- 1Department of Emergency, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Child Rare Diseases in Infection and Immunity, Chongqing, China
- 3Department of Respiratory, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Infection, Children’s Hospital of Chongqing Medical University, Chongqing, China
Objectives: This study aimed to examine the clinical characteristics and risk factors associated with the development of chronic critical illness (CCI) in children with sepsis.
Methods: A retrospective analysis was conducted on children diagnosed with sepsis and admitted to the Pediatric Intensive Care Unit (PICU) at Chongqing Medical University Affiliated Children's Hospital between January 2015 and December 2022. Patients were categorized into two groups based on clinical outcomes: CCI group, defined by an ICU stay ≥14 days with persistent organ dysfunction, and non-CCI group, including patients with rapid recovery or early death. Data on baseline demographics, clinical characteristics, and diagnostic and therapeutic differences were collected and analyzed.
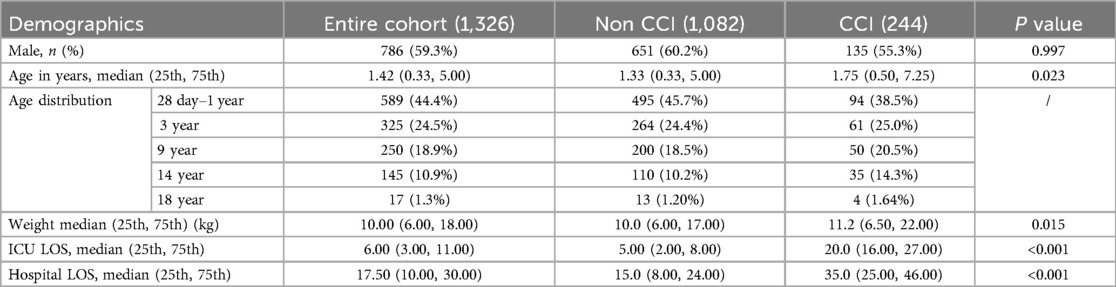
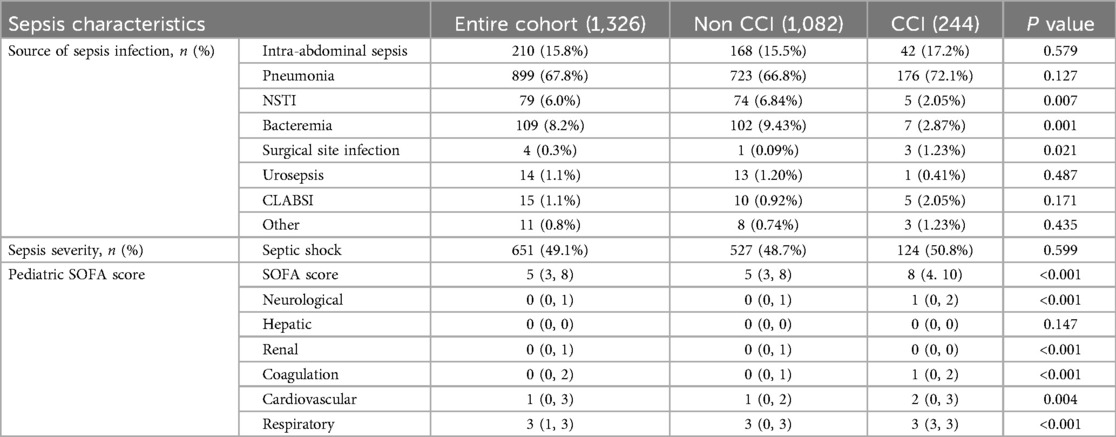
Results: Among 1,326 children with sepsis, 244 were classified in the CCI group (135 males, 109 females) and 1,082 were classified in the non-CCI group (651 males, 431 females), including 163 cases in the early death group and 919 cases in the rapid recovery group. No significant differences were observed between the groups in terms of sex, age distribution, or prevalence of septic shock. Respiratory and gastrointestinal infections were the predominant sources of infection in both groups. Compared to the non-CCI group, the CCI group exhibited significantly higher weights, pediatric sequential organ failure assessment (pSOFA) scores, rates of underlying respiratory diseases, trauma, surgical interventions, mechanical ventilation duration, ICU stay, total hospital stay, and secondary infection rates. Multivariate logistic regression identified pSOFA score, underlying respiratory diseases, trauma, prolonged mechanical ventilation, surgical interventions, and secondary infections as independent risk factors for the development of CCI in children with sepsis. Based on ROC analysis, the AUC of the established CCI prediction model was 0.902 (95% CI: 0.873–0.928). Secondary infections were also a prominent clinical feature of CCI cases.
Conclusions: CCI in pediatric sepsis is associated with underlying respiratory diseases, trauma, elevated pSOFA scores, surgical procedures, prolonged mechanical ventilation and secondary infections. These factors contribute to extended hospital stays, elevated secondary infection rates, and poor clinical outcomes. The persistence of pro-inflammatory mediators and subsequent immunosuppression may underlie the development of CCI in this population.
