- 1Department of Immunity, Beijing Children’s Hospital, National Center for Children’s Health, Capital Medical University, Beijing, China
- 2Department of Pharmacy, Beijing Children’s Hospital, National Center for Children’s Health, Capital Medical University, Beijing, China
- 3School of Basic Medicine and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China
- 4Ministry of Education Key Laboratory of Major Diseases in Children
- 5Department of Nephrology and Rheumatology, Children’s Hospital of Xinjiang Uygur Autonomous Region, Xinjiang Hospital of Beijing Children’s Hospital, Xinjiang, China
Objectives: The prevalence of glucocorticoids (GCs) administration in pediatric populations has resulted in numerous adverse reactions, notably osteoporosis. Given its role in managing glucocorticoid-induced osteoporosis, the efficacy and safety of bisphosphonates hold considerable importance. This study conducted a meta-analysis by systematically reviewing and incorporating relevant literature on the efficacy and safety of bisphosphonates in the management of osteoporosis or bone infarction induced by GCs therapy in pediatric populations. Additionally, the analysis of potential adverse reactions was augmented by utilizing real-world data from the FAERS database. The primary objective of this study is to offer insights and guidance for the treatment of glucocorticoid induced osteoporosis in pediatric patients.
Methods: A meta-analysis was performed on existing literature to assess the efficacy and safety of bisphosphonates for managing glucocorticoid-induced osteoporosis. Additionally, a retrospective pharmacovigilances study was carried out to investigate adverse reactions and medication variations in pediatric patients with glucocorticoid-induced osteoporosis, using data from the FDA Adverse Event Reporting System (FAERS) database between Q1 2004 and Q4 2023.
Results: The meta-analysis incorporated a total of 14 articles encompassing 572 patients. The findings of this study indicate that bisphosphonate therapy is more effective in enhancing bone mineral density (BMD) and BMD Z-scores in children compared to the control group, albeit with a heightened risk of adverse reactions. Furthermore, there was no significant disparity observed between the impact of bisphosphonate treatment and control groups on fracture outcomes. Subsequently, in the ensuing Pharmacovigilance investigation, 668 instances of adverse reactions associated with bisphosphonates are analyzed. The findings indicated that the most prevalent adverse reactions, as evidenced by the highest number of positive signals were various examinations, musculoskeletal and connective tissue diseases, injuries, poisoning and operational complications, as well as systemic diseases and reactions at the administration site.
Conclusions: This study conducted a comprehensive analysis of the efficacy and safety of bisphosphonates in the treatment of osteoporosis caused by GCs use in pediatric patients, laying the groundwork for future research. Nevertheless, the constraints of retrospective studies highlight the need for additional investigation through prospective studies.
1 Introduction
Osteoporosis is a condition characterized by heightened bone fragility and susceptibility to fractures. The study of secondary osteoporosis in pediatric populations is gaining prominence alongside primary hereditary osteoporosis. The primary objectives of treatment include the prevention of fractures, enhancement of bone mass, augmentation of trabecular and cortical thickness, restoration of vertebral fractures, correction of skeletal deformities, and enhancement of mobility, independence, and quality of life (1).
Glucocorticoids (GCs) are frequently recognized as a primary contributor to secondary osteoporosis. The resultant skeletal disorder characterized by decreased bone density and an elevated risk of fractures due to prolonged glucocorticoid therapy is referred to as glucocorticoid-induced osteoporosis. Although physiological doses of glucocorticoids are essential for osteoblast differentiation, high doses of glucocorticoids can promote osteoclast activity and suppress the function of both osteoblasts and osteoclasts. This results in reduced bone density and compromised bone microarchitecture. In pediatric and adolescent populations, these medications are primarily prescribed for the management of conditions such as asthma, rheumatologic diseases, and autoimmune disorders. Adolescents, being in the critical phase of peak bone mass acquisition, are consequently more vulnerable to the detrimental skeletal effects associated with glucocorticoid therapy. This is especially true for those receiving long-term glucocorticoid therapy or systemic glucocorticoid treatment during puberty, as they are at an elevated risk of developing glucocorticoid-induced osteoporosis (2–6). Presently, the primary therapeutic modalities encompass both non-pharmacologic and pharmacologic interventions. Non-pharmacological strategies consist of nutritional supplementation, adherence to a balanced diet, and exercise management. Pharmacological treatments predominantly consist of bisphosphonates, selective estrogen receptor modulators, calcitonin, and molecularly targeted drugs (7), which primarily function by suppressing osteoclast activity and decreasing bone turnover. Moreover, osteonecrosis is a recognized complication associated with glucocorticoid therapy. This condition is characterized by the necrosis of bone component cells resulting from an interruption in blood supply. Pharmacological interventions, particularly the administration of bisphosphonates,constitute a fundamental therapeutic approach for managing this condition. In clinical practice, numerous pediatric patients have encountered the adverse reaction of bone infarction subsequent to glucocorticoid administration. Given that bone infarction is a form of osteonecrosis, the effectiveness and safety of pharmacological interventions for this condition have become a focal point of our investigation.
Bisphosphonates are the most commonly utilized among the aforementioned drugs (8, 9). Their mechanism of action involves binding to bone minerals, uptake by osteoclasts during bone resorption, and subsequent inhibition of osteoclast activity (10). Bisphosphonates, a category of pharmaceutical agents, function by impeding bone resorption through various pathways. Extensive research has demonstrated the safety and efficacy of bisphosphonates, as evidenced by their ability to increase bone mineral density and decrease fracture risk, ultimately leading to a reduction in mortality rates and enhancement of overall quality of life (11).
Bisphosphonates possess the capacity to impede osteoclast activity through the inhibition of farnesyl pyrophosphate synthetase within osteoclasts, thereby disrupting the geranylation of geranyl and resulting in osteoclast inactivation. This mechanism is accountable for suppressing the nitrogen-containing bisphosphonates (N-BP) of bone resorption by osteoclasts and decreasing bone turnover in order to mitigate the risk of fractures (12, 13). A systematic review comprising 48 studies involving adult patients found that long-term administration of alendronate and zoledronate could effectively decrease the fracture risk in women diagnosed with osteoporosis (14). Nevertheless, the potential adverse effects of bisphosphonates, including gastrointestinal issues, kidney damage, bone necrosis, and eye inflammation, have garnered significant attention in the medical community (15–17). Currently, there is no approved indication for bisphosphonate use, yet it continues to be utilized off-label. The decision to prescribe this medication necessitates a thorough evaluation of the trade-off between its therapeutic benefits and potential adverse effects.
This study utilized a meta-analysis of prior research to investigate the effectiveness and safety of bisphosphonates in treating osteoporosis or bone infarction resulting from hormone use in pediatric patients. Additionally, real-world pharmacovigilance data from the Food and Drug Administration's adverse event reporting system (FAERS) was employed to examine reported incidents of adverse reactions associated with bisphosphonate use in clinical settings.
2 Methods
2.1 Research design
Initially, a meta-analysis was performed on extant literature to examine the effectiveness and safety of bisphosphonates for managing glucocorticoid-induced osteoporosis and Bone Infarction. Furthermore, retrospective data mining analysis utilizing the FDA FAERS database was conducted to address limitations in current studies regarding adverse reactions and variations among different medications.
