- 1Department of Epidemiology, School of Public Health, Southern Medical University, Guangzhou City, China
- 2Department of Rehabilitation Medicine, The First People’s Hospital of Foshan City, Foshan City, China
Objective: To investigate the influencing factors associated with feeding disorders in preterm infants and to construct a prediction model.
Methods: 314 cases of preterm infants admitted to our hospital from January 2019 to December 2022 were retrospectively analyzed and divided into feeding disorder group and non-feeding disorder group according to the presence of feeding disorder at 37 weeks of corrected gestational age. Statistical analysis of children's general information, hospitalization measures, laboratory tests, feeding time, etc. Multifactorial Logistic regression analysis of the occurrence of feeding disorders related to the influence of factors, and the use of subjects to make a work characteristic curve to analyze the predictive value of the relevant factors on feeding disorders.
Results: Multifactorial logistic regression analysis suggested that lower birth gestational age, birth weight, white blood cell count, absolute value of monocytes, blood calcium value, Apgar score at 1 min after birth, and longer duration of noninvasive ventilation were risk factors for feeding disorders in preterm infants. ROC curve analysis suggested that the area under the curve of the feeding disorders was predicted by the combination of the above seven indexes to construct the feeding disorders prediction model The AUC was 0.866 (P < 0.001, 95% CI 0.801–0.932), and it had a maximum Yoden index of 0.699, an optimal cutoff value of 0.169, a sensitivity of 85.4%, a specificity of 84.5%, and a prediction accuracy of 91.4%.
Conclusions: Lower birth gestational age, birth weight, white blood cell count, absolute monocyte value, blood calcium value, low Apgar score at 1 min after birth, and prolonged noninvasive ventilation are risk factors for feeding disorders in preterm infants, and the present prediction model is a good predictor of the occurrence of feeding disorders in preterm infants.
Introduction
Preterm labor (births at a gestational age of <37 weeks) (1) is an important issue for neonatal health worldwide. According to the World Health Organization, approximately 15 million preterm babies are born globally each year (2), and the preterm birth rate is approximately 11% and continues to increase (3). Preterm birth is the leading cause of death in children under 5 years of age (4), and its associated complications have a particular impact on quality of survival.
The universal global standard for discharge from the neonatal intensive care unit (5) is the infant's ability to take nutrition orally, known as full oral feeding (FOF) (6). Feeding disorders can affect the process of full oral feeding. Feeding Disorders are defined as the inability of a child to complete oral intake on his or her own, dependence on intravenous nutrition or gastrostomy tube feeding, or a single oral feeding lasting longer than 30 min (7).
The incidence of feeding disorders in preterm infants is as high as 82% due to immature development of sucking-swallowing-breathing coordination (8, 9), which is significantly higher than that of term infants. Such children are prone to gastrostomy tube dependence, leading to reduced tongue muscle activity (10), which in turn triggers degradation of swallowing function, delayed oral transportation and lagged pharyngeal phase initiation, increasing the risk of aspiration pneumonia (11). Both feeding intolerance and feeding disorders can lead to gastrostomy tube dependence, but most of the existing studies focus on the pathomechanisms of feeding intolerance (12, 13), neglecting the feeding disorders caused by deficiencies in the ability to feed by mouth. Feeding disorders not only prolong the duration of gastrostomy tube retention and interfere with the maturation of gastrointestinal dynamics, but also may lead to prolonged hospitalization and increased healthcare costs (12, 13), and even affect the neurodevelopment and social cognitive ability of children (14), which may be life-threatening in severe cases.
Despite the prevalence and consequences of preterm infant feeding problems globally, there are no harmonized guidelines as the gold standard of care (9). Due to the complexity of the mechanism of oral feeding and the immaturity of the preterm infant's organism leading to large individual differences at different months of age, it is difficult to assess the oral feeding function of preterm infants at small months of age. Most of the commonly used methods of assessment are based on scale assessment, which has a certain degree of subjectivity, while the methods used for the objective assessment of preterm infants are limited, and swallowing imaging and swallowing laryngoscopy are not applicable to a wide range of applications due to specific requirements. The smart pacifier system studied by Akbarzadeh (15) and others is expensive equipment and has some limitations in clinical application in preterm infants. Therefore, the construction of a prediction model for feeding disorders in preterm infants may be a new idea to solve the above clinical problems. Due to the fact that there are fewer influencing factors analyzed for feeding disorders in preterm infants, there are no reports on the prediction of feeding disorders model for preterm infants so far. In most studies, low birth gestational age, low birth weight, and concomitant etiologies associated with preterm labor are factors that influence infant feeding and swallowing (16–18). Pre-discharge or perinatal brain injury has not been identified as one of the risk factors for persistent feeding disorders after discharge (19).
To this end, we determined that there is a need to explore relevant evidence to support good predictive modeling to guide clinicians in the early identification of preterm infants with a poor prognosis for feeding disorders, integrating early interventions to increase rehabilitative therapies, as well as to inform caregivers in providing a supportive approach to feeding.
Hypothesis and objectives
We hypothesized that the maturity of the preterm infant, the degree of perinatal damage to the infant, and the organism's function would be related to the development of the preterm infant's feeding function.
The aim of this study is to investigate the influencing factors of feeding disorders in preterm infants and to construct a prediction model of feeding disorders in preterm infants, so as to provide a scientific basis for further shortening the gastric tube retention time.
Secondary aims
• Analyze factors related to the development of feeding function in premature infants.
• Evaluate oral feeding function:
○ Whether there is dependence on gastric tube.
○ Correct the ability to eat orally at a gestational age of 37 weeks.
○ Safe oral intake.
Methods
Infant recruitment
Retrospective analysis of 314 cases of preterm infants treated in the neonatology department of the First People's Hospital of Foshan City during the period of January 2019-December 2022 were divided into the feeding obstacle group (n = 50) and the non-feeding obstacle group (n = 264) according to the presence or absence of feeding obstacles at the time of correcting the gestational age of 37 weeks of the child. Inclusion criteria: (1) neonates born at a gestational age of <37 weeks. (2) complete clinical data. Exclusion criteria: (1) Combination of diseases requiring surgery and fasting. (2) necrotizing enterocolitis (NEC). (3) gastrointestinal malformations. (4) serious respiratory tract infections. This study was reviewed by the Ethics Committee of the First People's Hospital of Foshan City [Lun Audit Research (2024) No. 79].