Background
Sepsis is a complex clinical syndrome resulting from a dysregulated host response to infection, leading to life-threatening organ dysfunction and posing a significant threat to pediatric health. Approximately 25 million children are affected by sepsis each year, resulting in over 3 million deaths (1). In resource-limited regions, particularly in Africa and Asia, sepsis is the leading cause of mortality among children under the age of 5, accounting for 36.7% of all childhood deaths (2). While significant advancements in diagnostic and therapeutic strategies have reduced in-hospital mortality rates, with age-standardized sepsis mortality declining by 52.8% (47.7%–57.5%) between 1990 and 2017 (1), this reduction has not translated to improved long-term outcomes. Rather, increased short-term survival among sepsis patients has been linked to an increased incidence of chronic critical illness (CCI), with up to 33% of survivors developing this condition (3, 4). Patients with CCI frequently experience late complications of sepsis, which significantly contribute to mortality. However, much of the current understanding of CCI stems from studies on adult populations, leaving substantial gaps in the epidemiological and clinical characterization of CCI in children following sepsis.
CCI refers to patients who survive the initial hyperinflammatory phase of sepsis, commonly referred to as the “cytokine storm”, but fail to achieve full recovery, requiring prolonged critical care. Although a universally accepted definition is lacking, CCI is broadly characterized by persistent organ dysfunction necessitating extended hospitalization and substantial resource utilization, including prolonged intensive care unit (ICU) stays (5, 6). A 2-week ICU stay, as defined by length-of-stay data from Shands UF Health, serves as the standard for identifying CCI (7). Evidence suggests that patients with CCI experience higher rates of secondary infections, longer hospital stays, and poorer outcomes (8). Approximately 60% of CCI patients are readmitted within six months (9), predominantly due to recurrent infections, with mortality rates reaching 40% within this period (10). Alarmingly, 70% of CCI patients succumb to their illness before the cessation of treatment (11). These findings underscore the severe burden of CCI and its significant implications for clinical management and outcomes.
CCI is partially attributed to a malfunctioning host response to prolonged activation of pattern recognition receptors (PRRs), leading to deregulated hematopoiesis, persistent inflammation, T cell decline, and an increase in immunosuppressive cell activities. These attributes hint that CCI might constitute a unique pathophysiological state with distinct mechanisms (12). The core pathology of sepsis encompasses immune dysregulation, with studies in adults revealing that CCI patients frequently enter a prolonged immunosuppressive phase, marked by heightened vulnerability to secondary infections and elevated mortality risks. Although there's a prevalent clinical belief linking CCI to chronic immunosuppression, solid clinical evidence supporting this link is still scant. Currently, the underlying mechanisms of immunosuppression and inflammation in sepsis remain elusive, hindering efforts to prevent secondary infections and enhance long-term outcomes. To bridge these knowledge gaps, this study compared the clinical profiles of pediatric sepsis patients with prolonged vs. swift recovery, assessed risk factors for CCI development, and delved into the biological and pathophysiological features and mechanisms underlying the diverse clinical courses.
Subjects and methods
Study population
This retrospective observational study analyzed 1,353 pediatric sepsis patients admitted to the Pediatric Intensive Care Unit (PICU) of Chongqing Medical University Affiliated Children's Hospital between January 2015 and December 2022. Comprehensive data were collected for each patient, including demographic characteristics, clinical manifestations, underlying diseases, laboratory test results, secondary infections, treatment interventions, and final outcomes.
Inclusion and exclusion criteria
The inclusion criteria for the study included: (1) Patients admitted to the ICU; (2) Age range 0–18 years; (3) Clinically diagnosed with sepsis or septic shock, representing the patient's first episode of sepsis; and (4) Patients enrolled in the sepsis clinical management protocol. The exclusion criteria for the study included: (1) Patients not admitted to the PICU or not meeting the diagnostic criteria for sepsis; (2) Severe traumatic brain injury, evidenced by a Glasgow Coma Scale (GCS) score <8 and confirmed neurological injury on CT imaging; and (3) Patients with incomplete medical records.
Approval for conducting the study was granted by the Ethics Committee of Chongqing Medical University Affiliated Children's Hospital (Approval Number: 534/2022). Guardians of all participants were informed and provided their consent, adhering to ethical guidelines for research.
Data collection and methods
Data collection
Clinical data were obtained retrospectively through the hospital's electronic medical record system and supplemented by follow-up visits. Demographic variables included sex, age, and weight. Clinical characteristics included the primary source of infection, severity of sepsis (whether complicated by septic shock), organ dysfunction scores, and presence of underlying diseases. Laboratory tests encompassed essential indicators like white blood cell count, neutrophil count, lymphocyte count, hemoglobin levels, platelet count, procalcitonin, bilirubin, creatinine, albumin, and D-dimer levels. The treatment-related data documented the use of vasoactive medications, cardiopulmonary resuscitation, the duration of mechanical ventilation, surgical interventions, blood transfusions, blood purification therapies, length of ICU stay, and overall hospital stay. Additionally, secondary infection records were reviewed, noting the occurrence and specific anatomical sites of infections acquired during hospitalization. These comprehensive datasets provided a robust basis for subsequent analysis.