2.2 Systematic evaluation procedures
2.2.1 Access to literature
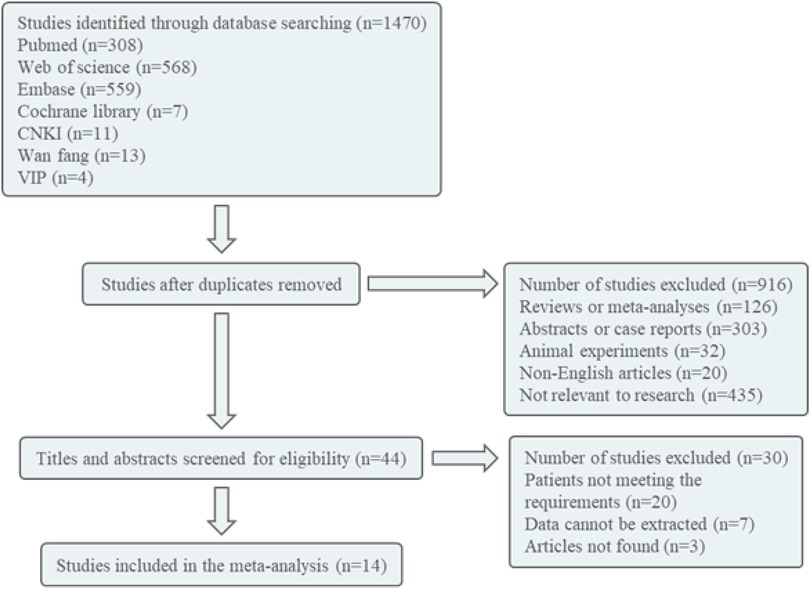
The retrieval database utilized in this study includes Pubmed, Embase, Cochrane Library, Web of Science, HowNet, VIP, and Wanfang. The English search terms employed are Diphosphates Bisphosphonates, Bisphosphonate, Alendronate, Etidronate, Zoledronate, Clodronate, Pamidronate, Tiludronate, Neridronate, Olpadronate, Risedronate, Ibandronate, Child, Children, Osteoporosis, bone loss, and bone infarct. The Chinese search term used is bisphosphonate, children, osteoporosis/bone loss/bone infarction, with the search conducted on October 7, 2023. The literature is initially screened by reviewing the title and abstract. Following this preliminary screening process, any literature deemed unsuitable will be excluded after a thorough examination of the full text, with the remaining literature being included in the study (Figure 1).
2.2.2 Criteria for inclusion and exclusion of literature
(1) Inclusion criteria.
A. Subjects: Patients with a documented history of glucocorticoid use within the past year, who subsequently received a diagnosis of osteoporosis or bone infarction following such use, were included in the study.
B. Intervention mode: bisphosphonate, including Alendronate Etidronate, Zoledronate, Clodronate, Pamidronate, Tiludronate, Neridronate, Olpadronate, Risedronate, Ibandronate, etc. The control group is the placebo group or the drug itself before and after control.
C. Outcome: (a) Bone mineral density (BMD) and BMD z-scores; (b) Frame Outcomes; (c) Paint and mobility outcomes; (d) Quality of Life; (e) Muscle strength, etc.
D. Research type: RCT or cohort study.
(2) Exclusion criteria.
A. The patients exhibited primary osteoporosis and Primary Bone Infarction, encompassing conditions such as osteogenesis imperfecta, adolescent idiopathic osteoporosis, Spontaneous Bone Infarction, Posttraumatic Bone Infarction, and other hereditary diseases.
B. Secondary osteoporosis caused by non-GCs treatment, such as too little exercise, muscle weakness, chemotherapy drugs and other bone destruction, Secondary osteoporosis.
C. Research on incomplete data or inability to extract data;
D. Meta, review, Meeting summary, animal test, case report;
E. Non-Chinese/English literature.
2.2.3 Quality assessment and data extraction
The modified Jadad scoring scale was utilized to evaluate the quality of randomized controlled trials, based on four criteria: random sequence generation, allocation concealment, blinding procedures, and participant attrition. The total score of the randomized controlled trials was 7 points, with scores ranging from 1 to 3 indicating low quality and scores from 4 to 7 indicating high quality. The Newcastle-Ottawa Scale (NOS) was employed to assess the quality of cohort and case-control studies across three dimensions: selection of the study population, comparability between groups, and measurement of the outcomes, with a total possible score of 9 points, categorized as poor (0–3), fair (4–6), or good (7–9). For cross-sectional studies, the Joanna Briggs Institute (JBI) scale, published by the Australian Centre for Evidence-Based Nursing, was used. This scale comprises 10 items, with a total possible score of 20 points.
Literature screening and data extraction: Two researchers conducted a comprehensive review of the literature, independently gathering data, evaluating quality, and verifying results. Any discrepancies were addressed through discussion and resolution, or by involving a third researcher for input. Data extraction encompassed various elements, such as basic information (e.g., authors, publication date, case count), interventions (e.g., program details, treatment regimen), and outcome indicators.
2.2.4 Statistical processing
The data analysis was conducted using Stata15.1 software, utilizing the Weighted Mean Difference (WMD) and Hazard Ratio (RR) as statistical measures, with effect sizes expressed through 95% confidence intervals (CI). Heterogeneity testing was performed on each outcome, with the random effect model utilized if the I2 statistic was greater than or equal to 50%, and the fixed effect model used otherwise. Sensitivity analysis was conducted for all outcomes, and publication bias was assessed through the Begg's test. The observed discrepancy was found to be statistically significant at a significance level of P < 0.05.
2.3 Pharmacovigilance study procedures
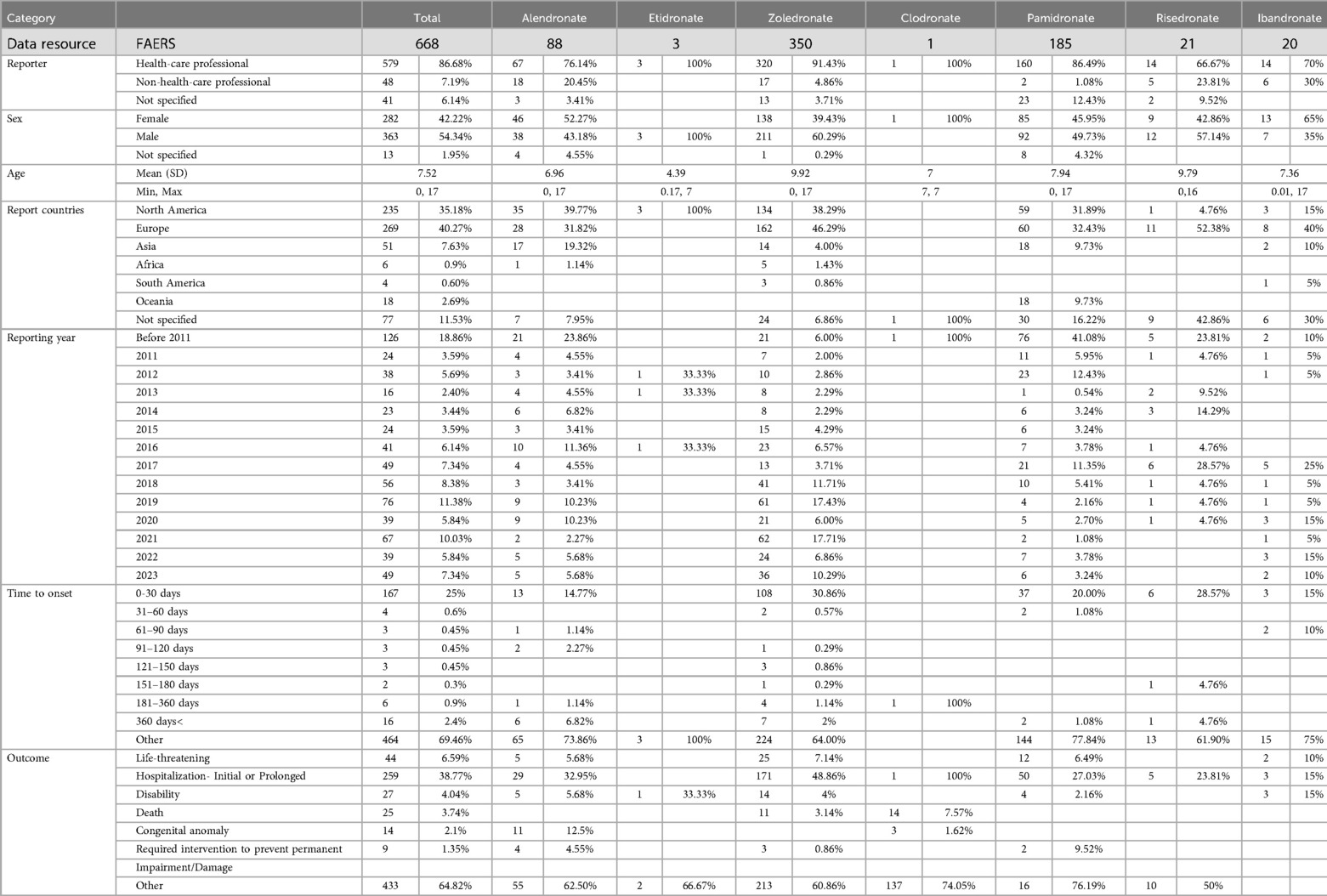
Search the real world pharmacovigilance data related to bisphosphonates in FAERS from January 1, 2004 to December 31, 2023. The common names of bisphosphonates (Alendronate, Etidronate, Zoledronate, Clodronate, Pamidronate, Risedronate, Ibandronate) are used to identify cases reported as suspected bisphosphonate. Furthermore, the study collected clinical characteristics data such as the reporter's profession (health care professional or non-health care professional), gender, age, reporting country, reporting year, and additional data as outlined in Table 1. Reports in the FAERS database are categorized using preferred terms (PTs) from the MedDRA hierarchy, including high-level terms (HLT), high-level group terms (HLGT), and system organ class (SOC) levels. For the purposes of this study, the SOC level was chosen to classify adverse events related to bisphosphonates.
