- 1Department of Pediatrics, University of Alberta, Edmonton, AB, Canada
- 2Department of Pediatrics, University of British Columbia, Vancouver, BC, Canada
- 3Department of Pediatrics, University of Toronto, Toronto, ON, Canada
- 4Department of Pediatrics, University of California at San Francisco, San Francisco, CA, United States
- 5Department of Obstetrics & Gynecology, University of British Columbia, Vancouver, BC, Canada
Despite a significant reduction in neonatal mortality due to advances in neonatal care, preterm birth (PTB) continues to pose a challenge due to the escalating incidence of long-term complications, which refer to health issues that persist or emerge beyond the immediate neonatal period. The impact of PTB, particularly in extremely preterm infants born before 28 weeks of gestational age, is not confined to the early years but extends across the lifespan, influencing physical, cognitive, and social development, as well as long-term health outcomes. These complications, which often persist from childhood into adulthood, span multiple systems and create a broad spectrum of health concerns. This comprehensive narrative review of literature delves into the breadth of well-characterized long-term complications associated with PTB, including neurodevelopmental, respiratory, cardiovascular, renal, gastrointestinal, and endocrine system disorders. By providing health care providers with a holistic understanding of the potential complications following PTB, this review aims to summarize the current literature and underscore the value of long-term monitoring strategies and proactive evaluations of this population. Our objective is to foster a clinical approach that anticipates these complications, enabling early interventions and better management of these at-risk infants.
1 Introduction
Preterm birth (PTB), defined as birth occurring prior to 37 weeks of gestation, encompasses various stages contingent on gestational age (GA): moderate to late preterm (32–37 weeks GA), very preterm (28 to less than 32 weeks GA), and extremely preterm (before 28 weeks GA) (1). In 2020, an estimated 13.4 million babies were born preterm, accounting for about 9.9% of all births worldwide (2). Notably, 15% of these preterm births occurred before 32 weeks of gestation, with 4.2% before 28 weeks and 10.4% between 28 and 32 weeks (2). The burden of PTB is disproportionately high in low- and middle-income countries (LMICs), particularly in Southern Asia and sub-Saharan Africa, which together contribute to 65% of global PTBs (2). Southern Asia, for instance, had the highest PTB rate globally (13.2%), with Bangladesh reaching a national rate of 16.2%, among the highest worldwide (2). Malawi also reported a high PTB rate of 14.5% (2). In contrast, PTB rates in high-income countries (HICs) are relatively lower, with Canada (8.0%) aligning closely with England (7.4%) and the USA (10.0%), while China reports a rate of 6.1% (2–5).
Similar to the differences in PTB rates between LMICs and HICs, there are notable disparities in survival outcomes (6). According to a global analysis by Cao et al., the age-standardized mortality rate of neonatal preterm birth declined in HICs between 1990 and 2019 (6), reflecting advancements in neonatal care, including improved respiratory support, surfactant therapy, and infection control measures (7). This declining trend in mortality rate was particularly evident in Bahrain, Greece, Japan, the UK, and the USA (6). However, in LMICs, particularly in Southern Sub-Saharan Africa, age-standardized mortality rate increased, indicating worsening neonatal survival despite global efforts to improve perinatal care (6). The progression of neonatal care, especially in HICs, has resulted in a global reduction in neonatal mortality associated with PTB by 47.7% from 1.27 million in 1990 to 0.66 million newborn deaths in 2019 (6). Consequently, a significant number of preterm infants can now survive into adulthood (8, 9). However, this decline has contributed to growing morbidity and long-term health risks for survivors (10, 11), as reinforced by population-based studies showing the lasting impact of PTB across the lifespan (12–16). For instance, a Norwegian cohort study following over 900,000 individuals found that those born extremely preterm had significantly higher risks of cerebral palsy, intellectual disability, and reliance on disability pensions in adulthood (14). Similarly, a German study of adolescents born before 27 weeks reported higher rates of psychological problems and lower health-related quality of life (13). A longitudinal cohort study from New Zealand also revealed that individuals born preterm were slower to reach their genetically determined height compared to those born full-term (16). The findings from these population-based studies highlight the persistent challenges faced by preterm survivors, emphasizing the need for long-term support and intervention.
Generally, the earlier the GA at birth, the more pronounced the medical, economic, and social consequences (14, 17). Hence, long-term consequences of PTB become a pressing public health concern. It is important to recognize that health outcomes extend beyond the immediate postnatal period, and that health is a continuum from intrauterine to postnatal conditions (11). Children and adolescents born prematurely also exhibit greater demand for specialized outpatient services, leading to higher long-term health care costs compared to their term-born peers (18). In the USA, the total estimated cost for PTBs in 2016 was approximately $25 billion and each PTB case incurred an average of $64,815 more in expenses compared to each term or post-term birth (19). In fact, during the first year of life, preterm children incur higher medical expenses compared to term infants due to the likelihood of various morbidities that require specialized care (20, 21).
While the initial hospitalization costs of preterm infants are well-documented (20, 21), emerging evidence highlights the sustained economic burden associated with PTB beyond infancy. A systematic review of economic consequences documented an inverse relationship between GA and healthcare costs, extending into childhood (22). Similarly, EPICure study showed that extremely preterm infants incurred higher public sector costs at age 11 compared to term-born peers (23). These findings are further supported by a European cohort study reporting an increased societal costs at age five for children born before 26 weeks (24). A UK model estimated that the incremental cost per preterm child up to 18 years was US$ 35, 471, with substantially higher costs for very preterm infants (17). Additionally, a German claims data analysis highlighted that ambulatory treatment costs for early preterm infants remained elevated during first three years of life (25). These findings collectively emphasize that the economic consequences of preterm birth persist well into childhood and beyond.
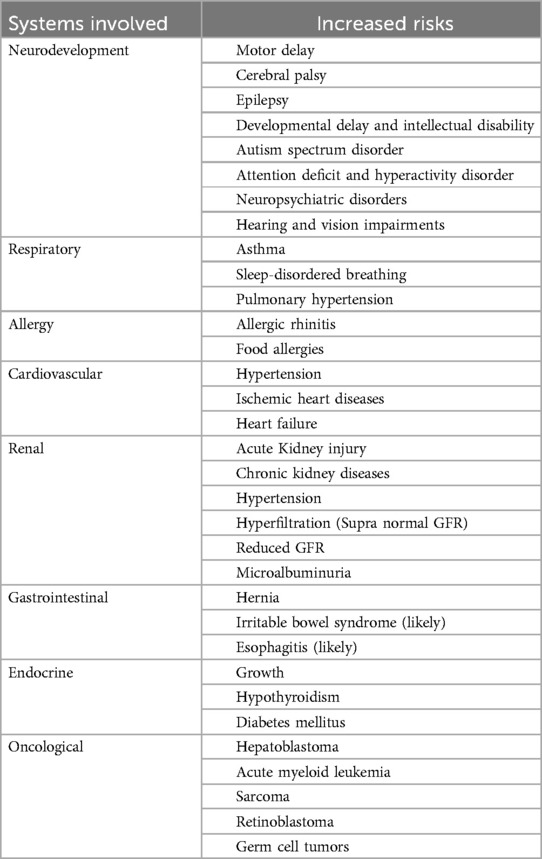
In Canada, efforts to reduce neonatal and childhood morbidity include the National Quality Improvement Project of Evidence-Based Practice for Improving Quality (EPIQ-3), which has shown sustainable improvements in outcomes of preterm neonates (26). Recognizing the long-term complications of PTB is essential for ensuring clinical follow-up that enables active prevention, monitoring, and early treatment of health sequelae. This narrative review aims to discuss the common long-term sequelae of PTB affecting various body systems (Table 1), by evaluating existing knowledge and evidence in scientific literature, with a particular focus on their progression and impact during early childhood, adolescence, and adulthood.
2 Long-term consequences of prematurity
2.1 Neurodevelopmental outcomes
The correlation between reduced gestational age and the risk of developing neurodevelopmental disabilities, such as learning problems, poor motor skills, cognitive, hearing, and visual impairments during childhood, is well-documented, particularly among children born extremely preterm (27–31). This association is not surprising, considering that cerebral corticogenesis, the process responsible for the formation of the cerebral cortex, is most active during the third trimester of pregnancy (32, 33). The more preterm, the higher the chance of brain injury and altered trajectory of brain development (i.e., dysmaturation) (34). In contemporary cohorts, despite lower prevalence of severe brain injury such as cystic periventricular leukomalacia, more commonly seen diffuse white matter injury and encephalopathy of prematurity result in abnormal brain maturation (35). For preterm infants, oligodendrocyte precursors which are the predominant cell type in the pre-myelinated white matter, are particularly vulnerable to oxidative stress, inflammation (such as from infection) and hypoxic-ischemic insults (34). The vulnerable developing brain is susceptible to injury during specific windows of development, especially during 23–32 weeks gestation when oligodendrocyte precursors are the most abundant (34). White matter injury of prematurity is also a significant contributor as arrested and altered development of oligodendrocytes result in reduced and altered connectivity of the developing brain (34). Consequently, children born very preterm often exhibit decreased brain volume, surface area, and cortical thickness (32). Additionally, there can be a reduction in neuronal cells due to central nervous system infections or hypoxemic-ischemic insults, both of which can trigger pro-inflammatory cytokines, e.g., interleukin (IL)-6, IL-8, and IL-1β, which are also associated with spontaneous PTB (36–40). Elevated levels of IL-6 may be linked to abnormal brain development and autism, while IL-1β may contribute to neuronal damage (39). Thus, preterm infants confront an increased susceptibility to a range of disorders spanning from motor and developmental to cognitive, visual and hearing loss, epileptic, behavioral, sensory, and neuropsychiatric conditions (31, 41–44). The direct consequences of preterm birth on neurodevelopment necessitate robust clinical monitoring and early interventions to minimize potential risks and optimize long-term health outcomes. Although this section highlights many conditions for which preterm infants have a high risk of developing, it is important to keep in mind that identification of these conditions can often improve functional outcomes through targeted supports and early interventions.
2.1.1 Motor outcomes
Challenges in motor development are common and well known in populations of children born preterm, particularly those born extremely preterm, ranging from delays in sitting or walking to developmental coordination disorder and more significant neuromotor impairment, such as cerebral palsy (CP) (27, 45). CP is a nonprogressive disorder of motor development caused by disturbances in the developing brain. Brain injury in premature infants such as intraventricular hemorrhage (IVH), periventricular hemorrhagic infarction and periventricular leukomalacia increase the risk of motor impairment (46). Most preterm born children who have CP are ambulatory with some assistance or equipment such as orthotics with those more severely affected being non-ambulatory and requiring mobility devices (47). Motor impairments such as developmental coordination disorder are associated with impacts beyond motor aspects of cognitive and behavioral functioning, including associations with decreased academic achievement and higher rates of anxiety (48).
The prevalence of CP is strongly correlated with the severity of prematurity, with approximately 10%–20% of individuals born extremely preterm being affected (27, 45, 49). A population-based study in France examining preterm infants at 2 years of age found that the most common presentation of CP is bilateral CP with spastic diplegia, and the prevalence of CP was 12.4% in those born at GA 24–26 weeks and 2.4% in those born at GA 32–34 weeks (50). Additionally, findings from a systematic review and meta-analysis (12 studies) demonstrated a significant positive association between preterm birth and CP [pooled Odds ratio (OR): 2.9; 95% CI: 2.1; 3.9] (51).
2.1.2 Cognitive outcomes
Children born extremely preterm have increased risk of challenges in cognitive abilities, which include but are not limited to issues with intellectual abilities, working memory, visuomotor integration, self-regulation and executive functions (52). As a result of challenges in these areas, they can have increased difficulties with academic achievement in several areas, including mathematics, reading, and spelling, which may persist throughout their schooling (42, 53). On average, they score 11–13 points lower on IQ scales compared to term-born children (42, 53). Neurodevelopmental outcomes in preterm infants depend on a variety of prenatal, perinatal, neonatal, postnatal and environmental factors, such as their general health, with poorer outcomes observed in very low birth weight (VLBW) infants (54, 55). A longitudinal cohort study in the Netherlands followed the neurodevelopmental trajectories of 705 infants born very preterm (GA <32 weeks) for 19 years (55). The study analyzed separate cohorts of very preterm (VP) infants with VLBW, VP infants without VLBW, and VLBW infants without VP for outcomes such as IQ score, neuromotor function, hearing, behavioral and emotional functioning, and educational achievement. Among the three groups, VP infants without VLBW had the best outcomes, while VLBW infants without VP had the worst outcomes (55). A meta-analysis of 14 studies also found that VP infants with neonatal sepsis were at a higher risk of neurodevelopmental impairment compared to VP infants without sepsis (54).
