- Department of Pediatric, Liaocheng People’s Hospital, Liaocheng, Shandong, China
Reports on congenital Mycoplasma pneumoniae (MP) pneumonia are rare in the medical literature. In this study, we presented a male neonatal case of severe congenital MP pneumonia complicated with atelectasis and macrolide resistance. Empirical treatment with conventional antibiotics proved ineffective and the ventilator could not be weaned. After undergoing five rounds of tracheal intubation, the neonate was successfully weaned off ventilator support after treatment with fluoroquinolone antibiotics combined with bronchoscopic lavage. The diagnoses were based on the early onset of respiratory distress, atypical pneumonia findings on pulmonary imaging, characteristic mucous plugs associated with MP observed during bronchoscopy, and positive MP nucleic acid and IgG antibodies in both the neonate and the mother. Importantly, this case report provides clinical evidence for the treatment of congenital MP infection with atelectasis and macrolide resistance by administering fluoroquinolones at appropriate dosages and durations, along with bronchoscopic lavage as an effective option to clear airway obstruction and improve pulmonary ventilation.
Introduction
Mycoplasma pneumoniae (MP) is a common pathogen responsible for respiratory tract infections worldwide. Since 2023, MP has escalated to epidemic proportions in China, particularly affecting younger children, with more severe clinical manifestations and high resistance to macrolide antibiotics (1). To date, MP-associated neonatal pneumonia has been rarely reported, with congenital cases even less so. A review of English-language, peer-reviewed literature revealed only four reported cases of congenital MP pneumonia in neonates (2–5). To enrich our knowledge of this condition, we report a severe case of congenital MP infection in a neonate, complicated by atelectasis and macrolide resistance.
Case report
A male neonate weighing 1,970 g at birth was delivered at 31 weeks of gestation by natural delivery after a 9-h rupture of membranes, without administration of lung maturation treatment. At birth, the infant exhibited cyanosis, weak crying, and signs of respiratory distress. His Apgar scores were 5 and 8 at 1 and 5 min, respectively. He was promptly transferred to the neonatal intensive care unit, where he required tracheal intubation and mechanical ventilation. Bovine surfactant (210 mg) was administered into the trachea according to standard protocol. Empirical antibiotic therapy with meropenem and penicillin was initiated for suspected infection. The neonate was successfully extubated on day 6 of life; however, he soon developed a respiratory distress syndrome. Nasal continuous positive airway pressure (CPAP) was commenced and used during the second round of tracheal intubation the next day. Given that the neonate’s serum mycoplasma IgG titer exceeded 300 U/ml, oral azithromycin was administered at a dose of 10 mg/kg and continued for 6 days. Despite treatment, extubation failed at the end of the azithromycin course due to left lung atelectasis, as confirmed by chest radiographs (Figure 1A). After a third tracheal intubation, the neonate was transferred to our department on day 13 of life with ventilatory support via a transport ventilator.
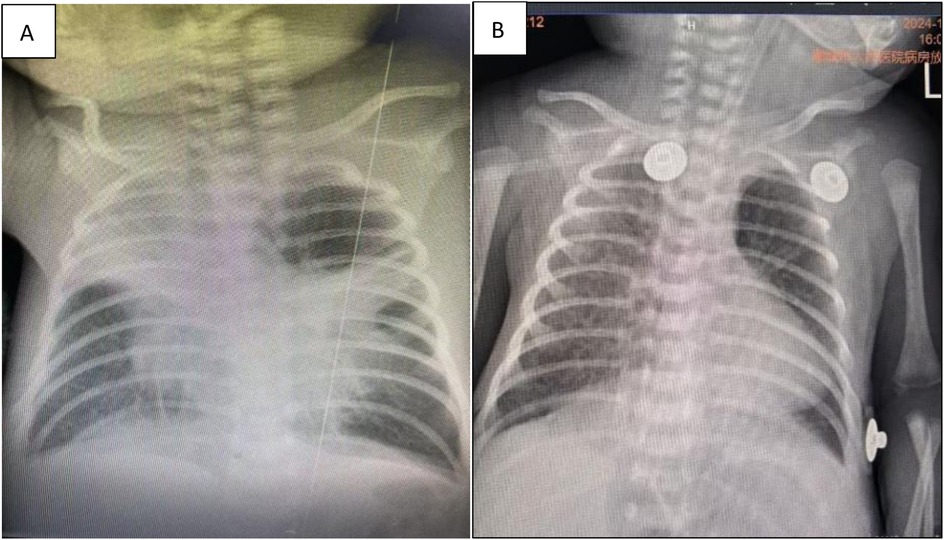
Figure 1. (A) Atelectasis of the left lung in chest radiographs. (B) Atelectasis after bronchoscopic lavage treatment during follow-up in chest radiographs.