2.4 The role of financial resources
The funding entity did not participate in the formulation of the research design, data collection, data analysis, data interpretation, or report composition.
3 Results
3.1 Results of the systematic evaluation
3.1.1 The findings of the literature search and the fundamental aspects of literature acquisition
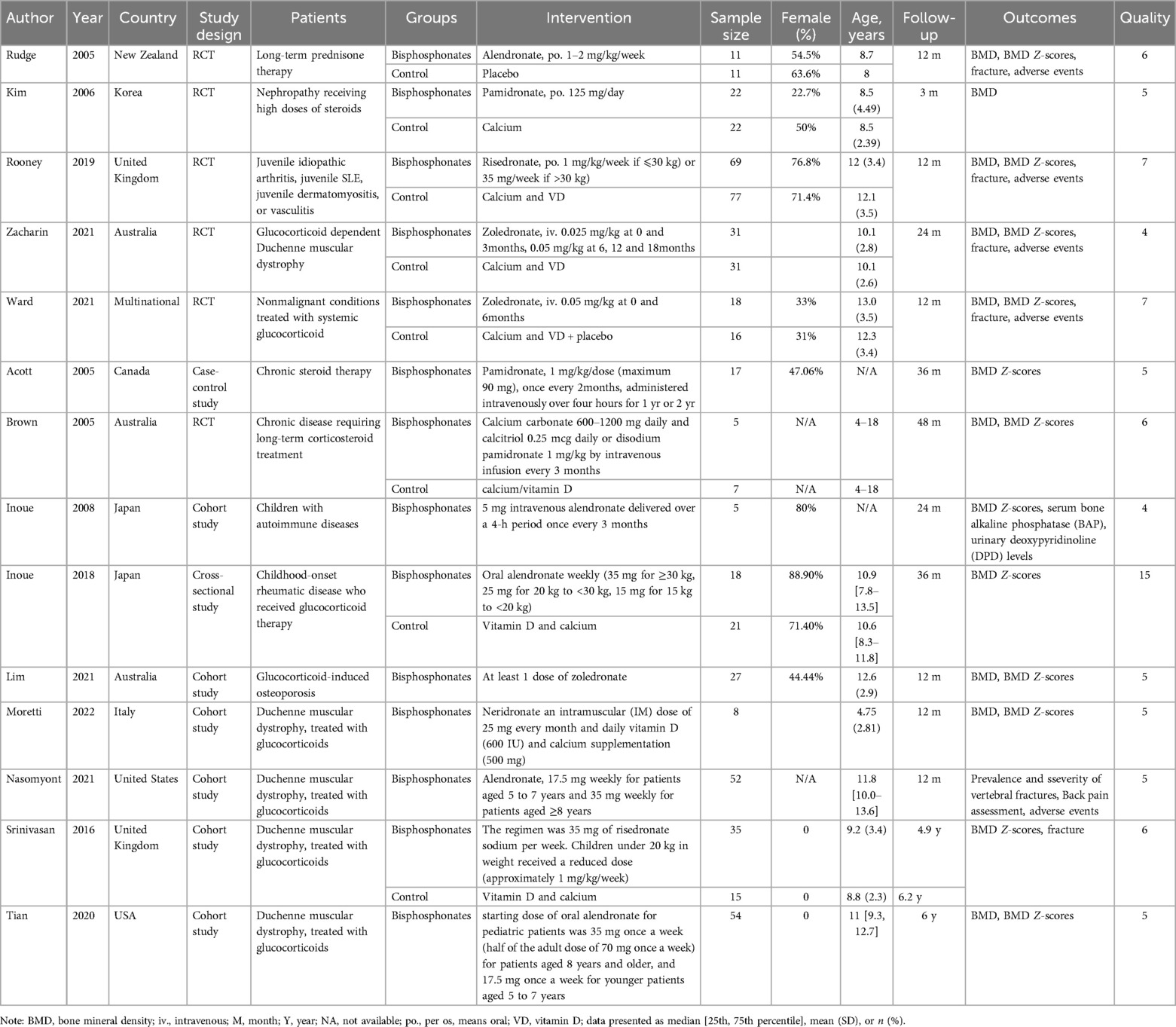
Following the implementation of the search strategy in both Chinese and English databases, a total of 1,470 articles were identified. These articles underwent screening based on predetermined inclusion and exclusion criteria, resulting in the inclusion of 14 articles for further analysis (18–31). The document screening flow chart 1 illustrates the specific process, which included 6 randomized controlled trials (RCTs), 6 cohort studies, 1 case-control study, and 1 cross-sectional study. Among the included articles, seven were deemed of high quality and seven were of medium quality. A total of 572 patients were involved in the study, as indicated in Table 2.
3.1.2 Changes in BMD
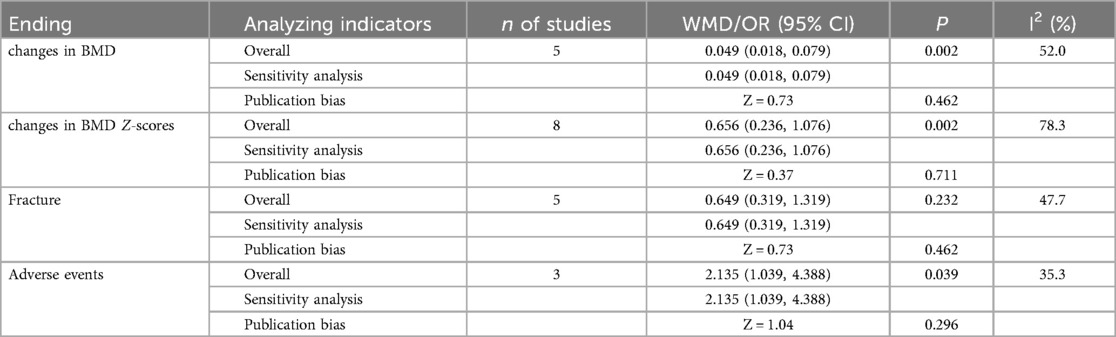
A total of five articles were incorporated into the analysis,each employing dual-energy x-ray absorptiometry (DXA) to assess of bone mineral density (BMD). The studies included a variety of bisphosphonates, specifically alendronate, pamidronate, risedronate, and zoledronate, with follow-up time spanning from 3 to 48 months. The heterogeneity test revealed statistically significant results (I2 = 52.0%). Therefore, a random effects model was employed for the combined analysis (Figure 2a). The weighted mean difference (WMD) was 0.049, with a 95% confidence interval (CI) of 0.018–0.079, and a P-value of 0.002. The results indicated statistically significant differences among the combined studies, suggesting that the improvement in bone mineral density (BMD) in pediatric patients treated with bisphosphonate was superior to that of the control group.