2.1.3 Communication, social-emotional and behavior conditions: autism spectrum disorder, attention deficit and hyperactivity disorder, and other neuropsychiatric disorders
Language delay is one of the most common challenges that preterm infants face (56). In addition to clinical risk factors, it important to highlight those sociodemographic and structural factors such as racism, lower socioeconomic status, lower caregiver education are associated with negative impact on outcomes on language development (56).
Autism spectrum disorder (ASD), a neurodevelopmental disorder, is likely caused by multiple prenatal, perinatal, and postnatal factors (57, 58). A national Swedish cohort study of over 4 million infants born between 1973 and 2013 found that the prevalence of ASD in all preterm births was 2.1%, with the highest prevalence (6.1%) observed in those born extremely preterm (59).
Another common childhood neurodevelopmental problem is attention deficit and hyperactivity disorder (ADHD). Studies have shown that PTB is a risk factor for ADHD, and the risk increases with each declining week of gestation (60, 61). These findings emphasize the importance of early evaluation, timely detection, and treatment, as well as long-term follow-up for ASD and ADHD, particularly in individuals born extremely preterm.
PTB has been associated with an increased susceptibility to psychiatric conditions, including affective disorders (such as depression and bipolar disorder) and psychosis, in young adults born prematurely (62, 63). Among 1.3 million young patients (>16 years old) recruited in Sweden, those born premature at GA <32 weeks have a higher likelihood of developing psychotic disorders than those born at GA >32 weeks GA (hazard ratio of 2.5 vs. 1.6) (63). These findings align with a Swedish cohort study, which demonstrated a threefold increase in the likelihood of antipsychotic medication prescriptions among 635,933 adults (aged 25.5–34) born extremely preterm (23–27 weeks GA) (64).
2.1.4 Sensory impairments (hearing, vision, and sensory processing)
Children born prematurely have a high risk of hearing impairment, ranging from mild hearing loss to hearing loss requiring cochlear implants (65, 66). Risk factors for hearing impairment include exposure to mechanical ventilation, commonly used ototoxic medications such as furosemide, or aminoglycosides (e.g., gentamicin) (67, 68). Common vision problems in preterm infants include myopia, reduced visual acuity, refractive error, strabismus, abnormal stereopsis, and retinal detachment (65, 69). Although PTB infants are also at increased risk of developing retinopathy of prematurity, problems with vision can still occur in the absence of history of retinopathy of prematurity (70). Additionally, sensory processing challenges, including hypo- and hypersensitivities, are frequently observed in preterm children (71, 72). While trends in both hearing and visual impairments have improved over time due to advancements in neonatal care, screening for these sensory difficulties, followed by interventions such as appropriate feeding strategies and occupational therapies, can significantly improve the child's functional outcomes.
2.1.5 Epilepsy
PTB has been associated with an increased risk of epilepsy in children and young adults, regardless of neurodevelopmental comorbidities (73). A Finland cohort of over 1 million infants, born between 1991 and 2008, found the incidence of epilepsy decreases with increased weeks of gestation (2.5% in the very preterm, 1.1% in the moderate preterm, 0.7% in the late preterm and 0.5% in the term group) (74). The study also documented that the risk of epilepsy was 1.5–4.5 times higher in PTB compared to term infants, followed until seven years after birth or up to 2009 (74). The increased risk of epilepsy due to PTB is long-term, as a Swedish cohort study of over 600,000 adults (age 25–37) found a five-fold increase in the risk of hospitalization due to epilepsy in individuals born extremely preterm compared to those born full-term (75).
2.2 Respiratory conditions
Preterm newborns often require respiratory support due to their immature lung development, which can lead to subsequent reduced lung function in early childhood and adulthood (76). Adolescents born preterm have been found to exhibit both reduced lung function and modifications in lung structure (77, 78). A study in Australia, involving over 200 children aged 9–11 years, reported multiple alterations in lung structure and function among children born preterm, including pulmonary obstruction, hyperinflation, diminished forced expiratory volume in one second (FEV1) Z-scores, and structural lung changes on CT scans in 92% of preterm children (79). Evidence of airway obstruction was also demonstrated in a Norwegian study of approximately 130 youth aged 10–18 years born extremely preterm, particularly in those who had experienced neonatal bronchopulmonary dysplasia (BPD) (78). Infants with a history of BPD exhibit severely reduced expiratory airflow, which can persist into adulthood (80).
2.2.1 Asthma
Asthma is a common chronic respiratory disease characterized by reversible airway obstruction (81). The most common asthma phenotype in childhood is atopic or Th2-high asthma (81); however it is unclear whether children born preterm who are diagnosed with asthma have a different underlying pathophysiology. A cross-sectional study in the USA, involving 90,721 children aged between 0 and 17 years, revealed that preterm birth was significantly associated with a 1.6 (95% CI: 1.4–1.8) times higher likelihood of developing asthma (15). A study in Norway with 46 subjects reported a higher prevalence of asthma and use of asthma inhalers among adults born extremely preterm (35.6% and 17.4%) (compared to those born at term (6.7% and 2.2%) (82). A large Swedish national cohort study examining asthma medication records of 622,616 patients aged 25–35 years found that young adults born extremely preterm had more than a two-fold increase in the risk of being prescribed asthma medications compared to those born at term (83). A meta-analysis of 147,252 European children up to 10 years old found a significant association between PTB and school-age asthma (pooled OR: 1.4; 95% CI: 1.2, 1.8) (84). Of note, a retrospective cohort study in the USA, examining 6,193 children aged 2–83 months born late preterm, did not find a significant association between late preterm birth and asthma (85).
2.2.2 Sleep-disordered breathing
Obstructive sleep-disordered breathing (SDB) is a condition characterized by intermittent upper airway obstruction during sleep, ranging from primary snoring to obstructive sleep apnea (OSA), with untreated severe OSA potentially leading to right heart failure (86). During early childhood, preterm infants, especially those born before 32 weeks of gestation, are at an increased risk of sleep apnea compared to term-born children (87). A large Swedish cohort study involving over 4 million infants found an inverse association between gestational age at birth and the risk of SDB from childhood to mid-adulthood, indicating that PTB is associated with a greater than 40% increased risk of developing SDB (88). Extremely preterm individuals had a more than two-fold increased risk of SDB in adulthood (88).
2.2.3 Pulmonary hypertension
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that frequently affects preterm infants, particularly those born at <32 weeks of gestation (89). A major complication associated with moderate to severe BPD is pulmonary hypertension (PH) (90), a condition defined by a mean pulmonary arterial pressure > 20 mm Hg, applicable to infants older than 3 months (91). Infants with BPD are at an increased risk of developing PH, with approximately 25% of those with moderate to severe BPD progressing to PH (92). PH in preterm infants with BPD can have profound clinical consequences, including altered ventricular function and heart failure (93). Moreover, PH is associated with adverse outcomes, including impaired somatic growth and neurodevelopmental delays (94, 95). These complications highlight the critical need for early detection, careful monitoring, and proactive management of PH in this vulnerable population (88).
2.3 Allergic conditions
PTB has an impact on immune system maturation and can influence the development of immune-mediated diseases, including allergy-related conditions (96, 97). Allergy-related diseases, such as allergic rhinitis and food allergies, are often preceded by immunoglobulin E (IgE) sensitization (98). A longitudinal population-based cohort study that followed 3,522 children prospectively up to 24 years of age found an inverse association between PTB and overall sensitization to common food and inhalant allergens (99). Similarly, a large Swedish national cohort study involving over 600,000 infants, followed up until the ages of 25–37 years to assess the prescription of nasal corticosteroids and oral antihistamines for treating allergic rhinitis, also found a negative association between preterm birth and the risks of developing allergic rhinitis (100). One possible explanation for these findings is that preterm infants may be exposed to allergens earlier, leading to a shift from a TH2 to a TH1 lymphocyte response, which promotes allergen tolerance. However, there is no clear evidence to demonstrate an association of adverse food reactions and PTB. For example, recent studies from Sweden (n = 3,522), Italy (n = 165), and Japan (n = 1,197) identified an inverse relationship between PTB and allergies (99, 101, 102). In contrast, a larger Swedish study involving over one million children found no association between moderate preterm birth and food allergies but reported a negative association between very preterm birth and food allergies (103). Similarly, a Canadian retrospective cohort study involving 13,980 children confirmed the absence of any association between gestational age and food allergies (104). These null findings are supported by a case control study of 160 infants admitted to emergency clinical hospital for children in Romania and a retrospective analysis of 2,116 neonates conducted in Japan (105, 106). In contrast, studies conducted in the USA (n = 51,748), China (n = 3,437), and Portugal (n = 1,217) found a strong positive relationship between PTB and food allergies (107–109). The current evidence does not establish a definitive association between PTB and allergic conditions, particularly food allergies.
Existing studies present conflicting findings, with some suggesting an increased risk of food allergies among preterm infants, while others report a decreased risk. This inconsistency likely stems from limitations in sample sizes within the available literature, which are insufficient to draw robust conclusions. Therefore, future research with significantly larger cohorts of preterm infants is essential to clarify the relationship between PTB and allergic outcomes and to provide a more comprehensive understanding of this potential association.
2.4 Cardiovascular conditions
Cardiovascular function is often compromised in PTB due to various factors, including excessive metabolic demands resulting from infection or sepsis, and specific physiological changes (110, 111). Preterm infants commonly exhibit diastolic dysfunction, characterized by impaired relaxation of the heart during the filling phase, even when their systolic left ventricular systolic function is normal. There is an increased dependence on atrial contraction and a compensatory increase in the heart rate in preterm infants. These findings are likely contributors to the known predisposition to cardiovascular disease later in life (112–114).
A study conducted on a cohort of 72 adolescents with a mean age of 13.3 years found that individuals born preterm had statistically significant smaller ventricular size and decreased cardiac mass compared to those born at term (115). Additionally, rapid childhood weight gain observed in preterm infants born with a low ponderal index (body weight-to-height ratio) can place an additional burden on the cardiovascular system and increase the risk of cardiovascular diseases (116). A study in Canada involving nearly 2,000 children with a mean age of 5.6 years found an association between preterm birth and an increase in the composite cardiometabolic risk score (taking into account factors such as waist circumference, triglyceride level, glucose level, systolic blood pressure, and high-density lipoprotein cholesterol level) (116). Lipid disorders (such as elevated cholesterol or triglycerides) have been found to be associated with PTB in a large Swedish national cohort study of more than 2 million people, with a 14% reduction in the risk of lipid disorders for each increment of five weeks of gestation (117).
2.4.1 Hypertension
Hypertension is one of the cardiovascular conditions commonly associated with preterm birth. A large Swedish national cohort study that followed over 600,000 infants into adulthood found that young adults born extremely preterm had a 2.5-fold increased risk of using antihypertensive medications per year compared to those born at term (118). A meta-analysis of 10 studies involving 1,342 infants born preterm (GA 28.8–34.1 weeks) or VLBW (mean birth weight 1,280 g), with their systolic blood pressure measured at age 6.3–22.4 years old, found that patients born preterm or VLBW had higher systolic blood pressure (SBP) by 2.5 mm Hg (95% CI: 1.7, 3.3 mm Hg) than those born term (119). Another meta-analysis of nine cohorts with blood pressure measurements in adults born preterm (GA <37 weeks) at VLBW (birth weight <1,500 g) found that these populations had SBP and diastolic blood pressure 3.4 mm Hg and 2.1 mm Hg higher than adults born term, respectively (120). One multicenter longitudinal study in Finland, involving over 1,700 individuals, found that those born preterm with small-for-gestational age had SBP 7.2 mm Hg higher (95% CI: 2.3, 12.2 mm Hg) than those born at term at the 31-year follow-up (121). The pathophysiology of hypertension in individuals born preterm is not fully understood but may involve inadequate elastin synthesis and altered endothelial cell function associated with PTB (122). Another study of 600 adults in Finland evaluating heart rate variability found that adults born preterm (GA <36 weeks) had decreased vagal control compared to those born term, postulating that such altered autonomic regulatory control may contribute to impaired vascular function and hypertension in adults born preterm (123). It is crucial to emphasize the need for regular BP monitoring in premature survivors of the neonatal intensive care units (NICUs), given the increased risk of developing hypertension later in life. The American Academy of Pediatrics recommends follow-up for hypertension in high-risk populations, including preterm infants, to detect and manage any early signs of elevated BP (124). While office BP measurements in childhood may appear normal, studies have shown that subtle abnormalities, such as a lack of nighttime dipping or increased BP load, can be detected using 24-hour ambulatory blood pressure monitoring (125). These more sensitive measurements may identify early signs of hypertension before it becomes clinically apparent, enabling timely interventions and better long-term cardiovascular outcomes for premature survivors (125).
