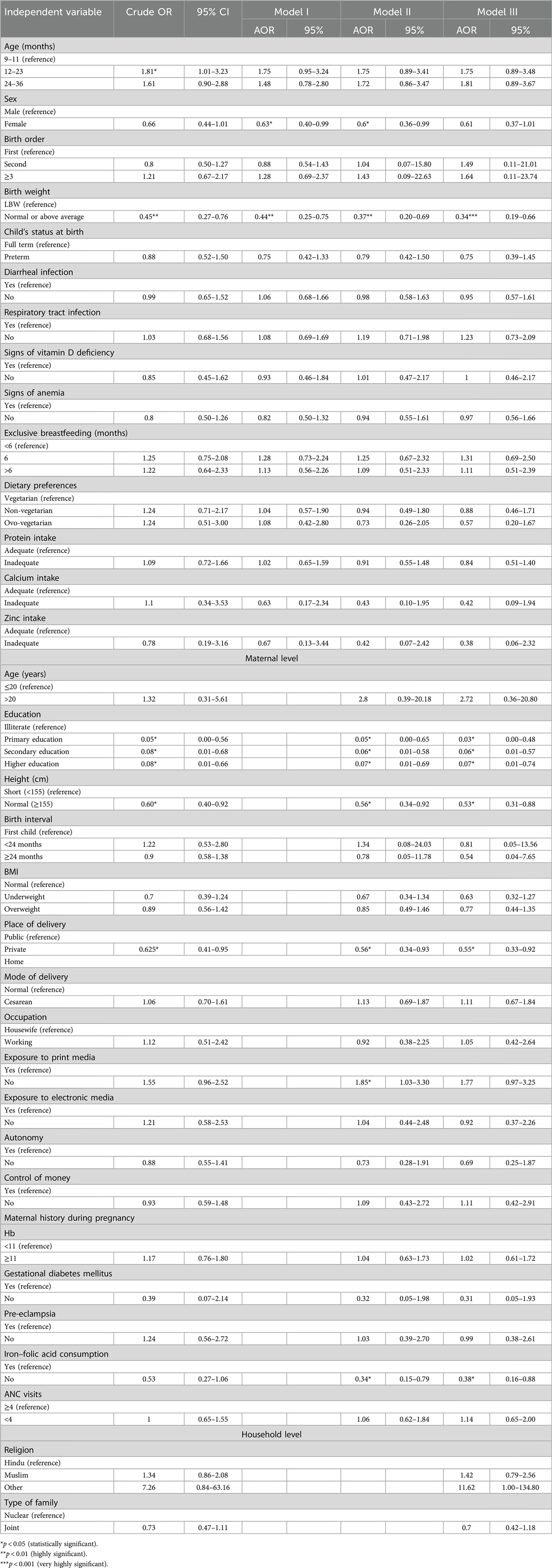
Table 4 presents the unadjusted crude and adjusted odds ratios (AORs) for the association between stunting and various child, maternal, and household-level characteristics.
Unadjusted estimates showed that children aged 12–23 months had a significantly higher risk of stunting (crude OR: 1.81, 95% CI: 1.01–3.23), but this association lost its significance after adjusting for other factors. Interestingly, sex was not significantly associated with stunting in the bivariate analysis, but in the adjusted model, being female was associated with a lower risk of stunting (AOR: 0.60, 95% CI: 0.36–0.99).
Children with normal or above-average birth weight consistently had a lower risk of stunting, as shown by both the crude (crude OR: 0.45, 95% CI: 0.27–0.76) and adjusted odds ratios (model 3—AOR: 0.34, 95% CI: 0.19–0.66). Regarding maternal characteristics, children of mothers with higher or secondary education were significantly less likely to be stunted (AOR: 0.06–0.07, 95% CI: 0.01–0.74), compared to mothers who were illiterate. Similarly, children of mothers with normal height were less likely to be stunted (AOR: 0.53, 95% CI: 0.31–0.88) compared to those with shorter mothers. Children delivered in private hospitals had a 45% lower risk of being stunted. A unique finding was the influence of maternal exposure to print media: in the adjusted models, children of mothers who lacked such exposure were more likely to be stunted (AOR: 1.85, 95% CI: 1.03–3.30), though this was not observed in the crude estimates. In addition, children of mothers who did not consume IFA tablets during pregnancy paradoxically showed a lower risk of stunting (AOR: 0.38, 95% CI: 0.16–0.88). This was a result that only became significant in the adjusted analyses, warranting further exploration.
