- Department of Pediatrics, The First People’s Hospital of Jiashan, Jiashan County, Jiaxing, Zhejiang, China
Background: Kawasaki Disease (KD) is an acute vasculitis primarily affecting children, with intravenous immunoglobulin (IVIG) being the standard treatment and leading to increased risk of coronary artery abnormalities.
Objective: This systematic review and meta-analysis aim to evaluate the prevalence of IVIG resistance in KD and identify potential predictors and outcomes associated with this resistance.
Methods: A comprehensive search of PubMed, Medline, Embase, and other relevant databases was conducted to identify studies reporting IVIG resistance in KD patients. Data on prevalence rates, patient demographics, and associated factors were extracted and analyzed.
Results: The analysis included 26 studies with a total of 46,461 patients. The overall prevalence of IVIG resistance was found to be 14% (95% CI: 12%–16%), and The prevalence among males and females was 9% (95% CI; 7% to 10%) and 4% (95% CI; 3.7% to 4.3%), respectively.
Conclusion: IVIG resistance remains a significant challenge in the management of KD. Identifying patients at higher risk for IVIG resistance and developing alternative treatment strategies are crucial for improving outcomes in this population.
Introduction
Kawasaki Disease (KD) is an acute systemic vasculitis primarily affecting children under the age of five. It is the leading cause of acquired heart disease in children, with coronary artery lesions (CALs) being the most common and life-threatening complication. If left untreated, CALs can lead to severe outcomes such as coronary artery dilatation, aneurysm formation, lumen stenosis, occlusion, and potentially fatal myocardial infarction (1).
KD triggers the release of various inflammatory factors, resulting in a cascade amplification effect. Although KD is a self-limited inflammatory process, it can become life-threatening depending on the extent of cardiac involvement. Diagnosis is based on clinical criteria, including fever, exanthema, conjunctivitis, changes in extremities, erythema of oral mucosa and lips, and cervical lymphadenopathy. Early recognition and treatment are crucial to lower the risk of cardiac complications, the etiology of KD remains unknown, though clinical, laboratory, and epidemiological features suggest an infectious origin or trigger. Immune system activation is a notable feature of KD, with elevated concentrations of proinflammatory cytokines and chemokines being studied for their potential to improve future anti-inflammatory therapies (2).
The standard treatment for KD involves high-dose intravenous immunoglobulin (IVIG) at 2 g/kg combined with aspirin, reducing the incidence of CALs from 20%–25% to 2%–4%. However, initial IVIG treatment fails in 7.5%–26.8% of KD patients, who continue to experience inflammatory reactions and remain at risk for CALs. Severe complications such as Kawasaki disease shock syndrome or macrophage activation syndrome can occur, posing significant threats to patient survival. Thus, in-depth analysis of factors influencing IVIG insensitivity, early prediction, and timely intervention are critical to reducing cardiac damage in IVIG-resistant KD (1). The American Heart Association (AHA) recommends early administration of IVIG to significantly reduce CAL incidence. Yet, up to 20% of KD patients exhibit IVIG resistance, strongly associated with CAL occurrence. Recent literature has focused on identifying predictors for IVIG resistance, aiming to implement additional therapeutic measures to reduce CAL incidence through early diagnosis. Research indicates various predictors, including male sex, high C-reactive protein (CRP) levels, decreased platelet counts, and elevated neutrophil ratios (3).
Therefore, early identification of IVIG resistance in KD patients is vital for initiating intensive treatments such as corticosteroids, potentially benefiting these patients by reducing the risk of severe cardiac complications (4).
This systematic review and meta-analysis seek to determine the prevalence of IVIG resistance in KD, assess the associated risk factors, and provide insights into the potential for early prediction and intervention. Understanding these elements is vital for optimizing treatment strategies and improving the prognosis for children affected by this complex disease.
Method
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy
A comprehensive search was conducted in the following electronic databases: PubMed, Cochrane Library, Embase, Web of Science, Google Scholar, Semantic Scholar, and ResearchRabbit from January 2008 to October 31, 2024. The search terms (Mesh) included (“Kawasaki Disease” AND “IVIG resistance”) AND (prevalence OR incidence). Additional relevant articles were obtained by searching the reference lists of the articles. The search was restricted to articles published in English.
Inclusion criteria (PIOS)
Population: Children diagnosed with Kawasaki disease.
Intervention: Initial treatment with intravenous immunoglobulin (IVIG).
Outcomes: Studies reporting the prevalence of IVIG resistance.
Study Design: Cohort studies published in peer-reviewed journals.
Exclusion criteria
Studies were excluded from the review if they were non-original research (e.g., case reports), lacked sufficient data on IVIG resistance prevalence, involved non-cohort designs, included adult populations or mixed-age groups without separate pediatric data, used treatments other than standard-dose IVIG, were not published in English, or represented duplicate data from the same patient population.