Diagnostic criteria
The diagnosis of sepsis was established using the pediatric sequential organ failure assessment (pSOFA) score, which assesses dysfunction across various organ systems, including respiratory, coagulation, liver, and kidney functions. A pSOFA score of 2 or higher was deemed indicative of sepsis, in line with established guidelines (13). Septic shock was characterized as severe infection leading to cardiovascular dysfunction, manifested by hypotension, the need for vasoactive drug treatment, or signs of perfusion abnormalities. Additionally, the “2024 International Consensus Standards for Pediatric Sepsis and Septic Shock” were adopted, incorporating the Phoenix Sepsis Score (PSS) for a comprehensive evaluation. The PSS assesses cardiovascular, respiratory, neurological, and coagulation functions, with a score of 2 or higher confirming sepsis in children with suspected or confirmed infections. Further, septic shock was defined as sepsis accompanied by a PSS cardiovascular score of at least 1 (14).
Group classification
Pediatric patients diagnosed with sepsis or septic shock in our hospital's PICU were categorized into three groups based on their clinical courses: rapid acute progression (RAP, comprising 919 cases), early death (163 cases), and complex chronic immunosuppression (CCI, 244 cases). The CCI group was distinguished by clinical immunosuppression, often manifested through secondary infections following sepsis.
The CCI group encompassed patients with an ICU stay of at least 14 days and persistent organ dysfunction, as determined by a cardiovascular pSOFA score of ≥1 or any other organ system pSOFA score of ≥2 at day 14. Additionally, patients with an ICU stay of less than 14 days who were transferred to another hospital, a long-term acute care facility, or hospice while still exhibiting organ dysfunction at discharge were also included in the CCI group. Patients who did not meet the CCI criteria were placed in the non-CCI group, which encompassed those in the rapid recovery and early death categories.
Statistical analysis
The data were analyzed using SPSS version 26.0 statistical software. Categorical variables were presented as frequencies (n) and percentages (%), with group differences tested using the Chi-square (χ2) test. Non-normally distributed data were described using medians with interquartile ranges (Q1, Q3), and group comparisons were conducted using the Mann–Whitney U test. To identify factors linked to study outcomes, univariate analysis was initially used to screen potential risk factors. Subsequently, Lasso regression was applied to select more robust predictive variables, retaining only those with statistical significance (P < 0.05). Multivariate logistic regression analysis, utilizing the backward elimination method, was employed to quantify the risks associated with the selected predictive variables. The predictive performance of the final model was assessed using the area under the receiver operating characteristic curve (AUROC). Statistical significance was established at a two-tailed P < 0.05.
Results
Patient demographics and sepsis characteristics
This study included 1,326 pediatric sepsis patients, comprising 244 cases in the CCI group (135 males and 109 females), including 28 cases in the CCI mortality group and 216 in the survival group. The remaining 1,082 cases were classified in the non-CCI group (651 males and 431 females), including 163 cases in the early death group and 919 in the rapid recovery group. The CCI group accounted for 18.4% of pediatric sepsis patients, with a mortality rate of 11.5%, both significantly lower than the proportions found in adult studies (3). In contrast, the rapid recovery group accounted for 69.3% of the cohort, indicating a generally favorable prognosis for the majority of pediatric sepsis patients. Children in the CCI group were older and had higher body weights compared to those in the non-CCI group. Additionally, both hospital and PICU stays were significantly longer for patients in the CCI group (P < 0.05, Table 1).
Clinical characteristics of CCI
In the CCI group, respiratory infections were the most common source of sepsis, followed by gastrointestinal infections, with the overall distribution of infection sources resembling that of the control group. Slight differences were observed in the prevalence of other sources of infection between the groups. Regarding sepsis severity, the incidence of septic shock was comparable between the CCI and control groups. However, patients in the CCI group exhibited significantly higher pSOFA scores across most parameters, except for liver function, as well as higher total pSOFA scores (P < 0.05, Table 2). These findings suggest a strong association between the degree of organ dysfunction and both disease outcome and the development of CCI.