Wasting
Table 5 highlights the factors associated with wasting. Children aged 24–36 months had a significantly higher risk of wasting in all the models, with an AOR of 4.04 (95% CI: 1.19–13.64). Among maternal characteristics, a lack of exposure to print media was associated with an increased risk of wasting in children (AOR: 3.18, 95% CI: 1.05–9.59). In addition, children of mothers without gestational diabetes had a lower risk of being wasted (AOR: 0.06, 95% CI: 0.01–0.52). Furthermore, the adjusted model indicates that children of mothers lacking autonomy had a decreased risk of being wasted (AOR: 0.1, 95% CI: 0.02–0.50).
Underweight
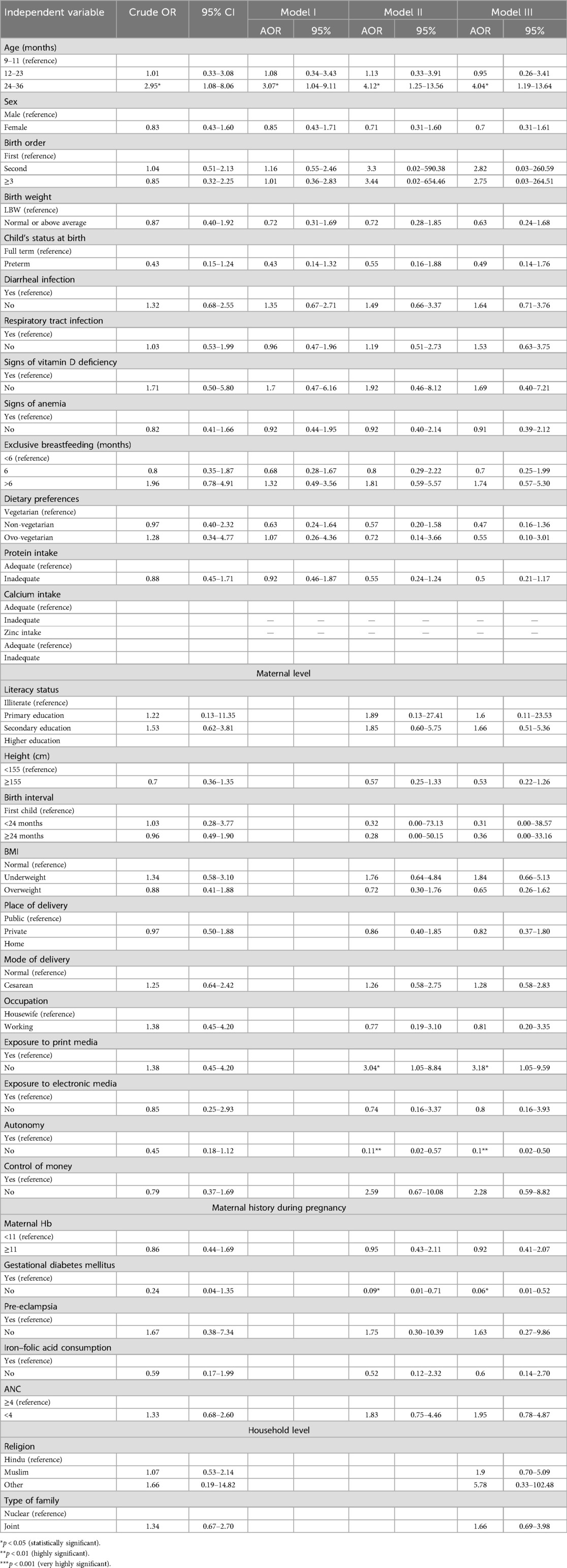
Table 6 shows the results for the factors associated with underweight. Estimates obtained from the final model (model 4) show that the children aged 12–23 and 24–36 months had a significantly higher risk of being underweight (95% CI: 1.82–12.39). Children with normal or above-average birth weight had a lower risk of being underweight (AOR: 0.43, 95% CI: 0.22–0.86) compared to those with low birth weight. Maternal characteristics of normal height and exposure to print media were protective against underweight (AOR: 0.35, 95% CI: 0.19–0.66; and AOR: 2.16, 95% CI: 1.07–4.38, respectively). Children of mothers who did not consume iron–folic acid tablets during pregnancy had a lower risk of being underweight (AOR: 0.29, 95% CI: 0.09–0.94). However, mother's autonomy was associated with an increased risk of the child being underweight (AOR: 0.20, 95% CI: 0.06–0.66), whereas a maternal lack of control over money led to a fourfold increased risk for the child to be underweight (AOR: 4.05, 95% CI: 1.34–12.25). Interestingly, children from non-Hindu and non-Muslim religious backgrounds had a dramatically increased risk of underweight (AOR: 10.79, 95% CI: 1.23–94.74), though this association was not apparent in the unadjusted analyses.
