- 1Department of Pediatric Surgery, Affiliated Hospital of Zunyi Medical University, Zunyi, China
- 2Department of Pediatric Surgery, Guizhou Children’s Hospital, Zunyi, Guizhou, China
- 3Department of General Surgery–Hernia and Pediatric Surgery, Northern Jiangsu People’s Hospital, Yangzhou University, Yangzhou, Jiangsu, China
- 4Department of Gastrointestinal Surgery, The Second Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, China
Objective: To explore early surgical indications and clinical predictive factors for neonatal necrotizing enterocolitis (NEC) to improve the prognosis of affected infants.
Methods: A retrospective analysis was conducted on the clinical data of 146 infants diagnosed with NEC at the Affiliated Hospital of Zunyi Medical University from January 2015 to December 2020. The infants were divided into two groups: the surgical treatment group (56 cases) and the non-surgical treatment group (90 cases). Maternal perinatal conditions, general infant characteristics, clinical manifestations, comorbidities, laboratory tests, and imaging findings were statistically analyzed. Significant factors were further analyzed using multivariate logistic regression, and predictive indicators were assessed by the receiver operating characteristic (ROC) curve and Youden's index.
Results: Statistically significant differences were observed between the two groups in birth weight, gestational age, abdominal wall erythema, absent bowel sounds, lethargy, fever, peritonitis, septic shock, metabolic acidosis, neonatal acute respiratory distress syndrome, and asphyxia (P < 0.05). No significant differences were found in maternal perinatal conditions, sex, feeding method, age at onset, abdominal distention, bloody stool, vomiting, gastric retention, apnea, neonatal pneumonia, neonatal hyperbilirubinemia, sepsis, electrolyte disturbances, or respiratory failure (P > 0.05). Laboratory and imaging markers such as prealbumin, IL-6, PCT, CRP, WBC, pneumoperitoneum, bowel wall gas, and portal venous gas showed statistically significant differences (P < 0.05). Multivariate logistic regression identified peritonitis (OR = 95.635), IL-6 (OR = 1.001), and portal venous gas (OR = 22.551) as independent risk factors for early surgery in NEC (P < 0.05). ROC curve analysis revealed that IL-6 (AUC = 0.875) and PCT (AUC = 0.798) demonstrated good predictive performance for early surgical intervention. The optimal cutoff values were 476 pg/ml for IL-6 (sensitivity 80.4%, specificity 85.6%) and 1.53 ng/ml for PCT (sensitivity 83.9%, specificity 70%).
Conclusion: Peritonitis and portal venous gas are independent risk factors for early surgery in NEC. IL-6 and PCT are reliable predictive markers for determining the need for early surgical intervention in NEC.
Introduction
Neonatal necrotizing enterocolitis (NEC) is one of the most common and life-threatening gastrointestinal emergencies in neonates, characterized by rapid progression and high mortality, with fatality rates reported as high as 35% (1). In recent years, advances in neonatal intensive care have significantly improved the survival rates of extremely low birth weight (ELBW) and ultra-low birth weight (ULBW) infants. However, this improvement has also been accompanied by a rising incidence of NEC, particularly among high-risk neonates (2).
The early clinical manifestations of NEC are typically nonspecific and insidious, making timely diagnosis and intervention extremely challenging. Many infants are diagnosed only after disease progression has occurred, and approximately 50% eventually require surgical treatment (3). However, the clinical course of NEC is highly variable, and significant heterogeneity exists in its pathophysiology among affected infants. Determining the optimal timing for surgical intervention remains a major clinical dilemma: delayed surgery can lead to extensive intestinal necrosis and worsened outcomes, whereas premature surgery may expose neonates to unnecessary risks.
Previous studies have attempted to identify predictive markers of surgical necessity in NEC, including abnormal vital signs, inflammatory biomarkers, and radiographic findings. Nevertheless, there is currently no widely accepted or quantitatively validated set of early predictors to guide surgical decision-making, particularly in the early stages of the disease when symptoms are subtle or atypical.
We hypothesize that a distinct set of clinical signs, laboratory parameters, and radiographic features observed at the time of NEC diagnosis may serve as independent risk factors to predict the need for surgical intervention.