2.4.2 Ischemic heart diseases and heart failure
PTB is associated with an increased risk of ischemic heart disease and heart failure in adulthood. A large national cohort study in Sweden, involving more than 2 million individuals born preterm between 1973 and 1994, found an inverse association between gestational age and ischemic heart disease in adulthood (126). The study also showed that preterm male and female infants had a 1.4- and 2.0-times higher risk, respectively, of developing ischemic heart disease compared to full-term infants, particularly in older age groups (126). This increased risk is consistent with the presence of cardiometabolic risk factors associated with preterm birth, such as hypertension, lipid disorders, and diabetes mellitus (117, 118, 127). PTB has also been associated with an increased risk of heart failure (3.6-fold in those born 28–31 weeks GA), extending from childhood to adulthood, in large Swedish national cohort studies involving over 2 million people (128). Similarly, another large Swedish cohort study with more than 4 million people found 4.7-fold increased risk in heart failure in adults born extremely preterm (22–27 weeks GA) (129).
2.5 Renal conditions
PTB has a significant impact on kidney development (130). The accelerated phase of nephrogenesis, responsible for 60% of nephron formation, occurs during the third trimester of pregnancy and nephrogenesis stops at 36 weeks of GA (130). Therefore preterm neonates have lower nephron endowment, sometimes resulting in smaller kidneys in terms of length and volume compared to full-term neonates (131–134). Autopsy studies revealed that nephrogenesis can continue for approximately 40 days after PTB (135). However, the nephrons developed during this period often exhibit histological abnormalities, such as poor glomerular capillarization, indicating impaired vascularization and non-functionality (136–138).
The compensatory mechanism for the reduced number of functional nephrons is hyperfiltration, which can cause glomerular enlargement. Single-nephron glomerular hyperfiltration, an early pathophysiological feature of chronic kidney disease (CKD), can result in either absolute hyperfiltration with elevated glomerular filtration rate (GFR) or relative hyperfiltration with a normal or reduced GFR (139). However, long-term consequences of this adaptation include sodium retention, hypertension, nephron loss due to sclerosis, and ultimately, the development of CKD due to high glomerular pressure from hyperfiltration can lead to pathological changes including secondary focal segmental glomerulosclerosis, which can manifest as mild albuminuria and subtle reductions in GFR in childhood (140–142). Other complications associated with PTB, such as hemodynamic instability leading to hypoperfusion, the use of nephrotoxic medications (e.g., gentamicin, aminoglycosides, and non-steroidal anti-inflammatory drugs), and systemic or urinary tract infections resulting in acute kidney injury, can further interfere with proper kidney development (143, 144).
Evidence from a large multicenter, multinational retrospective cohort study (Canada, Australia, USA, and India) found that the incidence of acute kidney injury (AKI) varied by gestational age (145). Specifically, 48% of patients with AKI were born between 22 and 29 weeks of gestation, compared to 18% and 37% of patients born between 29 and 36 and ≥36 weeks, respectively (145). Similarly, findings from Preterm Erythropoietin Neuroprotection Trial (PENUT) found an inverse relationship between AKI and GA (24 weeks: 27.8%; 25 weeks: 21.9%; 26 weeks: 13.6%; and 27 weeks: 9.4%) (146). However, studies on long-term renal outcomes in individuals born preterm are limited because renal problems may not manifest until later in life. A systematic review on the effects of prematurity on long-term renal health found that GFR is more affected when populations with preterm birth are measured at longer-term follow-ups, between 7.5 and 20 years of age (147). Similarly, hypertension was found to be associated with preterm birth when populations were followed into adulthood (147). A large Swedish cohort study involving over 4 million people found that prematurity (<37 weeks) overall had a 1.3-fold increased risk of new-onset hypertension at ages 18–29 years, and the risk increased to 2.4-fold (95% CI: 1.8, 3.3) in the population born at extremely preterm gestation (22–27 weeks GA) (148). Another large Swedish national cohort study by Crump et al. involving over 4 million infants, found that adults born preterm had a two-fold increased risk of CKD from childhood to mid-adulthood compared to those born at term, with the association between CKD and preterm birth being strongest during early childhood (0–9 years) (149). Interestingly, adults born at early term (37–38 weeks of gestation) also had a 1.3-fold increased risk of CKD compared to those born at term (149). Likewise, the FANCY (Follow-up of Acute Kidney Injury in Neonates during Childhood Years) study indicates that by age 5, VLBW patients with AKI had a 4.5-fold increased risk of renal dysfunction (95% CI: 1.2, 17.1) compared to VLBW patients without AKI (150). Evidence also shows that preterm infants may exhibit early signs such as mild albuminuria, reduced renal volume and GFR, and elevated blood pressure in early childhood (151–153). Since early renal disease is often asymptomatic, continuous monitoring of BP, serum creatinine, and albuminuria is critical in these high-risk infants (154). Additionally, persistent high BP and albuminuria are risk factors for the progression of CKD (154, 155), and antihypertensive medications like angiotensin-converting enzyme inhibitors can help slow this progression (156).
2.6 Gastrointestinal conditions
PTB has a significant impact on the development of the gastrointestinal (GI) tract, leading to various abnormalities and disorders (157). Feeding problems are commonly observed in preterm infants and often persist into childhood, with a higher incidence compared to term-born infants (158, 159). These feeding difficulties may arise from the extended stay in NICUs, and the therapeutic interventions received, which can delay positive oral experiences and hinder the development of a nurturing relationship with the caregiver (158, 159). These feeding difficulties can have adverse effects on the growth and development of preterm infants (159). In connection with managing PTB in NICUs, multiple factors (e.g., prolonged hospital microbial exposure, antibiotic use, and feeding choices) contribute to dysbiosis which can cause immune imbalance (160, 161), an increased risk of gastrointestinal diseases, and interference with other physiological systems (162).
A population-based cohort study in Israel has recruited over 200,000 patients (up to 18 years of age) born between 1991 and 2014 to analyze the digestive morbidity associated with prematurity (163). Digestive morbidities under study included esophageal diseases, diseases of appendix, hernia (inguinal, umbilical, and abdominal wall), inflammatory bowel diseases (IBD), irritable bowel syndrome (IBS) and celiac diseases (163). The study findings revealed a notably higher number of hospitalizations for digestive morbidities among PTB compared to those born at term, with the cumulative incidence rates increasing as gestational age declined (163). However, the authors did not find a statistically significant difference in the occurrence of different types of digestive morbidities, except for hernia (163). Another study (n = 2,737) suggested a positive and significant relationship between PTB and IBD (OR of 1.5 for Crohn's disease and 1.3 for ulcerative colitis) (164). Another study that recruited more than 7,000 cases of esophagitis found an association between prematurity and esophagitis in populations diagnosed at 9 years or younger (OR: 6.8; 95% CI: 4.6, 10.0), but the association was not as clear in populations diagnosed at 20 years of age (165). Similarly, a nested case control study in the USA found a positive association between PTB and eosinophilic esophagitis among study subjects aged 18 years and above; however, the results were not statistically significant (OR: 2.6; 95% CI: 0.5, 13.8) (166). The reasons for the association between PTB and esophagitis in early childhood, but not in adulthood, are not clear and warrant further investigation to elucidate the underlying mechanisms for this relationship. Overall, the long-term effects of preterm birth on the GI system are still being studied, and further research is needed to fully understand the specific gastrointestinal conditions and their association with PTB.
2.7 Endocrine conditions
2.7.1 Linear growth velocity
Growth is a complex process being affected by intrinsic factors such as chronic health status, nutrition, hormonal profile, medications, socioeconomic status and prolonged hospitalization (167). Although suboptimal growth is a common concern in children born preterm, the relationship between PTB and growth outcomes is complex, with varied and sometimes conflicting findings in the literature. For instance, Hollanders et al. emphasize the importance of distinguishing between VP infants and VLBW infants, highlighting that these groups follow distinct growth trajectories and achieve different final height outcomes (168). Their study suggests that prematurity and low birth weight independently impact growth patterns, emphasizing the need for stratified analyses to elucidate these effects (168). In contrast, Ferguson et al. focus on preterm infants born appropriate-for- gestational-age (AGA), revealing that while growth in this subset may initially lag, catch-up growth is typically observed over time (169). These findings underscore the protective role of AGA birth weight against some of the adverse growth effects of prematurity. These insights align with additional findings from a longitudinal study in New Zealand, which demonstrated that preterm AGA individuals (aged 2–20 years) were slower in reaching their genetically determined height potential compared to those born at term (16). Similarly, a large-scale study involving over 3.2 million children identified an increased risk of stunted growth at 12 and 24 months among late preterm AGA infants compared to those born at term (170). However, a United Kingdom (UK) cohort study involving 204 preterm AGA children and 50 term-born controls found no significant differences in adult height, though preterm children exhibited prolonged catch-up growth periods before attaining their final height (169). Together, these studies demonstrate the variability in growth outcomes influenced by the interplay of gestational age, birth weight, and environmental and genetic factors. Recognizing the multifaceted effects of PTB on growth requires careful interpretation of these findings, particularly considering birth weight appropriateness and the timing of growth assessments. Future research should prioritize examining these interactions.
In addition, there is a complex interplay between prematurity-associated factors and the occurrence of obesity in both childhood and adulthood (171). A systematic review of 19 studies, including 169,439 children, revealed that preterm infants were significantly more likely to develop childhood obesity compared to those born full-term (OR = 1.19, 95% CI: 1.13, 1.26) (172). This increased likelihood of obesity in preterm survivors can be attributed to several factors, including fetal nutrient deprivation, which can lead to metabolic alterations that predispose individuals to obesity later in life (173–175). Furthermore, accelerated weight gain during the neonatal period, often as a compensatory response to early growth deficits, is also a contributing factor (176, 177). The findings from the same review suggest that preterm infants who experience rapid weight gain from birth to the first year of life had a 2.69 times greater risk of developing obesity by ages 8–11 (172), a finding that is consistent with other research showing that fast weight gain in infancy is linked to a 2–4 fold higher risk of obesity (178–181). However, breast milk feeding has been shown to offer a protective effect, reducing the incidence of obesity in preterm infants (182). The nutrients in breast milk support healthy growth and regulate metabolism, potentially mitigating the risk of excessive weight gain in these vulnerable infants (183). These findings underscore the need for careful monitoring of growth patterns in preterm infants and the promotion of breast milk feeding to reduce the long-term risks of obesity.
2.7.2 Thyroid hormone
Thyroid hormones play a crucial role in growth and development during the fetal, neonatal and early childhood period (184, 185). In term births, thyroid hormone levels show distinct elevations and peaks, which are not observed in preterm births (186). Preterm infants, particularly those born very preterm (<32 weeks gestation), are at increased risk of transient hypothyroxinemia, characterized by low serum thyroxine (T4) levels without an elevation in thyroid-stimulating hormone (TSH) (187). This condition arises from the immaturity of the hypothalamic-pituitary-thyroid axis, reduced thyroidal iodine stores, and decreased conversion of T4 to the active triiodothyronine (T3) due to underdeveloped deiodinase activity, resulting in decreased T3 and T4 levels (187). Biochemically, serum T3, total T4 and free T4 are reduced in proportion to the degree of prematurity but without marked elevation of TSH levels; this phenomenon is known as hypothyroxinemia of prematurity (186–188). Reduced T3 and T4 levels in preterm newborns with less than 28 weeks of gestation usually take two to three weeks to reach the peak levels seen in term infants (186–188). This biochemical property differentiates it from congenital hypothyroidism, in which reduced T3 and T4 levels are accompanied by persistently elevated TSH. Distinguishing between the two conditions can be done by measuring persistently low T4 levels with marked elevation of TSH in repeated thyroid function tests at 4 weeks and 8 weeks after birth for early and extremely preterm infants (186–188).
The suppressed thyroid hormone levels in preterm infants could be attributed to several factors, including but not limited to immature hypothalamic-pituitary-thyroid regulation, loss of maternal thyroid hormone transfer in PTB, disease states in which cytokine elevation inhibiting the thyroid function, dopamine use for inotropic support, repeated iodine-based contrast computer-tomography use in infants with congenital heart diseases, and inadequate iodine intake postnatally (188, 189).