Methodology
Gather clinically relevant information about the child by reviewing medical records and test results:
(1) General information: gestational age at birth, birth weight, gender, mode of delivery, whether multiple births, length of hospitalization, Apgar score. (2) Hospitalization measures: time of invasive ventilation, non-invasive ventilation, high-flow oxygen, central oxygen. (3) Laboratory tests: serum albumin, serum protein, direct bilirubin, indirect bilirubin, serum calcium, calcitonin, basophil absolute value, eosinophils absolute value, lymphocyte count, red blood cell count, white blood cell count, monocyte count, platelet count, interleukin-6. (4) Feeding time: months after the start of oral feeding Absolute value of basophils, absolute value of eosinophils, lymphocyte count, red blood cell count, white blood cell count, monocyte count, platelet count, interleukin-6. (5) Feeding time: the age of the month of the start of oral feeding, the time of removal of the gastric tube.
Statistical analysis
SPSS25.0 software was used for statistical analysis. Measurement data were tested for data normality by the Kolmogorov–Smirnov method, and data that conformed to normal distribution were described by mean ± standard deviation, and independent samples t-test was performed; data that did not conform to normal distribution were expressed as median (interquartile spacing) [M ± (Q1, Q3)], and Mann–Whitney U test was performed. Count data were expressed as actual number of cases with percentages, and the χ² test or Fisher's exact probability method test was performed. Factors associated with feeding disorders in preterm infants were analyzed using univariate analysis, and binary logistic multifactorial regression analysis was used to screen the risk factors and protective factors for the emergence of feeding disorders in preterm infants at corrected gestational age of 37 weeks, and to assess the risk factors and protective factors for the development of feeding disorders in preterm infants at corrected gestational age of 37 weeks by using a receiver operating characteristic (ROC) curve made by the subjects, and assessing the risk factors and protective factors with the area under the curve (area under curve (AUC) was used to assess the ability of the relevant factors to predict feeding disorders, and the critical value was set to the value corresponding to the maximum Jordon index (sensitivity + specificity - 1). All statistical tests were performed with P < 0.05 as the difference being significant.
Results
Analysis of the distribution of clinical characteristics of preterm infants and baseline values
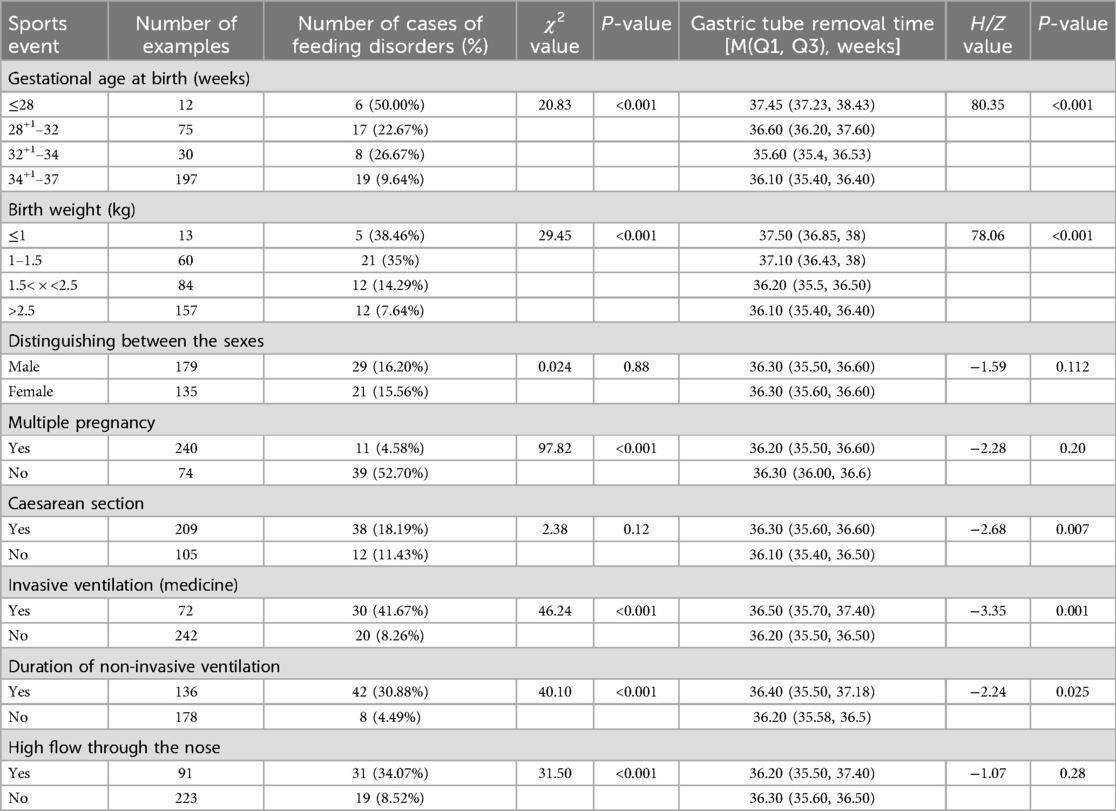
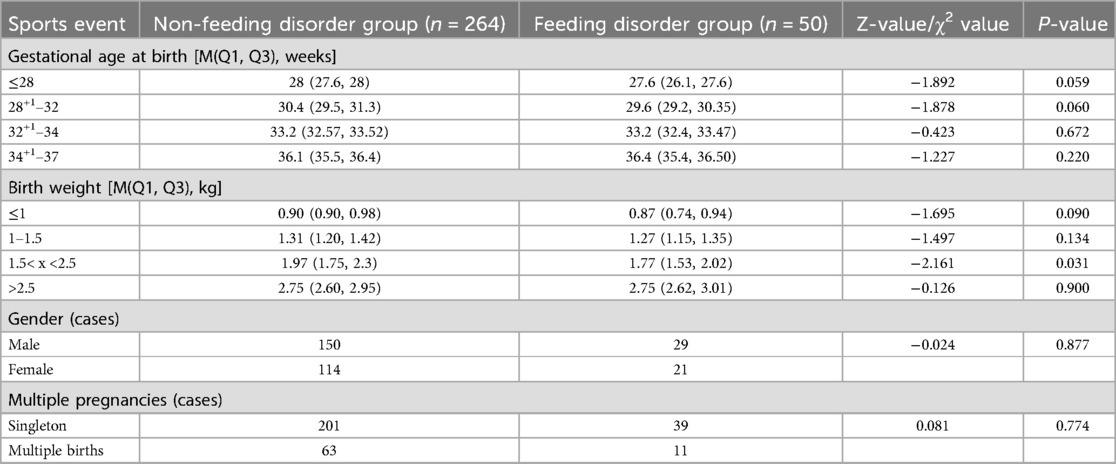
The gestational age of 314 preterm infants was (33.92 ± 2.85) weeks; birth weight was (2.22 ± 0.71) kg. 314 preterm infants corrected the incidence of feeding disorders at a gestational age of up to 37 weeks was 15.92% (50/314), and the time of gastrostomy tube removal was (36.26 ± 1.04) weeks, with a minimum of 34+1 weeks and a maximum of 42+3 weeks. The results showed that the incidence of feeding disorders was higher (P < 0.05) for lower birth weight, lower gestational age at birth, history of cesarean section, application of invasive positive pressure ventilation, and duration of noninvasive ventilation. There was no significant difference in the gestational age and gender of the children in both groups (P > 0.05) (Tables 1, 2).