Discussion
The article aimed to determine the prevalence of stunting, wasting, and underweight among an understudied population of children aged 9–36 months residing in the urban slums of Belagavi, Karnataka, and examined the key determinants of undernutrition (18–21). The findings revealed a high prevalence of stunting compared to the national estimates and state averages of Karnataka, while the prevalence of wasting and underweight was lower (5). These disparities may reflect an increased availability and accessibility of calorie-dense foods to prevent underweight and wasting, but the overall quality of foods remains poor, leading to stunting (22, 23). Previous studies in the literature report similar findings in urban slums, reflecting the unique environmental and economic conditions present in slums (24–29).
Table 7 summarizes all statistically significant determinants of undernutrition after adjusting for child, maternal, and household-level characteristics, along with their corresponding odds ratios.
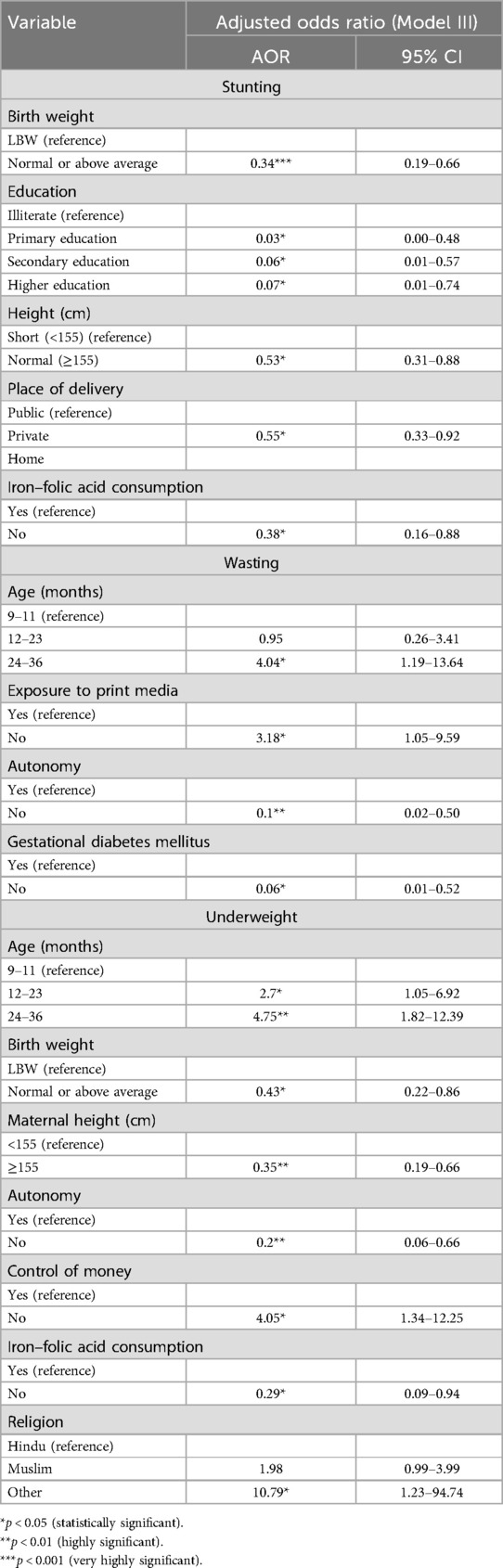
Table 7. Determinants of undernutrition after adjusting for child, maternal, and household-level characteristics.