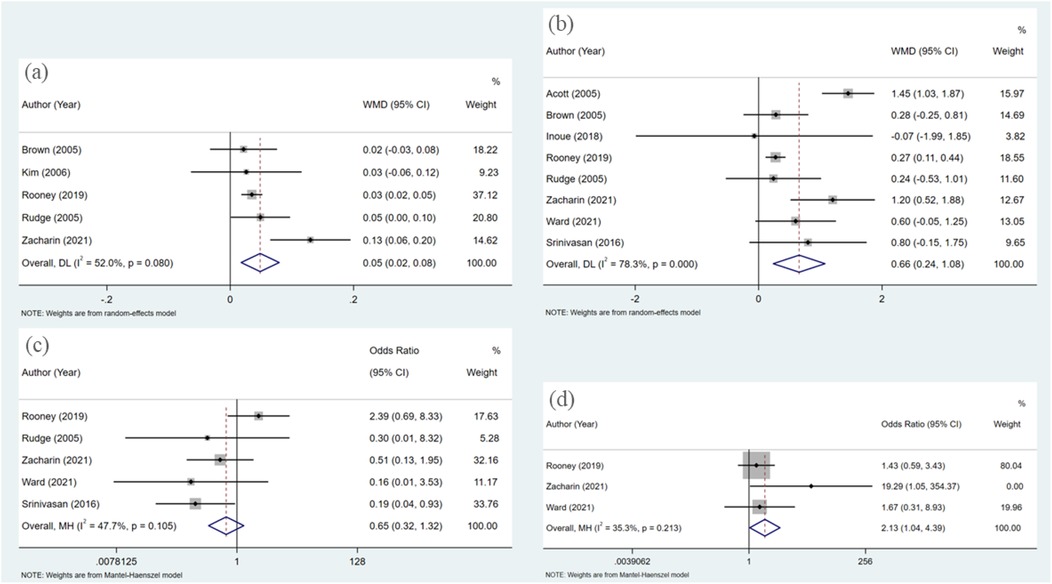
Figure 2. Forest plot of meta-analysis: (a) changes in BMD; (b) changes in BMD Z-scores; (c) fracture; (d) adverse events.
3.1.3 Changes in BMD Z-scores
In the eight studies analyzed, the Z-score, a critical metric in the assessment of BMD, was evaluated using dual-energy x-ray absorptiometry (DXA), akin to the measurement of BMD itself. The bisphosphonates investigated within these studies included alendronate, pamidronate, risedronate, and zoledronate, with follow-up durations ranging from 6 to 48 months. The heterogeneity test yielded statistically significant results (I2 = 78.3%), necessitating the application of a random effects model for the aggregated analysis (Figure 2b).The weighted mean difference (WMD) was found to be 0.656, with a 95% confidence interval of (0.236, 1.076) and a P-value of 0.002. Significant differences were observed among the combined studies, indicating that the efficacy of bisphosphonate in improving BMD Z-scores of pediatric patients is superior to that of the control group.
3.1.4 Fracture
The analysisincorporated a total of five articles, each of which documented the incidence of new fractures in patients during follow-up periods to evaluate the efficacy of bisphosphonate treatment in preventing fractures. The heterogeneity test revealed no statistically significant differences among these studies (I2 = 47.7%). Therefore, the fixed effect model was utilized for the combined analysis (Figure 2c). The odds ratio was 0.649 with a 95% confidence interval of 0.319–1.319, and a p-value of 0.232. These findings suggest that there was no statistically significant difference among the combined studies, indicating that the effects of bisphosphonate treatment and control groups on fracture outcomes cannot be considered to be different.
3.1.5 Adverse events
Three articles were included in the analysis, and the heterogeneity test results did not show any statistically significant differences (I2 = 35.3%). Therefore, the fixed effect model was utilized for the combined analysis (Figure 2d). The pooled odds ratio was 2.135 with a 95% confidence interval of (1.039, 4.388) and a p-value of 0.039, indicating statistically significant differences among the included studies. These findings suggest that the risk of adverse reactions in pediatric patients treated with bisphosphonate is higher compared to those in the control group.
3.1.6 Sensitivity analysis and publication bias
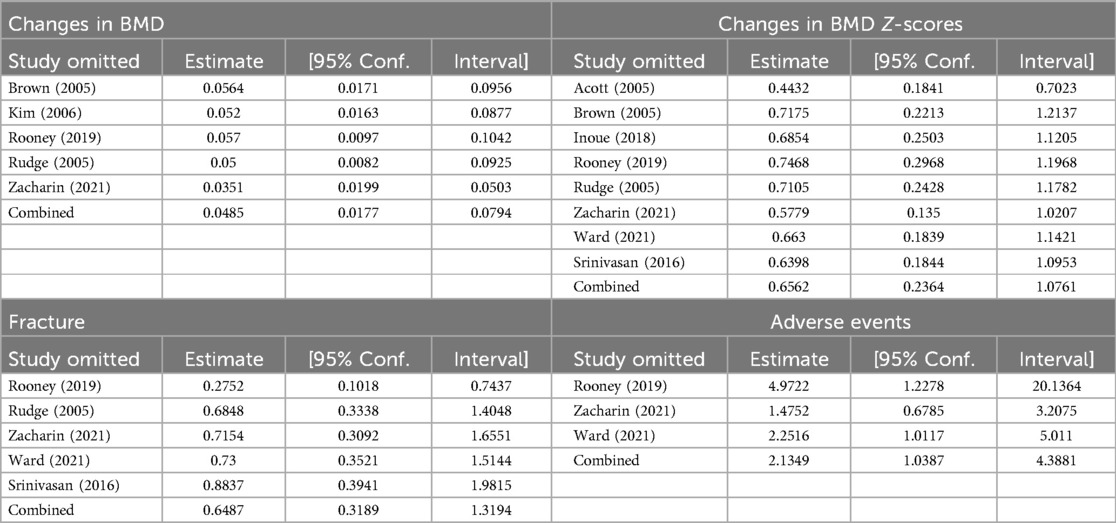
A sensitivity analysis was conducted to evaluate the potential influence of excluding individual studies on the overall findings of the study. The results indicated that none of the studies had a substantial impact on the overall results, demonstrating the stability of the findings (Table 3). Furthermore, the results of Begg's publication bias test revealed no evidence of publication bias in any of the outcomes (P < 0.05) (Table 4).
3.2 Pharmacovigilance study procedures
Between January 1, 2004, and December 31, 2023, a total of 668 cases of adverse reactions associated with bisphosphonates were documented. Specifically, there were 88 cases involving alendronate, 3 cases involving etidronate, 350 cases involving zoledronate, 1 case involving clodronate, 185 cases involving pamidronate, 21 cases involving risephosphate, and 20 cases involving ibandronate. Furthermore, it is noteworthy that all patients included in the study were minors, under the age of 18. The demographic details of the patients are outlined as follows. The majority of cases were documented by healthcare professionals (86.68%). The mean age at which symptoms first appeared was 7.52 years. The prevalence of male cases slightly exceeded that of female cases (54.34% vs. 42.22%). Since 2016, there has been a rise in the number of reported cases, with Europe, Asia, and North America being the primary regions of concern. The predominant reason for hospitalization was adverse reactions, occurring in the majority of patients within one month of initiating the drug (Table 1).
