- 1Department of Pediatrics, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
- 2Graduate School, Inner Mongolia Medical University, Hohhot, Inner Mongolia, China
Background and objective: This study aims to analyze the clinical characteristics of anti-GABABR encephalitis in pediatric patients. Due to its rarity and diagnostic challenges in children, we compare clinical features between adult and pediatric cases.
Materials and methods: Using the key words “anti-GABABR encephalitis, children, autoimmune encephalitis, limbic encephalitis”, we conduct a comprehensive literature review of all studies related to anti-GABABR encephalitis published from January 2010 to January 2024. A total of 207 cases are identified globally, including 14 pediatric cases.
Results: We report a case of an 8-year-and-6-month-old child with anti-GABABR encephalitis presenting with abnormal mental behavior (irritability, hallucinations), sleep disorders, and paroxysmal involuntary limb movements. Serum anti-GABABR antibodies were positive, and clinical symptoms improved significantly after corticosteroid treatment. Analysis reveal that children presented with mental/behavioral abnormalities as the initial symptom (85.71%), while adults presented with epileptic seizures as the initial symptom (76.71%). Main symptoms include epilepsy in adults (78.24%) and sleep disorders (26.67%) and involuntary limb movements (33.33%) in children. Neuroimaging shows higher involvement of the basal ganglia (55.56%), cerebellar hemispheres (22.22%), and brainstem (22.22%) in children compared to adults. Video electroencephalogram (EEG) analysis indicates more frequent abnormal EEG in adults, but epileptic waves are more common in children with abnormal EEG. Cerebrospinal fluid (CSF) cytology is not specific, with mild lymphocytic increases (adults 57.98% vs. children 33.33%, P = 0.2054). Despite higher prodromal fever rates in children (66.67% vs. 23.44%, P = 0.0228), they respond better to immunotherapy. No tumor-related issues are observed in pediatric cases, contrasting with 58.09% tumor comorbidity in adults.
Conclusions: This study suggests that the clinical phenotypes of anti-GABABR encephalitis in children and adults may differ: children are more likely to present with mental and behavioral abnormalities (the initial symptom trend), sleep disorders and involuntary movements (the main symptoms), and their brain imaging is more likely to involve regions such as the basal ganglia and brainstem, and they respond better to immunotherapy. Notably, due to the small sample size of pediatric cases (n = 15) compared to adult cases (n = 193), these comparative findings should be interpreted with caution despite the statistical significance indicated by P-values. However, long-term follow-up remains essential.
1 Introduction
Autoimmune encephalitis refers to a group of encephalitis conditions triggered by autoimmune reactions. Since the initial identification of autoimmune encephalitis, various forms have been recognized based on the specific antibodies involved. These antibodies target different neuronal antigens, leading to different clinical manifestations and usually responding to immunotherapy (1).
Since the first report of anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis in 2007 (2), people's understanding of it has gradually become clear. It is the most common form of autoimmune encephalitis, affecting both children and adults. It is characterized by a broad range of symptoms, which often evolves in a characteristic multiphasic pattern. Early symptoms are frequently psychiatric in nature, including acute behavioral changes, agitation, hallucinations, and delusions. As the disease progresses, patients may develop seizures, dyskinesias, movement disorders, and autonomic instability. Further progression can result in a decreased level of consciousness and even coma if not recognized and treated promptly (3). The diagnosis of anti-NMDAR encephalitis relies on the detection of NMDAR antibodies in the cerebrospinal fluid (CSF) or serum, along with clinical and neuroimaging findings. The main treatment measures for anti-NMDAR encephalitis are immunotherapy and tumor resection, and commonly used first-line immunotherapy methods include corticosteroids, immunoglobulins, and plasma exchange (4).
Other forms of autoimmune encephalitis have also been identified, each associated with specific antibodies. Anti-leucine-rich glioma inactivated protein antibody (anti-LGI1) encephalitis is characterized by limbic symptoms, such as memory loss and seizures, and often presents with hyponatremia. Anti-alpha-amino-3-hydroxy-5-methyl-4-isoxazolpropionic acid receptor (anti-AMPAR) encephalitis typically manifests with seizures, status epilepticus, and psychiatric symptoms. The diagnosis of these conditions relies on the detection of the respective antibodies in the CSF or serum, along with clinical and neuroimaging findings. Similar to anti-NMDAR encephalitis, the treatment involves immunotherapy, with the choice of therapy depending on the severity of the disease and the patient's response to initial treatment (1).