Data extraction
A standardized data extraction form was developed to systematically collect relevant information from each included study. Extracted data included study characteristics such as first author, publication year, sample size, participant demographics (including age), Kawasaki disease type, laboratory parameters, and reported prevalence of IVIG resistance.
Statistical analysis
The meta-analysis was conducted using STATA Ver. 17 and the Dichotomous outcomes were analyzed using risk ratios (OR) with 95% confidence intervals (CI), and continuous outcomes were analyzed using standardized mean differences (SMD) with 95% CI.
The random or fixed effect models were used to pool the data. The Q test and I2 index were used to assess the heterogeneity of the studies. If the I2 value was greater than 50%, the heterogeneity was considered substantial, and the sources of heterogeneity were explored (5). The random or fixed effect models were used to pool the data. A funnel plot was used to assess the publication bias. Meta-regression and the Eger test were used to explore the significance of publication bias (6).
Results
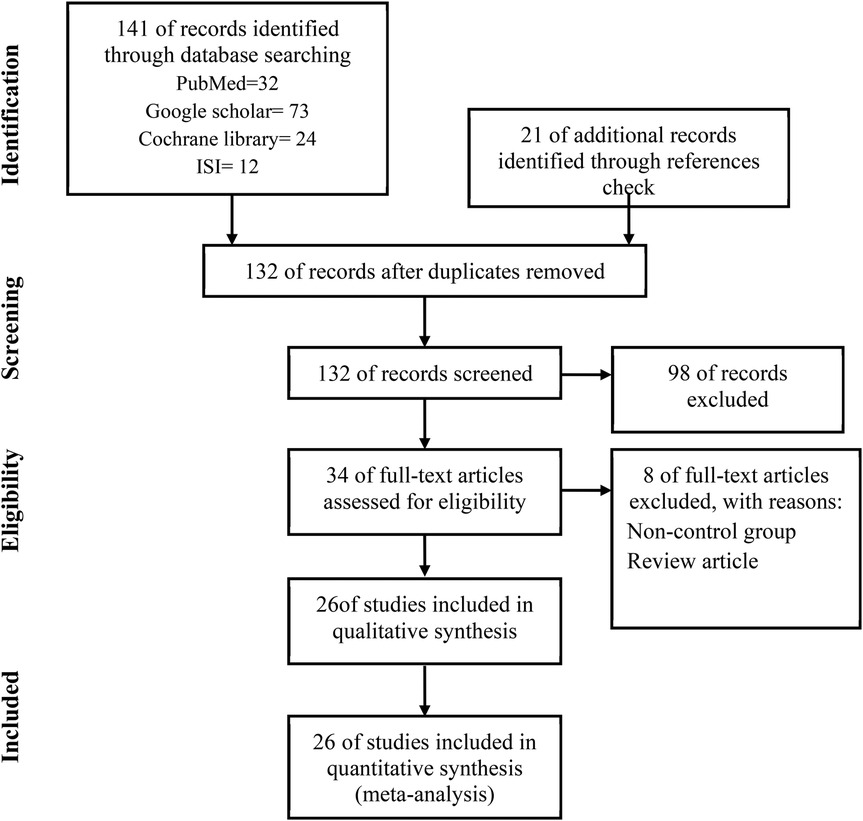
141 related studies were found by searching in the databases, 32 studies in PubMed, 73 in Google Scholar, 24 studies in Cochrane, and 12 studies in Web of Sciences. 21 studies were found by checking the references, after removing 30 duplicate studies, 132 articles were screened and reviewed. Out of 132 studies, 98 studies were excluded based on non-relevant titles or abstracts, 8 studies were excluded for the following reasons:
No original research, insufficient data, and no relevant outcomes. A total of 26 articles met our inclusion criteria and were finally included in this meta-analysis (Figure 1).
Characteristics of included studies
In 26 studies, the sample size was 46,461 people. While most studies included both complete and incomplete KD, a few explicitly reported data on atypical KD forms, which appear to be associated with a similar or potentially higher risk of IVIG resistance (4, 12). The general characteristics of the studies included in the meta-analysis are given in Table 1.
