- 1Data Center, Wuhan Children's Hospital (Wuhan Maternal and Child Health Care Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Institute of Maternal and Child Health, Wuhan Children's Hospital (Wuhan Maternal and Child Health Care Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Department of Obstetrics, Wuhan Children's Hospital (Wuhan Maternal and Child Health Care Hospital), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 4Department of Maternal and Child Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Objective: Mitochondrial DNA copy number (mtDNAcn), an indicator of mitochondrial damage and dysfunction, is widely used in research related to growth and metabolic health. While fetal intrauterine growth has been reported to impact further metabolic health, there is limited evidence regarding the relationship between fetal growth patterns and newborn mtDNAcn, especially in infants with normal birth weights, where varying fetal growth patterns can occur despite having the same birth weight. Therefore, this study aimed to examine the association between fetal growth trajectory and neonatal mtDNAcn among normal birth weight infants.
Methods: A total of 556 mother–infant pairs from a birth cohort in Wuhan, China, were included in the study. Ultrasound measurements (biparietal diameter, head circumference, abdominal circumference, and femoral length) were taken at 16, 24, 30, and 37 weeks of pregnancy and converted to Z-scores per WHO standards, and the fetal growth trajectory was fitted by the group-based multi-trajectory model. Cord blood was collected at birth, and mtDNAcn in cord blood was quantified via real-time fluorescent quantitative PCR. A generalized linear model was used to explore the associations of fetal growth pattern or birth weight with neonatal mtDNAcn.
Results: Three distinct patterns of fetal growth trajectory were identified, namely, “consistently low” (n = 144, 25.9%), “moderate” (n = 304, 54.7%), and “high-falling” (n = 108, 19.4%). Compared with the “moderate” intrauterine growth pattern, the “consistently low” intrauterine growth pattern was associated with lower neonatal mtDNAcn among male newborns, with a reduction of 22.55% (95% CI: −39.19%, −1.37%; p = 0.039). No significant association was detected between the intrauterine growth pattern and mtDNAcn among girls.
Conclusions: Our findings indicate that different intrauterine growth patterns are present in fetuses with normal birth weights. In male infants, the “consistently low” intrauterine trajectory pattern was associated with decreased neonatal mtDNAcn. The effective detection of and intervention in fetal intrauterine growth patterns may help prevent metabolic health events early in life.
1 Introduction
Birth weight is widely recognized as a key indicator of fetal intrauterine growth and has been established as an important marker for assessing postnatal health risks (1). Importantly, however, infants with the same birth weight may have different fetal growth trajectories (2, 3). Relying solely on a single measurement at birth may not fully capture the complexity of fetal growth trajectories and their implications for research findings (2). Epidemiological studies have demonstrated that inadequate fetal growth and development not only impact health at birth but also increase susceptibility to multiple metabolic health-related diseases later in life (4, 5). Serial ultrasound examinations conducted throughout pregnancy are vital for ensuring accurate gestational age determination while evaluating fetal growth (6). These examinations are gradually being used to study dynamic fetal growth patterns alongside their associated health outcomes.
Mitochondria are crucial organelles in eukaryotic cells, play pivotal roles in energy supply and cell proliferation, and are closely associated with intrauterine and postnatal growth and metabolic health (7–9). Furthermore, mitochondria are a significant source of intracellular reactive oxygen species (ROS) and are particularly vulnerable to oxidative stress (10, 11). Owing to the absence of protective histones and an efficient DNA repair system, mitochondrial DNA (mtDNA) is highly susceptible to oxidative damage and has a high mutation rate (10). Mitochondria counteract these mutations by increasing the rate and quantity of mitochondrial gene duplication. However, when the extent of damage exceeds the compensatory capacity, it ultimately leads to a decline in mtDNA content (9, 10). Consequently, alterations in mtDNA content can serve as indicators of mitochondrial impairment and dysfunction. Recently, DNA copy number (mtDNAcn), an indicator of mitochondrial DNA content, has been extensively utilized in clinical and epidemiological studies (11, 12). While a few studies (13–19) have reported an association between birth weight and cord blood or placental mtDNAcn, these studies had small sample sizes and inconsistent findings. Furthermore, these studies focused primarily on infants with low birth weights or intrauterine growth restrictions rather than those with normal birth weights, who constitute the majority of new-borns.
On the basis of a birth cohort, this study aimed to identify the intrauterine fetal growth trajectory pattern on the basis of routine ultrasound parameters and to explore the associations between different patterns and mtDNAcn in the cord blood of newborns.
2 Methods
2.1 Study design and population
All participants were recruited at Wuhan Children's Hospital (Wuhan Maternal and Child Health Hospital). Between July 2014 and October 2015, a total of 565 participants met the inclusion criteria for this study: (1) singleton pregnancy and recruitment at less than 16 weeks of gestation; (2) prenatal care, including at least three ultrasound examinations at the study hospital; and (3) term delivery (37–41 weeks) at the study hospital with umbilical cord blood donation. Participants were excluded if they had a diagnosis of low birth weight (<2,500 g), intrauterine growth retardation (IUGR, defined as birth weight < 3rd percentile) (n = 6), or poor-quality cord blood testing (n = 3). Finally, 556 participants were included in the analysis. Of these, 290 (52.2%) completed all four scheduled ultrasound examinations (16, 24, 30, and 37 weeks). Missing data patterns were as follows: 92 (16.5%) missed the 16-week measurement, 69 (12.4%) missed the 24-week measurement, 97 (17.4%) missed the 30-week measurement, and 8 (1.4%) missed the 37-week measurement.
Informed consent was obtained from each participant at the time of recruitment into the study. The research protocol was approved by the ethics committees of Wuhan Children's Hospital (Wuhan Maternal and Child Healthcare Hospital) and Tongji Medical College, Huazhong University of Science and Technology (approval number: 2010009).
2.2 Fetal ultrasound measurements during pregnancy
Each participant typically underwent ultrasound examinations conducted by a professional ultrasound doctor at approximately 16 (range, 13–18) weeks, 24 (range, 21–25) weeks, 30 (range, 28–32) weeks, and 37 (range, 36–40) weeks of gestation. The fetal ultrasound parameters measured included the biparietal diameter (BPD), head circumference (HC), abdominal circumference (AC), and femur length (FL). The gestational age was calculated on the basis of the last menstrual period (LMP). If the gestational age determined by ultrasound differed from that based on the LMP by more than 7 days, the ultrasound-corrected gestational age was used.