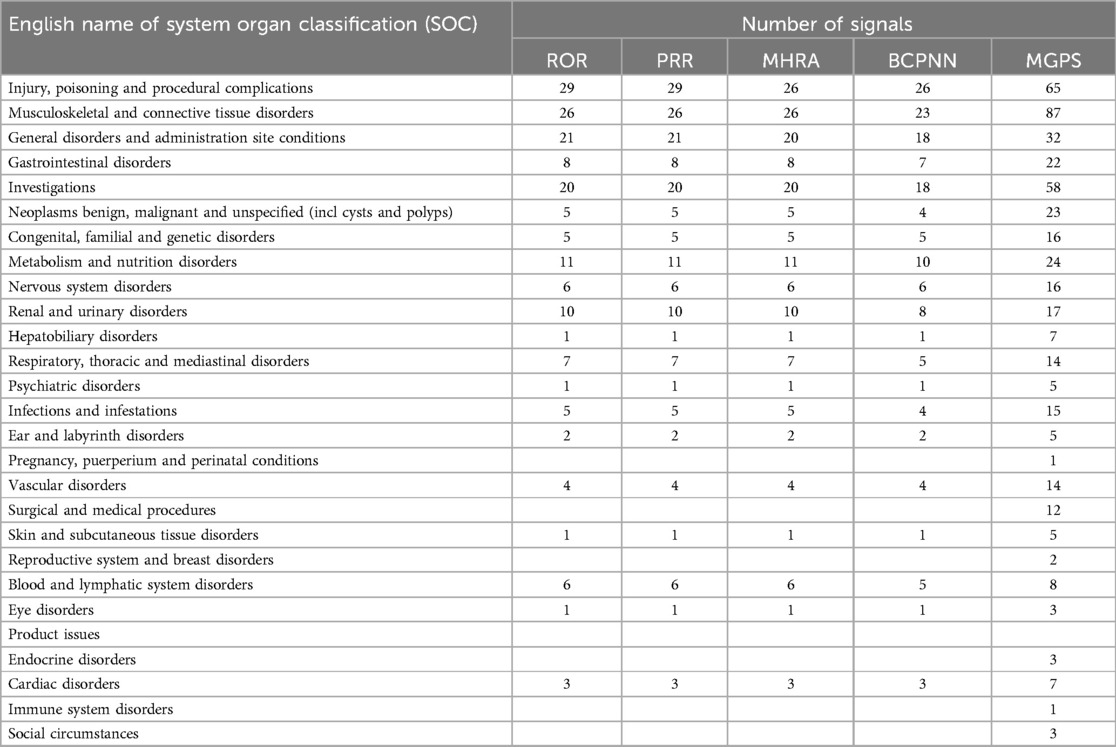
In this investigation, a systematic review of the literature was performed to identify specific clinical cases associated with adverse events (AEs) reported for seven bisphosphonates through a search of the System Organ Class (SOC) database. The results showed that the four adverse reactions with the highest number of positive signals were various examinations (ROR: 20; PRR: 20; MHRA: 20; BCPNN: 18; MGPS: 58), various musculoskeletal and connective tissue diseases (ROR: 26; PRR: 26; MHRA: 26; BCPNN:23; MGPS: 86), various injuries, poisoning and operational complications (ROR: 29; PRR: 29; MHRA: 26; BCPNN: 26; MGPS: 65) and systemic diseases and various reactions at the administration site (ROR: 21; PRR: 21; MHRA: 20; BCPNN: 18; MGPS: 32) (Table 5).
Two data mining techniques, specifically the proportional reporting ratio (ROR) and the Bayesian confidence propagation information component neural network (IC), are employed for conducting disproportionate analysis. All other drugs/events were documented for comparative analysis. Statistical findings indicate that pamidronate exhibited significant signals across various examinations (Figure 3a). Alendronate and ibandronate demonstrated high signal intensity for incidents such as diverse injuries, poisoning, and surgical complications (Figure 3b). For various musculoskeletal and connective tissue diseases, all bisphosphonates express strong signals, especially alendronate (Figure 3c). Systemic diseases and various reactions at the administration site are strongly signaled by zoledronate, pamidronate and ibandronate (Figure 3d).
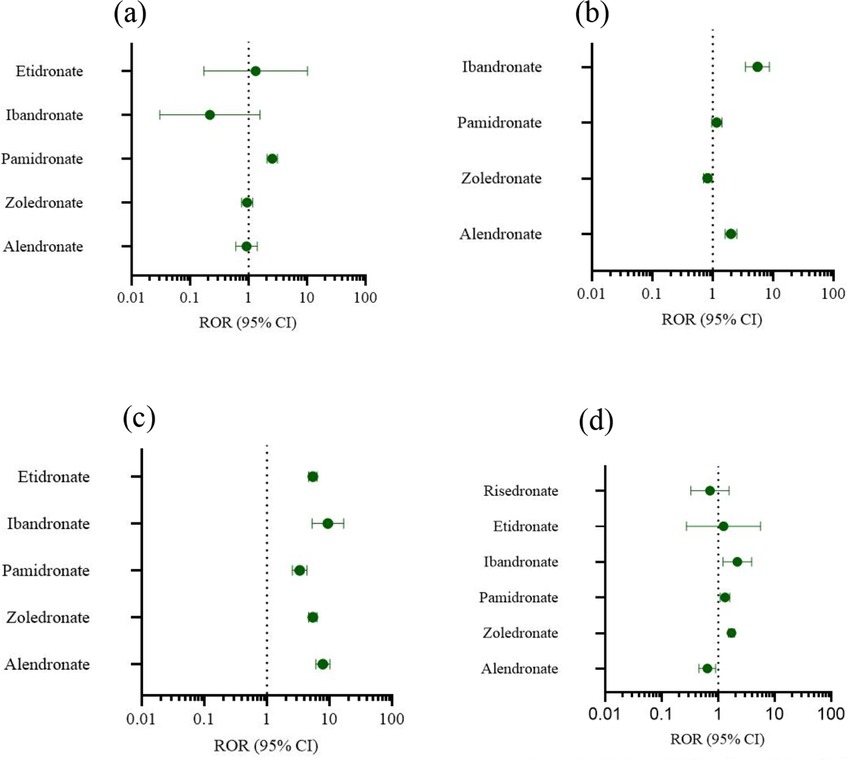
Figure 3. Forest plot of ROR. (a) Various examinations. (b) Injuries, poisoning and operational complications. (c) Musculoskeletal and connective tissue diseases. (d) Systematic diseases and reactions at the administration site.
4 Discussion
The widespread use of bisphosphonate has been supported by numerous studies demonstrating its efficacy, yet also revealing an increasing number of adverse reactions. This study represents the largest and most comprehensive analysis to date of the effectiveness and safety profile of bisphosphonates in the pediatric population. Data gathered from existing research and the FAERS database indicate that bisphosphonate exhibits superior therapeutic benefits compared to the control drug, but also carries certain safety risks.
This study is grounded in clinical observations indicating that glucocorticoids are implicated in the induction of osteoporosis and are frequently associated with adverse effects related to osteonecrosis. Notably, some pediatric patients exhibit both conditions simultaneously (32). Although the efficacy of bisphosphonates in treating pediatric osteonecrosis has been examined, it is unfortunate that our literature search concerning glucocorticoid-induced bone diseases, incorporating terms related to bone infarct, did not produce relevant studies (33). As a result, this study is limited to exploring glucocorticoid-induced osteoporosis.
Despite children's robust bone reconstruction ability (34), there remains a concerning and increasing prevalence of vertebral fractures in this population. Research indicates that approximately 10% of children screened within the first year exhibit vertebral fractures, with nearly half of these cases being asymptomatic (35). Furthermore, children experiencing stunted growth, older children with limited residual growth potential, and those at ongoing risk for compromised bone health are less likely to undergo vertebral body remodeling following a spinal fracture. Failure to initiate treatment promptly may result in lasting deformities of the vertebral bodies (36). Bisphosphonates are commonly utilized in managing osteoporosis induced by hormone therapy in pediatric patients. Previous research has demonstrated the effectiveness of bisphosphonates in enhancing bone density and preventing osteolysis, aligning with the findings of our study (37–39).