On the day of admission, deep sputum samples were collected via endotracheal intubation and analyzed using pathogen-targeted next-generation sequencing (tNGS). MP was the only pathogen detected; no other microorganisms were identified. Since the neonate had already been intermittently treated with azithromycin, no other antibiotics were administered; however, respiratory support was provided. After a 4-day interval, the second round of oral azithromycin was administered, combined with a low dose of methylprednisolone (2 mg/kg/day). On day 16 of life, the attempt to remove the tracheal tube failed and intubation was performed for the fourth time. Persistent respiratory distress with no signs of improvement raised concerns about a possible congenital airway anomaly in addition to MP infection. On day 21 of life, after extubation, electronic bronchoscopy was performed. There was no evidence of structural abnormalities in the airway; however, mucous plugs positive for MP were observed in the basal segments of the left upper and lower lobe bronchus. Bronchoscopic lavage was carried out, resulting in significant improvement in pulmonary ventilation (Figure 2). The day after electronic bronchoscopy, the neonate developed severe dyspnea and required a fifth intubation. Even after two full courses of azithromycin, the neonate remained dependent on ventilatory support.
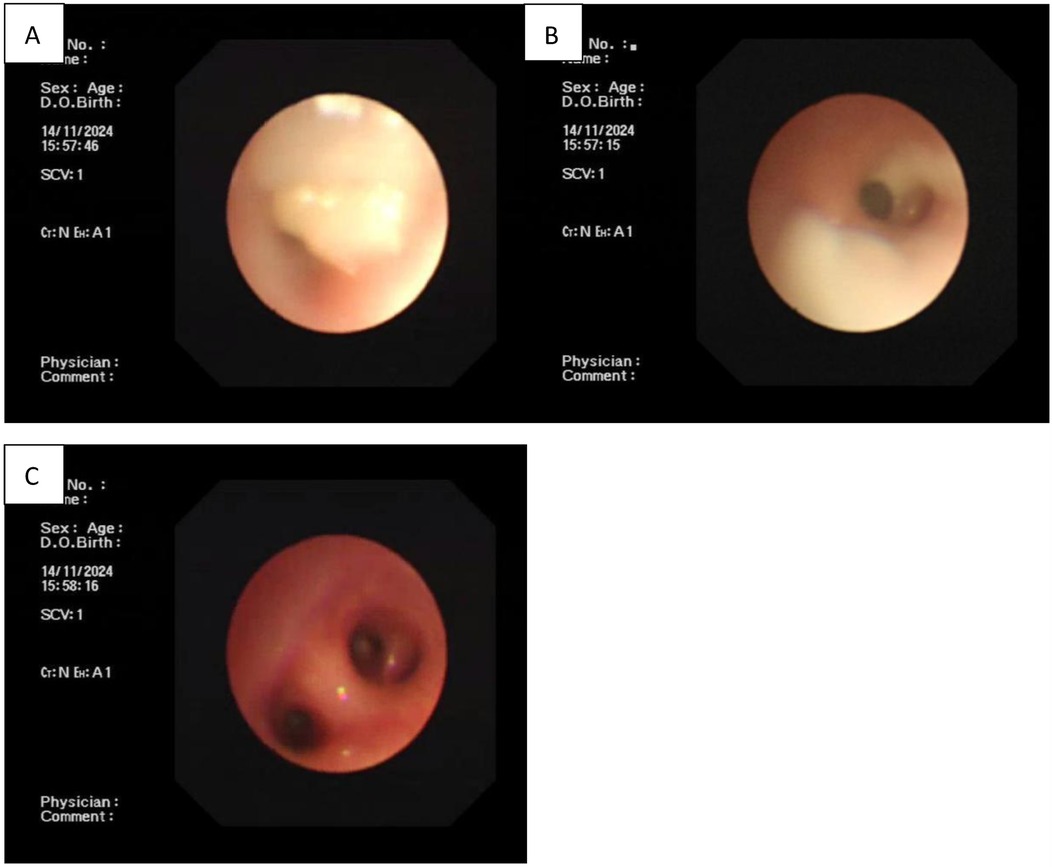
Figure 2. (A,B) Complete airway obstruction in the left upper and lower lobe bronchi as shown by electronic bronchoscopy. (C) Unblocked airway after bronchoscopic lavage treatment.