2.3 Assessment of mtDNA content
Umbilical cord blood was collected immediately after delivery via ethylenediaminetetraacetic acid (EDTA) tubes. The samples were centrifuged to separate the plasma and blood cells, which were then frozen at −80°C for further analysis. DNA extraction from umbilical cord blood leukocytes was performed via a Wizard® Genomic DNA Purification Kit (Promega Corporation, Madison, WI, USA). The concentration and purity of the DNA samples were assessed via a NanoDrop™ 1,000 Spectrophotometer (Thermo Fisher Scientific, USA), with a standard A260/A280 ratio of 1.8–2.0.
The relative content of mtDNA in umbilical cord blood was determined via real-time quantitative polymerase chain reaction (qPCR) as described in a published study with some modifications (20). The relative content of mtDNA was calculated by dividing the mitochondrial gene copy number (ND1) by the single-copy nuclear control gene [human β-globulin (hbg)]. qPCR was performed on a 384-well plate using the ViiA™ 7 Dx Real-Time PCR System (Applied Biosystems, USA). The sequences of the primers used were as follows: forward primer ND1-F, 5′-CCCTAAAACCCGCCACATCT-3′; reverse primer ND1-R, 5′-GAGCGATGGTGAGAGCTAAGGT-3′; forward primer hbg-f, 5′-GTGCACCTGACTCCTGAGGAGA-3′; reverse primer hbg-r, 5′-CCTTGATACCAACCTGCCCAG-3′. Each reaction mixture, totaling 10 μl, was composed of 5.0 μl of KAPA SYBR® FAST qPCR Kit Master MIX 2×, 1.0 μl of 10 ng/μl genomic DNA, 0.2 μl of 10 μM ND1-F (hbg-f), 0.2 μl of 10 μM ND1-R (hbg-r), and 3.6 μl of RNase-free water. Each sample was tested in triplicate, and standard deviations for the cycle threshold (Ct) value were maintained at or below 0.30. The measurements were repeated if the coefficient of variation (CV) of the Ct value exceeded 0.30.
The amplification conditions were as follows: a prerun step of 50°C for 2 min and 95°C for 3 min to activate DNA polymerase, followed by 40 cycles of denaturation at 95°C for 3 s and annealing/extension at 60°C for 30 s. Melt curve analysis was conducted at the end of each run to confirm amplification specificity. To generate the standard curve, reference DNA derived from a pooled sample of 50 randomly selected samples was used, with five-point DNA concentrations ranging from 0.4 ng/μl to 104 ng/μl achieving a coefficient of determination (R2) > 0.99. All the samples were tested in triplicate, and the coefficients of variation (CVs) for interplate and intraplate values calculated from the Ct values of the single-copy genes were 3.8% and 2.8%, respectively.
2.4 Covariates
During recruitment, participants completed a structured questionnaire, including demographic information, lifestyle information, obstetric history, and health information. Information regarding gestational diabetes mellitus (GDM), pregnancy-induced hypertension (PIH), and newborn birth was extracted from hospital electronic medical records. Pre-pregnancy BMI (kg/m2) was calculated as pre-pregnancy weight (kg) divided by the square of pre-pregnancy height (m2). Maternal passive smoking during pregnancy was self-reported and was defined as exposure to passive smoking during the six months before pregnancy. The covariates analyzed in this study included maternal age (years), pre-pregnancy BMI (kg/m2), education level (college or above/high school/junior high school or below), parity (nulliparous/multiparous), GDM (yes/no), PIH (yes/no), passive smoking during pregnancy (yes/no), newborn sex and gestational age at birth.
2.5 Statistical analysis
Group-based multi-trajectory modelling (GBMTM), a specialized form of finite mixture modeling, is designed to identify subgroups of individuals with similar trajectories of the measure of interest in multivariate longitudinal data (21, 22), which has been increasingly used in clinical studies in recent years (22), including identifying fetal growth trajectories (23, 24). In this study, the GBMTM was applied to identify subgroups of participants with similar trajectories of fetal ultrasound measurements, which can capture the correlations and potential synergistic effects of BPD, HC, AC, and FL. According to World Health Organization (WHO) standards (25), all fetal ultrasound parameters are converted to Z-scores on the basis of gestational age at the time of measurement and then fitted to the multi-trajectory model. The optimal number of trajectory subgroups and trajectory morphology were satisfied according to the following criteria (21, 22, 26): (1) smaller value in the Akaike information criterion and Bayesian information criterion; (2) each trajectory group comprising more than 5% of the total sample size; (3) average posterior probability for each trajectory group exceeding 0.7; and (4) interpretability of the trajectory.
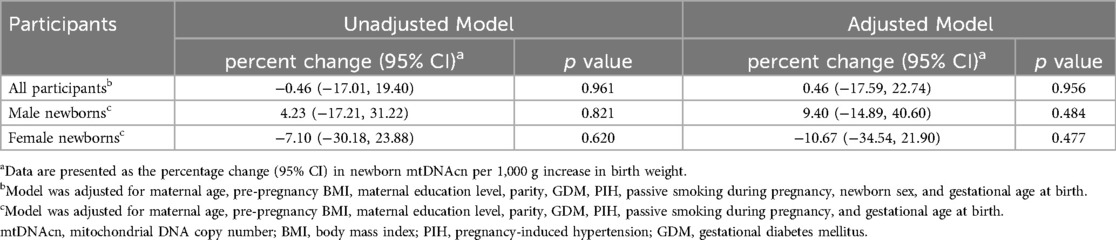
Continuous variables with a normal distribution are expressed as the means ± standard deviations (SDs) and were compared via t tests. Continuous variables with a skewed distribution are expressed as medians (interquartile ranges, IQRs) and were compared via the Wilcoxon rank sum test. Categorical variables are expressed as frequencies (percentages) and were compared via chi-square tests or Fisher's exact tests. Analysis of variance (ANOVA) was used to compare the differences in ultrasonic parameters or birth weight among the three trajectory patterns. Pairwise comparisons of birth weight among the three fetal growth trajectory groups were performed using post-hoc tests with Bonferroni correction. The generalized linear model was used to assess the association of fetal growth trajectory patterns with neonatal mtDNAcn. The fetal trajectory pattern of moderate growth accounted for the largest proportion and was used as the reference group. Owing to its skewed distribution, Lg-transformed mtDNAcn was included in the regression model as an outcome variable. The beta coefficient (β) and 95% confidence intervals (CIs) were estimated for mtDNAcn according to the trajectory patterns. For easier interpretation of the model, we used (10β—1) × 100% to estimate the percentage change in mtDNAcn for different fetal trajectory patterns compared with the reference group and (10β±1.96 ×SE—1) × 100% to estimate the 95% CI. In addition, newborn birth weight was analyzed as an outcome variable to assess the association of birth weight (per 1,000 g increase) with neonatal mtDNAcn. The multivariate logistic regression model was employed to assess the association between fetal growth trajectory patterns and mtDNAcn tertile categories (first, second, and third tertile), and to compute odds ratios (OR) along with their 95% CI. Covariates such as maternal age, pre-pregnancy BMI, maternal education level, parity, GDM, PIH, passive smoking during pregnancy, newborn sex, and gestational age at birth were selected according to previous studies (27–29) and adjusted for in the analysis. In addition, we performed stratified analysis to evaluate the associations of fetal growth trajectory with and newborn mtDNAcn across different sexes, and found no statistically significant interaction between sex and trajectory patterns in all the models.