In this meta-analysis, a synthesis of 14 studies was conducted to assess the effectiveness and safety of bisphosphonate therapy in managing steroid-induced osteoporosis in pediatric patients. Notably, this study represents a novel contribution by specifically focusing on children who have received hormone therapy, distinguishing it from prior analyses. Our analysis included six randomized controlled trials involving 572 patients. Given the unique physiological functions of children and the specific characteristics of drug-induced osteoporosis, our study may offer valuable insights for the management of osteoporosis resulting from hormone therapy in pediatric patients. In this research, a random effect model was utilized to compare the efficacy of bisphosphonate with that of the control group, revealing a significant improvement in bone mineral density (BMD) and BMD Z-scores with bisphosphonate treatment. Furthermore, a fixed effect model was employed for analysis, indicating no significant disparity in the efficacy of bisphosphonate compared to the control group post-fracture treatment. Additionally, concerning safety considerations, the incidence of adverse reactions was found to be higher in children treated with bisphosphonate compared to those in the control group.
According to Kan SL et al.'s study, bisphosphonate therapy is more effective in preventing and treating osteoporosis in rheumatic patients compared to calcium, vitamin D, or calcitonin (40). Consistent with prior research findings, our study also demonstrates that bisphosphonates are efficacious in preventing and treating hormone-induced alterations in bone mineral density. However, our analysis reveals no statistically significant disparity in fracture risk between the treatment and control groups (41). Prior research has primarily focused on adult patients, prompting our investigation into the applicability of these findings to children. Subsequent studies have indicated that bisphosphonates may offer greater benefits compared to active vitamin D3 analogues in mitigating the risk of glucocorticoid-induced fractures (42). Furthermore, a number of studies have demonstrated the impact of bisphosphonates in mitigating the likelihood of fractures (16, 43–45). However, our findings do not align with this assertion, as it is posited that certain bisphosphonates exhibit a diminished efficacy in reducing fracture risk compared to control group preparations (42), likely attributable to the combined effects of various bisphosphonates. Consequently, further research is warranted to explore the efficacy of bisphosphonates in fracture treatment.
The potential adverse effects of bisphosphonates have garnered significant attention. These effects encompass gastrointestinal disturbances, musculoskeletal discomfort, and acute phase reactions. Additionally, in rare instances, bisphosphonates may precipitate atrial fibrillation, atypical fractures, delayed fracture healing, osteonecrosis of the jaw, hypersensitivity reactions, and renal impairment (46). In the research conducted by Edwards BJ et al., a notable temporal association was observed between bisphosphonate use and atypical femoral fractures, with the incidence of fractures also demonstrating a significant correlation with the duration of bisphosphonate therapy. Furthermore, several studies have reported instances of induced fractures and osteosclerosis in pediatric populations (47–49). Our study observed a significant risk of adverse reactions associated with bisphosphonates in comparison to the control group when used for treating glucocorticoid-induced osteoporosis in Pediatric Patients. To enhance our understanding of the adverse reaction risk of bisphosphonates and address the absence of specific adverse reactions in the Meta analysis, we incorporated cases from the FAERS database. The majority of case reporters are professionals, thereby enhancing the credibility of the data. Our analysis revealed that a significant proportion of patients experienced adverse reactions within one month of initiating bisphosphonate therapy, thereby strengthening the association between adverse reactions and bisphosphonates to a certain degree. This also indicates a likelihood of adverse reactions occurring in the short term, underscoring the importance of vigilant monitoring for any abnormal patient responses. To mitigate potential adverse reactions, we conducted a comprehensive analysis of adverse events categorized by System Organ Class (SOC). Specifically, we identified four prominent categories of adverse reactions: various examinations, musculoskeletal and connective tissue diseases, injuries, poisoning and operational complications, and systemic diseases and reactions at the administration site. Notably, within the FAERS database, various examinations encompassed endocrine, cardiovascular, blood, and other examinations exhibiting abnormal values. Based on the statistical findings, it was determined that Pamidronate showed notable indications in the category of various examinations for adverse reactions. Previous literature has documented adverse reactions associated with bisphosphonates, including hypocalcemia and secondary hyperparathyroidism. Our study indicates that pamidronate is particularly pertinent to these atypical laboratory findings (50). Various injuries, poisonings, and operational complications primarily encompass fractures, medication errors, and other accidents, with alendronate and ibandronate showing high signal strength for such incidents. Musculoskeletal and connective tissue diseases are predominantly associated with arthritis, osteonecrosis, muscle weakness, and related conditions. All bisphosphonates, particularly alendronate, have exhibited robust signals, which we attribute to the constraints of the FAERS database, which cannot clearly establish the causal relationship between diseases and adverse reactions. The high prevalence of bisphosphonate use in diseases commonly treated with glucocorticoids underscores the significance of these findings. Alendronate constitutes 84% of oral bisphosphonates, as reported in the literature (51). Systemic diseases and various reactions at the administration site, such as injection site reactions, fever, treatment ineffectiveness, and other events, are prominently observed in the adverse event profiles of zoledronate, pamidronate, and ibandronate. The intravenous administration of bisphosphonates may result in a transient acute phase reaction characterized by symptoms such as bone and muscle pain, fever, myalgia, fatigue, and lymphopenia. The severity of these reactions may be dose-dependent and should be carefully monitored during the infusion of these medications (52).
While our study demonstrated the safety and efficacy of bisphosphonates for treating osteoporosis induced by hormone therapy in children, we acknowledge that potential biases may have arisen due to variations in factors such as route of administration, dosage, disease management, gender, and duration of treatment. Furthermore, our research primarily involves a comparison of bisphosphonates with a control group, thereby limiting our ability to make definitive conclusions regarding individual drug efficacy. A significant constraint of utilizing the FAERS database is the incomplete data and potential for selection and reporting biases (53), which hinders our ability to accurately determine the incidence of adverse reactions associated with each specific drug. Moreover, the causal relationship between bisphosphonates and adverse reactions may be uncertain, and there is a possibility of duplicate reports. Therefore, further high-quality research is necessary to thoroughly investigate the safety profile of bisphosphonates.
In summary, our research offers recommendations for managing osteoporosis fatalities resulting from hormone therapy in pediatric patients. While bisphosphonates demonstrate notable effectiveness, they also carry inherent risks of adverse reactions. Therefore, when choosing medications, a comprehensive evaluation should be conducted to mitigate potential risks by regulating the duration of drug administration.
5 Conclusion
This meta-analysis demonstrates that bisphosphonates can enhance bone mineral density (BMD) and BMD Z scores in Pediatric patients with GCs-induced osteoporosis. However, it also carries a risk of adverse reactions, highlighting the importance of considering this factor in clinical drug selection. Nevertheless, due to limitations in the current research, further evaluation through high-quality randomized controlled trials is warranted.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: These data can be found at: (1) https://research.cchmc.org/aers/explore.jsp (2) https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis.