Thyroid dysfunction in preterm infants, characterized by suppressed thyroid function, is often present during the neonatal period but may also persist into adulthood. For instance, Delange et al. observed a transient thyroid function deficit in preterm Belgian infants, influenced by prematurity and relative iodine deficiency, which generally resolved by 5–7 weeks, though some developed transient hypothyroidism (190). Similarly, Kim et al. observed that nearly one-fifth of preterm infants born before 32 weeks gestation required levothyroxine treatment, with maternal pregnancy-induced hypertension as a significant risk factor. Notably, almost half of those treated had normal initial thyroid function tests, emphasizing the importance of serial monitoring to identify late-onset dysfunction (191). Furthermore, a large Swedish cohort study involving over 600,000 individuals revealed a 1.60-fold increased risk of medically treated hypothyroidism in young adults born extremely preterm (23–31 weeks gestation), suggesting that the impact of PTB on thyroid function may have long-term implications. These findings emphasize the importance of early screening and monitoring of thyroid function across the lifespan of individuals born preterm, while future research should explore the underlying mechanisms of persistent thyroid dysfunction (192).
2.7.3 Diabetes mellitus
Pancreatic beta cells, primarily formed during the third trimester of pregnancy, may have reduced numbers and functionality in PTB, potentially leading to long-term metabolic consequences (193). An inverse relationship between gestational age and plasma insulin levels has been observed at birth and in early childhood (194). Supporting this, a large Swedish national cohort study involving over 4 million individuals demonstrated that those born preterm had an increased risk of diabetes mellitus (DM) from childhood (1.2-fold increased risk for both type 1 and type 2 DM) to mid-adulthood (1.2-fold increase in type 1 DM and 1.5-fold increase in type 2 DM) compared to those born term (127). The association between PTB and type 2 DM is particularly stronger in adult women (127).
Preterm birth has been associated with an increased risk of both type 1 and type 2 DM multiple studies, though findings vary by gestational age and diabetes type. A Swedish cohort study of 3.6 million children found a 10%–20% increased risk of type 1 diabetes among those born at 33–36 or 37–38 weeks, but a lower risk in those born at <33 weeks, a finding requiring further investigation (195). Conversely, a UK cohort of 3.8 million children and an Australian cohort of 558,633 children reported 15%–30% and 1.4–1.2-fold increased risks of type 1 DM, respectively, among preterm or early-term births (196, 197). For type 2 diabetes, a Finnish cohort of 12,813 adults found a 1.68-fold risk for those born <35 weeks (198), while Swedish and Scottish studies reported 1.6–2.0-fold risks in adults born very preterm (<33 weeks) (199, 200). A meta-analysis of ∼2.2 million participants reported pooled odds ratios of 1.2 (95% CI 1.1–1.2) for type 1 DM and 1.51 (95% CI 1.3–1.7) for type 2 DM, highlighting the consistent association between preterm birth and long-term diabetes risk (201). These consistent findings emphasize the need for continued research to elucidate mechanisms and preventive strategies.
2.8 Oncological conditions
Childhood cancer is a serious and devastating condition that affects children and their families (202, 203). Several factors associated with PTB increase the risk of developing certain types of childhood cancer. During their stay in the NICUs, preterm infants are exposed to an array of toxic insults including mechanical ventilation-induced oxidative stress, radiation exposure from diagnostic radiographs, treatment with nitric oxide for pulmonary hypertension, and treatment with oxygen (204–206). A Finnish population-based study found an elevated risk of childhood cancer in those born preterm, particularly in the early preterm cohort, with increased rates of acute myeloid leukemia (AML), germ cell tumors, and retinoblastoma (207). A systemic review on cancer risk in children and young adults born preterm found a consistent association between PTB and an increased risk of hepatoblastoma across multiple studies (208). Low birth weight has also been associated with hepatoblastoma in most studies (209–211), although the underlying mechanisms are unclear. Two meta-analyses of observational studies on prematurity and acute leukemia in childhood suggested that preterm birth is associated with an increased risk of AML (212, 213). The risks of other childhood cancers related to PTB appear to be study- or region-dependent. Some childhood cancers that have been proposed, but not definitively linked to PTB, include soft tissue sarcomas, retinoblastomas, and other gliomas (211). Further research is needed to better understand the underlying mechanisms and to identify effective screening strategies.
2.9 Genetic and epigenetic links between preterm birth and long-term morbidity
Emerging evidence suggests that genetic and epigenetic factors may play a critical role in both the etiology of PTB and the long-term health outcomes of survivors (214, 215). Genome-wide association studies (GWAS) have identified genetic variants associated with PTB (215, 216), while epigenetic studies have demonstrated that PTB can lead to lasting changes in DNA methylation and other regulatory mechanisms (217, 218). These genetic and epigenetic alterations may contribute to the risk of PTB and potentially play an independent role in the development of long-term multisystemic diseases (219, 220). Additionally, intergenerational studies indicate that mothers born preterm are more likely to deliver preterm infants, suggesting a heritable component that may also shape long-term health trajectories (221–224). However, whether these same genetic and epigenetic factors directly predispose preterm survivors to multisystemic diseases—such as cardiovascular, metabolic, and neurodevelopmental disorders—remains an area of further exploration. Future GWAS and epigenetic studies are needed to elucidate these complex interactions, which may provide insights into targeted interventions aimed at improving long-term health outcomes in this vulnerable population.
3 Discussion
This narrative review comprehensively examines the extensive long-term complications associated with PTB, revealing significant findings across multiple health domains. Our analysis highlights the critical need for continuous follow-up and targeted interventions for individuals born preterm, given their increased susceptibility to a range of health challenges including neurodevelopmental, respiratory, allergic, cardiovascular, renal, gastrointestinal, and endocrine system disorders. The novelty of our findings lies in the comprehensive scope of complications covered, offering a holistic understanding of the long-term health outcomes of PTB.
It is crucial to proactively prevent, monitor, and treat long-term consequences of PTB. By implementing specific practices for preterm infants, such as delivering them in perinatal centers equipped to handle high-risk cases, better short-term outcomes can be achieved compared to transferring them after birth (225). Regular clinical follow-up in pediatric clinics plays a vital role in evaluating and managing the various conditions related to PTB. These evaluations should encompass neurodevelopmental, respiratory, allergic, cardiovascular, renal, gastrointestinal, endocrine, and potential oncological conditions, with particular attention given to extremely preterm infants who are at higher risk. Such comprehensive monitoring allows for early detection and intervention, optimizing the long-term health and well-being of preterm individuals. The holistic approach should extend into the transition from pediatric to adult care, ensuring continuity of care and sustained efforts to manage and minimize the long-term consequences of PTB.
Current long-term follow-up programs for preterm infants often prioritize neurodevelopmental outcomes, but a more holistic, multidisciplinary approach is essential to better support these children and their families. Parents have expressed that their concerns extend beyond neurodevelopmental impairments, highlighting the importance of addressing a broader range of health and developmental factors. A study on parental perspectives regarding the outcomes of very preterm infants found that parents value a balanced approach, encompassing not only neurodevelopment but also other physical and psychological aspects of their child's health (226). This insight underscores the need to shift toward a more inclusive surveillance model that captures a wider spectrum of outcomes, ultimately benefiting the long-term well-being of preterm survivors. Further research is needed to deepen our understanding of pathophysiology underlying the long-term consequences of PTB. By gaining more insights into the mechanisms involved, researchers can develop more effective preventive strategies and targeted treatments to mitigate the impact of PTB on health outcomes.
In summary, our findings advocate for the implementation of targeted interventions and continuous follow-up programs tailored to address the specific health needs of this vulnerable population. A proactive approach that emphasizes prevention, monitoring, and treatment is essential for addressing the long-term consequences of PTB. By implementing specific practices, conducting regular clinical follow-ups, promoting holistic care, and advancing research, it is possible to mitigate the impact of PTB and improve the overall health and quality of life of individuals born preterm.
Author contributions
FG: Writing – original draft, Writing – review & editing. SA: Writing – original draft, Writing – review & editing. MH: Writing – original draft, Writing – review & editing. LR: Writing – review & editing. EC: Writing – review & editing. CY: Writing – review & editing. EK: Writing – review & editing. CM: Writing – review & editing. AR: Writing – review & editing. KK: Writing – review & editing. JW: Writing – review & editing. SR: Writing – review & editing. MC: Writing – review & editing. KH: Writing – review & editing. JL: Writing – review & editing. CL: Writing – review & editing. NC: Writing – review & editing. SL: Writing – review & editing. JT: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the Canadian Institutes of Health Research (CIHR) Operating Grant 2021 and Start-up Funding from Department of Pediatrics, University of Alberta under senior author (JYT). SAA and SL are supported by Alberta Innovates Postdoctoral Recruitment Fellowship and CIHR (grant PJT-183598), respectively. The funding agencies have no role in the development, review and approval of this manuscript.
Acknowledgments
We acknowledge the support from the funding agencies in supporting this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Preterm birth 2022. Available online at: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (Accessed May 13, 2023).
2. Ohuma EO, Moller A-B, Bradley E, Chakwera S, Hussain-Alkhateeb L, Lewin A, et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: a systematic analysis. Lancet. (2023) 402(10409):1261–71.37805217
3. Centers for Disease Control and Prevention. Preterm Birth 2022. Available online at: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm (Accessed June 03, 2023).
4. Office for National Statistics. Birth characteristics in England and Wales: 2020. 2021. Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2020#gestational-age (Accessed July 20, 2023).
5. Shah PS, Ye XY, Yang J, Campitelli MA. Preterm birth and stillbirth rates during the COVID-19 pandemic: a population-based cohort study. Can Med Assoc J. (2021) 193(30):E1164. doi: 10.1503/cmaj.210081
6. Cao G, Liu J, Liu M. Global, regional, and national incidence and mortality of neonatal preterm birth, 1990–2019. JAMA Pediatr. (2022) 176(8):787–96. doi: 10.1001/jamapediatrics.2022.1622
7. World Health Organization. WHO recommendations on interventions to improve preterm birth outcomes. Published November 1, 2015. Available online at: https://www.who.int/publications/i/item/9789241508988 (Accessed March 10, 2025).
8. Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc Health. (2019) 3(6):408–17. doi: 10.1016/S2352-4642(19)30108-7
9. Crump C, Winkleby MA, Sundquist J, Sundquist K. Prevalence of survival without Major comorbidities among adults born prematurely. JAMA. (2019) 322(16):1580–8. doi: 10.1001/jama.2019.15040
10. Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. (2012) 379(9814):445–52. doi: 10.1016/S0140-6736(11)61577-8
11. Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. (2008) 371(9608):261–9. doi: 10.1016/S0140-6736(08)60136-1
12. de Kleine MJ, den Ouden AL, Kollée LA, Ilsen A, van Wassenaer AG, Brand R, et al. Lower mortality but higher neonatal morbidity over a decade in very preterm infants. Paediatr Perinat Epidemiol. (2007) 21(1):15–25. doi: 10.1111/j.1365-3016.2007.00780.x
13. Stahlmann N, Eisemann N, Thyen U, Herting E, Rapp M. Long-term health outcomes and health-related quality of life in adolescents from a cohort of extremely premature infants born at less than 27 weeks of gestation in northern Germany. Neuropediatrics. (2016) 47(06):388–98. doi: 10.1055/s-0036-1593373
14. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. (2008) 359(3):262–73. doi: 10.1056/NEJMoa0706475
15. Zhang J, Ma C, Yang A, Zhang R, Gong J, Mo F. Is preterm birth associated with asthma among children from birth to 17 years old? -A study based on 2011–2012 US national survey of children’s health. Ital J Pediatr. (2018) 44(1):151. doi: 10.1186/s13052-018-0583-9
16. Rowe DL, Derraik JG, Robinson E, Cutfield WS, Hofman PL. Preterm birth and the endocrine regulation of growth in childhood and adolescence. Clin Endocrinol (Oxf). (2011) 75(5):661–5. doi: 10.1111/j.1365-2265.2011.04116.x
17. Mangham LJ, Petrou S, Doyle LW, Draper ES, Marlow N. The cost of preterm birth throughout childhood in England and Wales. Pediatrics. (2009) 123(2):e312–27. doi: 10.1542/peds.2008-1827
18. Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD. Community supports after surviving extremely low-birth-weight, extremely preterm birth: special outpatient services in early childhood. Arch Pediatr Adolesc Med. (2008) 162(8):748–55. doi: 10.1001/archpedi.162.8.748
19. Waitzman NJ, Jalali A, Grosse SD. Preterm birth lifetime costs in the United States in 2016: an update. Semin Perinatol. (2021) 45(3):151390. doi: 10.1016/j.semperi.2021.151390
20. McLaurin KK, Hall CB, Jackson EA, Owens OV, Mahadevia PJ. Persistence of morbidity and cost differences between late-preterm and term infants during the first year of life. Pediatrics. (2009) 123(2):653–9. doi: 10.1542/peds.2008-1439
21. Lai K-C, Lorch SA. Healthcare costs of major morbidities associated with prematurity in US children’s hospitals. J Pediatr. (2023) 256:53–62.e4. doi: 10.1016/j.jpeds.2022.11.038
22. Petrou S, Yiu HH, Kwon J. Economic consequences of preterm birth: a systematic review of the recent literature (2009–2017). Arch Dis Child. (2019) 104(5):456–65. doi: 10.1136/archdischild-2018-315778
23. Petrou S, Abangma G, Johnson S, Wolke D, Marlow N. Costs and health utilities associated with extremely preterm birth: evidence from the EPICure study. Value Health. (2009) 12(8):1124–34. doi: 10.1111/j.1524-4733.2009.00580.x
24. Kim SW, Andronis L, Seppänen A-V, Aubert AM, Zeitlin J, Barros H, et al. Economic costs at age five associated with very preterm birth: multinational European cohort study. Pediatr Res. (2022) 92(3):700–11. doi: 10.1038/s41390-021-01769-z
25. Jacob J, Lehne M, Mischker A, Klinger N, Zickermann C, Walker J. Cost effects of preterm birth: a comparison of health care costs associated with early preterm, late preterm, and full-term birth in the first 3 years after birth. Eur J Health Econ. (2017) 18(8):1041–6. doi: 10.1007/s10198-016-0850-x
26. Shah PS, Dunn M, Aziz K, Shah V, Deshpandey A, Mukerji A, et al. Sustained quality improvement in outcomes of preterm neonates with a gestational age less than 29 weeks: results from the evidence-based practice for improving quality phase 3 (1). Can J Physiol Pharmacol. (2019) 97(3):213–21. doi: 10.1139/cjpp-2018-0439
27. Pierrat V, Marchand-Martin L, Marret S, Arnaud C, Benhammou V, Cambonie G, et al. Neurodevelopmental outcomes at age 5 among children born preterm: ePIPAGE-2 cohort study. Br Med J. (2021) 373:n741. doi: 10.1136/bmj.n741
28. Kiechl-Kohlendorfer U, Simma B, Berger A, Urlesberger B, Wald M, Haiden N, et al. Two-year neurodevelopmental outcome in extremely preterm-born children: the Austrian preterm outcome study group. Acta Paediatr. (2024) 113(6):1278–87. doi: 10.1111/apa.17187
29. Chen Z, Xiong C, Liu H, Duan J, Kang C, Yao C, et al. Impact of early term and late preterm birth on infants’ neurodevelopment: evidence from a cohort study in Wuhan, China. BMC Pediatr. (2022) 22(1):251. doi: 10.1186/s12887-022-03312-3
30. Mitha A, Chen R, Razaz N, Johansson S, Stephansson O, Altman M, et al. Neurological development in children born moderately or late preterm: national cohort study. Br Med J. (2024) 384:1–11. doi: 10.1136/bmj-2023-075630
31. Hemmingsen D, Moster D, Engdahl BL, Klingenberg C. Sensorineural hearing impairment among preterm children: a Norwegian population-based study. Arch Dis Child Fetal Neonatal Ed. (2025) 110(1):68–74. doi: 10.1136/archdischild-2024-326870
32. Lax ID, Duerden EG, Lin SY, Mallar Chakravarty M, Donner EJ, Lerch JP, et al. Neuroanatomical consequences of very preterm birth in middle childhood. Brain Struct Funct. (2013) 218(2):575–85. doi: 10.1007/s00429-012-0417-2
33. Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, et al. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. (2013) 33(2):411–23. doi: 10.1523/JNEUROSCI.4445-12.2013
34. Schneider J, Miller SP. Preterm brain injury: white matter injury. Handb Clin Neurol. (2019) 162:155–72. doi: 10.1016/B978-0-444-64029-1.00007-2
35. Back SA, Miller SP. Brain injury in premature neonates: a primary cerebral dysmaturation disorder? Ann Neurol. (2014) 75(4):469–86. doi: 10.1002/ana.24132
36. Pandey M, Chauhan M, Awasthi S. Interplay of cytokines in preterm birth. Indian J Med Res. (2017) 146(3):316–27. doi: 10.4103/ijmr.IJMR_1624_14
37. Schultz C, Rott C, Temming P, Schlenke P, Möller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. (2002) 51(3):317–22. doi: 10.1203/00006450-200203000-00009
38. Vilotić A, Nacka-Aleksić M, Pirković A, Bojić-Trbojević Ž, Dekanski D, Jovanović Krivokuća M. IL-6 and IL-8: an overview of their roles in healthy and pathological pregnancies. Int J Mol Sci. (2022) 23(23):14574. doi: 10.3390/ijms232314574
39. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. (2018) 49(3):397–412. doi: 10.1016/j.immuni.2018.07.017
40. Leitner K, Al Shammary M, McLane M, Johnston MV, Elovitz MA, Burd I. IL-1 receptor blockade prevents fetal cortical brain injury but not preterm birth in a mouse model of inflammation-induced preterm birth and perinatal brain injury. Am J Reprod Immunol. (2014) 71(5):418–26. doi: 10.1111/aji.12216
41. Jin JH, Lee SH, Youk TM, Yoon SW. Long-term outcomes of preterm infants in the first 6 years of life: a nationwide population-based study in Korea. Eur J Pediatr. (2023) 182(2):641–50. doi: 10.1007/s00431-022-04728-w
42. Allotey J, Zamora J, Cheong-See F, Kalidindi M, Arroyo-Manzano D, Asztalos E, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG. (2018) 125(1):16–25. doi: 10.1111/1471-0528.14832
43. Anderson PJ, de Miranda DM, Albuquerque MR, Indredavik MS, Evensen KAI, Van Lieshout R, et al. Psychiatric disorders in individuals born very preterm/very low-birth weight: an individual participant data (IPD) meta-analysis. EClinicalMedicine. (2021) 42:928–39. doi: 10.1016/j.eclinm.2021.101216
44. McGowan EC, Hofheimer JA, O’Shea TM, Kilbride H, Carter BS, Check J, et al. Analysis of neonatal neurobehavior and developmental outcomes among preterm infants. JAMA Netw Open. (2022) 5(7):e2222249. doi: 10.1001/jamanetworkopen.2022.22249
45. Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. (2008) 50(5):334–40. doi: 10.1111/j.1469-8749.2008.02047.x
46. Ream MA, Lehwald L. Neurologic consequences of preterm birth. Curr Neurol Neurosci Rep. (2018) 18(8):48. doi: 10.1007/s11910-018-0862-2
47. Paul S, Nahar A, Bhagawati M, Kunwar AJ. A review on recent advances of cerebral palsy. Oxid Med Cell Longevity. (2022) 2022(1):2622310. doi: 10.1155/2022/2622310
48. Cameron KL, FitzGerald TL, McGinley JL, Allison K, Cheong JL, Spittle AJ. Motor outcomes of children born extremely preterm; from early childhood to adolescence. Semin Perinatol. (2021) 45(8):151481. doi: 10.1016/j.semperi.2021.151481
49. Hafström M, Källén K, Serenius F, Maršál K, Rehn E, Drake H, et al. Cerebral palsy in extremely preterm infants. Pediatrics. (2018) 141(1):1–10. doi: 10.1542/peds.2017-1433
50. Ancel PY, Livinec F, Larroque B, Marret S, Arnaud C, Pierrat V, et al. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. (2006) 117(3):828–35. doi: 10.1542/peds.2005-0091
51. Chen D, Huang M, Yin Y, Gui D, Gu Y, Zhuang T, et al. Risk factors of cerebral palsy in children: a systematic review and meta-analysis. Transl Pediatr. (2022) 11(4):556. doi: 10.21037/tp-22-78
52. Synnes A, Hicks M. Neurodevelopmental outcomes of preterm children at school age and beyond. Clin Perinatol. (2018) 45(3):393–408. doi: 10.1016/j.clp.2018.05.002
53. Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. (2018) 172(4):361–7. doi: 10.1001/jamapediatrics.2017.5323
54. Cai S, Thompson DK, Anderson PJ, Yang JY. Short- and long-term neurodevelopmental outcomes of very preterm infants with neonatal sepsis: a systematic review and meta-analysis. Children (Basel). (2019) 6(12):131. doi: 10.3390/children6120131
55. Hollanders JJ, Schaëfer N, van der Pal SM, Oosterlaan J, Rotteveel J, Finken MJJ. Long-Term neurodevelopmental and functional outcomes of infants born very preterm and/or with a very low birth weight. Neonatology. (2019) 115(4):310–9. doi: 10.1159/000495133
56. Chan NH-M, Synnes A, Grunau RE, Colby L, Petrie J, Elfring T, et al. Impact of differing language background exposures on Bayley-III language assessment in a national cohort of children born less than 29 Weeks’ gestation. Children. (2022) 9(7):1048. doi: 10.3390/children9071048
57. Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. (2011) 128(2):344–55. doi: 10.1542/peds.2010-1036
58. Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore). (2017) 96(18):e6696. doi: 10.1097/MD.0000000000006696
59. Crump C, Sundquist J, Sundquist K. Preterm or early term birth and risk of autism. Pediatrics. (2021) 148(3):1–12. doi: 10.1542/peds.2020-032300
60. Robinson R, Girchenko P, Pulakka A, Heinonen K, Lähdepuro A, Lahti-Pulkkinen M, et al. ADHD symptoms and diagnosis in adult preterms: systematic review, IPD meta-analysis, and register-linkage study. Pediatr Res. (2023) 93(5):1399–409. doi: 10.1038/s41390-021-01929-1
61. Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, et al. Preterm birth and poor fetal growth as risk factors of attention-Deficit hyperactivity disorder. Pediatrics. (2015) 136(3):e599–608. doi: 10.1542/peds.2015-1043
62. Lindström K, Lindblad F, Hjern A. Psychiatric morbidity in adolescents and young adults born preterm: a Swedish national cohort study. Pediatrics. (2009) 123(1):e47–53. doi: 10.1542/peds.2008-1654
63. Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, et al. Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry. (2012) 69(6):E1–8. doi: 10.1001/archgenpsychiatry.2011.1374
64. Crump C, Winkleby MA, Sundquist K, Sundquist J. Preterm birth and psychiatric medication prescription in young adulthood: a Swedish national cohort study. Int J Epidemiol. (2010) 39(6):1522–30. doi: 10.1093/ije/dyq103
65. Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, et al. Visual and hearing impairments after preterm birth. Pediatrics. (2018) 142(2):e20173888. doi: 10.1542/peds.2017-3888
66. van Dommelen P, Verkerk PH, van Straaten HL, Baerts W, Von Weissenbruch M, Duijsters C, et al. Hearing loss by week of gestation and birth weight in very PretermáNeonates. J Pediatr. (2015) 166(4):840–3.e1. doi: 10.1016/j.jpeds.2014.12.041
67. Chant K, Bitner-Glindzicz M, Marlow N. Cumulative risk factors contributing to hearing loss in preterm infants. Arch Dis Child Fetal Neonatal Ed. (2023) 108(5):464–70. doi: 10.1136/archdischild-2022-324331
68. Eras Z, Konukseven O, Aksoy HT, Canpolat FE, Genç A, Sakrucu ED, et al. Postnatal risk factors associated with hearing loss among high-risk preterm infants: tertiary center results from Turkey. Eur Arch Otorhinolaryngol. (2014) 271:1485–90. doi: 10.1007/s00405-013-2653-3
69. Leung MP, Thompson B, Black J, Dai S, Alsweiler JM. The effects of preterm birth on visual development. Clin Exp Optom. (2018) 101(1):4–12. doi: 10.1111/cxo.12578
70. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. (2013) 74(1):35–49. doi: 10.1038/pr.2013.205
71. Bröring T, Königs M, Oostrom KJ, Lafeber HN, Brugman A, Oosterlaan J. Sensory processing difficulties in school-age children born very preterm: an exploratory study. Early Hum Dev. (2018) 117:22–31. doi: 10.1016/j.earlhumdev.2017.12.003
72. Niutanen U, Harra T, Lano A, Metsäranta M. Systematic review of sensory processing in preterm children reveals abnormal sensory modulation, somatosensory processing and sensory-based motor processing. Acta Paediatr. (2020) 109(1):45–55. doi: 10.1111/apa.14953
73. Li W, Peng A, Deng S, Lai W, Qiu X, Zhang L, et al. Do premature and postterm birth increase the risk of epilepsy? An updated meta-analysis. Epilepsy Behav. (2019) 97:83–91. doi: 10.1016/j.yebeh.2019.05.016
74. Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, et al. The incidence and risk factors of epilepsy in children born preterm: a nationwide register study. Epilepsy Res. (2017) 138:32–8. doi: 10.1016/j.eplepsyres.2017.10.005
75. Crump C, Sundquist K, Winkleby MA, Sundquist J. Preterm birth and risk of epilepsy in Swedish adults. Neurology. (2011) 77(14):1376. doi: 10.1212/WNL.0b013e318231528f
76. Tana M, Tirone C, Aurilia C, Lio A, Paladini A, Fattore S, et al. Respiratory management of the preterm infant: supporting evidence-based practice at the bedside. Children. (2023) 10(3):535. doi: 10.3390/children10030535
77. Bhandari A, Carroll C, Bhandari V. BPD following preterm birth: a model for chronic lung disease and a substrate for ARDS in childhood. Front Pediatr. (2016) 4:60. doi: 10.3389/fped.2016.00060
78. Vollsæter M, Røksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax. (2013) 68(8):767–76. doi: 10.1136/thoraxjnl-2012-202980
79. Simpson SJ, Logie KM, O'Dea CA, Banton GL, Murray C, Wilson AC, et al. Altered lung structure and function in mid-childhood survivors of very preterm birth. Thorax. (2017) 72(8):702–11. doi: 10.1136/thoraxjnl-2016-208985
80. Doyle LW, Irving L, Haikerwal A, Lee K, Ranganathan S, Cheong J. Airway obstruction in young adults born extremely preterm or extremely low birth weight in the postsurfactant era. Thorax. (2019) 74(12):1147–53. doi: 10.1136/thoraxjnl-2019-213757
81. Di Cicco M, D’Elios S, Peroni DG, Comberiati P. The role of atopy in asthma development and persistence. Curr Opin Allergy Clin Immunol. (2020) 20(2):131–7. doi: 10.1097/ACI.0000000000000627
82. Halvorsen T, Skadberg BT, Eide GE, Røksund OD, Carlsen KH, Bakke P. Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study. Acta Paediatr. (2004) 93(10):1294–300. doi: 10.1111/j.1651-2227.2004.tb02926.x
83. Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics. (2011) 127(4):e913–20. doi: 10.1542/peds.2010-2603
84. Sonnenschein-van der Voort AM, Arends LR, de Jongste JC, Annesi-Maesano I, Arshad SH, Barros H, et al. Preterm birth, infant weight gain, and childhood asthma risk: a meta-analysis of 147,000 European children. J Allergy Clin Immunol. (2014) 133(5):1317–29. doi: 10.1016/j.jaci.2013.12.1082
85. Abe K, Shapiro-Mendoza CK, Hall LR, Satten GA. Late preterm birth and risk of developing asthma. J Pediatr. (2010) 157(1):74–8. doi: 10.1016/j.jpeds.2010.01.008
86. Kaditis A, Kheirandish-Gozal L, Gozal D. Algorithm for the diagnosis and treatment of pediatric OSA: a proposal of two pediatric sleep centers. Sleep Med. (2012) 13(3):217–27. doi: 10.1016/j.sleep.2011.09.009
87. Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep. (2012) 35(11):1475–80. doi: 10.5665/sleep.2192
88. Crump C, Friberg D, Li X, Sundquist J, Sundquist K. Preterm birth and risk of sleep-disordered breathing from childhood into mid-adulthood. Int J Epidemiol. (2019) 48(6):2039–49. doi: 10.1093/ije/dyz075
89. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200(6):751–9. doi: 10.1164/rccm.201812-2348OC
90. Levy PT, Levin J, Leeman KT, Mullen MP, Hansmann G, Kourembanas S. Diagnosis and management of pulmonary hypertension in infants with bronchopulmonary dysplasia. Semin Fetal Neonatal Med. (2022) 27(4):101351. doi: 10.1016/j.siny.2022.101351
91. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53(1):1–13. doi: 10.1183/13993003.01913-2018
92. Hansmann G, Sallmon H, Roehr CC, Kourembanas S, Austin ED, Koestenberger M. Pulmonary hypertension in bronchopulmonary dysplasia. Pediatr Res. (2021) 89(3):446–55. doi: 10.1038/s41390-020-0993-4
93. Moore SS, De Carvalho Nunes G, Dancea A, Wutthigate P, Simoneau J, Beltempo M, et al. Early cardiac function and death, severe bronchopulmonary dysplasia and pulmonary hypertension in extremely preterm infants. Pediatr Res. (2024) 95(1):293–301. doi: 10.1038/s41390-023-02817-6
94. Nakanishi H, Uchiyama A, Kusuda S. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: a cohort study. J Perinatol. (2016) 36(10):890–6. doi: 10.1038/jp.2016.108
95. Choi EK, Shin SH, Kim EK, Kim HS. Developmental outcomes of preterm infants with bronchopulmonary dysplasia-associated pulmonary hypertension at 18–24 months of corrected age. BMC Pediatr. (2019) 19(1):26. doi: 10.1186/s12887-019-1400-3
96. Merid SK, Novoloaca A, Sharp GC, Küpers LK, Kho AT, Roy R, et al. Epigenome-wide meta-analysis of blood DNA methylation in newborns and children identifies numerous loci related to gestational age. Genome Med. (2020) 12(1):25. doi: 10.1186/s13073-020-0716-9
97. Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, et al. Stereotypic immune system development in newborn children. Cell. (2018) 174(5):1277–92.e14. doi: 10.1016/j.cell.2018.06.045
98. Westman M, Lupinek C, Bousquet J, Andersson N, Pahr S, Baar A, et al. Early childhood IgE reactivity to pathogenesis-related class 10 proteins predicts allergic rhinitis in adolescence. J Allergy Clin Immunol. (2015) 135(5):1199–206.e1–11. doi: 10.1016/j.jaci.2014.10.042
99. Mitselou N, Andersson N, Bergström A, Kull I, Georgelis A, van Hage M, et al. Preterm birth reduces the risk of IgE sensitization up to early adulthood: a population-based birth cohort study. Allergy. (2022) 77(5):1570–82. doi: 10.1111/all.15077
100. Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and risk of allergic rhinitis in young adulthood. J Allergy Clin Immunol. (2011) 127(5):1173–9. doi: 10.1016/j.jaci.2011.02.023
101. Pagano F, Conti MG, Boscarino G, Pannucci C, Dito L, Regoli D, et al. Atopic manifestations in children born preterm: a long-term observational study. Children (Basel). (2021) 8(10):1–11.
102. Tanaka K, Matsui T, Sato A, Sasaki K, Nakata J, Nakagawa T, et al. The relationship between the season of birth and early-onset food allergies in children. Pediatr Allergy Immunol. (2015) 26(7):607–13. doi: 10.1111/pai.12440
103. Mitselou N, Hallberg J, Stephansson O, Almqvist C, Melén E, Ludvigsson JF. Cesarean delivery, preterm birth, and risk of food allergy: nationwide Swedish cohort study of more than 1 million children. J Allergy Clin Immunol. (2018) 142(5):1510–4.e2. doi: 10.1016/j.jaci.2018.06.044
104. Liem JJ, Kozyrskyj AL, Huq SI, Becker AB. The risk of developing food allergy in premature or low-birth-weight children. J Allergy Clin Immunol. (2007) 119(5):1203–9. doi: 10.1016/j.jaci.2006.12.671
105. Tătăranu E, Diaconescu S, Ivănescu CG, Sârbu I, Stamatin M. Clinical, immunological and pathological profile of infants suffering from cow’s milk protein allergy. Rom J Morphol Embryol. (2016) 57(3):1031–5.
106. Morita Y, Iwakura H, Ohtsuka H, Kohno Y, Shimojo N. Milk allergy in the neonatal intensive care unit: comparison between premature and full-term neonates. Asia Pac Allergy. (2013) 3(1):35–41. doi: 10.5415/apallergy.2013.3.1.35
107. Gaspar-Marques J, Carreiro-Martins P, Papoila AL, Caires I, Pedro C, Araújo-Martins J, et al. Food allergy and anaphylaxis in infants and preschool-age children. Clin Pediatr (Phila). (2014) 53(7):652–7. doi: 10.1177/0009922814527502
108. Chandran U, Demissie K, Echeverria SE, Long JB, Mizan S, Mino J. Food allergy among low birthweight children in a national survey. Matern Child Health J. (2013) 17:165–71. doi: 10.1007/s10995-012-0960-8
109. Huang Z, Gan H, Huang Y, Zhu H, Liu T, Chen T, et al. Risk assessment of allergic diseases among preschool children in Guangzhou, China: a cross-sectional study. J Asthma Allergy. (2023) 16:501–13. doi: 10.2147/JAA.S405318
110. Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: a systematic review. Am J Obstet Gynecol. (2014) 210(4):285–97. doi: 10.1016/j.ajog.2013.09.020
111. Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. J Pediatr. (2019) 210:69–80.e5. doi: 10.1016/j.jpeds.2019.02.041
112. Crispi F, Bijnens B, Figueras F, Bartrons J, Eixarch E, Le Noble F, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation. (2010) 121(22):2427–36. doi: 10.1161/CIRCULATIONAHA.110.937995
113. Hirose A, Khoo NS, Aziz K, Al-Rajaa N, van den Boom J, Savard W, et al. Evolution of left ventricular function in the preterm infant. J Am Soc Echocardiogr. (2015) 28(3):302–8. doi: 10.1016/j.echo.2014.10.017
114. Mohlkert LA, Hallberg J, Broberg O, Rydberg A, Halvorsen CP, Liuba P, et al. The preterm heart in childhood: left ventricular structure, geometry, and function assessed by echocardiography in 6-year-old survivors of periviable births. J Am Heart Assoc. (2018) 7(2):1–9. doi: 10.1161/JAHA.117.007742
115. Goss KN, Haraldsdottir K, Beshish AG, Barton GP, Watson AM, Palta M, et al. Association between preterm birth and arrested cardiac growth in adolescents and young adults. JAMA Cardiol. (2020) 5(8):910–9. doi: 10.1001/jamacardio.2020.1511
116. Yoshida-Montezuma Y, Sivapathasundaram B, Brown HK, Keown-Stoneman C, de Souza RJ, To T, et al. Association of late preterm birth and size for gestational age with cardiometabolic risk in childhood. JAMA Netw Open. (2022) 5(5):e2214379. doi: 10.1001/jamanetworkopen.2022.14379