All the statistical analyses were conducted via SAS 9.4 (SAS Institute Inc., Cary, NC), with GBMTM performed via PROC TRAJ macros. P values < 0.05 were considered statistically significant.
3 Results
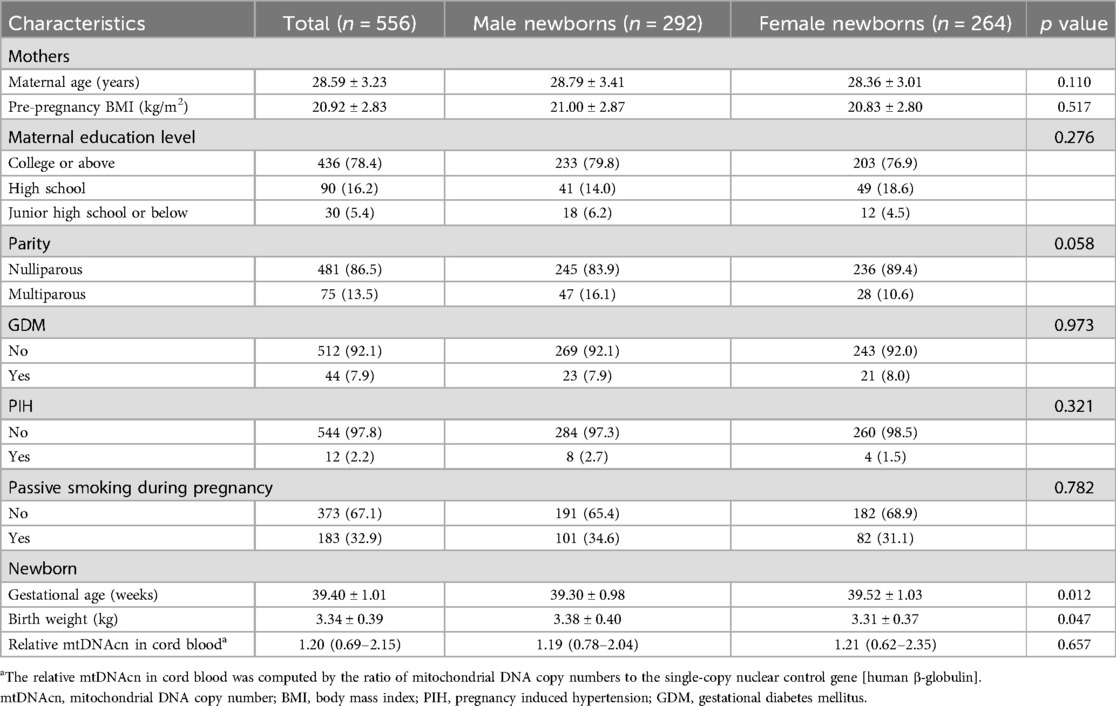
The study included 556 mother–newborn pairs (Table 1). The mean maternal age at delivery was 28.6 ± 3.2 years, and the pre-pregnancy BMI was 20.9 ± 2.8 kg/m2. Most of the mothers had a college degree or above (78.4%), were nulliparous (86.5%), and reported no passive smoking during pregnancy (67.1%). GDM was present in 7.9% of mothers, whereas PIH affected 2.2%. For the newborns, the gestational age at birth was 39.40 ± 1.01 weeks, the birth weight was 334 ± 0.39 kg, and the relative mtDNAcn in cord blood was 1.21 (0.69–2.16). There was a significant difference in gestational age (p = 0.012) and birth weight (p = 0.047) between male and female infants but not in mtDNAcn (p = 0.657).
Among the 556 subjects, the GBMTM identified three distinct fetal growth trajectories integrating BPD, HC, AC, and FL (Figure 1). Fetuses in the “consistently low” (n = 144, 25.9%) group showed consistently low intrauterine growth, with a trajectory predominantly below the Z-score of zero. The “moderate” group (n = 304, 54.7%) displayed an above-average growth level, serving as the reference group in subsequent analyses. The “high-falling” fetal growth trajectory (n = 108, 19.4%) was characterized by a high growth level (Z-scores > 2.0) at 16 weeks of gestation, gradually declining thereafter, still surpassing the “moderate” group at 37 weeks of gestation near delivery. The actual measured values, z-scores of ultrasound examination four times during pregnancy and birth weight by different fetal growth trajectory patterns are displayed in Table 2. There were statistically significant differences in each ultrasound measurement index across the different groups at the four pregnancy times. The average birth weights of the “consistently low”, “moderate”, and “high fitting” fetal growth trajectory groups were 3200.07 ± 353.41 g, 3339.24 ± 386.80 g, and 3552.04 ± 345.17 g, respectively, and there was a statistically significant difference in birth weight between any two groups (Table 2).