Anti-GABABR encephalitis, the focus of this study, is a relatively rare form of autoimmune encephalitis. It is characterized by seizures and psychiatric symptoms. GABABR is crucial for synaptic plasticity, which is related to neurotransmitter transmission, learning, memory and cognitive functions. Therefore, memory impairment, cognitive impairment and other conditions may also occur (5). The diagnosis is confirmed by the presence of GABABR antibodies in the CSF or serum, following the diagnostic criteria provided by Graus et al. (6). Neuroimaging may show abnormalities in the limbic system or other brain regions. Treatment typically involves immunotherapy, with corticosteroids and intravenous immunoglobulin (IVIG) being the first-line options. In some cases, second-line therapies such as rituximab or cyclophosphamide may be required.
As of January 2024, a total of 208 cases of anti- GABABR encephalitis have been reported globally in the literature. Among these, 14 are pediatric cases and 193 are adult cases. The present study describes an additional pediatric case, bringing the total number of reported pediatric cases to 15. This study aims to provide a reference for early clinical diagnosis and treatment by describing this new case and reviewing previously reported cases, while analyzing the similarities and differences between pediatric and adult anti-GABABR encephalitis.
2 Materials and methods
Data collection was conducted through searches in China's National Knowledge Infrastructure (CNKI) and the newly added PubMed search function. A total of 207 cases reported both domestically and internationally from January 2010 to January 2024 were collected. Combined with the cases included in this article, there were 15 children and 193 adults. The clinical characteristics of both the pediatric and adult groups were examined. Descriptive statistics, including mean ± standard deviation (x ± s) or median values, are used for continuous data, while categorical data are presented as rates or percentages. For group comparisons, either the chi-square test or Fisher's exact test was employed, with a significance level set at α = 0.05. Statistical significance is considered when P < 0.05.
3 Results
3.1 Case presentation
3.1.1 Medical history
An 8.5-year-old female patient was admitted in December 2023 with chief complaints of “abnormal mental behavior for 6 months, headache and dizziness for 4 months, and episodic involuntary limb movements for 1 week”.
Mental Behavior Phase (6 months prior): Non-specific onset of inattention, declining academic performance, irritability, crying spells, aggressive behavior, and sleep disturbances (insomnia, nightmares). Symptoms were intermittent and untreated.
Neurological Symptom Phase (4 months prior): Bilateral temporoparietal paroxysmal headaches (1–2 h/episode) with tinnitus and dizziness, exacerbated by exertion and relieved by rest, occurring daily. Occasional auditory hallucinations (mosquito buzzing, factory noise). Worsening 2 weeks prior included self-harm, aggression, and delusions of persecution (claiming bullying by teachers/peers).
Motor Dysfunction Phase (1 week prior): Nocturnal paroxysmal twitching and numbness in the right lower limb, progressing to 2–3 daily episodes (1–2 h/episode), disrupting sleep. Right upper limb tremors developed 4 days before admission; no fever, rash, or altered consciousness.
3.1.2 Personal, past medical, and family history
Full-term vaginal delivery (birth weight 2.35 kg), uncomplicated perinatal course. Normal growth/development; no prior illnesses. Negative family history for similar conditions.
3.1.3 Laboratory tests
Normal results for complete blood/urine/stool analyses, liver/kidney function, myocardial enzymes, electrolytes, glucose, ketones, lactate, ammonia, ceruloplasmin, homocysteine, vitamin D, parathyroid hormone, and thyroid function. Negative for lupus antibodies, ANCA, and infectious pathogens (mycoplasma, tuberculosis, streptococcus).
3.1.4 Other examination
Normal findings on echocardiography, gynecological ultrasound, chest/abdominal computerized tomography (CT), and urinary system imaging. Non-contrast medium magnetic resonance imaging (MRI) of the head showed no lesions. During the interictal period (when the patient did not exhibit involuntary limb movements), video electroencephalogram (EEG) monitoring revealed prominent spikes, spike-and-slow waves/sharp-and-slow waves predominantly in the frontal lobe area in front of the central sulcus during sleep. During the onset of symptoms (when the patient experienced involuntary limb movements), video EEG monitoring did not show epileptiform discharges.
3.1.5 CSF examination
Routine: Clear/colorless, negative Pandy's test, cell count 4 mm3. Biochemistry: LDH 21 U/L, glucose 3.21 mmol/L, protein 0.240 g/L, IgG 18.2 mg/L, chloride 124.1 mmol/L, lactate 1.63 mmol/L. Pathology/Immunology: Negative bacterial/tuberculosis stains, cryptococcal ink stain, viral antibodies, and cultures. Normal inflammatory cytokines (IL-2/4/6/10, TNF-α, IFN-γ, IL-17A). Serum anti-GABABR IgG positive (1:10); all other autoantibodies and the oligoclonal bands negative.