One-way logistic regression analysis
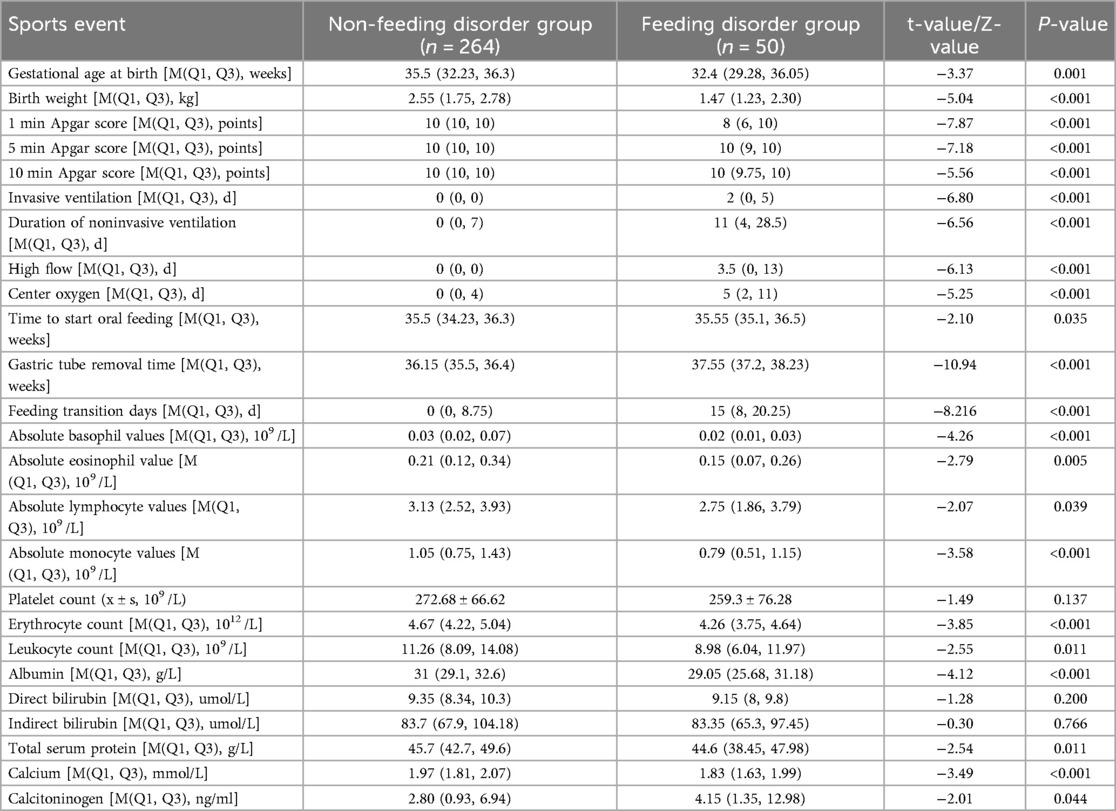
The children were categorized into a feeding-impaired group (n = 50) and a non-feeding-impaired group (n = 264) according to the presence or absence of a feeding disorder at 37 weeks of corrected gestational age. The results of univariate analysis showed that the preterm infants in both groups had the following characteristics in terms of birth gestational age, birth weight, Apgar score, invasive ventilation, duration of non-invasive ventilation, high-flow ventilation, duration of centralized oxygen, time of initiation of oral feedings, time of gastric tube removal, number of days of transition to feeding, absolute eosinophils, absolute eosinophilic granulocytes, absolute lymphocytes, absolute monocytes, erythrocyte count, leukocyte count, albumin, Comparisons in terms of total serum protein, calcium, and calcitoninogen showed statistically significant differences (P < 0.05) (Table 3).
Multifactor logistic regression analysis
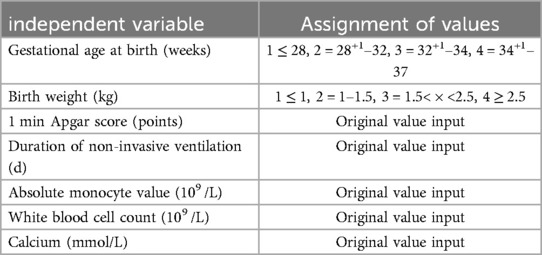
The presence of a feeding disorder at 37 weeks of corrected gestational age of the child was used as the dependent variable Y, and the 25 variables mentioned above were used as the independent variables X. The final prediction model incorporated seven clinical indicators as predictors: birth gestational age, birth weight, Apgar 1 min, time to noninvasive ventilation, absolute value of monocytes, leukocyte count, and calcium (Tables 4, 5).