Ethics statement
The studies involving humans were approved by Beijing Children's Hospital, National Center for Children's Health, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XX: Data curation, Formal Analysis, Funding acquisition, Investigation, Writing – original draft. LW: Writing – original draft. MY: Writing – review & editing. YL: Methodology, Writing – original draft. CY: Writing – review & editing. DM: Project administration, Resources, Writing – review & editing. PG: Project administration, Resources, Writing – review & editing. HM: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by the National Key R&D Program of China (2021YFC2702005, 2023YFC2706101), Beijing Hospitals Authority’s Ascent Plan (DFL20221001), Beijing Municipal Hospital Scientific Research and Cultivation Program (PX2023043), and Tianchi Talent Program of Xinjiang Uygur Autonomous Region. Furthermore, we would like to thank the anonymous reviewers who contributed to the paper's improvement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ciancia S, Högler W, Sakkers RJB, Appelman-Dijkstra NM, Boot AM, Sas TCJ, et al. Osteoporosis in children and adolescents: how to treat and monitor? Eur J Pediatr. (2023) 182(2):501–11. doi: 10.1007/s00431-022-04743-x
2. Urquiaga M, Saag KG. Risk for osteoporosis and fracture with glucocorticoids. Best Pract Res Clin Rheumatol. (2022) 36(3):101793. doi: 10.1016/j.berh.2022.101793
3. Hansen KE, Kleker B, Safdar N, Bartels CM. A systematic review and meta-analysis of glucocorticoid-induced osteoporosis in children. Semin Arthritis Rheum. (2014) 44(1):47–54. doi: 10.1016/j.semarthrit.2014.02.002
4. Sarinho ESC, Melo VMPP. Glucocorticoid-induced bone disease: mechanisms and importance in pediatric practice. Doença óssea induzida pelos glicocorticoides: mecanismos e importância na prática pediátrica. Rev Paul Pediatr. (2017) 35(2):207–15. doi: 10.1590/1984-0462;2017/;35;2;00007
5. Melo VMPP, Viana MT, Melo MMP. Is there an association between glucocorticoid use and fractures? A comparative study in a trauma hospital. Existe associação entre o uso de glicocorticoides e a presença de fraturas? Estudo comparativo em um hospital de trauma. Rev Paul Pediatr. (2019) 37(1):4–10. doi: 10.1590/1984-0462/;2019;37;1;00001
6. Aceto G, D'Addato O, Messina G, Carbone V, Cavallo L, Brunetti G, et al. Bone health in children and adolescents with steroid-sensitive nephrotic syndrome assessed by DXA and QUS. Pediatr Nephrol. (2014) 29(11):2147–55. doi: 10.1007/s00467-014-2834-3
7. Song S, Guo Y, Yang Y, Fu D. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol Ther. (2022) 237:108168. doi: 10.1016/j.pharmthera.2022.108168
8. Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. (2020) 16(8):437–47. doi: 10.1038/s41574-020-0341-0
9. Laurent MR, Goemaere S, Verroken C, Bergmann P, Body JJ, Bruyère O, et al. Prevention and treatment of glucocorticoid-induced osteoporosis in adults: consensus recommendations from the Belgian bone club. Front Endocrinol (Lausanne). (2022) 13:908727. doi: 10.3389/fendo.2022.908727
10. Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. (2022) 399(10329):1080–92. doi: 10.1016/S0140-6736(21)02646-5
11. Gehrke B, Alves Coelho MC, Brasil d’Alva C, Madeira M. Long-term consequences of osteoporosis therapy with bisphosphonates. Arch Endocrinol Metab. (2023) 68:e220334. doi: 10.20945/2359-4292-2022-0334
12. Reszka AA, Rodan GA. Bisphosphonate mechanism of action. Curr Rheumatol Rep. (2003) 5(1):65–74. doi: 10.1007/s11926-003-0085-6
13. Drake MT, Cremers SC. Bisphosphonate therapeutics in bone disease: the hard and soft data on osteoclast inhibition. Mol Interv. (2010) 10(3):141–52. doi: 10.1124/mi.10.3.5
14. Fink HA, MacDonald R, Forte ML, Rosebush CE, Ensrud KE, Schousboe JT, et al. Long-term drug therapy and drug discontinuations and holidays for osteoporosis fracture prevention: a systematic review. Ann Intern Med. (2019) 171(1):37–50. doi: 10.7326/M19-0533
15. Syversen U, Halse JI. Bisfosfonatbehandling av osteoporose og andre skjelettsykdommer. [Bisphosphonate treatment of osteoporosis and other skeletal diseases]. Tidsskr Nor Laegeforen. (2011) 131(3):244–7. doi: 10.4045/tidsskr.09.0470
16. de Roij van Zuijdewijn C, van Dorp W, Florquin S, Roelofs J, Verburgh K. Bisphosphonate nephropathy: a case series and review of the literature. Br J Clin Pharmacol. (2021) 87(9):3485–91. doi: 10.1111/bcp.14780
17. Krueger CD, West PM, Sargent M, Lodolce AE, Pickard AS. Bisphosphonate-induced osteonecrosis of the jaw. Ann Pharmacother. (2007) 41(2):276–84. doi: 10.1345/aph.1H521; Jakobsen TS, Funding M. Ugeskr Laeger. 2022;184(28):V03220169.17299010
18. Rudge S, Hailwood S, Horne A, Lucas J, Wu F, Cundy T. Effects of once-weekly oral alendronate on bone in children on glucocorticoid treatment. Rheumatology (Oxford). (2005) 44(6):813–8. doi: 10.1093/rheumatology/keh538
19. Kim SD, Cho BS. Pamidronate therapy for preventing steroid-induced osteoporosis in children with nephropathy. Nephron Clin Pract. (2006) 102(3–4):c81–7. doi: 10.1159/000089664
20. Rooney M, Bishop N, Davidson J, Beresford MW, Pilkington C, Donagh JM, et al. The prevention and treatment of glucocorticoid-induced osteopaenia in juvenile rheumatic disease: a randomised double-blind controlled trial. EClinicalMedicine. (2019) 12:79–87. doi: 10.1016/j.eclinm.2019.06.004
21. Zacharin M, Lim A, Gryllakis J, Siafarikas A, Jefferies C, Briody J, et al. Randomized controlled trial evaluating the use of zoledronic acid in Duchenne muscular dystrophy. J Clin Endocrinol Metab. (2021) 106(8):2328–42. doi: 10.1210/clinem/dgab302
22. Ward LM, Choudhury A, Alos N, Cabral DA, Rodd C, Sbrocchi AM, et al. Zoledronic acid vs placebo in pediatric glucocorticoid-induced osteoporosis: a randomized, double-blind, phase 3 trial. J Clin Endocrinol Metab. (2021) 106(12):e5222–35. doi: 10.1210/clinem/dgab458
23. Acott PD, Wong JA, Lang BA, Crocker JF. Pamidronate treatment of pediatric fracture patients on chronic steroid therapy. Pediatr Nephrol. (2005) 20(3):368–73. doi: 10.1007/s00467-004-1790-8
24. Brown JJ, Zacharin MR. Attempted randomized controlled trial of pamidronate versus calcium and calcitriol supplements for management of steroid-induced osteoporosis in children and adolescents. J Paediatr Child Health. (2005) 41(11):580–2. doi: 10.1111/j.1440-1754.2005.00720.x
25. Inoue Y, Shimojo N, Suzuki S, Arima T, Tomiita M, Minagawa M, et al. Efficacy of intravenous alendronate for the treatment of glucocorticoid-induced osteoporosis in children with autoimmune diseases. Clin Rheumatol. (2008) 27(7):909–12. doi: 10.1007/s10067-008-0864-6
26. Inoue Y, Mitsunaga K, Yamamoto T, Chiba K, Yamaide F, Nakano T, et al. Early use of alendronate as a protective factor against the development of glucocorticoid-induced bone loss in childhood-onset rheumatic diseases: a cross-sectional study. Pediatr Rheumatol Online J. (2018) 16(1):36. doi: 10.1186/s12969-018-0258-5