3.1.6 Diagnosis, treatment and outcome
Based on the clinical symptoms and serological anti-anti-GABABR IgG Ab, the patient was diagnosed as anti-GABABR encephalitis. We carried out treatment based on the 2022 Edition of the Expert Consensus on the Diagnosis and Treatment of Autoimmune Encephalitis in China (7). The diagnosis and treatment guidelines mention that first-line immunotherapy includes glucocorticoids, intravenous immunoglobulin, and plasma exchange. Therefore, methylprednisolone sodium succinate was given for treatment (dosage: 20 mg·kg−1·d−1), and omeprazole sodium, vitamin D, calcium, and potassium chloride sustained-release tablets were provided to prevent the side effects of methylprednisolone sodium succinate. Post-treatment improvements: resolved mood/sleep disturbances, no limb movements, normal EEG. Subsequently, the patient began to take prednisone as prescribed regularly and gradually reduced the dosage until the medication was discontinued six months after discharge; 6-month follow-up showed stable remission.
3.2 Literature review and comparative analysis
3.2.1 General demographic characteristics
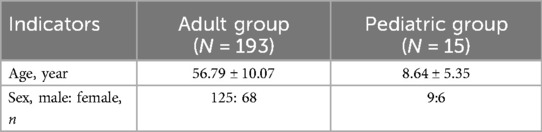
Among the 208 patients who meet the diagnostic criteria for anti-GABABR encephalitis, males predominate. The male-to-female ratio is 1.84:1 (125:68) in adult patients, with a mean age of 56.79 ± 10.07 years (range: 18–84 years). In the pediatric group, the ratio is 1.5:1 (9:6), with a mean age of 8.64 ± 5.35 years (range: 1–16 years) (Table 1).
3.2.2 Clinical manifestations
3.2.2.1 Prodromal symptoms
Among all the patients, a total of 70 patients (64 adults and 6 children) are recorded with prodromal symptoms, 19 (27.14%) experience prodromal symptoms of fever (19/70); 7 (10%) presented with headache as the prodromal symptom (7/70); 3 (4.29%) have diarrhea as a prodromal symptom (3/70); and 5 (7.14%) have cough as the prodromal symptom (5/70) (Table 2).
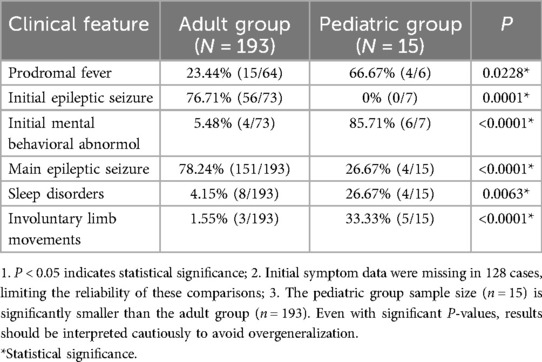
Table 2. Comparison of clinical characteristics between adults and children with anti-GABABR encephalitis.
In the adult group, febrile prodromal symptoms are present in 15 out of 64 cases, representing a prevalence rate of 23.44% (15/64). Headache occurs in 6 cases (9.38%, 6/64), while 2 cases of diarrhea, and 4 cases of cough, accounting for rates of 3.13% (2/64) and 6.25% (4/64), respectively. Among the children, four out of six have fever as a prodromal symptom, indicating a prevalence rate of 66.67% (4/6). One child experiences headache (16.67%, 1/6), one has diarrhea (16.67%, 1/6). Additionally, one patient exhibits cough (16.67%, 1/6).
Overall, fever was the most common prodromal symptom, with a higher proportion in children than in adults. Headache, diarrhea, and cough were less frequent overall, with similar patterns of age-related differences (Table 2).
3.2.2.2 Initial symptom
Among all the patients, a total of 80 patients are recorded with initial symptoms, the initial symptoms include epileptic seizures in 70% (56/80), mental behavior abnormalities in 12.5% (10/80), cognitive impairment in 21.25% (17/80), and consciousness disturbances in 6.25% (5/80).
Among the adults, the initial symptoms include epileptic seizures in 76.71% (56/73), mental and behavioral abnormalities in 5.48% (4/73), cognitive disorders in 23.29% (17/73), and consciousness disorders in 5.48% (4/73). In the pediatric group, the initial symptoms are mental and behavioral abnormalities in 85.71% (6/7), consciousness disturbances in 14.29% (1/7), with no cases of epileptic seizures or cognitive impairment as the initial symptoms (0/7).