Outcome
Prevalence rates
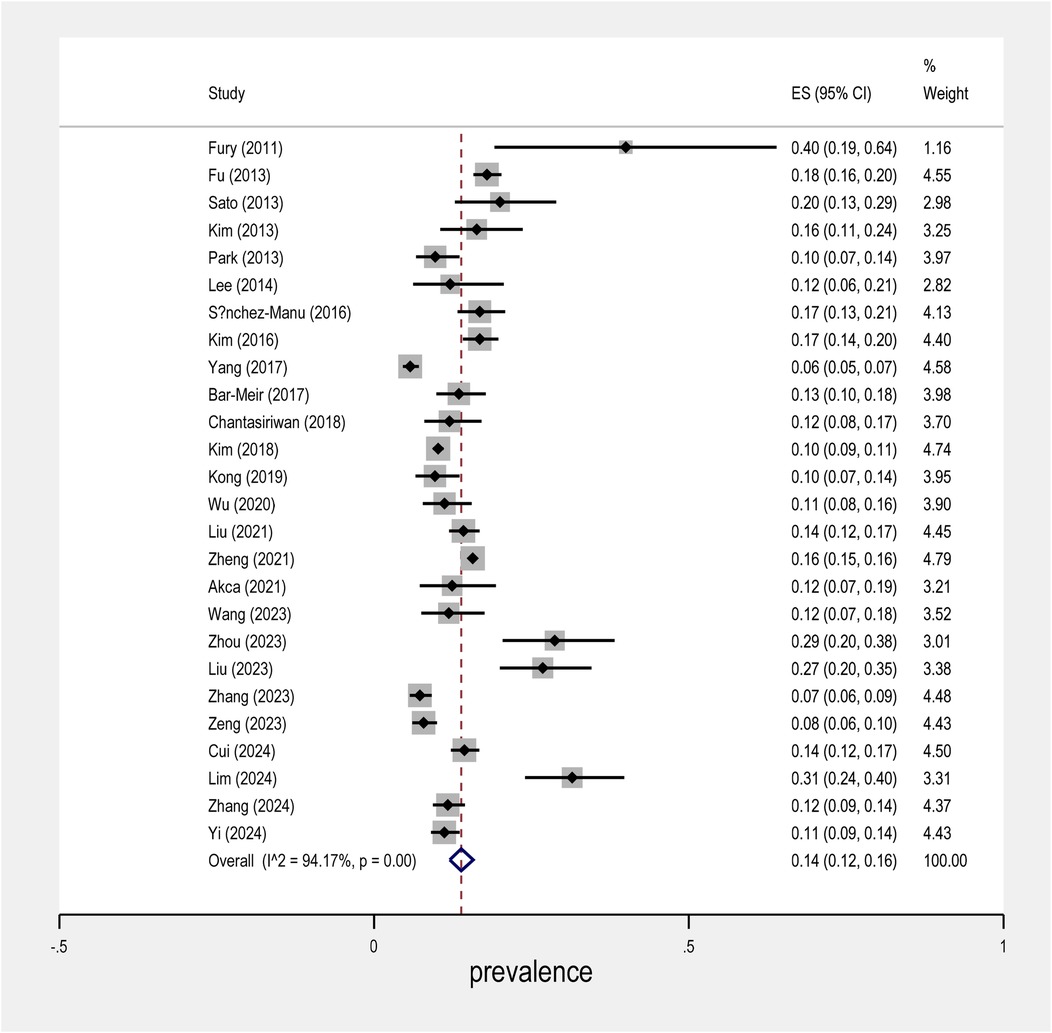
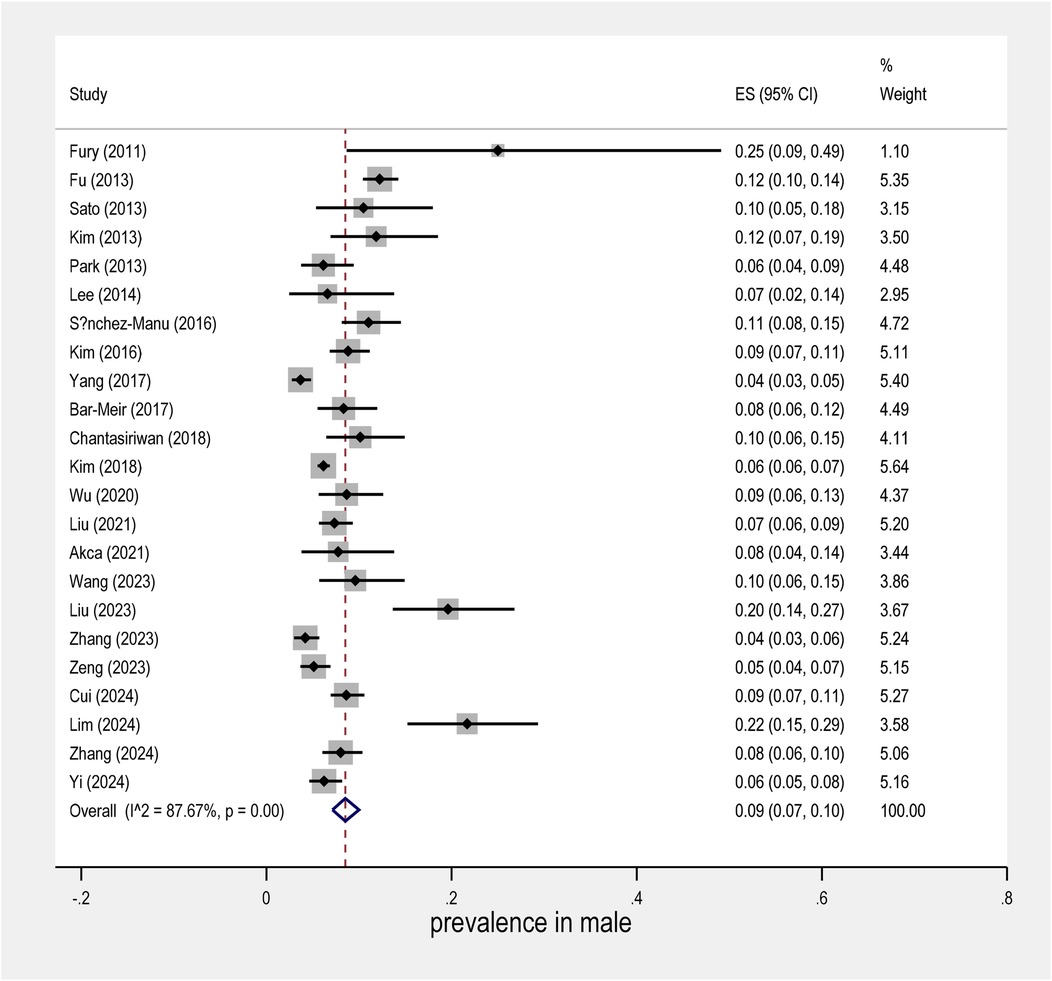
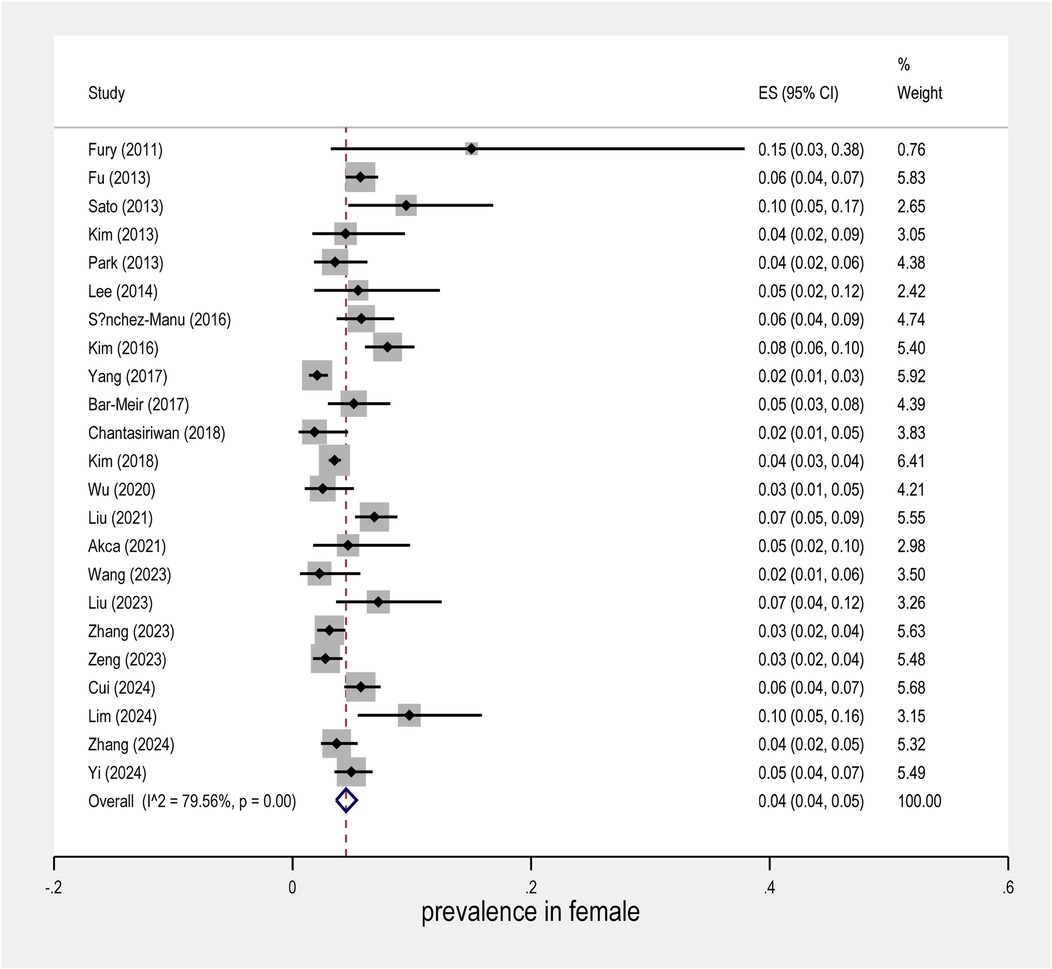
The meta-analysis conducted on the prevalence of intravenous immunoglobulin (IVIG) resistance in patients with Kawasaki Disease (KD) yielded important findings. The overall prevalence of IVIG resistance was found to be approximately 14%, with a 95% confidence interval (CI) ranging from 12% to 16% (Figure 2). This suggests that roughly one in seven KD patients may experience resistance to IVIG treatment. When examining the data by sex, it was observed that males had a prevalence rate of 9% (95% CI: 7% to 10%), as shown in Figure 3, while females exhibited a lower prevalence rate of 4% (95% CI: 4% to 5%) in Figure 4.