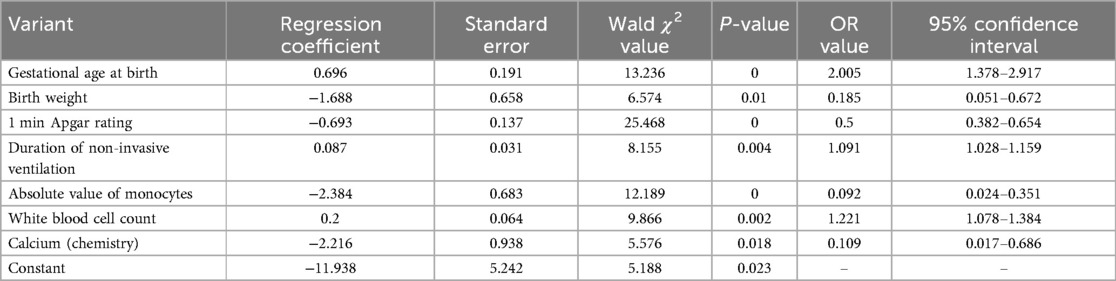
Table 5. Multifactorial logistic regression analysis of factors influencing feeding barriers in preterm infants.
Predictive modeling of feeding disorders in preterm infants
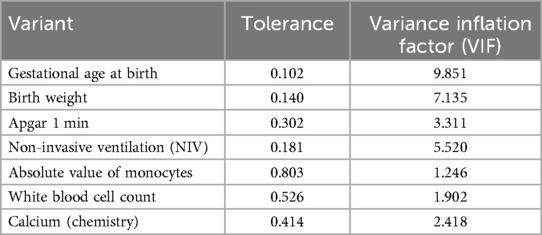
With the presence of feeding disorders at 37 weeks of corrected gestational age of the child as the dependent variable and the above seven indicators (birth gestational age, birth weight, Apgar 1 min, noninvasive ventilation time, absolute value of monocytes, leukocyte count, and calcium) as the independent variables, the regression equation was constructed as Logit (P) = −11.938 + 0.696 × birth gestational age—1.688 × birth weight—0.693 × 1 min Apgar score + 0.087 × duration of noninvasive ventilation—2.384 × absolute value of monocytes + 0.2 × leukocyte count—2.216 × calcium, and the predictive accuracy (accuracy, Acc) of this model was 91.401%, which indicates that the efficacy of the present model is better.The Hosmer-Lemeshow goodness-of-fit test showed that χ2 = 11.49, P = 0.175, P > 0.05, which indicates the goodness of fit of the model. The tolerance and variance inflation factor of the seven variables in the model were obtained through the linear regression function of SPSS25.0 statistical software(Table 6).The covariance diagnosis suggests that the tolerance of each variable is much greater than 0.1, and the variance inflation factor is less than 10, so it can be assumed that there is no multicollinearity among the variables.
Comparison of ROC curves of indicators on feeding disorders in preterm infants
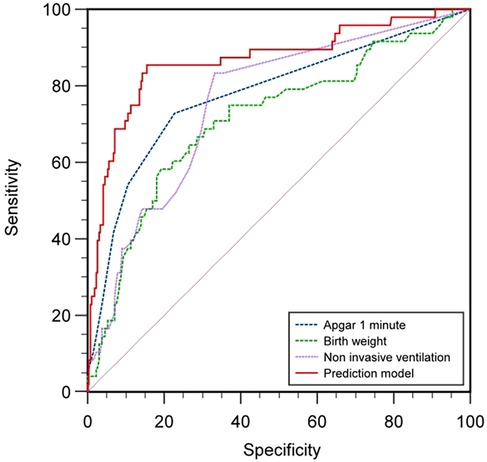
Based on the results of multifactorial binary logistic regression analysis, ROC curves were plotted and analyzed for the indicators that met P < 0.05 in the multifactorial analysis. The results showed that the AUC of birth weight for predicting feeding disorders was 0.725 (P < 0.001, 95% CI 0.642–0.808), with a maximum Yoden index of 0.407 and an optimal cut-off value of 1.555 kg, with a sensitivity of 60.0% and a specificity of 80.7%, and that the AUC of the Apgar 1-minute score for predicting feeding disorders was 0.787 (P < 0.001, 95% CI 0.709–0.865), with a maximum Jordon's index of 0.513, an optimal cutoff value of 9.5 points, a sensitivity of 74%, and a specificity of 77.3%; and the AUC for noninvasive ventilation time to predict feeding disorders was 0.765 (P < 0.001, 95% CI 0.694–0.836), with a maximum Jordon's index was 0.507, with an optimal cut-off value of 2.5 days, a sensitivity of 84%, and a specificity of 66.7%; suggesting that birth weight, 1-minute Apgar score, and noninvasive ventilation time all have some predictive ability for feeding disorders in preterm infants.
The area under the curve AUC for constructing a prediction model for feeding disorders to jointly predict feeding disorders was 0.866 (P < 0.001, 95% CI 0.801–0.932), which indicated that the prediction model had a better prediction ability for feeding disorders and its maximum Yoden index was 0.699, which at this time corresponded to a critical value of 0.169, with a sensitivity of 85.4% and a specificity of 84.5%. The results analyzed statistically showed that the AUC of the prediction model for predicting feeding disorders were all better than the single indicators of birth weight (Z = 3.584, P < 0.001), time of noninvasive ventilation (Z = 3.21, P = 0.001), and the Apgar 1-minute score (Z = 2.568, P = 0.010), and the difference was statistically significant (Table 7; Figure 1).
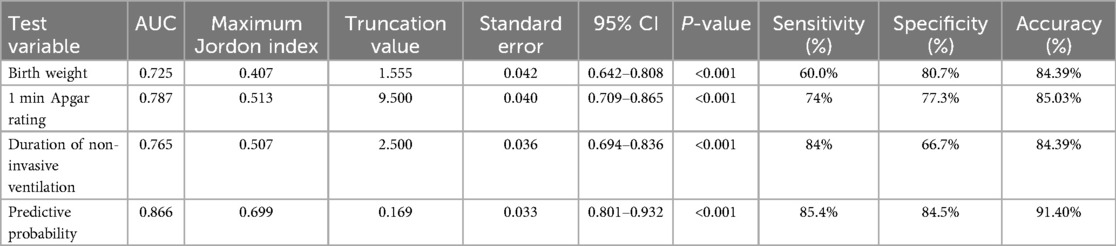
Table 7. Comparison of ROC curves and AUC values of indicators on feeding disorders in preterm infants.