Analysis of underlying diseases
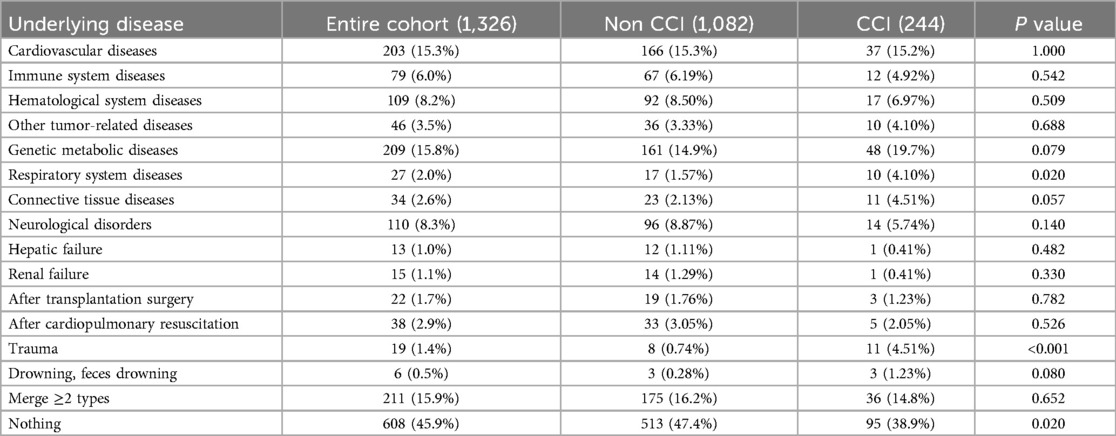
The proportion of patients with underlying diseases in the CCI group was significantly higher than that in the control group. Notably, patients with respiratory system disorders, such as bronchopulmonary dysplasia and congenital airway and lung maldevelopment, as well as those with trauma, demonstrated a significantly higher likelihood of progressing to CCI (P < 0.05, Table 3).
Laboratory parameters
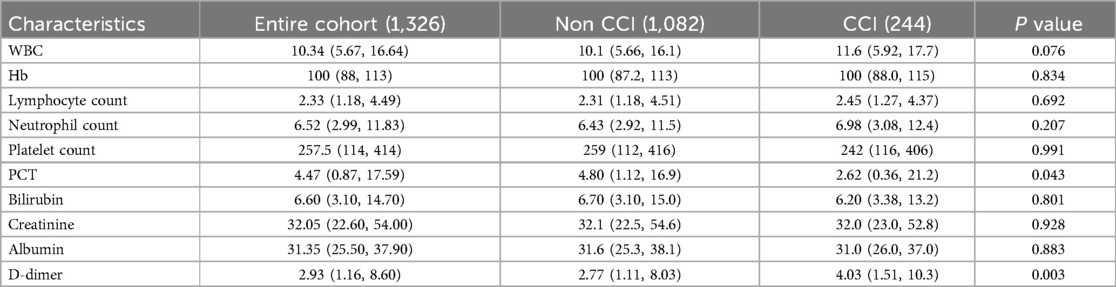
No significant differences were observed in white blood cell count (WBC), hemoglobin (Hb) levels, absolute lymphocyte count, absolute neutrophil count, platelet count, bilirubin levels, creatinine levels, or albumin levels between the CCI and control groups. However, procalcitonin (PCT) was significantly lower, while D-dimer levels were significantly higher in the CCI group compared to the control group (P < 0.05, Table 4).
Treatment and secondary infections
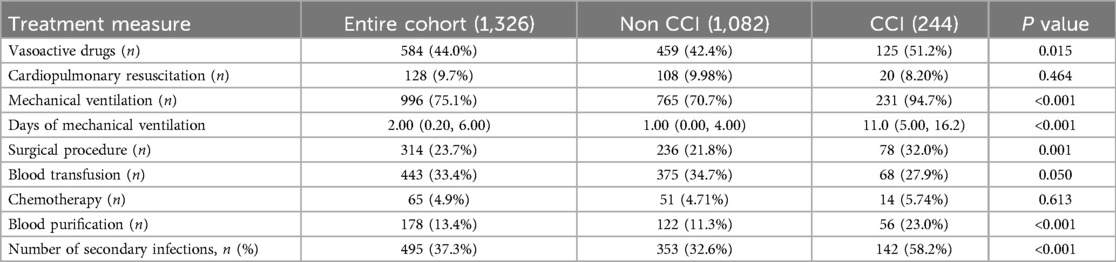
The CCI group demonstrated a significantly greater reliance on vasoactive drugs, along with significantly higher rates and durations of mechanical ventilation use, frequencies of surgical procedures, and use of blood purification therapies compared to the control group (P < 0.05, Table 5).
Secondary infections were more prevalent and numerous in the CCI group compared to the control group, with significantly higher rates of respiratory, urinary tract, surgical site, bloodstream, catheter-related, and fungal infections. These differences suggest a strong association between secondary infections and the progression of sepsis into CCI. Furthermore, the high incidence of secondary infections highlights their role as a key clinical characteristic of CCI, reflecting a greater degree of immunosuppression in this group.
Risk factors and diagnostic value analysis
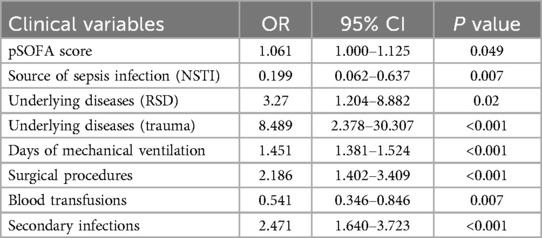
Univariate analysis was conducted to preliminarily screen potential risk factors, retaining those variables with statistical significance (P < 0.05). These variables were further refined using a Lasso regression model to identify robust predictors. Subsequently, multivariate logistic regression analysis was performed to assess the risk factors for CCI in pediatric sepsis patients (P < 0.05, Table 6). Multivariate logistic regression analysis identified pSOFA score, underlying diseases, trauma, days of mechanical ventilation, and surgical procedures as independent risk factors for the development of CCI, with necrotizing soft tissue infection (NSTI) and blood transfusions identified as potential protective factors (P < 0.05, Table 7). Additionally, secondary infections were found to function as both a significant risk factor and a defining clinical characteristic of CCI.