In terms of geographical variability, the study by Yang et al. reported the lowest prevalence of IVIG resistance, at only 6% (95% CI: 5% to 5%), suggesting that the occurrence of resistance may vary across different populations and clinical settings. In contrast, the study by Fury et al. showed a much higher rate of IVIG resistance, reaching over 40%. This marked difference highlights the influence of regional factors and the need for further investigation into the underlying causes of such disparities.
Further research is needed to fully understand the mechanisms behind IVIG resistance in KD and how best to tailor treatments to individual patients based on their risk profiles.
Overall, this meta-analysis underscores the complex nature of IVIG resistance in KD and calls for continued investigation into its prevalence, risk factors, and potential interventions.
Risk Factors and Predictors Across studies, predictors included:
High CRP and neutrophil ratio
Low albumin and platelet count
Elevated liver enzymes and bilirubin
Use of predictive scores (e.g., Egami, Kobayashi) with varied geographic accuracy.
Publication bias
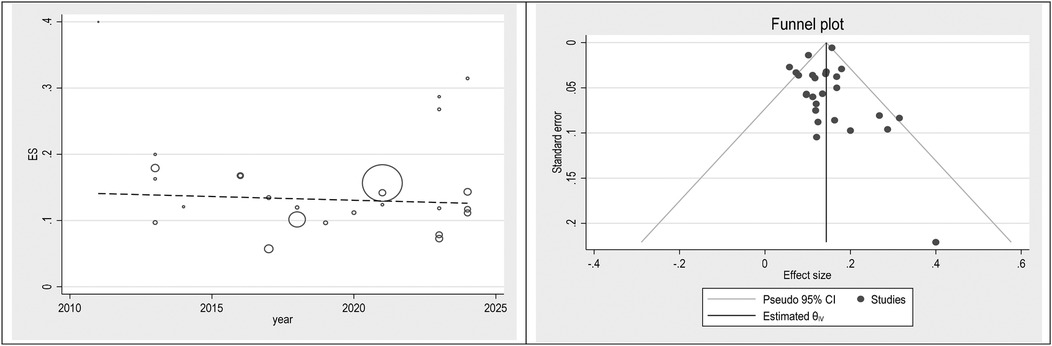
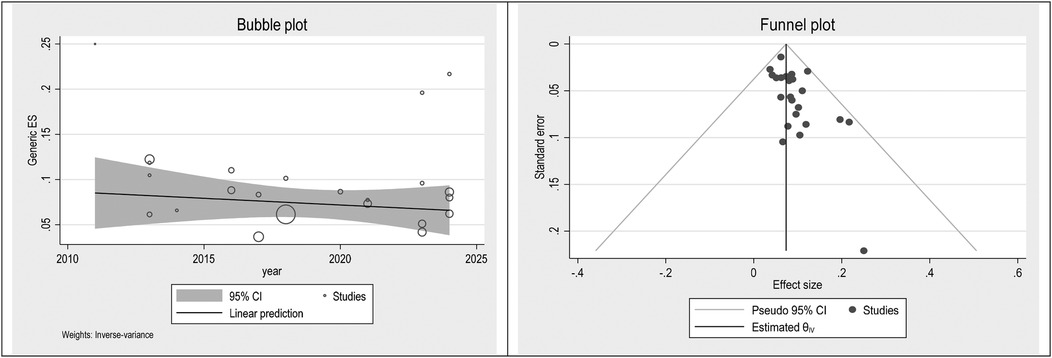
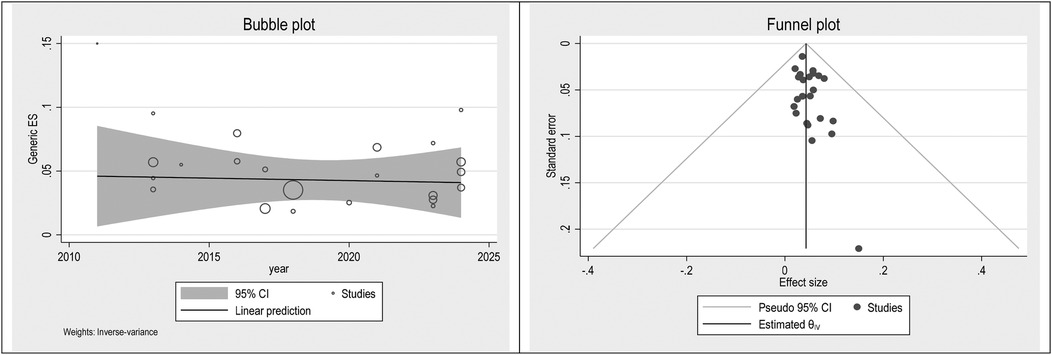
To check publication bias and small study, an effect funnel plot was used it demonstrated that the standard error of the majority of studies was low so the precision of included studies was high. bubble plot helps summarize the data in a way that highlights the relationships between multiple variables, the distribution of those relationships, and potential areas for deeper analysis. Figures 5–7 showed the effect of publication bias was not significant.