To test this hypothesis, we conducted a retrospective analysis of neonates diagnosed with NEC and hospitalized at the Affiliated Hospital of Zunyi Medical University between January 2015 and December 2020. By comparing the clinical data of patients in the surgical and non-surgical groups, we aimed to identify potential early surgical indicators and predictive biomarkers that may assist in determining the optimal timing for surgical intervention, ultimately improving patient outcomes.
Methods
Data sources
A total of 219 neonates diagnosed with NEC were admitted to the Neonatology and Pediatric Surgery Departments of the Affiliated Hospital of Zunyi Medical University between January 2015 and December 2020. Of these, 146 neonates met the inclusion criteria. This study adhered to the principles of the 2013 Declaration of Helsinki. Ethics approval number: KLL-2023-570.
Inclusion criteria
1. Met the diagnostic criteria for NEC (4).
2. Complete clinical data, including detailed records of maternal perinatal abnormalities, neonatal perinatal conditions, clinical manifestations, comorbidities, laboratory test results, abdominal x-ray findings, detailed surgical records, and discharge outcomes.
Exclusion criteria
1. Neonates who died within 48 h of hospitalization and did not undergo adequate clinical assessment or imaging/Laboratory evaluation to determine surgical eligibility.
2. Neonates requiring surgery but whose families refused surgical treatment.
3. Neonates discharged against medical advice before meeting the discharge criteria for conservative treatment.
4. Incomplete medical records, including missing imaging or laboratory tests on the day of NEC diagnosis.
Study design
A total of 146 neonates meeting the inclusion criteria were divided into a surgical treatment group (56 cases) and a non-surgical treatment group (90 cases). The surgical treatment criteria included a confirmed NEC diagnosis, failure of conservative treatment within 24–48 h, and progressive worsening of symptoms. In the surgical group, the extent of bowel lesions, surgical procedures, and outcomes were recorded. The following indicators were analyzed:
1. Maternal perinatal conditions: Including premature rupture of membranes, abnormal amniotic fluid, hypertensive disorders of pregnancy, placenta previa, amniotic cavity infection, and intrauterine distress.
2. Neonatal general characteristics: Including sex, birth weight, gestational age, feeding method, and age at onset.
3. Clinical manifestations: Including abdominal distention, vomiting, bloody stools, gastric retention, fever, lethargy, abdominal wall erythema, absent bowel sounds, and apnea.
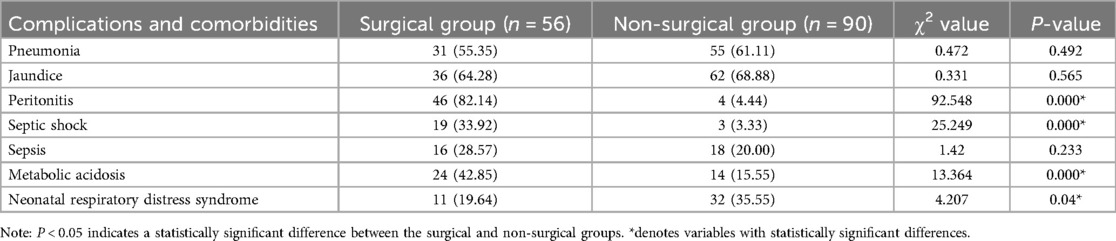
4. Comorbidities: Including peritonitis, septic shock, metabolic acidosis, pneumonia, neonatal hyperbilirubinemia, sepsis, electrolyte disturbance, respiratory failure, neonatal respiratory distress syndrome (NRDS), and asphyxia.
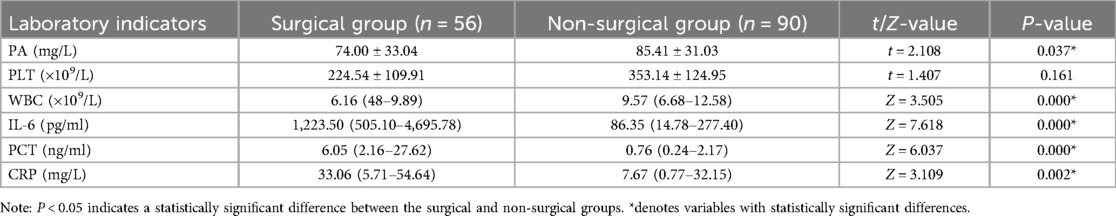
5. Laboratory tests: Including prealbumin (PA), interleukin-6 (IL-6), procalcitonin (PCT), C-reactive protein (CRP), white blood cell count (WBC), and platelet count (PLT).