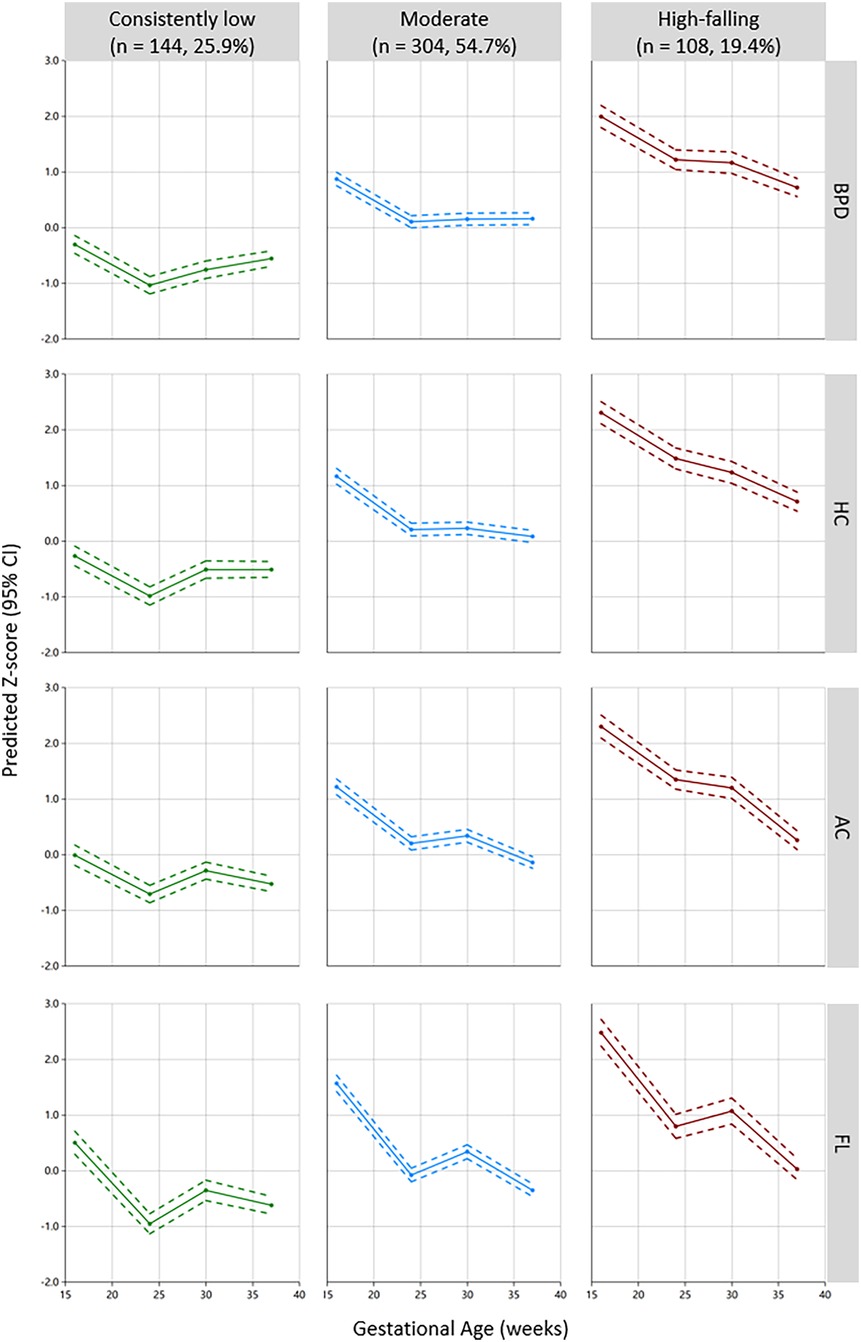
Figure 1. Three-group multi-trajectory model of fetal growth on the basis of the ultrasound parameters BPD, HC, AC, and FL. BPD, biparietal diameter; HC, head circumference; AC, abdominal circumference; FL, femur length.
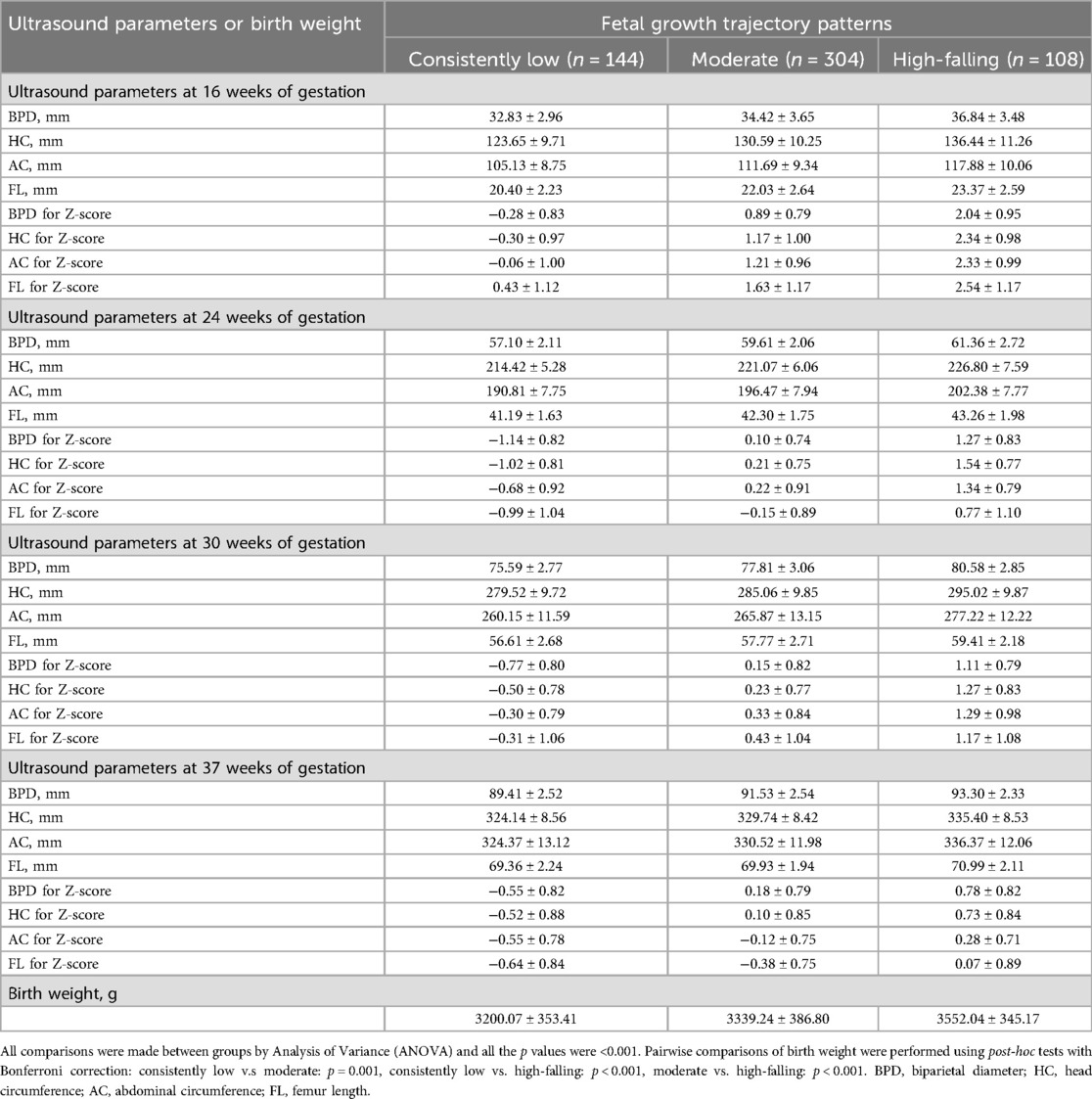
Table 2. Fetal ultrasound parameters during pregnancy and birth weight by different fetal growth trajectory patterns.
The associations between single birth weight measurements and neonatal mtDNAcn are presented in Table 3. No statistically significant associations of birth weight with the percentage change in newborn mtDNAcn were observed.