Key risk factors of stunting identified in the study included being male, aged 12–23 months, low birth weight, having an illiterate mother, shorter maternal stature, and maternal IFA consumption, while delivery in the private health sector emerged as a protective factor. The association with male children aligns with previous studies in the literature (29–32). This could be attributed to male children’s higher metabolic requirements and increased susceptibility to infections, leading to increased nutrient deficits that manifest as stunting. Moreover, children aged 12–23 months are at a critical stage of growth and development, having started weaning with increased physical activity, which places them at an increased risk of stunting, with established findings on growth faltering during this phase (33–35). Low birth weight emerged as a significant risk factor for stunting, emphasizing the importance of interventions targeting maternal health during the early phase of pregnancy (36, 37). The strong association between maternal education and stunting is consistent with a substantial body of literature (29, 38, 39). Maternal education is linked with improved health-seeking behavior, better child-caring practices, and utilization of healthcare services. Educated girls marry at an older age, bear a child later in life, have fewer children, and are better equipped with knowledge and resources to prevent malnutrition (29). Maternal stature was also identified as a key risk factor for stunting and is in line with available literature. Short maternal stature reflects suboptimal nutrition intake during their own childhood and adolescent phase and is linked with a higher risk of intra-uterine growth restriction, further adding to the intragenerational cycle of malnutrition (25, 40–42). Although our findings suggest that delivery in private facilities is associated with lower risk of stunting, this likely reflects underlying socioeconomic advantages rather than differences in care quality. Families choosing private care often have better access to resources, improving postnatal nutrition and childcare. Public facilities cater to more vulnerable populations and may be constrained by high patient loads. Additionally, the observed difference may be influenced by the relatively small sample size, which could limit the generalizability of the results. These findings highlight the need to strengthen postnatal support in the public sector.
In terms of wasting, a marker of the current nutritional status of the child (39), this study found that children aged 24–36 months were at increased risk of wasting, which is in contrast with previous studies that suggest an increased risk at an earlier age (43, 44). This observation of increased risk at an older age could reflect the effect of inadequate nutrition or repeated infections during this crucial phase, leading to wasting.
A history of gestational diabetes was associated with an increased risk of wasting, supporting the concept of “fetal programming,” wherein early exposure to certain adverse conditions can influence a child’s growth and lead to poor health outcomes later in life (45–47). Exposure to print media was found to be a protective factor, consistent with previous research showing that mothers with media exposure are better informed, and this can encourage adequate child-caring practices (47–49).
Underweight was significantly associated with low birth weight and older age, which is well documented in the literature (47, 50, 51). Among maternal risk factors were short maternal stature, IFA consumption, and maternal autonomy. Short maternal stature has been linked with increased risk of underweight in children in previous studies (52). In addition, this study revealed that children from non-Hindu and non-Muslim religions had an increased risk of being underweight, which is in contrast with findings from National Family Health Survey-4 (NFHS-4) data, where Hindu children had a higher risk of being underweight (53).
Protective factors against the child being underweight included maternal exposure to print media and control of money. Maternal exposure to media has been associated with a decreased risk of the child being underweight (19). Maternal control of money is one of the important dimensions of maternal autonomy and has been previously reported to be associated with better nutritional outcomes in children (54).
Contrary to expectation, maternal autonomy did not emerge as a protective factor for wasting or underweight. It is possible that the current study's definition of autonomy was not sufficiently elaborative to understand maternal autonomy (20, 54, 55). The association between IFA consumption and underweight, as with stunting, might reflect low adherence to IFA consumption, misreporting, or some confounding variables (56).
Table 8 presents the key determinants of undernutrition among young children residing in the urban slums of Belagavi city.

Table 8. Determinants of undernutrition in the urban slums of Belagavi among children aged 9–36 months
Strengths and limitations
The limitations of the present study include issues related to measurements of independent variables, such as maternal autonomy, which could have been captured with more detailed questions. The study's sample included children aged 9–36 months, whereas collecting data on all children under 5 years, along with a detailed history of past illnesses, would have increased the study’s comprehensiveness. In addition, maternal self-reporting on iron–folic acid use may have introduced recall bias. Moreover, including more household/environmental level characteristics could have provided deeper insights into the correlates of malnutrition.