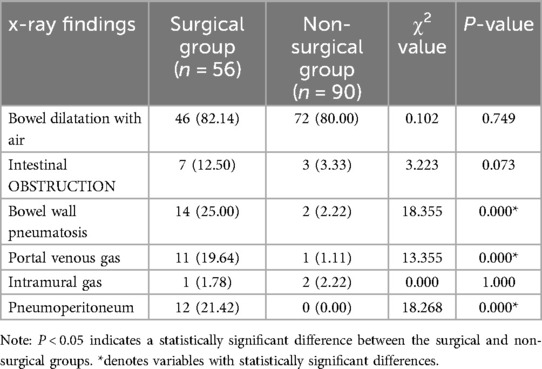
6. Imaging findings: Abdominal x-ray and ultrasound findings, including intestinal gas dilatation, bowel wall gas, bowel obstruction, interintestinal fluid, portal venous gas, and pneumoperitoneum.
Analytical methods
Univariate analysis was performed on the indicators listed above. Variables with statistical significance were subjected to multivariate logistic regression analysis. Laboratory indicators were evaluated using receiver operating characteristic (ROC) curves and Youden's index. The area under the curve (AUC) was calculated to assess predictive performance. An AUC >0.7 indicated good predictive performance, while an AUC <0.5 indicated no predictive value. The laboratory indicator with the largest AUC was considered to have the best predictive performance. The optimal cutoff values for laboratory indicators were determined using Youden's index (calculated as sensitivity + specificity−1).
Statistical analysis
Statistical analyses were conducted using SPSS software version 19.0. Categorical data were expressed as frequencies and percentages, while continuous data were presented as mean ± standard deviation (x¯ ± SD) for normally distributed variables or median (interquartile range) [M (P25–P75)] for non-normally distributed variables. Comparisons of categorical data between groups were performed using the χ2 test (or the χ2 correction formula for 1 ≤ T < 5). Normally distributed continuous variables were compared using the t-test, while non-normally distributed variables were analyzed using the Mann–Whitney U-test. Multivariate analysis was conducted using logistic regression, with results expressed as odds ratios (OR) and 95% confidence intervals (95% CI). A P-value <0.05 was considered statistically significant.
Results
General characteristics
Among the 219 neonates diagnosed with NEC, 146 cases were ultimately included in the analysis. A total of 73 cases were excluded for the following reasons:
1. Two neonates died within 48 h of admission due to rapid clinical deterioration and were excluded because they did not undergo sufficient clinical assessment, imaging, or laboratory evaluation to determine surgical eligibility.
2. Nineteen neonates who met surgical criteria were discharged against medical advice after their families declined surgical intervention, often opting for palliative care following a detailed explanation of the prognosis.
3. Twenty-eight neonates undergoing conservative treatment were discharged prematurely without meeting formal discharge criteria, typically due to family choice or socioeconomic constraints.
4. Twenty-four cases were excluded due to incomplete clinical data, including missing imaging or laboratory results.
Intraoperative findings and outcomes in the surgical group
Of the 56 neonates in the surgical group, intestinal perforation was observed in 31 cases (55.36%). Among these, 15 cases involved small bowel perforation (13 ileal perforations), and 16 cases involved colonic perforation (11 transverse colonic perforations). Thirteen neonates presented with perforations in more than two intestinal sites. All 56 neonates exhibited varying degrees of intestinal necrosis (100%), with the following distributions:
1. Small bowel necrosis: 39 cases (30 ileal necroses, 5 jejunal necroses, and 6 cases of extensive small bowel necrosis).
2. Colonic necrosis: 32 cases (25 cases of ascending and transverse colon necrosis, and 18 cases of extensive colonic necrosis or necrosis in more than two sites).