To sum up, epileptic seizures were the predominant initial symptom in adults, while mental and behavioral abnormalities were most common in children. Cognitive impairment was observed as an initial symptom in adults but not in children, and consciousness disturbances were rare in both groups (Table 2).
3.2.2.3 Main clinical manifestations
Among the patients, the main clinical manifestations include seizures in 74.52% (155/208), abnormal mental behavior in 51.92% (108/208), cognitive disorders in 45.67% (95/208), sleep disorders in 5.77% (12/208), and consciousness disturbances in 21.63% (45/208). Language disorders account for 7.69% (16/208), hallucinations for 8.65% (18/208), involuntary limb movements for 3.85% (8/208), inattention for 0.96% (2/208), and headaches for 3.37% (7/208).
In the adult group, 78.24% (151/193) of patients experience seizures, 51.81% (100/193) have mental and behavioral abnormalities, 47.15% (91/193) have cognitive disorders, 4.15% (8/193) have sleep disorders, and 22.23% (43/193) have consciousness disturbances. Language disorders are observed in 7.77% (15/193), hallucinations in 7.77% (15/193), involuntary limb movements in 1.55% (3/193), inattention in 0.52% (1/193), and headaches in 0.52% (5/193). In the pediatric group, 26.67% (4/15) of patients experience epilepsy, 53.33% (8/15) have abnormal mental behavior, 26.67% (4/15) have cognitive disorders, 26.67% (4/15) have sleep disorders, 13.33% (2/15) have consciousness disturbances, and 6.67% (1/15) have language disorders. Hallucinations are observed in 20.00% (3/15), involuntary limb movements in 33.33% (5/15), inattention in 6.67% (1/15), and headaches in 13.33% (2/15).
The main clinical manifestations varied between adult and pediatric patients. Seizures were far more common in adults, while children showed higher rates of sleep disorders, involuntary limb movements, and hallucinations. Both groups exhibited mental and behavioral abnormalities, though cognitive disorders were more prevalent in adults. Other symptoms such as language disorders, inattention, and headaches were relatively rare in both groups, with slight differences in their occurrence rates across age groups (Table 2).
3.2.3 Auxiliary inspection
3.2.3.1 CSF examination
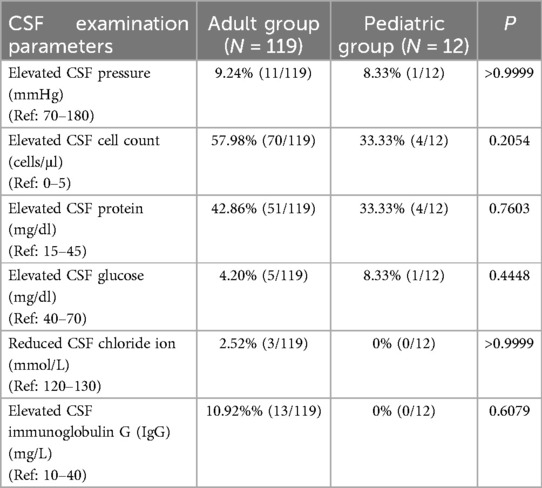
Lumbar puncture was performed in 131 patients (119 adults and 12 children), with assessments including CSF pressure, cell count, glucose, protein, chloride levels, and IgG. Overall, CSF parameters showed similar patterns between adults and children, with no statistically significant differences. Minor variations were observed—for example, adult patients had a slightly higher rate of increased cell count and elevated IgG, while pediatric cases showed no changes in chloride levels or IgG (Table 3).
3.2.3.2 Specific antibody detection and clinical characteristics
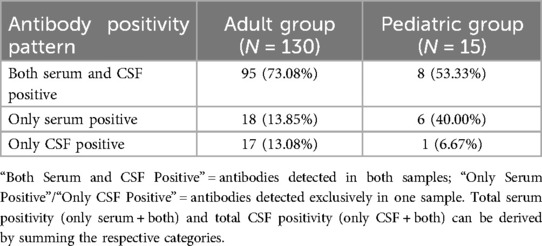
Antibody testing (serum and/or CSF) was conducted in 145 patients (130 adults and 15 children), with 63 cases lacking specific antibody data in the literature. The distribution of antibody positivity patterns differed between adults and children: adults more frequently tested positive for antibodies in both serum and CSF, while children showed a higher rate of exclusive serum positivity. Overall, serum antibody positivity was common in both groups, though CSF positivity rates were lower in children compared to adults (Table 4).