Table 4 shows the relationship between individual fetch ultrasound parameters and mtDNAcn. Only a positive correlation was found between FL Z-score and mtDNAcn at 37 weeks of pregnancy, meaning that for every unit increase in FL Z-score, mtDNAcn increased by 9.60% (95% CI: 0.55%, 19.47%; p = 0.037). In the results of sex stratification, only statistically significant results were found in boys, with AC Z-score (β = 15.59%; 95% CI: 2.11%, 30.85%; p = 0.022) and FL Z-score (β = 17.00%; 95% CI: 4.08%, 31.52%; p = 0.009) at 37 weeks of pregnancy positively correlated with mtDNAcn.
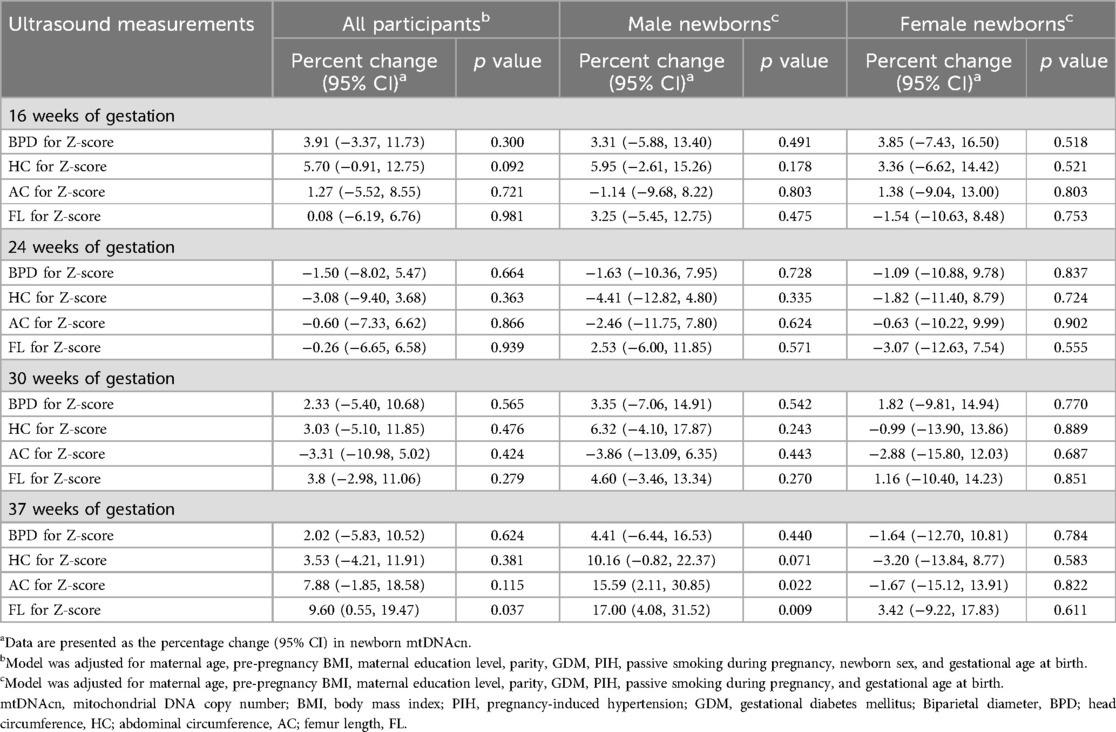
Table 4. Association of fetal ultrasound measurement Z-scores with the percentage change in newborn mtDNAcn in cord blood.
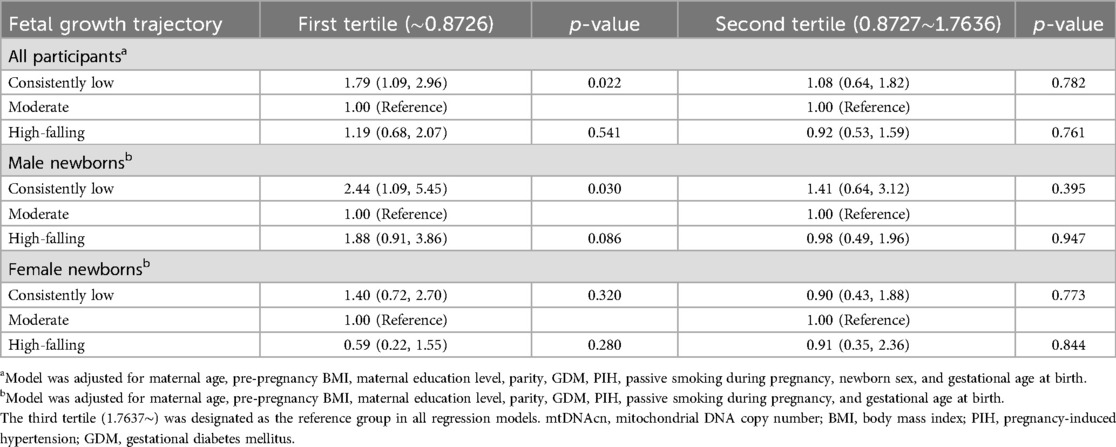
Table 5 shows the percentage change in mtDNAcn for the alternative fetal growth patterns compared with the “moderate” pattern. The association between the “consistently low” pattern and decreased mtDNAcn was observed exclusively in male newborns, with a 24.32% (95% CI: −40.57%, −3.62%; p = 0.026) reduction in mtDNAcn in the unadjusted model. This association remained stable in the adjusted models (β = −22.91%; 95% CI: −39.33%, −1.83%; p = 0.039). Table 6 shows the association of fetal growth trajectories and newborn mtDNAcn tertiles when mtDNAcn was divided into three groups (the first, second, and third tertile). When using the third tertile as a reference, boys exhibiting a “consistently low” pattern were more likely to fall within the first tertile of mtDNAcn compared to those with a “moderate” pattern (OR = 2.44; 95% CI: 1.09, 5.45; p = 0.030).
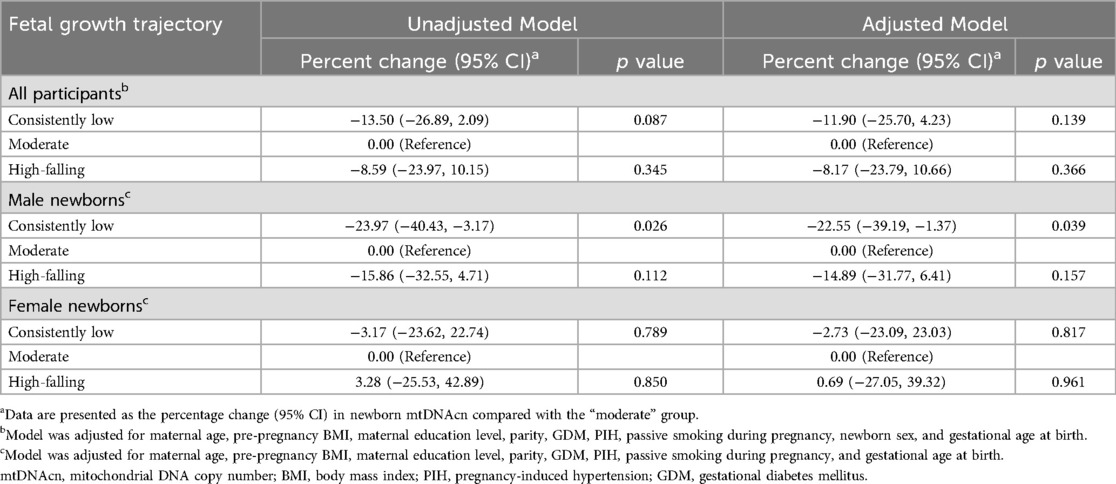
Table 5. Association of fetal growth trajectory with the percentage change in newborn mtDNAcn in cord blood.