Surgical procedures
1. Resection of necrotic bowel with primary anastomosis: 8 cases.
2. Resection of necrotic bowel with stoma creation: 41 cases.
3. Intestinal decompression: 1 case.
4. Extensive total small bowel necrosis: 6 cases (5 families opted for withdrawal of treatment, and 1 case underwent palliative peritoneal drainage).
The shortest resected necrotic bowel length was 3 cm, while the longest was 70 cm. Fourteen neonates had necrosis exceeding 20 cm, and 6 neonates had necrosis exceeding 40 cm.
Outcomes
Ten neonates died during hospitalization. Among them, six deaths were attributed to extensive intestinal necrosis, and four were due to complications. The remaining neonates were successfully treated and discharged.
Comparison of clinical data between groups
Comparison of Maternal Perinatal Factors see Table 1
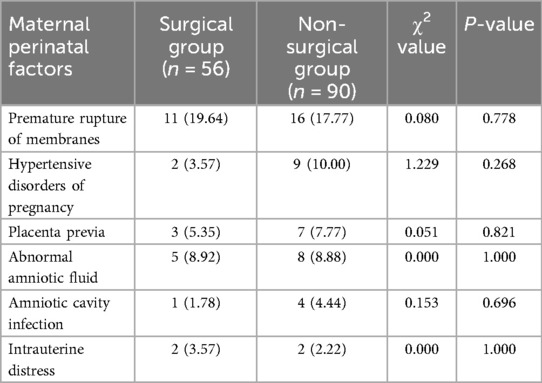
Table 1. Comparison of maternal perinatal factors between the surgical and non-surgical groups [n (%)].
Comparison of general perinatal characteristics between the two groups
Gestational age and birth weight showed statistically significant differences between the surgical and non-surgical groups (P < 0.05) see Table 2.
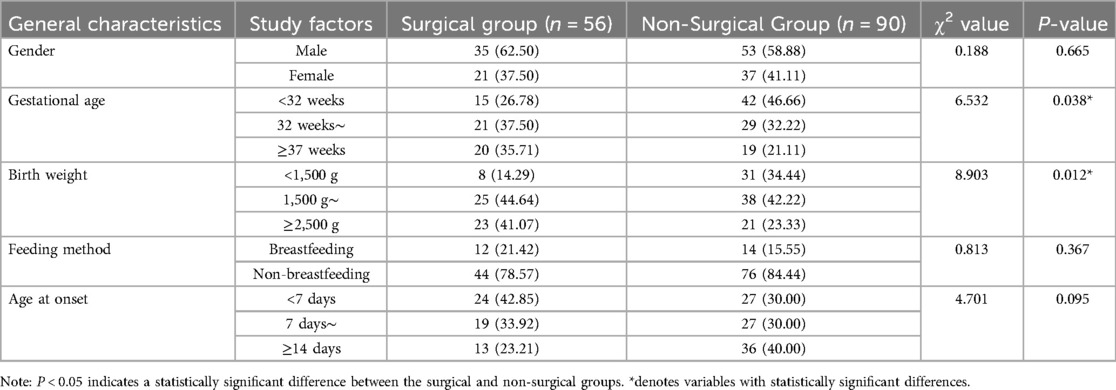
Table 2. Comparison of general perinatal characteristics between the surgical and non-surgical groups [n (%)].
Comparison of clinical manifestations between the two groups
Significant differences were observed between the surgical and non-surgical groups in poor response, fever, abdominal wall erythema, and absent bowel sounds (P < 0.05) see Table 3.
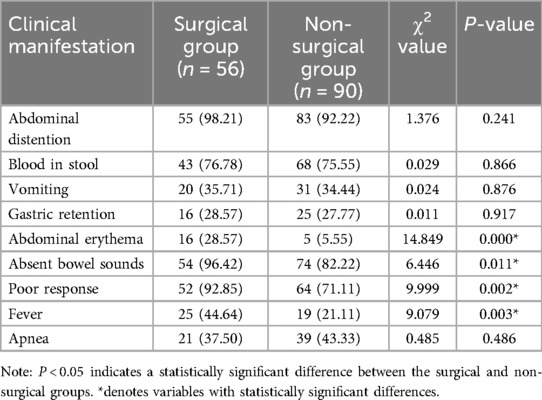
Table 3. Comparison of clinical manifestations between the surgical and non-surgical groups [n (%)].