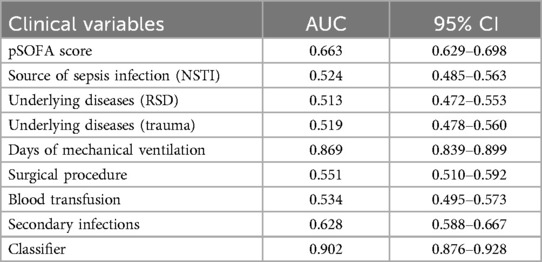
ROC curves were plotted for pSOFA score, source of sepsis infection (NSTI), underlying diseases, trauma, days of mechanical ventilation, surgical procedures, blood transfusion, and secondary infections (Figure 1), with AUC values of 0.663, 0.524, 0.513, 0.519, 0.869, 0.551, 0.534, and 0.628, respectively. The ROC curve for days of mechanical ventilation had the largest AUC, indicating the greatest predictive value for sepsis leading to CCI. Based on ROC analysis, the AUC of the established CCI prediction model was 0.902 (95% CI: 0.873–0.928).
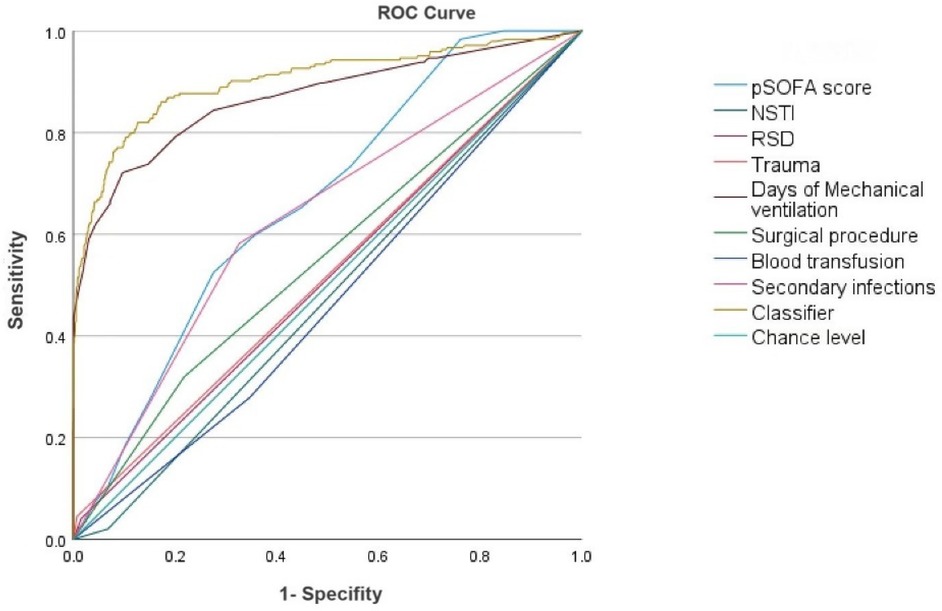
Figure 1. ROC curve analysis of independent predictors of CCI in children with sepsis. AUC: 0.902 (0.876–0.928).
Discussion
CCI following sepsis is an increasingly prominent issue in critical care medicine. Although standardized diagnostic criteria are lacking, CCI is widely defined as persistent organ dysfunction requiring an ICU stay exceeding 14 days, reflecting a transition from acute organ dysfunction to chronic failure with ongoing organ dysfunction (10, 15). Recent studies have highlighted several risk factors for the development of CCI following sepsis, including advanced age, male sex, and pre-existing conditions (16, 17). A key contributor to the progression of CCI is sepsis-induced immunosuppression, which markedly increases the risk of secondary infections (15, 18). This association underscores the critical interplay between immune dysregulation and the pathogenesis of CCI, with secondary infections not only complicating clinical management but also exacerbating long-term outcomes. Despite these insights, research on pediatric populations remains notably limited. Data on epidemiology, clinical characteristics, and mechanisms underlying CCI in children following sepsis are scarce, and the absence of pediatric-specific diagnostic criteria further complicates efforts to address this condition. This knowledge gap is a considerable obstacle to formulating effective prevention and treatment strategies. This study identified key independent risk factors for the development of CCI by comparing patients in the RAP and early death groups. These factors included higher pSOFA scores, underlying respiratory diseases, trauma, prolonged mechanical ventilation, surgical interventions, and secondary infections.