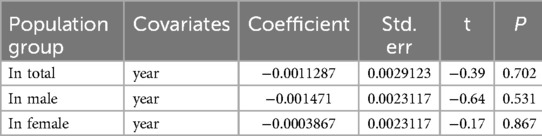
To explore source of heterogeneity and effect of year of publication on effect size multivariate meta-regression was applied, the results of meta regression showed that there were not significant association between year of publication and effects size (Table 2).
Discussion
The findings of this systematic review and meta-analysis provide a comprehensive understanding of the prevalence of intravenous immunoglobulin (IVIG) resistance in Kawasaki Disease (KD), a critical challenge in managing this pediatric vasculitis. Our analysis indicates that a significant proportion of KD patients do not respond to initial IVIG treatment, with prevalence rates ranging from 7.5% to 26.8% across various studies. This variability underscores the complexity of IVIG resistance and the need for tailored approaches in clinical practice. The high prevalence of IVIG resistance observed in KD patients has significant implications for clinical management. As CALs are the most severe complication associated with KD, timely and effective treatment is crucial to prevent long-term cardiac sequelae. Our findings align with previous studies that highlight the importance of early diagnosis and intervention to mitigate the risk of CALs (29, 30). Furthermore, understanding the factors contributing to IVIG resistance can guide the development of predictive models and personalized treatment strategies. Recent research has identified several predictors of IVIG resistance, including male gender, elevated C-reactive protein (CRP) levels, decreased platelet counts, and higher neutrophil ratios (31). These predictors can help clinicians identify patients at higher risk of resistance and consider alternative or adjunctive therapies, such as corticosteroids, in conjunction with IVIG (32).
Some studies also differentiated between complete, incomplete, and atypical KD, all of which are associated with IVIG resistance (4, 12). Several validated scoring systems have been developed to predict IVIG resistance in KD patients, including the Egami, Kobayashi, and Sano scores. These scores incorporate clinical and laboratory variables such as age, ALT levels, CRP, and neutrophil counts to stratify patients by risk. For example, the Kobayashi score includes seven variables and has been widely used in Japanese populations (11, 31). While these scoring systems show good sensitivity in Asian populations, their performance may vary in other regions (2). For patients resistant to initial IVIG therapy, second-line treatments are crucial. These may include corticosteroids, infliximab, or cyclosporine. Several studies in our review [e.g., Wu et al. (9); Cui et al. (4)] mentioned the use of adjunctive treatments, particularly corticosteroids, in refractory cases. According to AHA guidelines, early intensification of therapy in high-risk cases is recommended to reduce coronary complications (32).
The integration of such predictive models into clinical practice could enhance the precision of treatment plans and improve patient outcomes. The pathogenesis of KD and the mechanisms underlying IVIG resistance remain areas of active investigation. Further research into the molecular mechanisms driving IVIG resistance could uncover new therapeutic targets and improve the effectiveness of existing treatments. For instance, studies have suggested that genetic factors may influence an individual's response to IVIG, highlighting the potential for genetic screening in the future (33).
In addition to clinical and molecular research, epidemiological studies are essential to understand the broader impact of IVIG resistance in KD. Variations in resistance prevalence across different populations and regions suggest that environmental and genetic factors may play a role. This meta-analysis provides a global perspective on IVIG resistance, emphasizing the need for international collaboration in KD research.
In conclusion, this systematic review and meta-analysis have elucidated the prevalence and significant risk factors associated with IVIG resistance in KD. These findings emphasize the critical need for early identification and personalized treatment approaches to mitigate the risk of severe cardiac complications. Future research should continue to explore the molecular and genetic underpinnings of IVIG resistance, ultimately aiming to enhance therapeutic strategies and improve patient outcomes in KD.