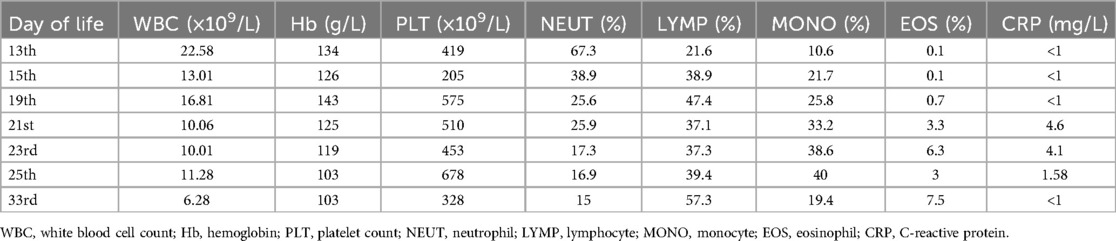
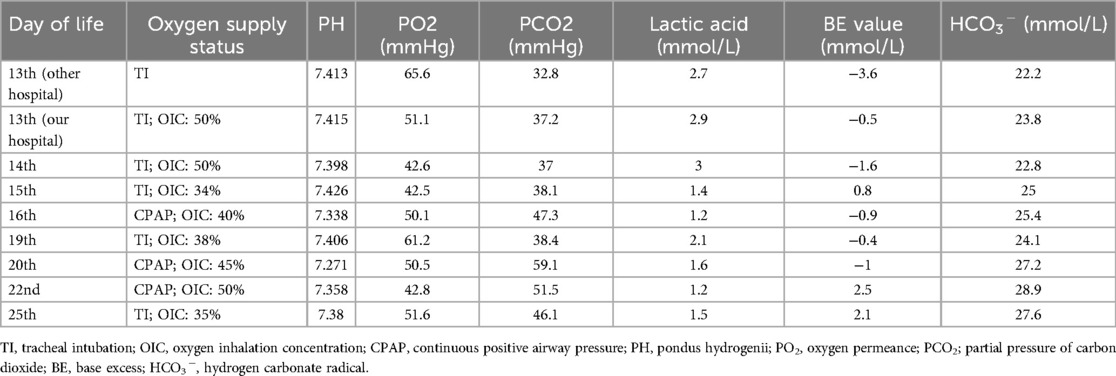
Based on the mutation assay results of the macrolide-resistant MP gene at locus A2063G, the present case was classified as severe macrolide-resistant MP pneumonia. After consulting with the hospital’s Department of Pharmacy and obtaining parental consent, treatment with moxifloxacin was initiated at a dosage of 10 mg/kg/day on day 23 of life. Meanwhile, the methylprednisolone dose was increased to 4 mg/kg/day. On day 25 of life, chest radiography revealed marked improvement in left lung atelectasis (Figure 1B). On day 28, the neonate was successfully extubated but still required nasal CPAP, followed by gradual transition to oxygen via a nasal catheter. On day 33, lung function testing showed that both the peak time ratio and peak volume ratio were only slightly below the lower normal limits, while all other parameters remained within the normal reference ranges. On day 41, the neonate was weaned from off nasal oxygen support and was discharged with a diagnosis of mild bronchopulmonary dysplasia (BPD). Changes in routine blood tests and blood gas analysis from day 13 to day 33 of life are presented in Tables 1, 2, respectively.