This study was conducted at the largest pediatric intensive care unit (PICU) in Southwest China, involving a retrospective and in-depth analysis of clinical outcomes in children with sepsis over nearly eight years. Analysis revealed that 12.3% of patients experienced early mortality, while 18.4% progressed to CCI. The remaining 69.3% were categorized into an early recovery group, demonstrating favorable prognoses. The proportion of children developing CCI (18.4%) was lower than that reported in adults (33%) (3), although it remains clinically significant. Notably, the mortality rate in the pediatric CCI group (11.5%) was also significantly lower than that observed in adults, potentially attributable to the unique physiological characteristics and enhanced immune recovery capacity of children, combined with the advanced treatment capabilities of our center.
Both patient groups were similar in age, weight, sepsis severity, and infection sources, primarily respiratory infections. Our findings further highlighted a strong correlation between higher pSOFA scores and the development of CCI, indicating that severe organ dysfunction plays a crucial role in both sepsis prognosis and CCI prediction. Additionally, underlying medical conditions emerged as a significant factor influencing patient outcomes. Our study showed a notable link between these conditions and the progression of sepsis to CCI, potentially due to the immune and physiological disturbances they cause. Notably, patients with pre-existing conditions, particularly respiratory diseases like bronchopulmonary dysplasia and congenital airway or lung anomalies, faced a substantially greater risk of CCI. Conversely, those without such conditions had a relatively lower risk. This discrepancy could be attributed to the increased likelihood and extended duration of mechanical ventilation in patients with respiratory comorbidities, both of which contribute to CCI development. Trauma was also identified as a contributing factor to CCI, potentially due to the exacerbated inflammatory response, local hemodynamic disturbances, and heightened physiological stress triggered by injury. These findings underscore the multifactorial nature of CCI development and highlight the importance of addressing underlying and contributing factors to mitigate its progression.
Regarding laboratory parameters, there were no notable differences between the CCI and control groups in terms of WBC count, Hb levels, absolute lymphocyte count, absolute neutrophil count, platelet count, bilirubin levels, creatinine levels, or albumin levels. However, the CCI group exhibited significantly lower PCT levels and notably higher D-dimer levels compared to the control group. D-dimer is a specific biomarker generated during fibrinolysis and fibrin degradation following thrombosis, playing a critical role in the diagnosis and assessment of sepsis (19, 20). These observations align with the findings of the recently published Pediatric Sepsis Phoenix Sepsis Score (PSS) study by Schlapbach et al. (14). The potential mechanisms underlying elevated D-dimer levels, such as hypercoagulability, hyperfibrinolysis, or endothelial injury, suggest that increased D-dimer is associated with adverse clinical outcomes (21). This study underscores the importance of D-dimer as both a diagnostic and prognostic indicator in sepsis, with its abnormal elevations closely linked to disease severity and the risk of CCI in sepsis patients. Procalcitonin (PCT), as a relatively specific inflammatory marker for sepsis, plays a significant role in assessing disease severity (22). The host response varies depending on the type of pathogen, leading to varying degrees of PCT elevation. Since the biomarkers we compared were based on laboratory results obtained within 24 h of admission to the pediatric intensive care unit (PICU), our findings suggest that early PCT levels are not directly associated with the development of chronic critical illness (CCI) following sepsis. In future studies, we aim to further explore the relationship between these biomarkers and the pathophysiology of CCI to elucidate the underlying clinical phenomena.
In terms of treatment, patients in the CCI group required substantially longer durations of mechanical ventilation and more frequent vasoactive drug therapy, surgical interventions, and blood purification procedures compared to those in the RAP group. Univariate analysis identified key factors such as prolonged mechanical ventilation and surgical interventions as significant contributors to CCI development. In addition to trauma, patients undergoing surgical procedures demonstrated a higher risk of progressing to CCI. This is consistent with findings from surgical ICU populations, where over one-third of patients with abdominal sepsis develop CCI, often accompanied by poor long-term prognoses (16). These observations underscore the heightened vulnerability of surgical ICU survivors to prolonged critical illness and pronounced immunosuppression, emphasizing the need for targeted management strategies to mitigate these risks. In this study, intergroup analysis revealed a relatively higher transfusion rate in the pediatric sepsis chronic critical illness (CCI) group. The primary reasons for this observation include prolonged ICU stays, recurrent infections, and frequent blood sampling, all of which contribute to an increased risk of anemia and, consequently, a higher likelihood of transfusion. Therefore, transfusion should not be considered a true protective factor but rather a consequence of chronic critical illness.
Gentile et al. identified and characterized the clinical phenotype of CCI as persistent inflammation-immunosuppression and catabolism syndrome (PICS) (4). This syndrome, a hallmark of various conditions, including sepsis, trauma, advanced malignancies, and chronic inflammatory diseases, provides a novel framework for understanding the complex pathophysiological mechanisms underlying CCI. PICS highlights the interplay between persistent inflammatory responses and profound immunosuppression as central drivers of CCI progression. This is consistent with our findings, which identified immune dysregulation as a pivotal factor in the occurrence and development of CCI. Immune imbalance, a defining feature of sepsis pathogenesis, manifests in CCI patients as a prolonged state of immunosuppression, substantially increasing their susceptibility to secondary infections and mortality. In the current study, immunosuppression was clinically determined by the occurrence of post-sepsis secondary infections, consistent with previous findings (15). Our research confirmed that immunosuppression is a central mechanism in the pathophysiology of post-sepsis CCI. Notably, secondary infections were markedly more prevalent in the CCI group compared to the non-CCI group, with the most common types including urinary, respiratory, gastrointestinal, and bloodstream infections (particularly catheter-related infections). These infections serve as both a driving risk factor and a clinical hallmark of CCI, indicating their dual significance. This study provided further evidence that immunosuppression following pediatric sepsis contributes to the onset of secondary infections, which play a pivotal role in progression to CCI. Early detection and effective management of immunosuppression are crucial for preventing progression from pediatric sepsis to CCI and improving patient outcomes and long-term prognosis.