Limitations
This review is subject to limitations inherent in meta-analyses, including publication bias and variability in study methodologies. Additionally, the heterogeneity among populations studied may affect the generalizability of findings.
Conclusion
The prevalence of IVIG resistance in Kawasaki disease remains a critical concern, affecting treatment outcomes and long-term cardiovascular health. Future research should focus on refining predictive scoring systems and exploring alternative treatment options for resistant cases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang S, Ding C, Zhang Q, Hou M, Chen Y, Huang H, et al. A novel model for predicting intravenous immunoglobulin-resistance in Kawasaki disease: a large cohort study. Front Cardiovasc Med. (2023) 10:1226592. doi: 10.3389/fcvm.2023.1226592
2. Sánchez-Manubens J, Antón J, Bou R, Iglesias E, Calzada-Hernandez J, Borlan S, et al. Role of the Egami score to predict immunoglobulin resistance in Kawasaki disease among a Western Mediterranean population. Rheumatol Int. (2016) 36:905–10. doi: 10.1007/s00296-016-3499-y
3. Zheng X, Li J, Yue P, Liu L, Li J, Zhou K, et al. Is there an association between intravenous immunoglobulin resistance and coronary artery lesion in Kawasaki disease?—current evidence based on a meta-analysis. PLoS One. (2021) 16(3):e0248812. doi: 10.1371/journal.pone.0248812
4. Cui Y, Zhang L, Liu X, Liu L, Zhou K, Hua Y, et al. The predictive value of fibrinogen-to-albumin ratio for predicting intravenous immunoglobulin resistance in Kawasaki disease: a prospective cohort study. Rev Cardiovasc Med. (2024) 25(11):421. doi: 10.31083/j.rcm2511421
5. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: q statistic or I² index? Psychol Methods. (2006) 11(2):193. doi: 10.1037/1082-989X.11.2.193
6. Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis. Publication bias in meta-analysis: Prevention, assessment and adjustments. (2005) 99–110.
7. Liu X, Shao S, Wang L, Zhang N, Wu M, Liu L, et al. Predictive value of the systemic immune-inflammation index for intravenous immunoglobulin resistance and cardiovascular complications in Kawasaki disease. Front Cardiovasc Med. (2021) 8:711007. doi: 10.3389/fcvm.2021.711007
8. Zhou Y, Wu Y, Yuan C, Yin W, Wang B, Ding Y. The expression of autophagy markers in IVIG-resistant Kawasaki disease and the establishment of prediction model. BMC Pediatr. (2023) 23(1):642. doi: 10.1186/s12887-023-04386-3
9. Wu S, Liao Y, Sun Y, Zhang C-Y, Zhang Q-Y, Yan H, et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in children. World J Pediatr. (2020) 16:607–13. doi: 10.1007/s12519-020-00348-2
10. Liu J, Ye B, Su D, Qin S, Zhao W, Pang Y. Evaluation of laboratory predictors for intravenous immunoglobulin resistance and coronary artery aneurysm in Kawasaki disease before and after therapy. Clin Rheumatol. (2023) 42(1):167–77. doi: 10.1007/s10067-022-06366-x
11. Lim YT, Kwon JE, Kim YH. Evaluating the performance of egami, kobayashi and sano scores in predicting IVIG resistance in infant kawasaki disease. BMC Pediatr. (2024) 24(1):606. doi: 10.1186/s12887-024-05035-z
12. Zhang R, Shuai S, Zhang H, Cai J, Cui N, Tang M, et al. Predictive value of albumin for intravenous immunoglobulin resistance in a large cohort of Kawasaki disease patients. Ital J Pediatr. (2023) 49(1):78. doi: 10.1186/s13052-023-01482-z
13. Zhang H, Cai J, Zhang R, Shuai S, Tang M, Ju R, et al. The role of serum lipid in predicting coronary artery lesions and intravenous immunoglobulin resistance in Kawasaki disease: a cohort study. J Int Med Res. (2024) 52(5):03000605241252115. doi: 10.1177/03000605241252115
14. Kong W-X, Ma F-Y, Fu S-L, Wang W, Xie C-H, Zhang Y-Y, et al. Biomarkers of intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. World J Pediatr. (2019) 15:168–75. doi: 10.1007/s12519-019-00234-6
15. Chantasiriwan N, Silvilairat S, Makonkawkeyoon K, Pongprot Y, Sittiwangkul R. Predictors of intravenous immunoglobulin resistance and coronary artery aneurysm in patients with Kawasaki disease. Paediatr Int Child Health. (2018) 38(3):209–12. doi: 10.1080/20469047.2018.1471381