3.2.3.3 Imaging examination
Cranial MRI or CT was performed in most patients (75.48%), with approximately half showing abnormal signals (excluding bleeding or infarction); these abnormalities were typically nonspecific hyperintensities on T2 and FLAIR sequences. Imaging rates and abnormal signal frequencies differed slightly by age: all pediatric patients underwent imaging, with a higher proportion showing abnormalities compared to adults (Table 5).
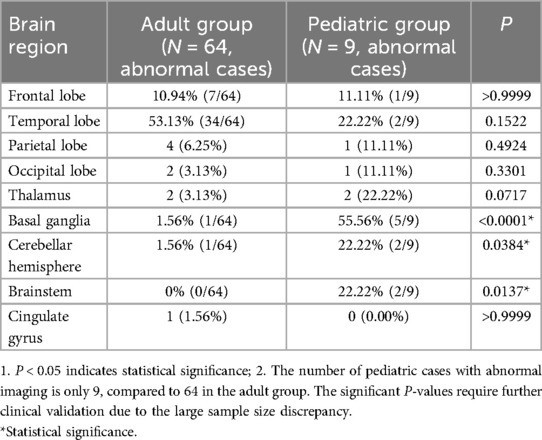
Notably, the distribution of involved brain regions varied significantly between age groups. Pediatric cases more frequently showed involvement of the basal ganglia, cerebellar hemispheres, and brainstem, whereas these regions were rarely affected in adults (Table 5).
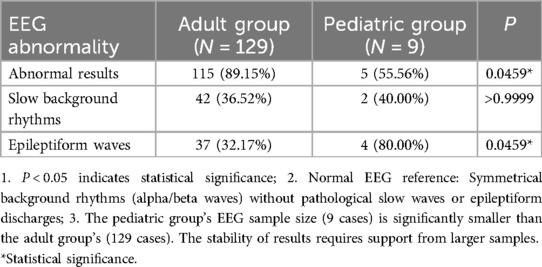
3.2.3.4 Electroencephalogram
EEG examinations were conducted in over half of the patients, with most showing abnormal results. Adults had a higher rate of abnormal EEG findings compared to children, though the pattern of abnormalities differed: epileptiform discharges were far more common in children with abnormal EEGs, whereas adults more frequently exhibited slow background rhythms. These age-related differences in EEG results are detailed in Table 6.
3.2.3.5 Tumor screening
Tumor imaging screening was performed in over 70% of patients, with notable differences between age groups. Adult patients showed a high rate of tumor detection, with lung cancer (predominantly small cell lung cancer) being the most common. In contrast, no tumors were identified in the pediatric group despite a high screening rate.
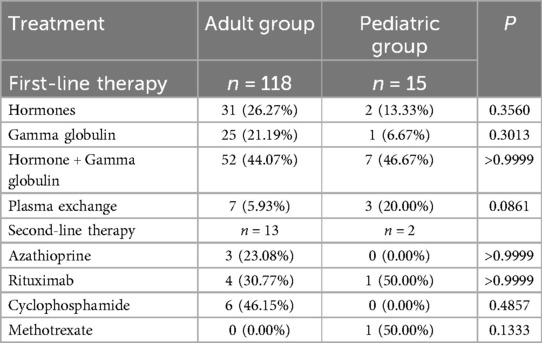
3.2.4 Therapy
Immunotherapy patterns differed between adults and children. Over 60% of patients overall received first-line immunotherapy, with children undergoing first-line treatment universally, compared to about 60% of adults. The most common first-line approaches included glucocorticoids, immunoglobulins, and their combination, with plasma exchange used more frequently in children. Second-line immunotherapy was administered to a small proportion of both groups (Tables 7, 8).
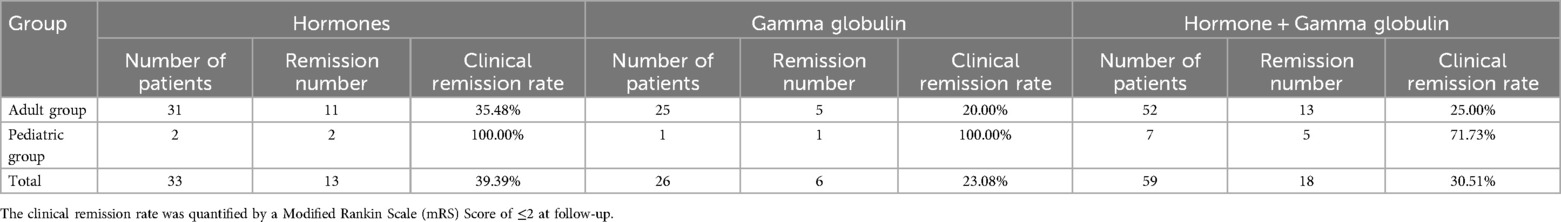
Clinical remission (defined as MRS ≤2 at follow-up) showed age-related variations in response to different immunotherapies: children generally had higher remission rates across treatment types, including monotherapy with glucocorticoids or immunoglobulins, and combination therapy, compared to adults (Tables 7, 8).