Multivariate logistic regression analysis identified several independent risk factors for the development of CCI in pediatric sepsis patients, including pSOFA score, respiratory system diseases, trauma, prolonged mechanical ventilation, surgical interventions, and secondary infections. Among these factors, prolonged mechanical ventilation demonstrated the highest predictive value, as evidenced by its largest AUC value. This strong predictive capacity may be linked to its association with critical illness myopathy (CIM), immunosuppression, and ICU-acquired weakness (ICU-AW). These complications increase the vulnerability of CCI patients to secondary infections, particularly ventilator-associated pneumonia, which can further prolong mechanical ventilation duration and exacerbate the risk of CCI. Thus, prolonged mechanical ventilation not only predicts the development of CCI but also plays a critical role in its pathogenesis. These findings highlight the importance of early recognition and targeted management of high-risk patients to mitigate the progression of sepsis to CCI. Immunosuppression, a central mechanism in this progression, significantly increases susceptibility to secondary infections, perpetuates organ dysfunction, delays recovery, and contributes to the chronic deterioration of the clinical condition. Understanding the role of immunosuppression in the development of CCI is crucial for advancing diagnostic and therapeutic strategies. Addressing this mechanism could lead to innovative treatment approaches aimed at improving the prognosis of sepsis patients. Furthermore, our study established immunosuppression as a hallmark of CCI, underscoring its pivotal role in shaping disease trajectory and outcomes.
This study has several limitations. As a single-center retrospective analysis, it is subject to potential selection bias, limiting the generalizability of its findings, and propose future multicenter prospective studies for validation. Additionally, the study included only a limited set of immune function evaluation markers, restricting the depth of immunological insights. The absence of dynamic follow-up data hindered the ability to track immunosuppression progression and its impact over time. Future research should explore the precise mechanisms underlying sepsis-induced immunosuppression and the development of strategies to modulate immune responses effectively. Such efforts are crucial to preventing and managing chronic critical illness, thereby reducing its incidence and improving long-term survival outcomes and quality of life for sepsis patients.
Our study indicated that CCI in pediatric sepsis was strongly linked to underlying respiratory diseases, trauma, elevated pSOFA scores, surgical procedures, and prolonged mechanical ventilation. These factors contributed to extended hospital stays, elevated secondary infection rates, and adverse clinical outcomes. The persistence of pro-inflammatory mediators and subsequent immunosuppression likely play a pivotal role in the progression to CCI within this population. By conducting a comprehensive analysis of the clinical features and risk factors related to CCI in pediatric sepsis, this study lays a crucial groundwork for the formulation of more targeted prevention and treatment approaches. This, in turn, holds the promise of substantially enhancing the outcomes for this susceptible patient group.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Chongqing Medical University Affiliated Children's Hospital (Approval Number: 534/2022). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
LY: Conceptualization, Data curation, Investigation, Writing – original draft, Writing – review & editing. NZ: Supervision, Writing – original draft. CL: Formal analysis, Writing – original draft. KP: Investigation, Software, Writing – original draft. YY: Methodology, Writing – original draft. EL: Project administration, Supervision, Writing – review & editing, Writing – original draft. LT: Resources, Validation, Writing – review & editing, Writing – original draft.