ROC Curves: Comparison between the combined model (red) and top individual predictors. Shaded areas represent 95% CIs from bootstrapping (1,000 iterations).
Threshold Markers: Optimal cutoff (0.65) balances sensitivity (85.4%) and specificity (84.5%) using Youden's index.
Discussion
Transoral feeding involves precise coordination of oral motor functions, respiratory and digestive systems. Compared to full-term infants, preterm infants often have to face more survival challenges such as respiratory distress, respiratory distress syndrome, sepsis, oxygen desaturation or bradycardia due to their immature development (20). In this study, we found that 15.92% of preterm infants corrected for a gestational age of 37 weeks had significant feeding problems requiring gastric tube-assisted feeding, despite being close to the physiologic status of full-term infants. This finding emphasizes that even when preterm infants reach a stage of relative maturity, their feeding problems cannot be ignored and require more careful and individualized interventions by the healthcare team.
Developmental maturity of preterm infants
Low birth gestational age is a risk factor for feeding disorders. The younger the gestational age, the higher the incidence of feeding disorders in preterm infants (21), susceptible to decreased oxygen saturation and apnea due to weak sucking, immature swallowing, and uncoordinated sucking-swallowing-breathing (22), and with low maturity of intestinal function, the higher the risk of FI. In this study, we found that the gestational age of the feeding disorder group was lower than that of the non-feeding disorder group (Z = −3.37, P = 0.001).
Low birth weight is a risk factor for feeding disorders in preterm infants. The higher the birth weight the more tolerant the child is and the more mature the organs are. At the same time, a heavier birth weight reduces feeding intolerance (FI) in preterm infants, suggesting better digestive function (23, 24). In the present study, we found that children in the feeding disorder group were lighter (P < 0.001) and the lower the weight, the greater the gestational age at which the gastrostomy tube was removed, suggesting that birth weight significantly affects feeding function in preterm infants.
Organizational functioning of preterm infants
Low white blood cell (WBC) count is a risk factor for feeding disorders in preterm infants and is associated with birth gestational age, immune function, and inflammatory response (25). In preterm infants, WBC rises with increasing birth weight and peaks within the first day of life. Neonatal WBC at birth was (15–28) × 109 /L (26) and 32–34 weeks preterm WBC was (13.01 ± 5.18) × 109 /L (27), which is in agreement with the present study. The leukocyte erythrocyte count ratio (LER) correlates intrapulmonary lesions (28–30) as a risk factor for aspiration pneumonia (31), and low WBC also correlates with brain injury and NEC (32, 33), with blood redistribution during brain injury, gastrointestinal hypoxia-ischemia is the first to occurs, inhibiting gastrointestinal motility (34). And brain function, gastrointestinal function, and lung function affect swallowing function (35). The present study showed that WBC [8.98 (6.04, 11.97)] was lower in the feeding disorder group than in the non-feeding disorder group [11.26 (8.09, 14.08)] (P < 0.001), suggesting that low WBC may be related to feeding disorder.
Low absolute monocyte values are a risk factor for feeding disorders in preterm infants. Monocytes are involved in the body's defense processes (36). They are greatly altered when the organism undergoes inflammation or other diseases (37). Studies have shown that monocytes and other peripheral immune cells may affect brain function (38) and that absolute monocyte values are reduced in the early stages of the onset of neonatal NEC (39) and are lowest at the time of NEC diagnosis (40). In the present study, we found that the absolute monocyte values of children in the feeding disorder group were lower than those in the non-feeding disorder group, and we consider that there may be an indirect relationship between the low absolute monocyte values and the initial intestinal inflammation, which may affect the feeding function of preterm infants.
Low blood calcium values are a risk factor for feeding disorders in preterm infants. Blood calcium is important for metabolic and physiologic functions and is associated with maternal and own calcium and phosphorus regulation (41). The lower the gestational age at birth and the lower the birth weight, the lower the blood calcium (42), which is associated with a shorter calcium reserve time and insufficient parathyroid response in preterm infants (43); therefore, preterm infants generally have low blood calcium in the first 24–36 h of life, which is consistent with the present study. Extracellular Ca is involved in the excitability of neurons and muscle cells (44). Hypocalcemia negatively affects contractility and motility of the gastrointestinal tract (45). The blood calcium level in the feeding disorder group was [1.83 (1.63, 1.99) mmol/L], which was lower than that in the non-feeding disorder group [1.97 (1.81, 2.07) mmol/L] (P < 0.05), suggesting that low blood calcium is associated with insufficient excitability of swallowing muscles and gastrointestinal dysfunction in preterm infants.
Extent of damage to preterm infants
A low Apgar score at 1 min after birth is a risk factor for feeding disorders in preterm infants. The score reflects the immediate health status of the preterm infant, with higher scores predicting healthier newborns and greater chances of survival (46). A low score may suggest that the child is at risk of hypoxia and asphyxia (47), which is strongly associated with secondary multi-organ damage (e.g., lung, gastrointestinal, brain) (48), which are critical for transoral feeding. The present study showed that 1-minute Apgar scores of children in the feeding-impaired group were lower than those in the non-feeding-impaired group (P < 0.001), suggesting that a former hypoxic state may be associated with feeding impairment in preterm infants.
Prolonged noninvasive ventilation is a risk factor for feeding disorders in preterm infants. For very preterm infants, respiratory disease is still the most important clinical problem because lung tissues are not yet mature (49), so mechanical ventilation is needed to assist respiration, but prolonged mechanical ventilation is prone to respiratory dependence, which inhibits spontaneous respiration to a certain degree (50) and affects the coordination of sucking, swallowing, and respiration (51), which results in the feeding process of the children This can lead to disorders of gas exchange, breath-holding, decreased oxygen saturation, and decreased heart rate (52), affecting feeding function and even aspiration (53). In addition, noninvasive ventilation increases gastrointestinal insufflation and intestinal wall dilatation, which reduces gastrointestinal blood flow and slows down gastrointestinal peristalsis (54), affecting gastrointestinal function, and the longer the duration of noninvasive ventilation, the more likely that feeding intolerance will occur (55), which will have a certain effect on transoral feeding. In the present study, we found that the duration of noninvasive ventilation was longer in the feeding-impaired group (P < 0.001), suggesting that poor lung function is associated with feeding impairment in preterm infants.