Comparison of comorbidities
Significant differences were observed between the surgical and non-surgical groups in peritonitis, septic shock, metabolic acidosis, and neonatal respiratory distress syndrome (P < 0.05) see Table 4.
Comparison of laboratory tests between the two groups
Significant differences were observed between the surgical and non-surgical groups for PA, IL-6, PCT, CRP, and WBC (P < 0.05) see Table 5.
Comparison of imaging findings
Significant differences were observed between the surgical and non-surgical groups in bowel wall gas, portal venous gas, and pneumoperitoneum (P < 0.05) see Table 6.
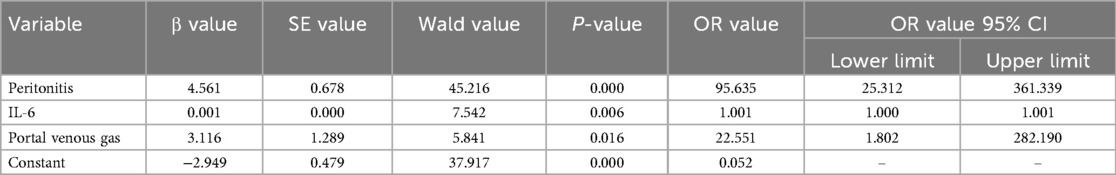
Multivariate logistic regression analysis
Peritonitis (OR = 95.635, 95% CI: 25.312\u2013361.339), IL-6 (OR = 1.001, 95% CI: 1.000\u20131.001), and portal venous gas (OR = 22.551, 95% CI: 1.802\u2013282.190) were identified as independent factors for predicting early surgical indications in NEC (P < 0.05) see Table 7.
ROC curve analysis of laboratory indicators
The results of the ROC curve analysis and Youden index indicated that IL-6 (AUC = 0.875) and PCT (AUC = 0.798) demonstrated good predictive performance for early surgical intervention in NEC, with IL-6 showing the best predictive efficacy, followed by PCT. CRP (AUC = 0.653) had moderate predictive efficacy, while WBC and PA had no predictive value. The optimal cutoff value for predicting surgical timing was 476 pg/ml for IL-6, 1.53 ng/ml for PCT, and 27.515 mg/L for CRP. Among these indicators, IL-6 had the highest specificity, PCT had the highest sensitivity, and CRP showed poor sensitivity and specificity see Figure 1 and Table 8.
Discussion
Currently, most scholars agree that the optimal timing for NEC surgery is when there is intestinal ischemia and necrosis but before overt perforation occurs (5). However, accurately determining this window—especially in the absence of definitive signs such as pneumoperitoneum or clinical deterioration—remains a key challenge in neonatal surgical decision-making. Although peritonitis and portal venous gas are recognized indicators for surgical intervention, they may appear only in advanced stages of NEC, limiting their utility for early decision-making. Therefore, this study aimed to identify earlier, quantifiable clinical and biochemical markers that can supplement traditional indicators and better inform surgical timing.
In our cohort, peritonitis, portal venous gas, and elevated levels of IL-6 and PCT were independently associated with the need for surgical intervention. While peritonitis and portal venous gas are not novel indicators, their independent statistical significance in this analysis reaffirms their importance in clinical practice. Rather than proposing new criteria, our intention was to assess whether newer biomarkers—particularly IL-6—can serve as early warning indicators in patients with subtle or equivocal signs, thus supporting more timely intervention.
The predictive role of IL-6 has been previously reported (6), and our study reinforces its utility in this context. Although the odds ratio (OR) for IL-6 was 1.001, this reflects its use as a continuous variable across a wide range of concentrations (from <100 to >5,000 pg/ml). As such, a per-unit OR may underestimate its actual clinical relevance when applied to larger magnitude changes. Statistical consultation confirmed the robustness of this result, and IL-6 showed the highest predictive performance (AUC = 0.875) among all parameters tested.
PCT also emerged as a useful biomarker, with an optimal cutoff of 1.53 ng/ml and good sensitivity and specificity (83.9% and 70%, respectively), consistent with earlier reports (7). IL-6 and PCT may jointly enhance early risk stratification in NEC. CRP was also elevated in surgical cases but showed relatively modest predictive value, aligning with existing literature (8, 9).