A 1-month follow-up was conducted after the neonate’s discharge, at a corrected gestational age of 41 weeks. His body weight was 3,350 g and length was 51 cm. He no longer required oxygen support, exhibited good feeding tolerance, and showed normal activity endurance. Given the diagnosis of BPD, ongoing evaluation of lung function was deemed necessary.
A subsequent follow-up was conducted 6 months after discharge. The infant was exclusively breastfed, with a length of 62 cm and weight of 5.5 kg. According to the Fenton growth curve, his length corresponded to the 15th–25th percentile, while his weight fell between the 1st–3rd percentiles for his age. Despite the suboptimal weight gain, the child did not require oxygen support, had no episodes of wheezing, tolerated feeding well, maintained normal activity levels, and showed completely normalized lung function upon re-examination. Due to the relatively low weight, enhanced nutritional support and ongoing monitoring height and weight were recommended.
Discussion
MP is the leading cause of community-acquired pneumonia in children aged under 5 years in China. Unlike bacteria and viruses, MP lacks a cell wall and is the smallest prokaryotic microorganism capable of independent survival (6). In contrast to urogenital mycoplasmas—such as Ureaplasma urealyticum, Mycoplasma genitalium, and Mycoplasma hominis—MP primarily colonizes the urogenital tract (7–9). Reports of congenital MP pneumonia in neonates are rare, with this case representing only the fifth described in the literature. Congenital MP pneumonia typically presents with early-onset symptoms, as all five reported cases—including the present one—exhibited signs of dyspnea on the day of birth. It is possible that congenital MP pneumonia is attributed to maternal infection of MP during pregnancy, which may lead to chorioamnionitis via the placenta and cause ascending intrauterine infection. Consequently, affected neonates frequently experience preterm birth, low birth weight, and abnormal fetal development. Aside from one full-term newborn, four of the five cases of congenital MP pneumonia (four published plus the present case) involved premature infants. It is worth noting that this is the first reported case of congenital MP pneumonia with confirmed macrolide resistance treated with quinolones. It is also the first case diagnosed as severe congenital MP infection using electronic bronchoscopy.
Globally, particularly in East Asian countries, the resistance rate of MP to macrolide antibiotics is over 80%, and this resistance is extremely serious (10). The exact mechanisms behind the macrolide resistance are not fully understood, but are though to involve a combination of genetic, biochemical, and environmental factors that reduce bacterial susceptibility. Proposed mechanisms include genetic mutations, methylation changes, and drug inactivation (11). There is evidence that macrolide-resistant MP (MRMP) strains harbor point mutations in domain V of the 23S rRNA gene. Among these, the A2063G mutation is the most common (12–14). This mutation impairs the binding of macrolides to the 23S rRNA V domain of the 50S ribosomal subunit, preventing inhibition of peptide chain elongation and protein synthesis, and ultimately resulting in resistance (11). In the present case, the A2063G mutation was detected in the MRMP gene, underscoring the importance of genetic information in guiding clinical classification and medication.
In addition to macrolide resistance, the present neonatal case met the diagnostic criteria for severe MP pneumonia, as evidenced by persistent respiratory distress, repeated failures to wean from mechanical ventilation, and obvious pulmonary atelectasis. Prior studies have identified several risk factors associated with severe MP pneumonia, including advanced age, persistent fever, wheezing, atelectasis, macrolide resistance, and elevated levels of D-dimer, lactate dehydrogenase, C-reactive protein, and erythrocyte sedimentation rate (15–18). In this case, there was no evidence of persistent fever, possibly due to the early administration of dexamethasone aimed at preventing bronchopulmonary dysplasia. Although MP pneumonia predominantly affects preschool- and school-aged children, the risk of developing severe MP pneumonia has been shown to increase with age (18). In recent years, the incidence of severe MP pneumonia in children has shifted toward younger age groups. We hence suggest that more attention should be directed to the management of neonates congenitally infected with severe MP pneumonia to reduce the pediatric burden.