ROC curve analysis of predictive models for prediction of feeding disorder outcomes in preterm infants
In this study, seven indicators were included in the prediction model, including “birth gestational age”, “duration of noninvasive ventilation”, “white blood cell count”, “birth weight”, “1-minute Apgar score”, “absolute monocyte value”, and “calcium”, which covered a wide range of clinical indicators of preterm infants. “1 min Apgar score”, “absolute value of monocytes”, and “blood calcium”, which cover a wide range of clinical indicators of preterm infants. Birth gestational age, birth weight and developmental maturity of preterm infants are related to each other, white blood cell count, absolute monocyte value, and blood calcium are related to the body function of preterm infants, and 1-minute Apgar score and noninvasive ventilation time are related to the degree of damage of preterm infants. In this study, the predictive model was found to be superior to single-indicator prediction with high sensitivity (85.4%) and specificity (84.5%), and a prediction accuracy of 91.4%.The sensitivity of 85.4% suggests that the model was able to identify more than 80% of the potentially feeding-disordered children, which is helpful in reducing the risk of underdiagnosis and in reducing delayed interventions. Children at high risk (positive model prediction) can be prioritized to receive bedside swallowing function assessment to shorten the diagnostic delay. The present predictive model integrates the developmental maturity, degree of organic function and impairment, and organic function of preterm infants, which facilitates better prediction of the prognosis of feeding disorders and is consistent with our hypothesis.
Conclusion
In this study, low birth gestational age, low birth weight, low white blood cell count, low absolute monocyte value, low blood calcium value, low Apgar score at 1 min after birth, and prolonged noninvasive ventilation were found to be risk factors for feeding disorders in preterm infants. This prediction model has high sensitivity, specificity, and predictive accuracy for prediction of feeding disorders in preterm infants, which can warn preterm infants of the risk of developing feeding disorders, and help clinicians to identify this group of infants and take active rehabilitative therapeutic interventions to improve the feeding function, which can provide clinical value for the early removal of gastrostomy tubes and shorten the length of hospitalization.
Limitations
The main limitation that limits this study is its retrospective design. Other limitations: Model validation was not performed. Potential confounding variables that may affect the outcome, such as maternal health status, antenatal care, initiation of breastfeeding and socioeconomic factors were not considered, and multicenter validation was lacking.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Foshan First People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LC: Conceptualization, Formal analysis, Methodology, Writing – original draft. HZ: Project administration, Supervision, Writing – review & editing. ZT: Data curation, Validation, Visualization, Writing – review & editing. HD: Formal analysis, Methodology, Project administration, Visualization, Writing – review & editing. ZL: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Guangdong Medical Research Fund Project (B2025017) and Foshan City Self-financing Category Science and Technology Innovation Project (2320001006957).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. (2016) 21(2):68–73. doi: 10.1016/j.siny.2015.12.011
2. Wang C, Huang Z. Research progress on early human auditory development and auditory management in preterm infants. Chin J Rehabil Med. (2024) 39(3):443–7. doi: 10.3969/j.issn.1001-1242.2024.03.023
3. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7(1):37–46. doi: 10.1016/S2214-109X(18)30451-0
4. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388(10063):3027–35. doi: 10.1016/S0140-6736(16)31593-8. Erratum in: Lancet. (2017) 389(10082):1884. doi: 10.1016/S0140-6736(17)31212-627839855
5. Committee on Fetus and Newborn. Hospital discharge of the high-risk neonate. Pediatrics. (2008) 122:1119–26. doi: 10.1542/peds.2008-2174
6. Bakker L, Jackson B, Miles A. Oral-feeding guidelines for preterm neonates in the NICU: a scoping review. J Perinatol. (2021) 41(1):140–9. doi: 10.1038/s41372-020-00887-6
7. Schädler G, Süss-Burghart H, Toschke AM, von Voss H, von Kries R. Feeding disorders in ex-prematures: causes–response to therapy–long term outcome. Eur J Pediatr. (2007) 166(8):803–8. doi: 10.1007/s00431-006-0322-x
8. Fucile S, Phillips S, Bishop K, Jackson M, Yuzdepski T, Dow K. Identification of a pivotal period in the oral feeding progression of preterm infants. Am J Perinatol. (2019) 36(5):530–6. doi: 10.1055/s-0038-1669947
9. Vizzari G, Morniroli D, D'Auria A, Travella P, Bezze E, Sannino P, et al. Feeding difficulties in late preterm infants and their impact on maternal mental health and the mother-infant relationship: a literature review. Nutrients. (2023) 15(9):2180. doi: 10.3390/nu15092180
10. Tang Z, An D, Wen H, Wen B, Dou Z. Quantitative relationship between tongue pressure and hyoid bone movement and pharyngeal phase activity in stroke patients with dysphagia. Chin J Phys Med Rehabil. (2019) 41(12):889–93. doi: 10.3760/cma.j.issn.0254-1424.2019.12.002
11. Zhang YW, Dou ZL, Zhao F, Xie CQ, Shi J, Yang C, et al. Neuromuscular electrical stimulation improves swallowing initiation in patients with post stroke dysphagia. Front Neurosci. (2022) 16:1011824. doi: 10.3389/fnins.2022.1011824
12. Huff K, Rose RS, Engle WA. Late preterm infants. Pediatr Clin North Am. (2019) 66:387–402. doi: 10.1016/j.pcl.2018.12.008
13. Sharma D, Padmavathi IV, Tabatabaii SA, Farahbakhsh N. Late preterm: a new high risk group in neonatology. Matern Fetal Neonatal Med. (2021) 34(16):2717–30. doi: 10.1080/14767058.2019.1670796
14. Zhiyong P, Wang M, Yang H, Zhu J, Li H, Dou Z, et al. Effect of high energy density enteral nutrition support on clinical outcomes in patients with dysphagia with aspiration pneumonia. Chin J Phys Med Rehabil. (2021) 43(12):1114–6. doi: 10.3760/cma.j.issn.0254-1424.2021.12.013
15. Akbarzadeh S, Farhoodi R, Lyu T, Awais M, Zhao X, Abbasi SF, et al. Evaluation of Apgar scores and non-nutritive sucking skills in infants using a novel sensitized non-nutritive sucking system. Annu Int Conf IEEE Eng Med Biol Soc. (2020) 2020:4282–5. doi: 10.1109/EMBC44109.2020.9176146
16. Dodrill P, Gosa MM. Pediatric dysphagia: physiology, assessment, and management. Ann Nutr Metab. (2015) 66:24–31. doi: 10.1159/000381372
17. Arvedson JC, Brodsky L. Pediatric Swallowing and Feeding: Assessment and Management. Albany, NY, USA: Singular Thomson Learning (2002).