Conclusion
The study underscores the persistent burden of undernutrition, particularly stunting, among young children in the urban slums of Belagavi, Karnataka. Key risk factors include individual-level characteristics such as male sex, low birth weight, and critical growth phases, such as at 12–23 months; maternal factors such as shorter stature, low education levels, and limited autonomy; and belonging to a non-Hindu or non-Muslim religion, a household-level determinant. Protective factors, including delivery in private health facilities and maternal exposure to print media, highlight actionable areas for interventions. Empowering mothers through education and increased autonomy is crucial for improving the nutritional status of children and reducing the prevalence of malnutrition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethical Committee (Human) for Ph.D. Research, KAHER, Belagavi. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
DO: Conceptualization, Data curation, Methodology, Formal analysis, Writing – original draft. MA: Conceptualization, Project administration, Data curation, Methodology, Investigation, Writing – review & editing. NM: Conceptualization, Methodology, Supervision, Writing – review & editing. MS: Formal analysis, Writing – original draft. SH: Data curation, Writing – original draft. MG: Conceptualization, Methodology, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely thank KLE Academy of Higher Education & Research for granting us the opportunity to conduct this study. We also extend our gratitude to the District Health Officer, Taluka Health Officer, and Medical Officers of Belagavi city, Karnataka, for their invaluable support. Our deepest appreciation goes to all the mothers and children who participated in the study. We are also grateful to Dr. Miguel San Sebastian and Dr. Masoud Vaezghasemi, Umeå University, for their guidance. Finally, we acknowledge the contributions of Dr. Ahilam Sayyed, Vinayak Narasinghshetti, Gouri Hongekar, Uzama Tolgi, and Aparna Singh, students of the Public Health Department, J.N. Medical College, for their assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI, Chat GPT, was used to assist in language editing and enhancing clarity.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Levels and trends in child malnutrition: UNICEF/WHO/World Bank Group joint child malnutrition estimates: key findings of the 2023 edition. Available at: https://www.who.int/publications-detail-redirect/9789240073791 (Accessed February 21, 2024).
2. Arora A. UNICEF DATA. 2020. Global Nutrition Report 2020. Available at: https://data.unicef.org/resources/global-nutrition-report-2020/ (Accessed July 27, 2024).
3. The Lancet Child & Adolescent Health. Child malnutrition: hungry for action. Lancet Child Adolesc Health. (2021) 5(7):459. doi: 10.1016/S2352-4642(21)00170-X
4. World Health Organization. SDG Target 2.2 Malnutrition. Available at: https://www.who.int/data/gho/data/themes/topics/sdg-target-2_2-malnutrition (Accessed February 22, 2024).
5. data.gov. Open Government Data (OGD) Platform India (2022). Available at: https://data.gov.in (Accessed February 22, 2024).
6. Siddiqui F, Salam RA, Lassi ZS, Das JK. The intertwined relationship between malnutrition and poverty. Front Public Health. (2020) 8:453. doi: 10.3389/fpubh.2020.00453
7. Victora CG, Christian P, Vidaletti LP, Gatica-Domínguez G, Menon P, Black RE. Revisiting maternal and child undernutrition in low-income and middle-income countries: variable progress towards an unfinished agenda. Lancet. (2021) 397(10282):1388–99. doi: 10.1016/S0140-6736(21)00394-9
8. Goudet S, Jayaraman A, Chanani S, Osrin D, Devleesschauwer B, Bogin B, et al. Cost effectiveness of a community based prevention and treatment of acute malnutrition programme in Mumbai slums, India. PLoS One. (2018) 13(11):e0205688. doi: 10.1371/journal.pone.0205688
9. Ezeh A, Oyebode O, Satterthwaite D, Chen YF, Ndugwa R, Sartori J, et al. The history, geography, and sociology of slums and the health problems of people who live in slums. Lancet. (2017) 389(10068):547–58. doi: 10.1016/S0140-6736(16)31650-6
10. Roy M. Under-five malnutrition in Indian slums. J Dr NTR Univ Health Sci. (2017) 6:270–1. doi: 10.4103/JDRNTRUHS.JDRNTRUHS_60_17
11. Gera T, Shah D, Sachdev HS. Zinc supplementation for promoting growth in children under 5 years of age in low- and middle-income countries: a systematic review. Indian Pediatr. (2019) 56(5):391–406. doi: 10.1007/s13312-019-1537-z
12. Mathur NB, Agarwal DK. Zinc supplementation in preterm neonates and neurological development: a randomized controlled trial. Indian Pediatr. (2015) 52(11):951–5. doi: 10.1007/s13312-015-0751-6
13. Radhika MS, Swetha B, Kumar BN, Krishna NB, Laxmaiah A. Dietary and nondietary determinants of nutritional status among adolescent girls and adult women in India. Ann N Y Acad Sci. (2018) 1416(1):5–17. doi: 10.1111/nyas.13599
14. Kozuki N, Katz J, Lee ACC, Vogel JP, Silveira MF, Sania A, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. (2015) 145(11):2542–50. doi: 10.3945/jn.115.216374
15. World Health Organization. Regional Office for the Western Pacific. The Asia-Pacific Perspective : Redefining Obesity and Its Treatment. Sydney: Health Communications Australia (2000). Available at: https://iris.who.int/handle/10665/206936 (Accessed August 29, 2024).