4 Discussion
In this study, we utilized serial ultrasound measurements throughout pregnancy to identify three distinct patterns of intrauterine fetal growth trajectory combined BPD, HC, FL, and AC. We found that the “consistently low” pattern was associated with reduced cord blood mtDNAcn in male newborns. Even after adjusting for potential confounding factors, this association remained significant.
Previous studies have suggested that neonates may exhibit distinct patterns of intrauterine growth despite having similar birth sizes (2, 3). Unlike birth weight, which indirectly reflects intrauterine growth, ultrasound measurements during pregnancy provide a direct reflection of fetal growth in utero. Serial fetal ultrasonography, as a routine procedure during prenatal care, offers a convenient and effective means of capturing fetal development at different gestational stages. As demonstrated by many epidemiological studies, the fetal growth trajectory derived from serial ultrasonic measurements presents a dynamic pattern of intrauterine growth, which has been recognized for its association with health outcomes not only in childhood (28, 30–33) but also throughout adulthood (34–38). For example, fetuses exposed to adverse intrauterine environments may adopt a subnormal growth trajectory to increase their chances of survival. However, such developmental reprogramming can increase the risk of metabolic diseases in adulthood (39, 40). The Raine Study conducted in Western Australia suggested that restricted fetal AC and HC trajectory patterns described as “low-falling” or “low-stable” patterns were associated with elevated systolic blood pressure (38) and insulin resistance (36) over a span of two decades. However, no studies have proposed early-life biomarkers associated with below-normal intrauterine growth patterns.
In this study, we conducted an association analysis between different fetal growth trajectories and mtDNAcn. Our findings indicated that, compared with fetuses exhibiting a “moderate growth” trajectory, male infants with a “consistently low” trajectory were more likely to have reduced mtDNAcn in cord blood. In the context of pregnancy, the mtDNA content in placental and umbilical cord blood has been studied as a potential biomarker for growth and development (13–19).
Several studies have explored the association between birth weight and placental or cord blood mtDNA content. For example, Gemma C et al. (14) used the “mtDNA/nuclear DNA ratio” as a research indicator and reported that the mtDNA content in cord blood was lower in small-for-gestational-age (SGA, birthweight-for-gestational-age is less than the 10th percentile) infants than in appropriate-for-gestational-age (AGA, birthweight-for-gestational-age is between the 10th and 90th percentiles) infants (18 ± 6 vs. 28 ± 4, p < 0.03). Findings from the INMA birth cohort (17, 18) suggested that exposure to air pollution during pregnancy was associated with lower birth weight (a decrease of 46.7 g in birth weight for each 10 µg/m3 increase in NO2), and placental mitochondrial content served as a mediator variable with a mediating proportion of 10% and an indirect effect of −5.2 g. Guitar-Mampel M. et al. (15) studied IUGR newborns and reported a 5.62% decrease in the mtDNA content in their cord blood compared with that of normal birth weight newborns. However, the difference was not statistically significant, and the study included a small sample size of 14 IUGR infants and 22 normal birth weight infants. In contrast, two studies reported increased placental mtDNA content in IUGR newborns (13, 16). The inconsistent results among these studies might be due to the pathological state of IUGR, where the placenta undergoes “reprogramming” in terms of energy metabolism to maximize intrauterine growth satisfaction for the fetus (41). In this study, we did not observe a statistically significant positive or negative association between cord blood mtDNAcn and birth weight. This could be attributed to the absence of IUGR cases with pathological conditions in our study population, as all the newborns had normal birth weights.
Considering that different fetal growth patterns can exist even among infants with the same birth weight (2, 3), we examined the impact of these patterns on neonatal mtDNAcn in a healthy population with birth weights ≥ 2,500 g. To the best of our knowledge, this is the first study to report a correlation between fetal growth patterns of “consistently low” and mtDNA depletion, which is considered a putative link between early-life health and adverse metabolic outcomes in adulthood (14). The potential underlying mechanism for this correlation is that alterations in mitochondrial function could serve as early indicators of impaired fetal growth resulting from adverse intrauterine conditions (42), even if these alterations do not result in low birth weight. Hence, on the basis of the accumulated evidence of fetal growth patterns and postnatal metabolic health (35–38), we hypothesize that mtDNA depletion may be a potential link between adverse fetal growth patterns and increased risk factors for metabolic disease later in life. It is noteworthy that statistically significant results were exclusively observed in males. This is attributed to the fact that male fetuses exhibit greater sensitivity to oxidative stress (43) and responses to DNA damage (44).
There are several strengths in our study. First, the findings of this research were progressive and novel in light of the existing body of literature, as this was the first study to explore the associations of fetal growth patterns with neonatal mtDNAcn. Our study examined fetal growth throughout pregnancy, not just birth weight. Second, we utilized standardized, continuous ultrasound measurements during pregnancy, which are considered integral components in endeavors aimed at preventing metabolic health-related diseases during early life stages (45, 46). Fetal ultrasound measurement during pregnancy is a convenient and accessible technique, even in underdeveloped areas (6). Thus, prenatal care interventions based on our findings can be applied to a greater extent. Third, we performed strict quality control for mtDNAcn detection, which made the results more credible.
However, this study also has several limitations. First, the participants were all from a single ethnic group, which limits the generalization of the research results to other ethnic groups. Second, although our model adjusted for several covariates, residual or unmeasured confounding factors (e.g., maternal dietary) may still influence our results. Finally, future research is needed to elucidate the mechanisms driving unique fetal growth patterns during pregnancy and understand how adverse patterns increase the risk of metabolic diseases.
5 Conclusions
In summary, our research identified three distinct fetal growth trajectories in normal birth weight infants and revealed that the “consistently low” trajectory was associated with reduced neonatal mtDNAcn in male infants. On the basis of accumulating evidence, we propose that mtDNA depletion serves as one of the presumptive links between adverse fetal growth patterns and an increased risk of metabolic diseases after birth. Our findings reaffirm the critical role of fetal growth and intrauterine development data in preventing metabolic health issues in early life.