117. Crump C, Sundquist J, Sundquist K. Association of preterm birth with lipid disorders in early adulthood: a Swedish cohort study. PLoS Med. (2019) 16(10):e1002947. doi: 10.1371/journal.pmed.1002947
118. Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am J Epidemiol. (2011) 173(7):797–803. doi: 10.1093/aje/kwq440
119. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. (2012) 59(2):226–34. doi: 10.1161/HYPERTENSIONAHA.111.181784
120. Hovi P, Vohr B, Ment LR, Doyle LW, McGarvey L, Morrison KM, et al. Blood pressure in young adults born at very low birth weight: adults born preterm international collaboration. Hypertension. (2016) 68(4):880–7. doi: 10.1161/HYPERTENSIONAHA.116.08167
121. Juonala M, Cheung MM, Sabin MA, Burgner D, Skilton MR, Kähönen M, et al. Effect of birth weight on life-course blood pressure levels among children born premature: the cardiovascular risk in young Finns study. J Hypertens. (2015) 33(8):1542–8. doi: 10.1097/HJH.0000000000000612
122. Ligi I, Grandvuillemin I, Andres V, Dignat-George F, Simeoni U. Low birth weight infants and the developmental programming of hypertension: a focus on vascular factors. Semin Perinatol. (2010) 34(3):188–92. doi: 10.1053/j.semperi.2010.02.002
123. Karvonen R, Sipola M, Kiviniemi A, Tikanmäki M, Järvelin M-R, Eriksson JG, et al. Cardiac autonomic function in adults born preterm. J Pediatr. (2019) 208:96–103.e4. doi: 10.1016/j.jpeds.2018.12.061
124. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140(3):1–72. doi: 10.1542/peds.2017-1904
125. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. (2014) 63(5):1116–35. doi: 10.1161/HYP.0000000000000007
126. Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of preterm birth with risk of ischemic heart disease in adulthood. JAMA Pediatr. (2019) 173(8):736–43. doi: 10.1001/jamapediatrics.2019.1327
127. Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia. (2020) 63(3):508–18. doi: 10.1007/s00125-019-05044-z
128. Carr H, Cnattingius S, Granath F, Ludvigsson JF, Edstedt Bonamy AK. Preterm birth and risk of heart failure up to early adulthood. J Am Coll Cardiol. (2017) 69(21):2634–42. doi: 10.1016/j.jacc.2017.03.572
129. Crump C, Groves A, Sundquist J, Sundquist K. Association of preterm birth with long-term risk of heart failure into adulthood. JAMA Pediatr. (2021) 175(7):689–97. doi: 10.1001/jamapediatrics.2021.0131
130. Faa G, Gerosa C, Fanni D, Monga G, Zaffanello M, Van Eyken P, et al. Morphogenesis and molecular mechanisms involved in human kidney development. J Cell Physiol. (2012) 227(3):1257–68. doi: 10.1002/jcp.22985
131. Drougia A, Giapros V, Hotoura E, Papadopoulou F, Argyropoulou M, Andronikou S. The effects of gestational age and growth restriction on compensatory kidney growth. Nephrol Dial Transplant. (2009) 24(1):142–8. doi: 10.1093/ndt/gfn431
132. Huang HP, Tsai IJ, Lai YC, Cheng CH, Tsau YK. Early postnatal renal growth in premature infants. Nephrology (Carlton). (2007) 12(6):572–5. doi: 10.1111/j.1440-1797.2007.00882.x
133. Kandasamy Y, Smith R, Wright IM, Lumbers ER. Extra-uterine renal growth in preterm infants: oligonephropathy and prematurity. Pediatr Nephrol. (2013) 28(9):1791–6. doi: 10.1007/s00467-013-2462-3
134. Schmidt IM, Chellakooty M, Boisen KA, Damgaard IN, Mau Kai C, Olgaard K, et al. Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney Int. (2005) 68(2):731–40. doi: 10.1111/j.1523-1755.2005.00451.x
135. Rodríguez MM, Gómez AH, Abitbol CL, Chandar JJ, Duara S, Zilleruelo GE. Histomorphometric analysis of postnatal glomerulogenesis in extremely preterm infants. Pediatr Dev Pathol. (2004) 7(1):17–25. doi: 10.1007/s10024-003-3029-2
136. Sutherland MR, Gubhaju L, Moore L, Kent AL, Dahlstrom JE, Horne RS, et al. Accelerated maturation and abnormal morphology in the preterm neonatal kidney. J Am Soc Nephrol. (2011) 22(7):1365–74. doi: 10.1681/ASN.2010121266
137. Gubhaju L, Sutherland MR, Black MJ. Preterm birth and the kidney: implications for long-term renal health. Reprod Sci. (2011) 18(4):322–33. doi: 10.1177/1933719111401659
138. Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol. (2009) 297(6):F1668–77. doi: 10.1152/ajprenal.00163.2009
139. Kanbay M, Copur S, Bakir CN, Covic A, Ortiz A, Tuttle KR. Glomerular hyperfiltration as a therapeutic target for CKD. Nephrol Dial Transplant. (2024):gfae027. doi: 10.1093/ndt/gfae027
140. Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens. (1988) 1(4 Pt 1):335–47. doi: 10.1093/ajh/1.4.335
141. Chong E, Yosypiv IV. Developmental programming of hypertension and kidney disease. Int J Nephrol. (2012) 2012:760580. doi: 10.1155/2012/760580
142. Stritzke A, Thomas S, Amin H, Fusch C, Lodha A. Renal consequences of preterm birth. Mol Cell Pediatr. (2017) 4(1):2. doi: 10.1186/s40348-016-0068-0
143. Carmody JB, Charlton JR. Short-term gestation, long-term risk: prematurity and chronic kidney disease. Pediatrics. (2013) 131(6):1168–79. doi: 10.1542/peds.2013-0009
144. Uber AM, Sutherland SM. Nephrotoxins and nephrotoxic acute kidney injury. Pediatr Nephrol. (2020) 35(10):1825–33. doi: 10.1007/s00467-019-04397-2
145. Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. The Lancet Child & Adolescent Health. (2017) 1(3):184–94. doi: 10.1016/S2352-4642(17)30069-X
146. Askenazi DJ, Heagerty PJ, Schmicker RH, Griffin R, Brophy P, Juul SE, et al. Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr Nephrol. (2020) 35:1737–48. doi: 10.1007/s00467-020-04563-x
147. Sangla A, Kandasamy Y. Effects of prematurity on long-term renal health: a systematic review. BMJ Open. (2021) 11(8):e047770. doi: 10.1136/bmjopen-2020-047770
148. Crump C, Sundquist J, Sundquist K. Risk of hypertension into adulthood in persons born prematurely: a national cohort study. Eur Heart J. (2020) 41(16):1542–50. doi: 10.1093/eurheartj/ehz904
149. Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. Br Med J. (2019) 365:l1346. doi: 10.1136/bmj.l1346
150. Harer MW, Pope CF, Conaway MR, Charlton JR. Follow-up of acute kidney injury in neonates during childhood years (FANCY): a prospective cohort study. Pediatr Nephrol. (2017) 32:1067–76. doi: 10.1007/s00467-017-3603-x
151. Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol. (2012) 8(5):265–74. doi: 10.1038/nrneph.2012.38
152. Bonamy AK, Källén K, Norman M. High blood pressure in 2.5-year-old children born extremely preterm. Pediatrics. (2012) 129(5):e1199–204. doi: 10.1542/peds.2011-3177
153. Harer MW, Charlton JR, Tipple TE, Reidy KJ. Preterm birth and neonatal acute kidney injury: implications on adolescent and adult outcomes. J Perinatol. (2020) 40(9):1286–95. doi: 10.1038/s41372-020-0656-7
154. Wickland J, Steven Brown L, Blanco V, Heyne R, Turer C, Rosenfeld CR. Persistent high blood pressure and renal dysfunction in preterm infants during childhood. Pediatr Res. (2023) 93(1):217–25. doi: 10.1038/s41390-022-02083-y
155. Sanderson KR, Chang E, Bjornstad E, Hogan SL, Hu Y, Askenazi D, et al. Albuminuria, hypertension, and reduced kidney volumes in adolescents born extremely premature. Front Pediatr. (2020) 8:230. doi: 10.3389/fped.2020.00230
156. van den Belt SM, Heerspink HJ, Gracchi V, de Zeeuw D, Wühl E, Schaefer F, et al. Early proteinuria lowering by angiotensin-converting enzyme inhibition predicts renal survival in children with CKD. J Am Soc Nephrol. (2018) 29(8):2225–33. doi: 10.1681/ASN.2018010036
157. Soyupak SK, Narli N, Yapicioğlu H, Satar M, Aksungur EH. Sonographic measurements of the liver, spleen and kidney dimensions in the healthy term and preterm newborns. Eur J Radiol. (2002) 43(1):73–8. doi: 10.1016/S0720-048X(01)00466-1
158. Ross ES, Browne JV. Developmental progression of feeding skills: an approach to supporting feeding in preterm infants. Semin Neonatol. (2002) 7(6):469–75. doi: 10.1053/siny.2002.0152
159. Kamity R, Kapavarapu PK, Chandel A. Feeding problems and long-term outcomes in preterm infants—a systematic approach to evaluation and management. Children. (2021) 8(12):1158. doi: 10.3390/children8121158
160. Groer MW, Luciano AA, Dishaw LJ, Ashmeade TL, Miller E, Gilbert JA. Development of the preterm infant gut microbiome: a research priority. Microbiome. (2014) 2:38. doi: 10.1186/2049-2618-2-38
161. Weng M, Walker WA. The role of gut microbiota in programming the immune phenotype. J Dev Orig Health Dis. (2013) 4(3):203–14. doi: 10.1017/S2040174412000712
162. Healy DB, Ryan CA, Ross RP, Stanton C, Dempsey EM. Clinical implications of preterm infant gut microbiome development. Nat Microbiol. (2022) 7(1):22–33. doi: 10.1038/s41564-021-01025-4
163. Ohana O, Wainstock T, Sheiner E, Leibson T, Pariente G. Long-term digestive hospitalizations of premature infants (besides necrotizing enterocolitis): is there a critical threshold? Arch Gynecol Obstet. (2021) 304(2):455–63. doi: 10.1007/s00404-021-06068-w
164. Sonntag B, Stolze B, Heinecke A, Luegering A, Heidemann J, Lebiedz P, et al. Preterm birth but not mode of delivery is associated with an increased risk of developing inflammatory bowel disease later in life. Inflamm Bowel Dis. (2007) 13(11):1385–90. doi: 10.1002/ibd.20206
165. Forssell L, Cnattingius S, Bottai M, Lagergren J, Ekbom A, Akre O. Risk of esophagitis among individuals born preterm or small for gestational age. Clin Gastroenterol Hepatol. (2012) 10(12):1369–75. doi: 10.1016/j.cgh.2012.09.014
166. Dellon ES, Shaheen O, Koutlas NT, Chang AO, Martin LJ, Rothenberg ME, et al. Early life factors are associated with risk for eosinophilic esophagitis diagnosed in adulthood. Dis Esophagus. (2021) 34(2):doaa074. doi: 10.1093/dote/doaa074
167. Boguszewski M, Cardoso-Demartini AA. Management of endocrine disease: growth and growth hormone therapy in short children born preterm. Eur J Endocrinol. (2017) 176(3):R111–22. doi: 10.1530/EJE-16-0482
168. Hollanders JJ, Van Der Pal SM, Van Dommelen P, Rotteveel J, Finken MJ. Growth pattern and final height of very preterm vs. Very low birth weight infants. Pediatr Res. (2017) 82(2):317–23. doi: 10.1038/pr.2017.63
169. Ferguson E, Wright N, Gibson A, Carney S, Wright A, Wales J. Adult height of preterm infants: a longitudinal cohort study. Arch Dis Child. (2017) 102(6):503–8. doi: 10.1136/archdischild-2016-310469
170. Santos IS, Matijasevich A, Domingues MR, Barros AJD, Victora CG, Barros FC. Late preterm birth is a risk factor for growth faltering in early childhood: a cohort study. BMC Pediatr. (2009) 9(1):71. doi: 10.1186/1471-2431-9-71
171. Brown LD, Hay WW. The nutritional dilemma for preterm infants: how to promote neurocognitive development and linear growth, but reduce the risk of obesity. J Pediatr. (2013) 163(6):1543–5. doi: 10.1016/j.jpeds.2013.07.042
172. Ou-Yang M-C, Sun Y, Liebowitz M, Chen C-C, Fang M-L, Dai W, et al. Accelerated weight gain, prematurity, and the risk of childhood obesity: a meta-analysis and systematic review. PLoS One. (2020) 15(5):e0232238. doi: 10.1371/journal.pone.0232238
173. Barker DJ. In utero programming of chronic disease. Clin Sci (Lond). (1998) 95(2):115–28. doi: 10.1042/cs0950115
174. Tosh DN, Fu Q, Callaway CW, McKnight RA, McMillen IC, Ross MG, et al. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. (2010) 299(5):G1023–9. doi: 10.1152/ajpgi.00052.2010