3.2.5 Outcome
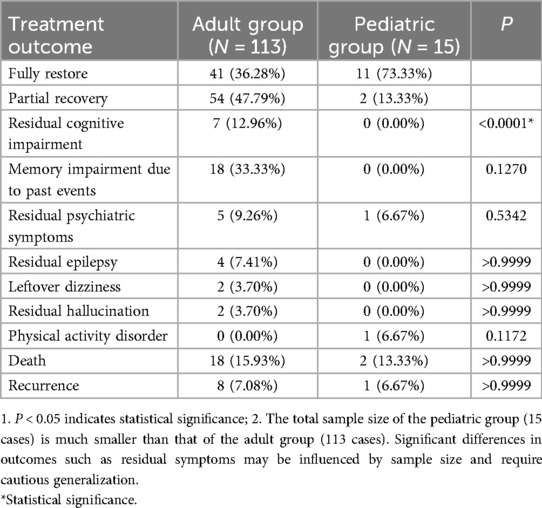
Treatment outcomes were analyzed in 128 patients (113 adults and 15 children), with variations observed between age groups. Overall, nearly half of the patients achieved full or significant recovery following immunotherapy, while a similar proportion showed partial recovery—though residual symptoms such as cognitive impairment, memory issues, and mental health problems were common in this subgroup. A smaller percentage of patients did not survive (Table 9).
Notably, children had a higher rate of full or substantial recovery compared to adults, with fewer residual symptoms. In contrast, adults more frequently experienced persistent cognitive impairment, and the mortality rate was higher in adults than in children. Detailed breakdowns of outcomes and residual symptoms are provided in Table 9.
4 Discussion
4.1 Core clinical differences and pathophysiological mechanisms
Anti-GABABR encephalitis demonstrates distinct clinical phenotypes between children and adults, likely rooted in developmental neuroanatomy and synaptic plasticity. In terms of the initial symptoms, the limited available data (80 cases, with 128 cases missing data) indicated an age-related trend: Epileptic seizures were more common in adult patients, while children mostly presented with behavioral abnormalities. However, due to the lack of detailed records of initial symptoms in approximately 61.5% (128/208) of the cases, the reliability of this trend is limited. It should be interpreted with caution and cannot be regarded as a definitive conclusion. In the analysis of main symptoms with relatively complete data, the proportions of sleep disorders and involuntary movements in pediatric patients were significantly higher than those in adult patients, and the incidence of epilepsy in adults was even higher (P < 0.0001). This divergence may relate to age-dependent GABA-B receptor distribution: pediatric cases exhibit higher involvement of basal ganglia (55.56%), cerebellar hemispheres (22.22%), and brainstem (22.22%) (Table 5), regions critical for motor coordination and inhibitory control, whereas adult limbic-cortical networks are more susceptible to seizure generation (8–10). Combined with the fact that febrile prodromes are more common in children (66.67% vs. 23.44% in adults, P = 0.0228), it suggests that pediatric cases may have a more obvious pre-immune activation prodromal period, and this characteristic deserves clinical attention.
Electrophysiologically, adults show higher rates of abnormal EEG (89.15% vs. 55.56%, P = 0.0459), but children with abnormal EEG display more frequent epileptiform waves (80.00% vs. 32.17%, P = 0.0459) (Table 6). This paradox may reflect developmental differences in neuronal network stability—children's immature circuits are prone to subclinical epileptiform discharges, while adults require more severe inhibition loss to manifest clinical seizures (10, 11).
4.2 Therapeutic response and prognostic influencing factors
Pediatric patients exhibit superior treatment outcomes, with 100% clinical remission rates using monotherapy (corticosteroids or gamma globulin) and 71.73% with combination therapy (Table 8). This efficacy contrasts with adults (23.08%–39.39% remission) and may relate to two key factors: (1) absence of tumor comorbidity in children (0% vs. 58.09% in adults, Table 9), eliminating paraneoplastic immune activation; (2) potentially reversible synaptic dysfunction in developing brains, as opposed to adult neurons with higher vulnerability to chronic inflammation (12, 13).