National guidelines recommend that macrolides, especially azithromycin, are the first-line treatment for mild MP pneumonia in children. In cases of severe MP pneumonia, tetracyclines are recommended for children aged ≥8 years, while macrolides remained the preferred choice for children aged <8 years. Fluoroquinolones are prescribed for off-label use in children under 18 years due to specific adverse effects, including tooth discoloration, enamel dysplasia, and cartilage damage (19). In the present case, the neonate failed to wean from ventilator support after two rounds of oral azithromycin, prompting the use of fluoroquinolone to combat the severe MP pneumonia. Despite the adverse effects, the off-label use of tetracyclines and fluoroquinolones in children has become more common, especially in the presence of macrolide resistance and severe MP pneumonia outbreaks.
The successful treatment of this case of congenital severe MP pneumonia was not solely attributed to the administration of fluoroquinolones, but also by the use of electronic bronchoscopy. Considering the neonate could not wean from the ventilator and that radiographs showed obvious atelectasis, bronchoscopy was prioritized as an alternative diagnostic intervention. The procedure revealed that the airway was blocked by several mucus plugs. After bronchoscopic lavage, pulmonary ventilation improved significantly, with no signs of atelectasis. Currently, there is no consensus regarding the timing for bronchoscopy in MP pneumonia. Although the majority of respiratory specialists recommend performing the procedure within 7–14 days of disease onset, others maintain that performing bronchoscopic lavage within 24 h of hospitalization can significantly shorten the length of hospital stay and reduce MP sequences (20). Further research is warranted to determine the ideal timing for bronchoscopy.
In summary, this severe neonatal case provides clinical evidence for the treatment of congenital MP infection complicated by atelectasis and macrolide resistance, for the administration of fluoroquinolones in proper dosages and courses, and for the adoption of bronchoscopic lavage as an effective treatment choice to clear airway obstruction and improve pulmonary ventilation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Liaocheng People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
DW: Conceptualization, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. CY: Conceptualization, Formal analysis, Software, Writing – original draft. DQ: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. JW: Methodology, Resources, Supervision, Validation, Writing – original draft. JZ: Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu K, Fu HM, Lu Q. Advancement in epidemiology of Mycoplasma pneumoniae pneumonia in children in China. Zhonghua Er Ke Za Zhi. (2024b) 62:696–9. doi: 10.3760/cma.j.cn112140-20240407-00247
2. Ursi D, Ursi JP, Ieven M, Docx M, Van Reempts P, Pattyn SR. Congenital pneumonia due to Mycoplasma pneumoniae. Arch Dis Child Fetal Neonatal Ed. (1995) 72:F118–120. doi: 10.1136/fn.72.2.F118
3. Srinivasjois RM, Kohan R, Keil AD, Smith NM. Congenital Mycoplasma pneumoniae pneumonia in a neonate. Pediatr Infect Dis J. (2008) 27:474–5. doi: 10.1097/INF.0b013e318165ebaf
4. Huber BM, Meyer Sauteur PM, Unger WWJ, Hasters P, Eugster MR, Brandt S, et al. Vertical transmission of Mycoplasma pneumoniae infection. Neonatology. (2018) 114:332–6. doi: 10.1159/000490610
5. Samonini A, Grosse C, Aschero A, Boubred F, Ligi I. Congenital pneumonia owing to Mycoplasma pneumoniae. J Pediatr. (2018) 203:460–460.e461. doi: 10.1016/j.jpeds.2018.06.055