16. de Onis M, Onyango A, Borghi E, Siyam A, Blössner M, Lutter C. Worldwide implementation of the WHO Child Growth Standards | Public Health Nutrition | Cambridge Core. Available at: https://www.cambridge.org/core/journals/public-health-nutrition/article/worldwide-implementation-of-the-who-child-growth-standards/F413605BFAB559159B434E029598BE58 (Accessed January 7, 2025).
17. World Health Organization. WHO Anthro Survey Analyser and other tools. Available at: https://www.who.int/tools/child-growth-standards/software (Accessed October 17, 2024).
18. United Nations International Children’s Emergency Fund (UNICEF). UNICEF Conceptual Framework on Maternal and Child Malnutrition. New York: UNICEF. Available at: https://www.unicef.org/reports/state-worlds-children-2019 (Accessed September 10, 2024).
19. Ahsan KZ, Arifeen SE, Al-Mamun MA, Khan SH, Chakraborty N. Effects of individual, household and community characteristics on child nutritional status in the slums of urban Bangladesh. Arch Public Health. (2017) 75:9. doi: 10.1186/s13690-017-0176-x
20. Arulampalam W, Bhaskar A, Srivastava N. Measuring maternal autonomy and its effect on child nutrition in rural India. Economica. (2024) 91(363):719–39. doi: 10.1111/ecca.12518
21. Yani D, Rahayuwati L, Sari C, Komariah M, Fauziah S. Family household characteristics and stunting: an update scoping review. Nutrients. (2023) 15:233. doi: 10.3390/nu15010233
22. Auma CI, Pradeilles R, Ohly H, Eymard-Duvernay S, Brizendine KA, Blankenship J, et al. Urban nutrition situation in the slums of three cities in Asia during the COVID-19 pandemic. Matern Child Nutr. (2023):e13543. doi: 10.1111/mcn.13543
23. Iannotti LL, Dulience SJL, Green J, Joseph S, François J, Anténor ML, et al. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid-based nutrient supplement. Am J Clin Nutr. (2014) 99(1):198–208. doi: 10.3945/ajcn.113.063883
24. Usmani G. Health status in India: a study of urban slum and non-slum population. Nurs Res Pract. (2018) 2(1):09–14.
25. Huey SL, Finkelstein JL, Venkatramanan S, Udipi SA, Ghugre P, Thakker V, et al. Prevalence and correlates of undernutrition in young children living in urban slums of mumbai, India: a cross sectional study. Front Public Health. (2019) 7:191. doi: 10.3389/fpubh.2019.00191
26. Das S, Chanani S, Shah More N, Osrin D, Pantvaidya S, Jayaraman A. Determinants of stunting among children under 2 years in urban informal settlements in mumbai, India: evidence from a household census. J Health Popul Nutr. (2020) 39(1):10. doi: 10.1186/s41043-020-00222-x
27. Patel K, Rawat R, Sindhu M. An epidemiological study of acute malnutrition in children of age 6 months to 5 years in an urban slum of Mumbai, Maharashtra. Available at: https://www.researchgate.net/publication/323413629_An_epidemiological_study_of_acute_malnutrition_in_children_of_age_6_months_to_5_years_in_an_Urban_Slum_of_Mumbai_Maharashtra (Accessed September 1, 2024).
28. Popat CN, Chaudhari AI, Mazumdar VS, Patel SV. A cross sectional study to measure the prevalence of malnutrition and factors associated with malnutrition among under five children of an urban slum of Vadodara city. Available at: https://www.jrmds.in/abstract/a-cross-sectional-study-to-measure-the-prevalence-of-malnutrition-and-factors-associated-with-malnutrition-among-under-f-1549.html (Accessed September 1, 2024).