175. Ellis PJ, Morris TJ, Skinner BM, Sargent CA, Vickers MH, Gluckman PD, et al. Thrifty metabolic programming in rats is induced by both maternal undernutrition and postnatal leptin treatment, but masked in the presence of both: implications for models of developmental programming. BMC Genomics. (2014) 15:1–18. doi: 10.1186/1471-2164-15-49
176. Lyons-Reid J, Albert BB, Kenealy T, Cutfield WS. Birth size and rapid infant weight gain—where does the obesity risk lie? J Pediatr. (2021) 230:238–43. doi: 10.1016/j.jpeds.2020.10.078
177. Han J, Jiang Y, Huang J, Zhang Y, Zhang Y, Zhang Y, et al. Postnatal growth of preterm infants during the first two years of life: catch-up growth accompanied by risk of overweight. Ital J Pediatr. (2021) 47:1–9. doi: 10.1186/s13052-020-00935-z
178. Halilagic A, Moschonis G. The effect of growth rate during infancy on the risk of developing obesity in childhood: a systematic literature review. Nutrients. (2021) 13(10):3449. doi: 10.3390/nu13103449
179. Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. (2018) 19(3):321–32. doi: 10.1111/obr.12632
180. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. Br Med J. (2005) 331(7522):929. doi: 10.1136/bmj.38586.411273.E0
181. Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. (2006) 95(8):904–8. doi: 10.1080/08035250600719754
182. Dzulkifli A, Nadhiroh SR, Syahdana AN. Breastfeeding on body composition in premature infants: a systematic review. Amerta Nutrition. (2024) 8(3):496–505. doi: 10.20473/amnt.v8i3.2024.496-505
183. Uwaezuoke SN, Eneh CI, Ndu IK. Relationship between exclusive breastfeeding and lower risk of childhood obesity: a narrative review of published evidence. Clin Med Insights Pediatr. (2017) 11:1179556517690196. doi: 10.1177/1179556517690196
184. Bernal J, Nunez J. Thyroid hormones and brain development. Eur J Endocrinol. (1995) 133(4):390–8. doi: 10.1530/eje.0.1330390
185. Walsh JP. Thyroid function across the lifespan: do age-related changes matter? Endocrinol Metab. (2022) 37(2):208–19. doi: 10.3803/EnM.2022.1463
186. van Wassenaer AG, Kok JH. Hypothyroxinaemia and thyroid function after preterm birth. Semin Neonatol. (2004) 9(1):3–11. doi: 10.1016/S1084-2756(03)00114-3
187. LaFranchi SH. Thyroid function in preterm/low birth weight infants: impact on diagnosis and management of thyroid dysfunction. Front Endocrinol (Lausanne). (2021) 12:666207. doi: 10.3389/fendo.2021.666207
188. Grob F. Approaching the diagnosis of thyroid disorders in preterm infants. Pediatr Res. (2022) 91(5):1021–2. doi: 10.1038/s41390-022-01951-x
189. LaFranchi SH. Thyroid function in preterm/low birth weight infants: impact on diagnosis and management of thyroid dysfunction. Front Endocrinol (Lausanne). (2021) 12:666207. doi: 10.3389/fendo.2021.666207
190. Delange F, Dalhem A, Bourdoux P, Lagasse R, Glinoer D, Fisher DA, et al. Increased risk of primary hypothyroidism in preterm infants. J Pediatr. (1984) 105(3):462–9. doi: 10.1016/S0022-3476(84)80030-X
191. Kim H-R, Jung YH, Choi CW, Chung HR, Kang M-J, Kim BI. Thyroid dysfunction in preterm infants born before 32 gestational weeks. BMC Pediatr. (2019) 19:1–8. doi: 10.1186/s12887-019-1792-0
192. Crump C, Winkleby MA, Sundquist J, Sundquist K. Preterm birth and risk of medically treated hypothyroidism in young adulthood. Clin Endocrinol (Oxf). (2011) 75(2):255–60. doi: 10.1111/j.1365-2265.2011.04034.x
193. Bloomfield FH. Impact of prematurity for pancreatic islet and beta-cell development. J Endocrinol. (2018) 238(3):R161–71. doi: 10.1530/JOE-18-0021
194. Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. (2014) 311(6):587–96. doi: 10.1001/jama.2014.1
195. Khashan AS, Kenny LC, Lundholm C, Kearney PM, Gong T, McNamee R, et al. Gestational age and birth weight and the risk of childhood type 1 diabetes: a population-based cohort and sibling design study. Diabetes Care. (2015) 38(12):2308–15. doi: 10.2337/dc15-0897
196. Goldacre RR. Associations between birthweight, gestational age at birth and subsequent type 1 diabetes in children under 12: a retrospective cohort study in England, 1998–2012. Diabetologia. (2018) 61:616–25. doi: 10.1007/s00125-017-4493-y
197. Haynes A, Bower C, Bulsara MK, Finn J, Jones T, Davis E. Perinatal risk factors for childhood type 1 diabetes in western Australia—a population-based study (1980–2002). Diabetic Med. (2007) 24(5):564–70. doi: 10.1111/j.1464-5491.2007.02149.x
198. Kajantie E, Osmond C, Barker DJ, Eriksson JG. Preterm birth—a risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care. (2010) 33(12):2623–5. doi: 10.2337/dc10-0912
199. Kaijser M, Edstedt Bonamy A-K, Akre O, Cnattingius S, Granath F, Norman M, et al. Perinatal risk factors for diabetes in later life. Diabetes. (2009) 58(3):523–6. doi: 10.2337/db08-0558
200. Lawlor D, Davey Smith G, Clark H, Leon D. The associations of birthweight, gestational age and childhood BMI with type 2 diabetes: findings from the Aberdeen children of the 1950s cohort. Diabetologia. (2006) 49:2614–7. doi: 10.1007/s00125-006-0408-z
201. Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. (2014) 15(10):804–11. doi: 10.1111/obr.12214
202. Patterson JM, Holm KE, Gurney JG. The impact of childhood cancer on the family: a qualitative analysis of strains, resources, and coping behaviors. Psychooncology. (2004) 13(6):390–407. doi: 10.1002/pon.761
203. Roser K, Erdmann F, Michel G, Winther JF, Mader L. The impact of childhood cancer on parents’ socio-economic situation-A systematic review. Psychooncology. (2019) 28(6):1207–26. doi: 10.1002/pon.5088
204. Martini S, Aceti A, Della Gatta AN, Beghetti I, Marsico C, Pilu G, et al. Antenatal and postnatal sequelae of oxidative stress in preterm infants: a narrative review targeting pathophysiological mechanisms. Antioxidants. (2023) 12(2):422. doi: 10.3390/antiox12020422
205. Iyer NP, Baumann A, Rzeszotarski MS, Ferguson RD, Mhanna MJ. Radiation exposure in extremely low birth weight infants during their neonatal intensive care unit stay. World J Pediatr. (2013) 9(2):175–8. doi: 10.1007/s12519-013-0417-1
206. Dixon F, Ziegler DS, Bajuk B, Wright I, Hilder L, Abdel Latif ME, et al. Treatment with nitric oxide in the neonatal intensive care unit is associated with increased risk of childhood cancer. Acta Paediatr. (2018) 107(12):2092–8. doi: 10.1111/apa.14436
207. Seppälä LK, Vettenranta K, Leinonen MK, Tommiska V, Madanat-Harjuoja LM. Preterm birth, neonatal therapies and the risk of childhood cancer. Int J Cancer. (2021) 148(9):2139–47. doi: 10.1002/ijc.33376
208. Paquette K, Coltin H, Boivin A, Amre D, Nuyt AM, Luu TM. Cancer risk in children and young adults born preterm: a systematic review and meta-analysis. PLoS One. (2019) 14(1):e0210366. doi: 10.1371/journal.pone.0210366
209. Heck JE, Meyers TJ, Lombardi C, Park AS, Cockburn M, Reynolds P, et al. Case-control study of birth characteristics and the risk of hepatoblastoma. Cancer Epidemiol. (2013) 37(4):390–5. doi: 10.1016/j.canep.2013.03.004
210. Ikeda H, Matsuyama S, Tanimura M. Association between hepatoblastoma and very low birth weight: a trend or a chance? J Pediatr. (1997) 130(4):557–60. doi: 10.1016/S0022-3476(97)70239-7
211. Spector LG, Puumala SE, Carozza SE, Chow EJ, Fox EE, Horel S, et al. Cancer risk among children with very low birth weights. Pediatrics. (2009) 124(1):96–104. doi: 10.1542/peds.2008-3069
212. Wang YF, Wu LQ, Liu YN, Bi YY, Wang H. Gestational age and childhood leukemia: a meta-analysis of epidemiologic studies. Hematology. (2018) 23(5):253–62. doi: 10.1080/10245332.2017.1396056
213. Huang Q-t, Gao Y-f, Zhong M, Yu Y-h. Preterm birth and subsequent risk of acute childhood leukemia: a meta-analysis of observational studies. Cell Physiol Biochem. (2016) 39(3):1229–38. doi: 10.1159/000447828
214. Mead EC, Wang CA, Phung J, Fu JY, Williams SM, Merialdi M, et al. The role of genetics in preterm birth. Reprod Sci. (2023) 30(12):3410–27. doi: 10.1007/s43032-023-01287-9
215. Jain VG, Monangi N, Zhang G, Muglia LJ. Genetics, epigenetics, and transcriptomics of preterm birth. Am J Reprod Immunol. (2022) 88(4):e13600. doi: 10.1111/aji.13600
216. Zhang G, Feenstra B, Bacelis J, Liu X, Muglia LM, Juodakis J, et al. Genetic associations with gestational duration and spontaneous preterm birth. N Engl J Med. (2017) 377(12):1156–67. doi: 10.1056/NEJMoa1612665
217. Parets SE, Bedient CE, Menon R, Smith AK. Preterm birth and its long-term effects: methylation to mechanisms. Biology (Basel). (2014) 3(3):498–513. doi: 10.3390/biology3030498
218. Cruickshank MN, Oshlack A, Theda C, Davis PG, Martino D, Sheehan P, et al. Analysis of epigenetic changes in survivors of preterm birth reveals the effect of gestational age and evidence for a long term legacy. Genome Med. (2013) 5:1–12. doi: 10.1186/gm500
219. Breton CV, Marsit CJ, Faustman E, Nadeau K, Goodrich JM, Dolinoy DC, et al. Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: the children’s environmental health and disease prevention research center’s epigenetics working group. Environ Health Perspect. (2017) 125(4):511–26. doi: 10.1289/EHP595
220. Tobi E, Slieker R, Luijk R, Dekkers K, Stein A, Xu K, et al. DNA Methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci Adv. (2018) 4:eaao4364. doi: 10.1126/sciadv.aao4364
221. Bhattacharya S, Raja EA, Mirazo ER, Campbell DM, Lee AJ, Norman JE, et al. Inherited predisposition to spontaneous preterm delivery. Obstet Gynecol. (2010) 115(6):1125–33. doi: 10.1097/AOG.0b013e3181dffcdb
222. Koire A, Chu DM, Aagaard K. Family history is a predictor of current preterm birth. Am J Obstet Gynecol MFM. (2021) 3(1):100277. doi: 10.1016/j.ajogmf.2020.100277
223. Boyd HA, Poulsen G, Wohlfahrt J, Murray JC, Feenstra B, Melbye M. Maternal contributions to preterm delivery. Am J Epidemiol. (2009) 170(11):1358–64. doi: 10.1093/aje/kwp324
224. Swamy GK, SkjŠrven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA. (2008) 299(12):1429–36. doi: 10.1001/jama.299.12.1429
225. Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. (2008) 358(16):1700–11. doi: 10.1056/NEJMra0707601
Keywords: preterm birth, neonate, perinatal epidemiology, health outcomes, narrative review
Citation: Gette F, Aziz Ali S, Ho MSP, Richter LL, Chan ES, Yang CL, Kieran E, Mammen C, Roberts A, Kang KT, Wong J, Rassekh SR, Castaldo M, Harris KC, Lee J, Lam CKL, Chan NH, Lisonkova S and Ting JY (2025) Long-term health outcomes of preterm birth: a narrative review. Front. Pediatr. 13:1565897. doi: 10.3389/fped.2025.1565897
Received: 23 January 2025; Accepted: 31 March 2025;
Published: 23 April 2025.
Edited by:
Qiuping Li, Bayi Children's Hospital, ChinaReviewed by:
Astri Maria Lang, Akershus University Hospital, NorwayRochelle Sequeira Gomes, Nemours Children's Hospital, United States
Copyright: © 2025 Gette, Aziz Ali, Ho, Richter, Chan, Yang, Kieran, Mammen, Roberts, Kang, Wong, Rassekh, Castaldo, Harris, Lee, Lam, Chan, Lisonkova and Ting. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph Y. Ting, am9zZXBoLnRpbmdAdWFsYmVydGEuY2E=
†These authors share first authorship
 Faith Gette1,†
Faith Gette1,† Sumera Aziz Ali
Sumera Aziz Ali Lindsay L. Richter
Lindsay L. Richter Edmond S. Chan
Edmond S. Chan Cherry Mammen
Cherry Mammen Jonathan Wong
Jonathan Wong Shahrad R. Rassekh
Shahrad R. Rassekh Michael Castaldo
Michael Castaldo Kevin C. Harris
Kevin C. Harris Sarka Lisonkova
Sarka Lisonkova Joseph Y. Ting
Joseph Y. Ting