Funding
The authors declare that this study received funding from Rural Science & Technology Department, Chongqing Municipal Science & Technology Bureau. Grant number: CSTB2024TIAD-KPX0034-2. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet (Lond Engl). (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
2. Bassat Q, Blau DM, Ogbuanu IU, Samura S, Kaluma E, Bassey I-A, et al. Causes of death among infants and children in the child health and mortality prevention surveillance (CHAMPS) network. JAMA Netw Open. (2023) 6:e2322494. doi: 10.1001/jamanetworkopen.2023.22494
3. Hawkins RB, Raymond SL, Stortz JA, Horiguchi H, Brakenridge SC, Gardner A, et al. Chronic critical illness and the persistent inflammation, immunosuppression, and catabolism syndrome. Front Immunol. (2018) 9:1511. doi: 10.3389/fimmu.2018.01511
4. Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. (2012) 72:1491–501. doi: 10.1097/TA.0b013e318256e000
5. Nelson JE, Cox CE, Hope AA, Carson SS. Chronic critical illness. Am J Respir Crit Care Med. (2010) 182:446–54. doi: 10.1164/rccm.201002-0210CI
6. Nomellini V, Kaplan LJ, Sims CA, Caldwell CC. Chronic critical illness and persistent inflammation: what can we learn from the elderly, injured, septic, and malnourished? Shock. (2018) 49:4–14. doi: 10.1097/SHK.0000000000000939
7. Prescott HC. Preventing chronic critical illness and rehospitalization: a focus on sepsis. Crit Care Clin. (2018) 34:501–13. doi: 10.1016/j.ccc.2018.06.002
8. Rosenthal MD, Kamel AY, Rosenthal CM, Brakenridge S, Croft CA, Moore FA. Chronic critical illness: application of what we know. Nutr Clin Pract. (2018) 33:39–45. doi: 10.1002/ncp.10024
9. Loss SH, Nunes DSL, Franzosi OS, Salazar GS, Teixeira C, Vieira SRR. Chronic critical illness: are we saving patients or creating victims? Rev Bras Ter Intensiva. (2017) 29:87–95. doi: 10.5935/0103-507X.20170013
10. Anna KG, Gabriela LG, Zhong KW, Tezcan OB, Steven LR, Robert TM, et al. The development of chronic critical illness determines physical function, quality of life, and long-term survival among early survivors of sepsis in surgical ICUs. Crit Care Med. (2019) 47(4):566–73. doi: 10.1097/CCM.0000000000003655
11. Unroe M, Kahn JM, Carson SS, Govert JA, Martinu T, Sathy SJ, et al. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann Intern Med. (2010) 153:167–75. doi: 10.7326/0003-4819-153-3-201008030-00007
12. Fenner BP, Darden DB, Kelly LS, Rincon J, Brakenridge SC, Larson SD, et al. Immunological endotyping of chronic critical illness after severe sepsis. Front Med (Lausanne). (2020) 7:616694. doi: 10.3389/fmed.2020.616694
13. Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. (2020) 21:e52–106. doi: 10.1097/PCC.0000000000002198
14. Schlapbach LJ, Watson RS, Sorce LR, Argent AC, Menon K, Hall MW, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. (2024) 331:665–74. doi: 10.1001/jama.2024.0179
15. Stortz JA, Murphy TJ, Raymond SL, Mira JC, Ungaro R, Dirain ML, et al. Evidence for persistent immune suppression in patients who develop chronic critical illness after sepsis. Shock. (2018) 49:249–58. doi: 10.1097/SHK.0000000000000981
16. Cox MC, Brakenridge SC, Stortz JA, Hawkins RB, Darden DB, Ghita GL, et al. Abdominal sepsis patients have a high incidence of chronic critical illness with dismal long-term outcomes. Am J Surg. (2020) 220:1467–74. doi: 10.1016/j.amjsurg.2020.07.016
17. Mankowski RT, Anton SD, Ghita GL, Brumback B, Cox MC, Mohr AM, et al. Older sepsis survivors suffer persistent disability burden and poor long-term survival. J Am Geriatr Soc. (2020) 68:1962–9. doi: 10.1111/jgs.16435
18. Guirgis FW, Brakenridge S, Sutchu S, Khadpe JD, Robinson T, Westenbarger R, et al. The long-term burden of severe sepsis and septic shock: sepsis recidivism and organ dysfunction. J Trauma Acute Care Surg. (2016) 81:525–32. doi: 10.1097/TA.0000000000001135
19. Gando S, Saitoh D, Ogura H, Fujishima S, Mayumi T, Araki T, et al. A multicenter, prospective validation study of the japanese association for acute medicine disseminated intravascular coagulation scoring system in patients with severe sepsis. Crit Care (lond Engl). (2013) 17:R111. doi: 10.1186/cc12783
20. Iba T, Levy JH, Warkentin TE, Thachil J, van der Poll T, Levi M, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thromb Haemost. (2019) 17:1989–94. doi: 10.1111/jth.14578
21. Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. (2017) 149:38–44. doi: 10.1016/j.thromres.2016.11.007
Keywords: pediatric sepsis, chronic critical illness (CCI), clinical characteristics, risk factors, immunosuppression
Citation: Yang L, Zang N, Liu C, Yang Y, Pu KB, Tan LP and Liu EM (2025) Clinical characteristics and risk factors of chronic critical illness in children with sepsis. Front. Pediatr. 13:1561044. doi: 10.3389/fped.2025.1561044
Received: 15 January 2025; Accepted: 31 March 2025;
Published: 2 May 2025.
Edited by:
Kenneth E. Remy, Case Western Reserve University, United StatesReviewed by:
Suneel Kumar Pooboni, Mediclinic City Hospital, United Arab EmiratesBo-tao Ning, Shanghai Children's Medical Center, China
Copyright: © 2025 Yang, Zang, Liu, Yang, Pu, Tan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Ping Tan, dGFubHAwODI1QDE2My5jb20=; En Mei Liu, ZW1saXUxODZAMTI2LmNvbQ==
 Lin Yang
Lin Yang Na Zang2,3
Na Zang2,3 Cong Liu
Cong Liu En Mei Liu
En Mei Liu