16. Kaya Akca U, Arslanoglu Aydin E, Aykan HH, Serin O, Sag E, Demir S, et al. Comparison of IVIG resistance predictive models in Kawasaki disease. Pediatr Res. (2022) 91(3):621–6. doi: 10.1038/s41390-021-01459-w
17. Lee SM, Lee JB, Go YB, Song HY, Lee BJ, Kwak JH. Prediction of resistance to standard intravenous immunoglobulin therapy in Kawasaki disease. Korean Circ J. (2014) 44(6):415–22. doi: 10.4070/kcj.2014.44.6.415
18. Fury W, Tremoulet AH, Watson VE, Best BM, Shimizu C, Hamilton J, et al. Transcript abundance patterns in Kawasaki disease patients with intravenous immunoglobulin resistance. Hum Immunol. (2010) 71(9):865–73. doi: 10.1016/j.humimm.2010.06.008
19. Fu P-P, Du Z-D, Pan Y-S. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J. (2013) 32(8):e319–e23. doi: 10.1097/INF.0b013e31828e887f
20. Sato S, Kawashima H, Kashiwagi Y, Hoshika A. Inflammatory cytokines as predictors of resistance to intravenous immunoglobulin therapy in Kawasaki disease patients. Int J Rheum Dis. (2013) 16(2):168–72. doi: 10.1111/1756-185X.12082
21. Yang S, Song R, Zhang J, Li X, Li C. Predictive tool for intravenous immunoglobulin resistance of Kawasaki disease in Beijing. Arch Dis Child. (2019) 104(3):262–7. doi: 10.1136/archdischild-2017-314512
22. Zeng Y, Chen F, Xu K-K, Ji L-F, Yang S-W. Comparison of different risk scoring systems for predicting intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. (2023).
23. Kim BY, Kim D, Kim YH, Ryoo E, Sun YH, Jeon I-S, et al. Non-responders to intravenous immunoglobulin and coronary artery dilatation in Kawasaki disease: predictive parameters in Korean children. Korean Circ J. (2016) 46(4):542–9. doi: 10.4070/kcj.2016.46.4.542
24. Kim MK, Song MS, Kim GB. Factors predicting resistance to intravenous immunoglobulin treatment and coronary artery lesion in patients with Kawasaki disease: analysis of the Korean nationwide multicenter survey from 2012 to 2014. Korean Circ J. (2018) 48(1):71–9. doi: 10.4070/kcj.2017.0136
25. Kim SY, Han MY, Cha S-H, Jeon YB. N-terminal pro-brain natriuretic peptide (NT proBNP) as a predictive indicator of initial intravenous immunoglobulin treatment failure in children with Kawasaki disease: a retrospective study. Pediatr Cardiol. (2013) 34:1837–43. doi: 10.1007/s00246-013-0724-2
26. Park HM, Lee DW, Hyun MC, Lee SB. Predictors of nonresponse to intravenous immunoglobulin therapy in Kawasaki disease. Korean J Pediatr. (2013) 56(2):75. doi: 10.3345/kjp.2013.56.2.75
27. Bar-Meir M, Kalisky I, Schwartz A, Somekh E, Tasher D, Group IK. Prediction of resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatric Infect Dis Soc. (2018) 7(1):25–9. doi: 10.1093/jpids/piw075
28. Yi C, Zhou Y-N, Guo J, Chen J, She X. Novel predictors of intravenous immunoglobulin resistance in patients with Kawasaki disease: a retrospective study. Front Immunol. (2024) 15:1399150. doi: 10.3389/fimmu.2024.1399150
29. Burns JC, Glodé MP. Kawasaki syndrome. Lancet. (2004) 364(9433):533–44. doi: 10.1016/S0140-6736(04)16814-1
30. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. (1991) 324(23):1633–9. doi: 10.1056/NEJM199106063242305
31. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. (2006) 113(22):2606–12. doi: 10.1161/CIRCULATIONAHA.105.592865
32. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135(17):e927–e99. doi: 10.1161/CIR.0000000000000484
Keywords: Kawasaki disease, IVIG resistance, prevalence, risk factors, randomized controlled trials, clinical outcomes
Citation: Zou S and Hu B (2025) Prevalence of IVIG resistance in Kawasaki disease: a systematic review and meta-analysis. Front. Pediatr. 13:1566590. doi: 10.3389/fped.2025.1566590
Received: 25 January 2025; Accepted: 16 June 2025;
Published: 4 July 2025.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TürkiyeReviewed by:
Figen Çakmak, Istanbul University, TürkiyeYusuf Ziya Varli, Kocaeli University, Türkiye
Copyright: © 2025 Zou and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuting Zou, em91c2h1dGluZzE2M0BvdXRsb29rLmNvbQ==
 Shuting Zou
Shuting Zou Bingyu Hu
Bingyu Hu