6. Kumar S, Kumar S. Mycoplasma pneumoniae: among the smallest bacterial pathogens with great clinical significance in children. Indian J Med Microbiol. (2023) 46:100480. doi: 10.1016/j.ijmmb.2023.100480
7. Ahmed J, Rawre J, Dhawan N, Khanna N, Dhawan B. Mycoplasma hominis: an under recognized pathogen. Indian J Med Microbiol. (2021) 39:88–97. doi: 10.1016/j.ijmmb.2020.10.020
8. Matasariu DR, Ursache A, Agache A, Mandici CE, Boiculese VL, Bujor IE, et al. Genital infection with Ureaplasma urealyticum and its effect on pregnancy. Exp Ther Med. (2022) 23:89. doi: 10.3892/etm.2021.11012
9. Yu J, Zhou Y, Luo H, Su X, Gan T, Wang J, et al. Mycoplasma genitalium infection in the female reproductive system: diseases and treatment. Front Microbiol. (2023) 14:1098276. doi: 10.3389/fmicb.2023.1098276
10. Kim K, Jung S, Kim M, Park S, Yang HJ, Lee E. Global trends in the proportion of macrolide-resistant Mycoplasma pneumoniae infections: a systematic review and meta-analysis. JAMA Netw Open. (2022) 5:e2220949. doi: 10.1001/jamanetworkopen.2022.20949
11. Yang HJ, Song DJ, Shim JY. Mechanism of resistance acquisition and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Korean J Pediatr. (2017) 60:167–74. doi: 10.3345/kjp.2017.60.6.167
12. Suzuki Y, Seto J, Shimotai Y, Ikeda T, Yahagi K, Mizuta K, et al. Development of an endpoint genotyping assay to detect the Mycoplasma pneumoniae 23S rRNA gene and distinguish the existence of macrolide resistance-associated mutations at position 2063. J Microbiol Methods. (2016) 131:130–4. doi: 10.1016/j.mimet.2016.10.017
13. Guo DX, Hu WJ, Wei R, Wang H, Xu BP, Zhou W, et al. Epidemiology and mechanism of drug resistance of Mycoplasma pneumoniae in Beijing, China: a multicenter study. Bosn J Basic Med Sci. (2019) 19:288–96. doi: 10.17305/bjbms.2019.4053
14. Jiang Y, Dou H, Xu B, Xu B, Zhou W, Wang H, et al. Macrolide resistance of Mycoplasma pneumoniae in several regions of China from 2013 to 2019. Epidemiol Infect. (2024) 152:e75. doi: 10.1017/S0950268824000323
15. Chang Q, Chen HL, Wu NS, Gao YM, Yu R, Zhu WM. Prediction model for severe Mycoplasma pneumoniae pneumonia in pediatric patients by admission laboratory indicators. J Trop Pediatr. (2022) 68:1–8. doi: 10.1093/tropej/fmac059
16. Qiu J, Ge J, Cao L. D-dimer: the risk factor of children’s severe Mycoplasma pneumoniae pneumonia. Front Pediatr. (2022) 10:828437. doi: 10.3389/fped.2022.828437
17. Liu F, Chen L, Wang MY, Shi WJ, Wang XP. Exploring high-risk factors for the prediction of severe mycoplasma pneumonia in children. Transl Pediatr. (2024a) 13:2003–11. doi: 10.21037/tp-24-293
18. Zhang X, Sun R, Jia W, Li P, Song C. A new dynamic nomogram for predicting the risk of severe Mycoplasma pneumoniae pneumonia in children. Sci Rep. (2024) 14:8260.38589453
19. Wang YS, Zhou YL, Bai GN, Li SX, Xu D, Chen LN, et al. Expert consensus on the diagnosis and treatment of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. World J Pediatr. (2024) 20:901–14. doi: 10.1007/s12519-024-00831-0
Keywords: Mycoplasma pneumoniae pneumonia, atelectasis, macrolide resistance, fluoroquinolone antibiotic, bronchoscopic lavage
Citation: Wang D, Yang C, Qin D, Wang J and Zhao J (2025) Case Report: A neonatal case of severe congenital Mycoplasma pneumoniae pneumonia with atelectasis and macrolide resistance. Front. Pediatr. 13:1561097. doi: 10.3389/fped.2025.1561097
Received: 15 January 2025; Accepted: 14 May 2025;
Published: 11 June 2025.
Edited by:
Luca Rastrelli, University of Salerno, ItalyReviewed by:
Alessandra Matsuno, Universidade de São Paulo, BrazilZhenguo Nie, Tsinghua University, China
Copyright: © 2025 Wang, Yang, Qin, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingcai Wang, d2FnamluY2lAMTYzLmNvbQ==; Jing Zhao, MTM1NjEyODc3NzlAMTYzLmNvbQ==
 Dan Wang
Dan Wang Jing Zhao
Jing Zhao