It is worth noting that in adult cases, there is one patient with small cell lung cancer who does not receive immunotherapy during chemotherapy (cisplatin + etoposide) (14). The follow-up lung imaging after 6 months shows that the tumor has completely disappeared. However, as no neurological assessment is conducted, we have no idea about the recovery of the patient's neurological symptoms. Therefore, we consider that when combined with tumors, tumor treatment and immunotherapy are sometimes in conflict. On the one hand, immunotherapy (such as glucocorticoids and rituximab) supposes the immune system, and the effect of myelosuppression or immunosuppression in tumor treatment (such as chemotherapy and radiotherapy) superimposes, increasing the risk of infection. On the other hand, some scholars have speculated that (13) immunosuppressive therapy may promote the metastasis of neuroendocrine tumors, which is presumed to be related to “inhibiting the anti-tumor immune response”.
It is worth noting that although the clinical remission rate of first-line treatment for children is relatively high, there were still 2 cases of children who received second-line treatment (Table 7). Among them, one child patient's symptoms worsened after using gamma globulin, and then improved after receiving high-dose methylprednisolone pulse therapy and infusion of rituximab (15). Long-term follow-up is essential, as 13.33% of children showed partial recovery with residual motor or psychiatric symptoms (Table 9).
4.3 Differential diagnosis with anti-NMDAR encephalitis
Regarding the main symptoms, anti-GABABR encephalitis is less stereotypical compared to the representative psychomotor symptoms seen in anti-NMDAR encephalitis. Additionally, the clinical phenotype of anti-GABABR encephalitis remains stable over time, whereas the clinical presentation of anti-NMDAR encephalitis evolves (16). In terms of tumors, anti-NMDAR encephalitis is often accompanied by ovarian teratoma in children (17, 18), while no tumors occur in the children of this study. In terms of EEG, epileptic waves are more common in children with anti-GABABR encephalitis, while diffuse slow waves are predominant in children with anti-NMDAR encephalitis (19). The above-mentioned differences are helpful for rapid clinical differentiation.
4.4 Limitations and future perspectives
The primary limitations are the small pediatric sample size (n = 15) and retrospective design, which may bias rare symptom analysis (e.g., consciousness disorders). Notably, the large discrepancy in sample size between pediatric and adult groups (15 vs. 193) undermines the robustness of comparative statistical analyses, even when significant P-values are reported. Prospective multicenter studies with ≥50 pediatric cases are needed to validate immune mechanisms and optimize treatment algorithms, particularly regarding the role of combination therapy in severe cases (16).
5 Conclusion
This study suggests that the clinical phenotypes of anti-GABABR encephalitis in children and adults may differ: children are more likely to present with mental and behavioral abnormalities (the initial symptom trend), sleep disorders and involuntary movements (the main symptoms), and their brain imaging is more likely to involve regions such as the basal ganglia and brainstem, and they respond better to immunotherapy. Notably, due to the small pediatric sample size (n = 15) compared to adults (n = 193), these findings—despite statistically significant P-values—remain preliminary and require validation in larger pediatric cohorts. It should be noted that the conclusion regarding the initial symptoms is limited by the lack of data. The overall conclusion still requires verification through studies with larger sample sizes and more complete data. The current findings can provide a reference for the identification and diagnosis of pediatric cases in clinical practice, but they cannot be used as an absolute diagnostic basis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Affiliated Hospital of Inner Mongolia Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JF: Writing – original draft. KJ: Writing – original draft. TL: Data curation, Writing – review & editing. ZQ: Conceptualization, Writing – review & editing. YJ: Methodology, Writing – review & editing. XY: Formal analysis, Writing – review & editing. HP: Formal analysis, Writing – review & editing. LZ: Formal analysis, Writing – review & editing. PL: Formal analysis, Writing – review & editing. MZ: Formal analysis, Writing – review & editing. GY: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the National Natural Science Foundation of China (Grant No. 8226050455), the Major Project of Inner Mongolia Medical University (Grant No. YKD2022MS032), and the Science and Technology Project of High-level Clinical Specialties Construction in the Capital City of Inner Mongolia Autonomous Region (Grant No. 2024SGGZ076).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Uy CE, Binks S, Irani SR. Autoimmune encephalitis: clinical spectrum and management. Pract Neurol. (2021) 21(5):412–23. doi: 10.1136/practneurol-2020-002567
2. Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61(1):25–36. doi: 10.1002/ana.21050
3. Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. (2014) 27(3):361–8. doi: 10.1097/wco.0000000000000087
4. Gong Z, Lao D, Huang F, Lv S, Mao F, Huang W. Risk factors and prognosis in anti-NMDA receptor encephalitis patients with disturbance of consciousness. Patient Relat Outcome Meas. (2023) 14:181–92. doi: 10.2147/prom.S411260
5. Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. (2004) 5(12):952–62. doi: 10.1038/nrn1556
6. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15(4):391–404. doi: 10.1016/s1474-4422(15)00401-9
7. The Neuroinfectious Diseases and Cerebrospinal Fluid Cytology Group of the Neurology Branch of the Chinese Medical Association. Expert consensus on diagnosis and treatment of autoimmune encephalitis in China (2022 edition). Chin J Neurol. (2022) 19:931–49. doi: 10.3760/cma.j.cn113694-20220219-00118
8. Benarroch EE. GABAB receptors: structure, functions, and clinical implications. Neurology. (2012) 78(8):578–84. doi: 10.1212/WNL.0b013e318247cd03
9. Maity B, Stewart A, Yang J, Loo L, Sheff D, Shepherd AJ, et al. Regulator of G protein signaling 6 (RGS6) protein ensures coordination of motor movement by modulating GABAB receptor signaling. J Biol Chem. (2012) 287(7):4972–81. doi: 10.1074/jbc.M111.297218
10. Bassetti D. Keeping the balance: GABA(B) receptors in the developing brain and beyond. Brain Sci. (2022) 12(4):419. doi: 10.3390/brainsci12040419
11. Nibber A, Mann EO, Pettingill P, Waters P, Irani SR, Kullmann DM, et al. Pathogenic potential of antibodies to the GABA(B) receptor. Epilepsia Open. (2017) 2(3):355–59. doi: 10.1002/epi4.12067
12. Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. (2015) 15(12):1391–419. doi: 10.1586/14737175.2015.1115720
13. André A, Félix A, Shamasna M, Nzwalo H, Basílio C. Neuroendocrine tumour metastatic brain disease during immunosuppressive treatment for paraneoplastic GABA(B) receptor antibodies encephalitis: is immunosuppression always beneficial? J Neuroimmunol. (2017) 310:66–8. doi: 10.1016/j.jneuroim.2017.06.009
14. Dogan Onugoren M, Rauschka H, Bien CG. Conjoint occurrence of GABAB receptor antibodies in Lambert-Eaton myasthenic syndrome with antibodies to the voltage gated calcium channel. J Neuroimmunol. (2014) 273(1–2):115–6. doi: 10.1016/j.jneuroim.2014.05.011
15. Yang L, Zhang D. Case report: coexistence of Labbe vein thrombosis and autoimmune encephalitis with two different antibodies. Front Neurol. (2023) 14:1170169. doi: 10.3389/fneur.2023.1170169
16. Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. (2013) 81(17):1500–6. doi: 10.1212/WNL.0b013e3182a9585f
17. Acién P, Acién M, Ruiz-Maciá E, Martín-Estefanía C. Ovarian teratoma-associated anti-NMDAR encephalitis: a systematic review of reported cases. Orphanet J Rare Dis. (2014) 9:157. doi: 10.1186/s13023-014-0157-x
18. Liang Z, Yang S, Sun X, Li B, Li W, Liu Z, et al. Teratoma-associated anti-NMDAR encephalitis: two cases report and literature review. Medicine (Baltimore). (2017) 96(49):e9177. doi: 10.1097/md.0000000000009177
Keywords: anti-GABABR encephalitis, children, autoimmune encephalitis, borderline encephalitis, clinical characteristics
Citation: Fu J, Jia K, Li T, Qiao Z, Jia Y, Yang X, Pan H, Zhao L, Li P, Zan M and Yang G (2025) A pediatric case of anti-GABABR encephalitis: case report and literature review. Front. Pediatr. 13:1563323. doi: 10.3389/fped.2025.1563323
Received: 21 January 2025; Accepted: 11 August 2025;
Published: 25 August 2025.
Edited by:
Alina Gonzalez-Quevedo, Instituto de Neurología y Neurocirugía, La Habana, CubaReviewed by:
Deepika Gandhi, NMC Royal Hospital, United Arab EmiratesYafang Hu, Southern Medical University, China
Copyright: © 2025 Fu, Jia, Li, Qiao, Jia, Yang, Pan, Zhao, Li, Zan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guanglu Yang, bm15Z2wxNUAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Junxian Fu1,†
Junxian Fu1,† Kairu Jia
Kairu Jia Guanglu Yang
Guanglu Yang