Previous studies have reported that NEC infants with intestinal perforation are predominantly preterm and of low birth weight, likely due to immature intestinal development and impaired perfusion, placing them at higher risk for requiring surgical intervention (2, 10). Consistent with prior studies (11–14), lower gestational age and birth weight were significantly more frequent in the surgical group, reaffirming their role as risk factors for disease progression and surgical intervention. Conversely, although 82.2% of NEC infants were not breastfed, feeding mode did not differ significantly between groups, suggesting a stronger association with disease onset rather than progression to surgical NEC (15, 16).
With respect to imaging, bowel wall gas and portal venous gas—but not all forms of bowel dilation—were significantly associated with surgery, in line with prior consensus (9, 17). Notably, 20% (3/15) of patients with surgically confirmed perforation had no radiographic evidence of pneumoperitoneum, emphasizing the limitation of imaging alone and supporting a multimodal approach incorporating biochemical and clinical findings.
Limitations
This study has several limitations. First, its single-center, retrospective design and moderate sample size limit generalizability and introduce potential selection bias. Second, the retrospective nature inherently limits the ability to establish causality, and the inclusion of known surgical indicators such as peritonitis may introduce confirmation bias. Third, the inflammatory markers evaluated—CRP, PCT, and IL-6—are non-specific and may be elevated in other infections or systemic inflammatory states. Therefore, interpretation must occur in conjunction with clinical and radiological assessments. Lastly, while IL-6 showed statistical significance, its low OR value should be interpreted cautiously due to the nature of the continuous variable; further prospective validation is needed.
Conclusion
This study identified peritonitis, portal venous gas, elevated IL-6, and elevated PCT as independent risk factors for early surgical intervention in neonates with NEC. Among these, IL-6 demonstrated the highest predictive accuracy, suggesting its value as an objective biomarker for surgical timing. Additionally, low gestational age and birth weight remain essential considerations for early risk identification.
These findings underscore the importance of a comprehensive, multimodal evaluation that incorporates clinical signs, laboratory markers, and imaging to guide timely and individualized surgical decision-making. While these results support preliminary clinical utility, prospective multicenter studies involving dynamic monitoring and disease-specific biomarkers are warranted to refine early surgical criteria and improve outcomes in NEC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Zunyi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Software, Validation, Visualization, Writing – review & editing. HW: Conceptualization, Formal analysis, Investigation, Writing – original draft. YL: Data curation, Methodology, Project administration, Supervision, Validation, Writing – original draft. XY: Visualization, Writing – review & editing, Software. LH: Visualization, Data curation, Validation, Writing – review & editing. HF: Software, Writing – original draft, Investigation, Visualization. LX: Resources, Writing – original draft, Methodology, Software. SX: Visualization, Conceptualization, Resources, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was conducted solely with institutional resources available at the Department of Pediatric Surgery, Affiliated Hospital of Zunyi Medical University, and Guizhou Children's Hospital. This work was supported by Guizhou Provincial Health Commission [gzwkj2024-431] and Guizhou Science and Technology Cooperation Achievement LC [2025] General No. 142. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hau EM, Meyer SC, Berger S, Goutaki M, Kordasz M, Kessler U. Gastrointestinal sequelae after surgery for necrotising enterocolitis: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2019) 104(3):F265–73. doi: 10.1136/archdischild-2017-314435
2. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20(1):344. doi: 10.1186/s12887-020-02231-5
3. Carr BD, Gadepalli SK. Does surgical management alter outcome in necrotizing enterocolitis? Clin Perinatol. (2019) 46(1):89–100. doi: 10.1016/j.clp.2018.09.008
4. Evidence-Based Professional Committee, Neonatology Branch of the Chinese Medical Doctor Association. Clinical guidelines for the diagnosis and treatment of neonatal necrotizing enterocolitis (2020). Chin J Contemp Pediatr. (2021) 23:1–11. doi: 10.7499/j.issn.1008-8830.2011145
5. Han JB, Yu MN, Liu G, Li G, Cao JY, Duan L, et al. Comparative study of postoperative outcomes in neonates with necrotizing enterocolitis with and without intestinal perforation. J Clin Pediatr Surg. (2022) 21:530–4. doi: 10.3760/cma.j.cn101785-202007046-006
6. Zheng ZB, Liu YM, Zhang F, Jin Z, Mao YC, Gao MJ, et al. Application of procalcitonin, interleukin-6, and C-reactive protein in determining surgical timing for neonatal necrotizing enterocolitis. J Clin Pediatr Surg. (2019) 18:361–7. doi: 10.3969/j.issn.1671-6353.2019.05.005
7. Gregory KE. Disease prediction strategies for necrotizing enterocolitis. J Perinat Neonatal Nurs. (2015) 29(1):5–7. doi: 10.1097/JPN.0000000000000088
8. Patel EU, Wilson DA, Brennan EA, Lesher AP, Ryan RM. Earlier re-initiation of enteral feeding after necrotizing enterocolitis decreases recurrence or stricture: a systematic review and meta-analysis. J Perinatol. (2020) 40(11):1679–87. doi: 10.1038/s41372-020-0722-1
9. Liu Y, Qingjuan S, Gao Z, Deng C, Wang Y, Guo C. Circulating fibrocytes are involved in inflammation and leukocyte trafficking in neonates with necrotizing enterocolitis. Medicine (Baltimore). (2017) 96(26):e7400. doi: 10.1097/MD.0000000000007400
10. Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med. (2015) 372(4):331–40. doi: 10.1056/NEJMoa1403489
11. Zhong WT, Lin TL, Chen JL, He QM, Tian Y, Ma ZY, et al. Risk factor analysis for surgical treatment in neonates with necrotizing enterocolitis. Chin J Neonatol. (2023) 38:29–33. doi: 10.3760/cma.j.issn.2096-2932.2023.01.007
12. Lü ZB, Sheng QF. Advances in the etiology and diagnosis of neonatal necrotizing enterocolitis. J Clin Pediatr Surg. (2019) 18:352–5. doi: 10.3969/j.issn.1671-6353.2019.05.003
13. Zhang ZB. Neonatal necrotizing enterocolitis: interpretation of bell staging and determination of surgical indications. J Clin Pediatr Surg. (2022) 21:306–9. doi: 10.3760/cma.j.cn101785-202201045-002
14. Qian T, Zhang R, Zhu L, Shi P, Yang J, Yang CY, et al. Necrotizing enterocolitis in low birth weight infants in China: mortality risk factors expressed by birth weight categories. Pediatr Neonatol. (2017) 58(6):509–15. doi: 10.1016/j.pedneo.2016.10.004
15. Chen Y, Koike Y, Chi L, Ahmed A, Miyake H, Li B, et al. Formula feeding and immature gut microcirculation promote intestinal hypoxia, leading to necrotizing enterocolitis. Dis Model Mech. (2019) 12(12):dmm040998. doi: 10.1242/dmm.040998
16. Singh DK, Miller CM, Orgel KA, Dave M, Mackay S, Good M. Necrotizing enterocolitis:bench to bedside approaches and d advancing our understanding of disease pathogenesis. Front Pediatr. (2023) 10:1107404. doi: 10.3389/fped.2022.1107404
Keywords: neonatal necrotizing enterocolitis, early surgery, peritonitis, IL-6, PCT, portal venous gas
Citation: Shang X, Wang H, Liu Y, Yang X, Huang L, Fu H, Xiang L and Xu S (2025) Analysis of early surgical indications and related factors in neonatal necrotizing enterocolitis. Front. Pediatr. 13:1571921. doi: 10.3389/fped.2025.1571921
Received: 6 February 2025; Accepted: 14 April 2025;
Published: 20 May 2025.
Edited by:
Simonetta Costa, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Laura Hollinger, Medical University of South Carolina, United StatesEric Rellinger, University of Kentucky, United States
Copyright: © 2025 Shang, Wang, Liu, Yang, Huang, Fu, Xiang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanmei Liu, eXVhbm1laTExNkBhbGl5dW4uY29t
 Xianhui Shang
Xianhui Shang Huinan Wang3
Huinan Wang3 Yuanmei Liu
Yuanmei Liu Xuefeng Yang
Xuefeng Yang Lu Huang
Lu Huang