Data availability statement
The datasets presented in this article are not readily available because Data are available on reasonable request. The data set is available from the corresponding author on reasonable request and if consistent with the projects's ethics approvals. Requests to access the datasets should be directed to Kai Chen,Y2hlbmthaUB6Z3doZmUuY29t.
Ethics statement
The studies involving humans were approved by The ethics committee of Wuhan Children's Hospital (Wuhan Maternal and Child Healthcare Hospital), Tongji Medical College, Huazhong University of Science and Technology. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
KC: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JL: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. LX: Data curation, Writing – review & editing. XF: Data curation, Writing – review & editing. ZC: Data curation, Writing – review & editing. LS: Supervision, Validation, Writing – review & editing. YW: Supervision, Validation, Writing – review & editing. CX: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing. AZ: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Knowledge Innovation Specialized Project of Wuhan Science and Technology Bureau (2023020201010198) and Open project of Key Laboratory of Environment and Health, Ministry of Education (2022GWKFJJ05).
Acknowledgments
We sincerely thank all participants and collaborators for their valuable contributions to this birth cohort and this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AC, abdominal circumference; BMI, body mass index; BPD, biparietal diameter; CI, confidence interval; FL, femur length; GBMTM, group-based multi-trajectory modelling; GDM, gestational diabetes mellitus; HC, head circumference; IUGR, intrauterine growth retardation; mtDNA, mitochondrial DNA; mtDNAcn, mitochondrial DNA copy number; OR, odds ratio; PCR, polymerase chain reaction; PIH, pregnancy-induced hypertension.
References
1. Hanson MA, Gluckman PD. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. (2014) 94(4):1027–76. doi: 10.1152/physrev.00029.2013
2. Bloomfield FH, Oliver MH, Harding JE. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed. (2006) 91(4):F299–304. doi: 10.1136/adc.2005.076646
3. Sato N, Miyasaka N. Heterogeneity in fetal growth velocity. Sci Rep. (2019) 9(1):11304. doi: 10.1038/s41598-019-47839-5
4. Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. (2007) 19(1):1–19. doi: 10.1002/ajhb.20590
5. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. (2008) 359(1):61–73. doi: 10.1056/NEJMra0708473
6. Sovio U, White IR, Dacey A, Pasupathy D, Smith G. Screening for fetal growth restriction with universal third trimester ultrasonography in nulliparous women in the pregnancy outcome prediction (POP) study: a prospective cohort study. Lancet. (2015) 386(10008):2089–97. doi: 10.1016/S0140-6736(15)00131-2
7. Lee HC, Wei YH. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int J Biochem Cell Biol. (2005) 37(4):822–34. doi: 10.1016/j.biocel.2004.09.010
8. Pejznochova M, Tesarova M, Honzik T, Hansikova H, Magner M, Zeman J. The developmental changes in mitochondrial DNA content per cell in human cord blood leukocytes during gestation. Physiol Res. (2008) 57(6):947–55. doi: 10.33549/physiolres.931246
9. Clay ML, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. (2009) 36(3):125–31. doi: 10.1016/S1673-8527(08)60099-5
10. Lee HC, Wei YH. Mitochondrial role in life and death of the cell. J Biomed Sci. (2000) 7(1):2–15. doi: 10.1007/BF02255913
11. Malik AN, Czajka A. Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion. (2013) 13(5):481–92. doi: 10.1016/j.mito.2012.10.011
12. Shoar Z, Goldenthal MJ, De Luca F, Suarez E. Mitochondrial DNA content and function, childhood obesity, and insulin resistance. Endocr Res. (2016) 41(1):49–56. doi: 10.3109/07435800.2015.1068797
13. Lattuada D, Colleoni F, Martinelli A, Garretto A, Magni R, Radaelli T, et al. Higher mitochondrial DNA content in human IUGR placenta. Placenta. (2008) 29(12):1029–33. doi: 10.1016/j.placenta.2008.09.012
14. Gemma C, Sookoian S, Alvarinas J, Garcia SI, Quintana L, Kanevsky D, et al. Mitochondrial DNA depletion in small- and large-for-gestational-age newborns. Obesity (Silver Spring). (2006) 14(12):2193–9. doi: 10.1038/oby.2006.257
15. Guitart-Mampel M, Juarez-Flores DL, Youssef L, Moren C, Garcia-Otero L, Roca-Agujetas V, et al. Mitochondrial implications in human pregnancies with intrauterine growth restriction and associated cardiac remodelling. J Cell Mol Med. (2019) 23(6):3962–73. doi: 10.1111/jcmm.14282
16. Naha R, Anees A, Chakrabarty S, Naik PS, Pandove M, Pandey D, et al. Placental mitochondrial DNA mutations and copy numbers in intrauterine growth restricted (IUGR) pregnancy. Mitochondrion. (2020) 55:85–94. doi: 10.1016/j.mito.2020.08.008
17. Barrett JR. Prenatal air pollution and reduced birth weight: decline in placental mitochondria as a potential mechanism. Environ Health Perspect. (2016) 124(5):A98. doi: 10.1289/ehp.124-A98
18. Clemente DB, Casas M, Vilahur N, Begiristain H, Bustamante M, Carsin AE, et al. Prenatal ambient air pollution, placental mitochondrial DNA content, and birth weight in the INMA (Spain) and ENVIRONAGE (Belgium) birth cohorts. Environ Health Perspect. (2016) 124(5):659–65. doi: 10.1289/ehp.1408981
19. Kupsco A, Bloomquist TR, Hu H, Reddam A, Tang D, Goldsmith J, et al. Mitochondrial DNA copy number dynamics and associations with the prenatal environment from birth through adolescence in a population of Dominican and African American children. Mitochondrion. (2023) 69:140–6. doi: 10.1016/j.mito.2023.02.008
20. Xing J, Chen M, Wood CG, Lin J, Spitz MR, Ma J, et al. Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst. (2008) 100(15):1104–12. doi: 10.1093/jnci/djn213
21. Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. (2018) 27(7):2015–23. doi: 10.1177/0962280216673085
22. Nagin DS, Jones BL, Elmer J. Recent advances in group-based trajectory modeling for clinical research. Annu Rev Clin Psychol. (2024) 20(1):285–305. doi: 10.1146/annurev-clinpsy-081122-012416