29. Murarkar S, Gothankar J, Doke P, Pore P, Lalwani S, Dhumale G, et al. Prevalence and determinants of undernutrition among under-five children residing in urban slums and rural area, Maharashtra, India: a community-based cross-sectional study. BMC Public Health. (2020) 20(1):1559. doi: 10.1186/s12889-020-09642-0
30. Kimani-Murage EW, Muthuri SK, Oti SO, Mutua MK, van de Vijver S, Kyobutungi C. Evidence of a double burden of malnutrition in urban poor settings in Nairobi, Kenya. PLoS One. (2015) 10(6):e0129943. doi: 10.1371/journal.pone.0129943
31. Otsuka Y, Agestika L, Widyarani Sintawardani N, Yamauchi T. Risk factors for undernutrition and diarrhea prevalence in an urban slum in Indonesia: focus on water, sanitation, and hygiene. Am J Trop Med Hyg. (2019) 100(3):727–32. doi: 10.4269/ajtmh.18-0063
32. Bork KA, Diallo A. Boys are more stunted than girls from early infancy to 3 years of age in rural Senegal. J Nutr. (2017) 147(5):940–7. doi: 10.3945/jn.116.243246
33. Akhade KS, Sankhe LR, Akarte SV. Magnitude of malnutrition among underfive children in urban slums of commercial capital of India and its multifactorial causation: a community-based study. J Fam Med Prim Care. (2019) 8(12):3865–70. doi: 10.4103/jfmpc.jfmpc_829_19
34. Singh J, Singh T, Lal M, Mahajan S, Ruchika Padda P. Morbidity profile of under-5 slum dwellers of Amritsar city: a descriptive cross-sectional study. J Fam Med Prim Care. (2021) 10(11):4131–6. doi: 10.4103/jfmpc.jfmpc_110_21
35. Islam MM, Sanin KI, Mahfuz M, Ahmed AMS, Mondal D, Haque R, et al. Risk factors of stunting among children living in an urban slum of Bangladesh: findings of a prospective cohort study. BMC Public Health. (2018) 18(1):197. doi: 10.1186/s12889-018-5101-x
36. Jeyakumar A, Babar P, Menon P, Nair R, Jungari S, Tamboli A, et al. Is infant and young child-feeding (IYCF) a potential double-duty strategy to prevent the double burden of malnutrition among children at the critical age? Evidence of association from urban slums in Pune, Maharashtra, India. PLoS One. (2022) 17(12):e0278152. doi: 10.1371/journal.pone.0278152
37. Upadhyay RP, Taneja S, Strand TA, Sommerfelt H, Hysing M, Mazumder S, et al. Early child stimulation, linear growth and neurodevelopment in low birth weight infants. BMC Pediatr. (2022) 22(1):586. doi: 10.1186/s12887-022-03579-6
38. Roja VR, Narayanan P, Chandra Sekaran VC, MG AK. Living environment and health of under-five children in urban slums of a coastal region in South India. Ghana Med J. (2020) 54(4):238–44. doi: 10.4314/gmj.v54i4.6
39. Singh SK, Srivastava S, Chauhan S. Inequality in child undernutrition among urban population in India: a decomposition analysis. BMC Public Health. (2020) 20(1):1852. doi: 10.1186/s12889-020-09864-2
40. Porwal A, Agarwal PK, Ashraf S, Acharya R, Ramesh S, Khan N, et al. Association of maternal height and body mass index with nutrition of children under 5 years of age in India: evidence from comprehensive national nutrition survey 2016–18. Asia Pac J Clin Nutr. (2021) 30(4):675–86. doi: 10.6133/apjcn.202112_30(4).0014
41. United Nations International Children’s Emergency Fund (UNICEF). Programme guidance on maternal nutrition. New York: UNICEF. Available at: https://www.unicef.org/documents/programme-guidance-maternal-nutrition (Accessed September 20, 2024).