27. Lim A, Simm PJ, James S, Lee SL, Zacharin M. Outcomes of zoledronic acid use in paediatric conditions. Horm Res Paediatr. (2020) 93(7-8):442–52. doi: 10.1159/000512730
28. Moretti A, Liguori S, Paoletta M, Gimigliano F, Iolascon G. Effectiveness of neridronate in the management of bone loss in patients with Duchenne muscular dystrophy: results from a pilot study. Adv Ther. (2022) 39(7):3308–15. doi: 10.1007/s12325-022-02179-1
29. Nasomyont N, Tian C, Hornung L, Khoury J, Hochwalt PM, Tilden JC, et al. The effect of oral bisphosphonate therapy on vertebral morphometry and fractures in patients with Duchenne muscular dystrophy and glucocorticoid-induced osteoporosis. Muscle Nerve. (2021) 64(6):710–6. doi: 10.1002/mus.27416
30. Srinivasan R, Rawlings D, Wood CL, Cheetham T, Moreno AC, Mayhew A, et al. Prophylactic oral bisphosphonate therapy in Duchenne muscular dystrophy. Muscle Nerve. (2016) 54(1):79–85. doi: 10.1002/mus.24991
31. Tian C, Wong BL, Hornung L, Khoury JC, Rybalsky I, Shellenbarger KC, et al. Oral bisphosphonate treatment in patients with Duchenne muscular dystrophy on long term glucocorticoid therapy. Neuromuscul Disord. (2020) 30(7):599–610. doi: 10.1016/j.nmd.2020.06.005
32. van Houten P, de Rooy J, van der Geest I, Netea-Maier R, van de Ven A. Spontaneous bone infarction of the distal femur in a patient with Cushing’s disease: a case report. Bone Rep. (2021) 14:100756. doi: 10.1016/j.bonr.2021.100756
33. Grimbly C, Escagedo PD, Jaremko JL, Bruce A, Alos N, Robinson ME, et al. Sickle cell bone disease and response to intravenous bisphosphonates in children. Osteoporos Int. (2022) 33(11):2397–408. doi: 10.1007/s00198-022-06455-2
34. Horton DB, Haynes K, Denburg MR, Thacker MM, Rose CD, Putt ME, et al. Oral glucocorticoid use and osteonecrosis in children and adults with chronic inflammatory diseases: a population-based cohort study. BMJ Open. (2017) 7(7):e016788. doi: 10.1136/bmjopen-2017-016788
35. Galindo-Zavala R, Bou-Torrent R, Magallares-López B, Mir-Perelló C, Palmou-Fontana N, Sevilla-Pérez B, et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr Rheumatol Online J. (2020) 18(1):20. doi: 10.1186/s12969-020-0411-9
36. Ward LM. Glucocorticoid-induced osteoporosis: why kids are different. Front Endocrinol (Lausanne). (2020) 11:576. doi: 10.3389/fendo.2020.00576
37. Lane NE. Glucocorticoid-Induced osteoporosis: new insights into the pathophysiology and treatments. Curr Osteoporos Rep. (2019) 17(1):1–7. doi: 10.1007/s11914-019-00498-x
38. Saag KG, Emkey R, Schnitzer TJ, Brown JP, Hawkins F, Goemaere S, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-induced osteoporosis intervention study group. N Engl J Med. (1998) 339(5):292–9. doi: 10.1056/NEJM199807303390502
39. Wallach S, Cohen S, Reid DM, Hughes RA, Hosking DJ, Laan RF, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. (2000) 67(4):277–85. doi: 10.1007/s002230001146
40. Feng Z, Zeng S, Wang Y, Zheng Z, Chen Z. Bisphosphonates for the prevention and treatment of osteoporosis in patients with rheumatic diseases: a systematic review and meta-analysis. PLoS One. (2013) 8(12):e80890. doi: 10.1371/journal.pone.0080890
41. Homik J, Cranney A, Shea B, Tugwell P, Wells G, Adachi R, et al. Bisphosphonates for steroid induced osteoporosis. Cochrane Database Syst Rev. (2000) (2):CD001347. doi: 10.1002/14651858.CD001347. Update in: Cochrane Database Syst Rev. (2016) 10:CD001347. doi: 10.1002/14651858.CD00134710796432
42. de Nijs RN, Jacobs JW, Algra A, Lems WF, Bijlsma JW. Prevention and treatment of glucocorticoid-induced osteoporosis with active vitamin D3 analogues: a review with meta-analysis of randomized controlled trials including organ transplantation studies. Osteoporos Int. (2004) 15(8):589–602. doi: 10.1007/s00198-004-1614-5
43. Everts-Graber J, Bonel H, Lehmann D, Gahl B, Häuselmann H, Studer U, et al. Comparison of anti-fracture effectiveness of zoledronate, ibandronate and alendronate versus denosumab in a registry-based cohort study. Osteoporos Int. (2023) 34(11):1961–73. doi: 10.1007/s00198-023-06863-y
44. Allen CS, Yeung JH, Vandermeer B, Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev. (2016) 10(10):CD001347. doi: 10.1002/14651858.CD001347.pub2
45. Zhao S, Zhao W, Du D, Zhang C, Zhao T, Zheng L, et al. Effect of bisphosphonate on hip fracture in patients with osteoporosis or osteopenia according to age: a meta-analysis and systematic review. J Investig Med. (2022) 70(3):837–43. doi: 10.1136/jim-2021-001961
46. Rizzoli R, Reginster JY, Boonen S, Bréart G, Diez-Perez A, Felsenberg D, et al. Adverse reactions and drug-drug interactions in the management of women with postmenopausal osteoporosis. Calcif Tissue Int. (2011) 89(2):91–104. doi: 10.1007/s00223-011-9499-8
47. Edwards BJ, Bunta AD, Lane J, Odvina C, Rao DS, Raisch DW, et al. Bisphosphonates and nonhealing femoral fractures: analysis of the FDA adverse event reporting system (FAERS) and international safety efforts: a systematic review from the research on adverse drug events and reports (RADAR) project. J Bone Joint Surg Am. (2013) 95(4):297–307. doi: 10.2106/JBJS.K.01181
48. Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, et al. Incidence of all atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. (2012) 27(12):2544–50. doi: 10.1002/jbmr.1719
49. Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res. (2008) 23(10):1698–707. doi: 10.1359/jbmr.080511. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. (2003) 349(5):457–6318505375
50. Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens). (2009) 8(2):96–110. doi: 10.14310/horm.2002.1226
51. Psimma C, Psimma Z, Willems HC, Klüter WJ, van der Maarel-Wierink CD. Oral bisphosphonates: adverse effects on the oral mucosa not related to the jaw bones. A scoping review. Gerodontology. (2022) 39(4):330–8. doi: 10.1111/ger.12590
52. Horikawa A, Miyakoshi N, Hongo M, Kasukawa Y, Kodama H, Shimada Y. A prospective comparative study of intravenous alendronate and ibandronate for the treatment of osteoporosis. Medicine (Baltimore). (2019) 98(6):e14340. doi: 10.1097/MD.0000000000014340
Keywords: bisphosphonates, osteoporosis, glucocorticoid, adverse reactions, meta-analysis, pediatrics
Citation: Xu X, Wang L, Yang M, Li Y, Yang C, Mei D, Guo P and Mao H (2025) Efficacy and safety of bisphosphonates in pediatric glucocorticoid-induced osteoporosis: a meta-analysis and pharmacovigilance study. Front. Pediatr. 13:1571381. doi: 10.3389/fped.2025.1571381
Received: 5 February 2025; Accepted: 17 June 2025;
Published: 7 July 2025.
Edited by:
Margherita Neri, University of Ferrara, ItalyReviewed by:
Maria Felicia Faienza, University of Bari Aldo Moro, ItalyOscar Brunetto, Hospital Pedro de Elizalde, Argentina
Copyright: © 2025 Xu, Wang, Yang, Li, Yang, Mei, Guo and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huawei Mao, bWFvaHdlaUBxcS5jb20=; Peng Guo, Mjk1OTU1OTk5QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiaolin Xu
Xiaolin Xu Luquan Wang
Luquan Wang Mengyang Yang1,†
Mengyang Yang1,† Yan Li
Yan Li Huawei Mao
Huawei Mao