18. Khan Z, Sitter C, Dunitz-Scheer M, Posch K, Avian A, Bresesti I, et al. Full oral feeding is possible BeforeDischarge even in extremely preterm infants. Acta Paediatr. (2019) 108:239–44. doi: 10.1111/apa.14478
19. Slana N, Hočevar-Boltežar I, Kornhauser-Cerar L. Risk factors for feeding and swallowing disorders in very low birth weight infants in their second year of life. Medicina (Kaunas). (2022) 58(11):1536. doi: 10.3390/medicina58111536
20. Zhang YR. Feeding and swallowing disorders in infants and young children—elements of management from tube feeding to oral feeding in newborns. Chin J Phys Med Rehabil. (2019) 41(12):949–51. doi: 10.3760/cma.j.issn.0254-1424.2019.12.017
21. Suan-Suan S, Mei-Qin Y. Research progress on support strategies for preterm infants with oral feeding difficulties. China Nurs Manag. (2022) 22(10):1569–73. doi: 10.3969/j.issn.1672-1756.2022.10.026
22. Meixia H, Yunyun L, Ping S. Effectiveness of an integrated program of progressive oral feeding combined with physical intervention in very preterm infants. Evid Based Nurs. (2024) 10(10):1860–4. doi: 10.12102/j.issn.2095-8668.2024.10.025
23. Fan M, Chen X, Zhang Z, Jia W. Study on clinical characteristics and related influencing factors of feeding intolerance in preterm infants. Chin Fam Med. (2022) 20(3):431–4. 449. doi: 10.16766/j.cnki.issn.1674-4152.002370
24. Dani C, Ciarcià M, Luzzati M, Nardecchia S, Petrolini C, Sarli WM, et al. Feeding intolerance during phototherapy in preterm infants. J Matern Fetal Neonatal Med. (2022) 35(25):6610–4. doi: 10.1080/14767058.2021.1918093
25. Xia D, Jingming H, Jian H, Lingxia W, Gong Q, Zhou X, et al. Analysis of risk factors and prediction model for associated pneumonia in patients with post-stroke dysphagia. Chin J Rehabil Med. (2022) 37(5):616–22. doi: 10.3969/j.issn.1001-1242.2022.05.008
26. Shao X-M, Ye H-M, Qu X-S. Practical Neonatology. 4th ed Beijing: People’s Health Publishing House (2011). p. 588.
27. Li Q, Zhao X, Bai R, Zeng J, Li Z. Dynamic changes of leukocyte and platelet counts in preterm infants and their influencing factors. Chin J Perinat Med. (2015) 18(12):921–6. doi: 10.3760/cma.j.issn.1007-9408.2015.12.008
28. Daikun H, Xueting S, Lina W, Zhigang P. Predictive value of baseline CRP, NLR and LER for the development of aspiration pneumonia in patients with acute visceral infarction. Chin J Emerg Med. (2022) 31(12):1635–41. doi: 10.3760/cma.j.issn.1671-0282.2022.12.012
29. Damar Çakırca T, Torun A, Çakırca G, Portakal RD. Role of NLR, PLR, ELR and CLR in differentiating COVID-19 patients with and without pneumonia. Int J Clin Pract. (2021) 75(11):e14781–91. doi: 10.1111/ijcp.14781
30. Li LL, Yang YQ, Qiu M, Wang L, Yuan HL, Zou RC. The clinical significance of neutrophil-lymphocyte ratio in patients treated with hemodialysis complicated with lung infection. Medicine (Baltimore). (202l) 100(29):e26591–601. doi: 10.1097/MD.0000000000026591
31. Hu J, Zhang X. Construction of nomogram prediction model for aspiration pneumonia within 1 month in elderly patients with swallowing disorder after cerebral infarction. Hebei Med Univ. (2024) 45(6):638–45. doi: 10.3969/j.issn.1007-3205.2024.06.004
32. Zhong XQ, Cui QL, Yu KH. Relationship between perinatal inflammation and brain injury in preterm infants. Chin J Perinat Med. (2015) 18(6):467–9. doi: 10.3760/cma.j.issn.1007-9408.2015.06.016
33. Li J, Chen Y, Yang M, Xu F, Dong H. Analysis of clinical characteristics and risk factors of necrotizing small bowel colitis in one of preterm twin fetuses. Chin J Neonatol. (2024) 39(4):193–8. doi: 10.3760/cma.j.issn.2096-2932.2024.04.001
34. Juan G. Retrospective analysis of complications in 526 preterm infants born to mothers with hypertensive syndrome of pregnancy. Chin J Pract Med. (2018) 45(16):12–4. 18. doi: 10.3760/cma.j.issn.1674-4756.2018.16.005
35. Yuan Y, Wang J, Huang X, Wu D. Central and peripheral neuromodulation mechanisms of swallowing function. Chin J Rehabil Med. (2018) 33(12):1479–82. doi: 10.3969/j.issn.1001-1242.2018.12.023
36. Zhuo C, Mao X, Long S. Clinical value of blood mononuclear cells in children with mycoplasma pneumoniae infection. Chin Commun Phys. (2021) 37(2):134–5. doi: 10.3969/j.issn.1007-614x.2021.02.066
37. Hu X, Chang Y, Li Z. Impact of feeding intolerance on recent outcomes of preterm infants. J Clin Pediatr. (2021) 39(5):355–9. doi: 10.3969/j.issn.1000-3606.2021.05.008
38. Bai M, Sun R, Cao B, Feng J, Wang J. Monocyte-related cytokines/chemokines in cerebral ischemic stroke. CNS Neurosci Ther. (2023) 29(12):3693–712. doi: 10.1111/cns.14368
39. Remon J, Kampanatkosol R, Kaul RR, Muraskas JK, Christensen RD, Maheshwari A. Acute drop in blood monocyte count differentiates NEC from other causes of feedingintolerance. J Perinatol. (2014) 34(7):549–54. doi: 10.1038/jp.2014.52