23. Bommarito PA, Cantonwine DE, Stevens DR, Welch BM, Davalos AD, Zhao S, et al. An application of group-based trajectory modeling to define fetal growth phenotypes among small-for-gestational-age births in the LIFECODES fetal growth study. Am J Obstet Gynecol. (2023) 228(3):331–4. doi: 10.1016/j.ajog.2022.08.041
24. Bommarito PA, Cantonwine DE, Stevens DR, Welch BM, Davalos AD, Zhao S, et al. Fetal growth trajectories of babies born large-for-gestational age in the LIFECODES fetal growth study. Am J Obstet Gynecol. (2023) 228(3):340–1. doi: 10.1016/j.ajog.2022.10.006
25. Kiserud T, Piaggio G, Carroli G, Widmer M, Carvalho J, Neerup JL, et al. The world health organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. Plos Med. (2017) 14(1):e1002220. doi: 10.1371/journal.pmed.1002220
26. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. (2010) 6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413
27. Zhang C, Hediger ML, Albert PS, Grewal J, Sciscione A, Grobman WA, et al. Association of maternal obesity with longitudinal ultrasonographic measures of fetal growth: findings from the NICHD fetal growth studies-singletons. JAMA Pediatr. (2018) 172(1):24–31. doi: 10.1001/jamapediatrics.2017.3785
28. Bartels HC, O'Connor C, Segurado R, Mason O, Mehegan J, Geraghty AA, et al. Fetal growth trajectories and their association with maternal and child characteristics. J Matern Fetal Neonatal Med. (2020) 33(14):2427–33. doi: 10.1080/14767058.2018.1554041
29. Zou JJ, Wei Q, Shi YY, Wang K, Zhang YH, Shi HJ. Longitudinal associations between maternal glucose levels and ultrasonographic fetal biometrics in a Shanghai cohort. Jama Netw Open. (2022) 5(4):e226407. doi: 10.1001/jamanetworkopen.2022.6407
30. Villar J, Ochieng R, Gunier RB, Papageorghiou AT, Rauch S, McGready R, et al. Association between fetal abdominal growth trajectories, maternal metabolite signatures early in pregnancy, and childhood growth and adiposity: prospective observational multinational INTERBIO-21st fetal study. Lancet Diabetes Endocrinol. (2022) 10(10):710–9. doi: 10.1016/S2213-8587(22)00215-7
31. Villar J, Gunier RB, Tshivuila-Matala C, Rauch SA, Nosten F, Ochieng R, et al. Fetal cranial growth trajectories are associated with growth and neurodevelopment at 2 years of age: iNTERBIO-21st fetal study. Nat Med. (2021) 27(4):647–52. doi: 10.1038/s41591-021-01280-2
32. Tideman J, Polling JR, Jaddoe V, Vingerling JR, Klaver C. Growth in foetal life, infancy, and early childhood and the association with ocular biometry. Ophthalmic Physiol Opt. (2019) 39(4):245–52. doi: 10.1111/opo.12630
33. Pike KC, Crozier SR, Lucas JS, Inskip HM, Robinson S, Roberts G, et al. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax. (2010) 65(12):1099–106. doi: 10.1136/thx.2010.134742
34. Dyer K, Sanfilippo PG, White SW, Guggenheim JA, Hammond CJ, Newnham JP, et al. Associations between fetal growth trajectories and the development of myopia by 20 years of age. Invest Ophthalmol Vis Sci. (2020) 61(14):26. doi: 10.1167/iovs.61.14.26
35. Rytter D, Bech BH, Frydenberg M, Henriksen TB, Olsen SF. Fetal growth and cardio-metabolic risk factors in the 20-year-old offspring. Acta Obstet Gynecol Scand. (2014) 93(11):1150–9. doi: 10.1111/aogs.12463
36. Yadav A, Beilin LJ, Huang RC, Newnham JP, White SW, Mori TA. Fetal growth trajectories and measures of insulin resistance in young adults. J Clin Endocrinol Metab. (2023) 108(9):e861–70. doi: 10.1210/clinem/dgad292
37. Yadav A, Beilin LJ, Huang RC, Vlaskovsky P, Newnham JP, White SW, et al. Relationships between intrauterine fetal growth trajectories and markers of adiposity and inflammation in young adults. Int J Obes. (2022) 46(10):1925–35. doi: 10.1038/s41366-022-01203-2
38. Yadav A, Beilin LJ, Huang RC, Vlaskovsky P, Newnham JP, White SW, et al. The relationship between intrauterine foetal growth trajectories and blood pressure in young adults. J Hypertens. (2022) 40(3):478–89. doi: 10.1097/HJH.0000000000003035
39. Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. (2002) 31(6):1235–9. doi: 10.1093/ije/31.6.1235
40. Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. (1992) 99(4):275–6. doi: 10.1111/j.1471-0528.1992.tb13719.x
41. Mayer C, Joseph KS. Fetal growth: a review of terms, concepts and issues relevant to obstetrics. Ultrasound Obstet Gynecol. (2013) 41(2):136–45. doi: 10.1002/uog.11204
42. Lu M, Sferruzzi-Perri AN. Placental mitochondrial function in response to gestational exposures. Placenta. (2021) 104:124–37. doi: 10.1016/j.placenta.2020.11.012
43. Allegra A, Caserta S, Genovese S, Pioggia G, Gangemi S. Gender differences in oxidative stress in relation to cancer susceptibility and survival. Antioxidants. (2023) 12(6):1255. doi: 10.3390/antiox12061255
44. Broestl L, Rubin JB. Sexual differentiation specifies cellular responses to DNA damage. Endocrinology. (2021) 162(11):bqab192. doi: 10.1210/endocr/bqab192
45. Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. (2012) 11:42. doi: 10.1186/1476-069X-11-42
Keywords: gestational ultrasound measurements, fetal growth trajectory, intrauterine growth pattern, mitochondrial DNA, mtDNA, mitochondrial DNA copy number, mtDNAcn
Citation: Chen K, Li J, Xu L, Fan X, Cao Z, Song L, Wang Y, Xiong C and Zhou A (2025) Association of fetal growth trajectory with mitochondrial DNA copy number in the cord blood of newborns: evidence from a birth cohort. Front. Pediatr. 13:1569702. doi: 10.3389/fped.2025.1569702
Received: 1 February 2025; Accepted: 19 May 2025;
Published: 9 June 2025.
Edited by:
Javier Diaz-Castro, University of Granada, SpainReviewed by:
Kripa Raghavan, United States Department of Agriculture (USDA), Washington, United StatesAalekhya Reddam, Office of Environmental Health Hazard Assessment, United States
Copyright: © 2025 Chen, Li, Xu, Fan, Cao, Song, Wang, Xiong and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Xiong, eGNwb3BvMTFAeWVhaC5uZXQ=; Aifen Zhou, YXByaWwxOTcyQDE2My5jb20=
†These authors have contributed equally to this work
 Kai Chen
Kai Chen Junwei Li3,†
Junwei Li3,† Xiaoxuan Fan
Xiaoxuan Fan