42. Khaliq A, Nambiar S, Miller YD, Wraith D. Assessing the relationship of maternal short stature with coexisting forms of malnutrition among neonates, infants, and young children of Pakistan. Food Sci Nutr. (2024) 12(4):2634–49. doi: 10.1002/fsn3.3945
43. Harding KL, Aguayo VM, Webb P. Factors associated with wasting among children under five years old in south Asia: implications for action. PLoS One. (2018) 13(7):e0198749. doi: 10.1371/journal.pone.0198749
44. Hossain MM, Abdulla F, Rahman A. Prevalence and determinants of wasting of under-5 children in Bangladesh: quantile regression approach. PLoS One. (2022) 17(11):e0278097. doi: 10.1371/journal.pone.0278097
45. Bazer FW, Spencer TE, Wu G, Cudd TA, Meininger CJ. Maternal nutrition and fetal development. J Nutr. (2004) 134(9):2169–72. doi: 10.1093/jn/134.9.2169
46. Calek E, Binder J, Palmrich P, Eibensteiner F, Thajer A, Kainz T, et al. Effects of intrauterine growth restriction (IUGR) on growth and body composition compared to constitutionally small infants. Nutrients. (2023) 15(19):4158. doi: 10.3390/nu15194158
47. Wali N, Agho K, Renzaho AM. Hidden hunger and child undernutrition in south Asia: a meta-ethnographic systematic review. Asia Pac J Clin Nutr. (2022) 31(4):713–39. doi: 10.6133/apjcn.202212_31(4).0014
48. Dhawan D, Pinnamaneni R, Viswanath K. Association between mass media exposure and infant and young child feeding practices in India: a cross-sectional study. Sci Rep. (2023) 13(1):19353. doi: 10.1038/s41598-023-46734-4
49. Seth P, Jain P. What are the key determinants of child malnutrition in India? Empirical evidence from NFHS-4. Indian Econ J. (2023) 71(4):729–47. doi: 10.1177/00194662221137853
50. Chowdhury MRK, Khan HTA, Rashid M, Kabir R, Islam S, Shariful Islam M, et al. Differences in risk factors associated with single and multiple concurrent forms of undernutrition (stunting, wasting or underweight) among children under 5 in Bangladesh: a nationally representative cross-sectional study. BMJ Open. (2021) 11(12):e052814. doi: 10.1136/bmjopen-2021-052814
51. Kundu RN, Hossain MG, Haque MA, Mahumud RA, Pal M, Bharati P. Burden of undernutrition among under-five Bengali children and its determinants: findings from demographic and health surveys of Bangladesh and India. PLoS One. (2024) 19(4):e0301808. doi: 10.1371/journal.pone.0301808
52. Li Z, Kim R, Vollmer S, Subramanian SV. Factors associated with child stunting, wasting, and underweight in 35 low- and middle-income countries. JAMA Netw Open. (2020) 3(4):e203386. doi: 10.1001/jamanetworkopen.2020.3386
53. Banerjee S, SubirBiswas Roy S, Pal M, Hossain MG, Bharati P. Nutritional and immunization status of under-five children of India and Bangladesh. BMC Nutr. (2021) 7(1):77. doi: 10.1186/s40795-021-00484-6
54. Paul P, Saha R. Is maternal autonomy associated with child nutritional status? Evidence from a cross-sectional study in India. PLoS One. (2022) 17(5):e0268126. doi: 10.1371/journal.pone.0268126
55. Arulampalam W, Bhaskar A, Srivastava N. Does greater autonomy among women provide the key to better child nutrition? Available at: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2742569 (Accessed September 12, 2024).
56. Nisar YB, Aguayo VM, Billah SM, Dibley MJ. Antenatal iron-folic acid supplementation is associated with improved linear growth and reduced risk of stunting or severe stunting in south Asian children less than two years of age: a pooled analysis from seven countries. Nutrients. (2020) 12(9):2632. doi: 10.3390/nu12092632
Keywords: undernutrition, urban slums, determinants, young children, undernutrition in India, stunting, wasting, maternal factors
Citation: Oswal D, Angolkar M, Mahantashetti NS, Singh M, Haritay S and Godbole M (2025) Prevalence and determinants of undernutrition in the urban slums of Belagavi: a cross-sectional study among young children. Front. Pediatr. 13:1559692. doi: 10.3389/fped.2025.1559692
Received: 13 January 2025; Accepted: 7 April 2025;
Published: 14 May 2025.
Edited by:
Sisay Abebe Debela, Suzhou University, ChinaReviewed by:
Marta Cristina Sanabria, National University of Asunción, ParaguayTeresa Abbattista, Senigallia Hospital, Italy
Copyright: © 2025 Oswal, Angolkar, Mahantashetti, Singh, Haritay and Godbole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deshna Oswal, ZGVzaG5hb3N3YWwxMTExQGdtYWlsLmNvbQ==
 Deshna Oswal
Deshna Oswal Mubashir Angolkar1
Mubashir Angolkar1