40. Qin AZ, Li ST, Zhuang GY, Qu LH, Xiao X. Dynamic changes and significance of absolute monocyte count in the peripheral blood of children with necrotizing small bowel colitis in newborns. Chin J Clin Phys. (2017) 45(8):110–2. doi: 10.3969/j.issn.2095-8552.2017.08.039
41. Teng Z, Jia Y, Jiang Y, Li X, Liu J, Chen J. Study on serum calcium, phosphorus, alkaline phosphatase and vitamin D status in 185 neonates. Chongqing Acad Med. (2017) 46(3):387–9. doi: 10.3969/j.issn.1671-8348.2017.03.032
42. Seymen-Karabulut G, Günlemez A, Gökalp AS, Hatun Ş, Kaya Narter F, Mutlu M, et al. Vitamin D deficiency prevalence in late neonatal hypocalcemia: a multicenter study. Clin Res Pediatr Endocrinol. (2021) 13(4):384–90. doi: 10.4274/jcrpe.galenos.2020.2021.0169
43. Mihatsch W, Thome U, Saenz de Pipaon M. Update on calcium and phosphorus requirements of preterm infants and recommendations for enteral mineral intake. Nutrients. (2021) 13(5):1470. doi: 10.3390/nu13051470
44. Han P, Trinidad BJ, Shi J. Hypocalcemia-induced seizure: demystifying the calcium paradox. ASN Neuro. (2015) 7(2). doi: 10.1177/1759091415578050
45. Wilkens MR, Nelson CD, Hernandez LL, McArt JAA. Symposium review: transition cow calcium homeostasis-health effects of hypocalcemia and strategies for prevention. J Dairy Sci. (2020) 103(3):2909–27. doi: 10.3168/jds.2019-17268
46. Jiangsu Provincial Neonatal Intensive Care Unit Breastfeeding Quality Improvement Clinical Research Collaborative Group. Risk factors for low Apgar score at 1 min after birth in very low/ultra low birth weight infants: a multicenter retrospective study. Chin J Contemp Pediatr. (2023) 25(9):909–14. doi: 10.7499/j.issn.1008-8830.2306139
47. Chen Z, Liu J. Interpretation of “recommendations on the criteria for the diagnosis and classification of neonatal asphyxia”. Chin J Contemp Pediatr. (2013) 15(1):2–4. doi: 10.7499/j.issn.1008-8830.2013.01.002
48. Qiong F, Jing M, Chunlei L, Dong C. Analysis of high-risk factors for neonatal asphyxia secondary to multiple organ injury. Chin J Clin Phys. (2020) 48(2):232–5. doi: 10.3969/j.issn.2095-8552.2020.02.035
49. Cai Z-Y, Liu J-D, Bian H-L, Cai J-L, Xu H, Zhang C-F, et al. Efficacy of the technique of “tracheal intubation-use of pulmonary surface active substances-removal of the tube and use of continuous positive pressure ventilation” at different times in high-risk preterm infants with respiratory distress syndrome. Chin Pract Pediatr Clin Misc. (2016) 2:101–104. doi: 10.3760/cma.j.issn.2095-428X.2016.02.006
50. Ye L-Q, Peng J-H, Chen S-B. Factors influencing the transition time of oral feeding in preterm infants in NICU. Prim Med Forum. (2022) 26(31):145–7. doi: 10.19435/j.1672-1721.2022.31.047
51. Wang S, He Z, Pei Y, Cai F, Liu Z, Zhou A, et al. Effects of breathing pattern intervention on oral feeding performance of preterm infants with sucking-swallowing-breathing coordination disorder. Chin J Phys Med Rehabil. (2021) 43(6):494–8. doi: 10.3760/cma.j.issn.0254-1424.2021.06.003
52. Reynolds EW, Grider D, Bell CS. Swallow-breath interacion and phase of respiration with swallow during non-nutritive suck in infant affected by neonatal abstinence syndrome. Front Pediatr. (2017) 5(214):1–7. doi: 10.3389/fped.2017.00214
53. Chuke L, Peng L, Zhiyong G, Chuke L, Peng L, Zhiyong G, et al. Effect of fluid food regiment on swallowing function in patients with dysphagia after radiotherapy for nasopharyngeal carcinoma. Chin J Rehabil Med. (2021) 36(8):968–72. doi: 10.3969/j.issn.1001-1242.2021.08.012
54. Chen ZJ, Fang JH. Construction and validation of a prediction model for feeding intolerance in preterm infants. Chin J Pract Nurs. (2024) 40(11):816–22. doi: 10.3760/cma.j.cn211501-20230920-00586
Keywords: preterm infants, feeding disorders, influencing factors, predictive modeling, ROC curves
Citation: Chen L, Zhou H, Tang Z, Deng H and Li Z (2025) Influential factors related to feeding disorders in preterm infants and the construction of predictive models. Front. Pediatr. 13:1562778. doi: 10.3389/fped.2025.1562778
Received: 18 January 2025; Accepted: 22 April 2025;
Published: 14 May 2025.
Edited by:
Georgios D. Floros, Aristotle University of Thessaloniki, GreeceReviewed by:
Luh Karunia Wahyuni, RSUPN Dr. Cipto Mangunkusumo, IndonesiaKee Hyun Cho, Kangwon National University, Republic of Korea
Copyright: © 2025 Chen, Zhou, Tang, Deng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihao Li, emhpaGFvbGkyMDEzQHNtdS5lZHUuY24=; Huichang Zhou, emhjaGFuZ0Bmc3l5eS5jb20=
 Lishan Chen
Lishan Chen Huichang Zhou2*
Huichang Zhou2* Haiyin Deng
Haiyin Deng Zhihao Li
Zhihao Li