- 1Maternal-Fetal Medicine Division, Instituto Nacional de Perinatologia, Mexico City, Mexico
- 2Department of Epidemiology and Public Health, Universidad Panamericana School of Medicine, Mexico City, Mexico
- 3Neonatology Division, Instituto Nacional de Perinatologia, Mexico City, Mexico
Introduction: Globally, an estimated 15.1 million preterm neonates are born annually, with 1% classified as extremely preterm (i.e., <28.0 weeks of gestation). The survival and outcomes of this vulnerable population are influenced by multiple factors, particularly gestational age, birth weight, and available medical resources. This study aimed to describe the hospital discharge survival of extremely preterm infants born in a middle-income setting. As a secondary objective, we assessed the neonatal morbidity associated with this group.
Material and methods: In this cross-sectional study of singleton pregnancies, neonatal survival following extremely preterm birth was determined using three different denominators and expressed as prevalence (i.e., percentages): (1) the total number of extremely preterm births, including intrapartum fetal deaths; (2) the total number of all live births, including neonatal deaths in the delivery room, and (3) the total number of preterm neonates admitted to the neonatal intensive care unit (NICU). Neonatal morbidity was assessed as a secondary outcome.
Results: There were no live births between 22.0 and 23.6 weeks of gestation. Overall mortality decreased with increasing gestational age, from 100% (22/22) at <24.0 weeks of gestation to 87% (14/16), 42% (16/38), and 21% (11/52) at a gestational age of 25, 26, and 27 weeks, respectively. The survival rate to NICU discharge among extremely preterm infants was 49% (65/132), 67% (65/97), and 69% (65/93), depending on whether survival was calculated based on all births, all live births, or NICU admissions, respectively. None of the neonates born before 24.6 weeks of gestation survived to discharge. Notably, 97.0% of NICU survivors were diagnosed with major morbidity.
Conclusion: The survival rate at NICU discharge exceeds 50% from 26 weeks onwards in a middle-income setting. Importantly, survival rates varied significantly depending on the denominator used, highlighting the need to carefully select inclusion criteria in neonatal survival analyses. Notably, survival after extremely preterm birth was associated with significant morbidity.
1 Introduction
Over the years, advances in perinatal care and management have led to sustained improvement in the survival rates and outcomes of preterm infants (1). Yet, prematurity remains one of the leading causes of neonatal mortality and long-term morbidity worldwide (2, 3). Globally, an estimated 15.1 million preterm neonates are born annually, with 1% classified as extremely preterm (i.e., <28.0 weeks of gestation) (4). Extremely preterm infants face the highest risk of mortality and morbidity, with an inverse correlation between gestational age and such risks (5). The survival and outcomes of this vulnerable population are influenced by multiple factors, particularly gestational age, birth weight, and available resources (6, 7).
The limit of viability is defined as the earliest gestational age at which a preterm neonate has a significant probability of survival with available medical technology (8). Over time, this threshold has changed, and the World Health Organization (WHO) currently defines it at 22 weeks of gestation, and/or 500 g of birth weight, and/or a birth length of 25 cm (8–10). However, this definition has inherent limitations, as the exact point at which viability occurs depends on multiple factors, including biological variability, environmental conditions, and the availability of specialized neonatal care (11, 12). These thresholds apply predominantly to high-resource settings with highly specialized technical capabilities.
Given these limitations, it is recommended that each center establishes its viability threshold to guide counseling, medical decision-making, and management of extremely preterm births. This study aimed to describe the hospital discharge survival rates of extremely preterm infants born at the Instituto Nacional de Perinatologia. As a secondary objective, we assessed neonatal morbidity in this population.
2 Material and methods
2.1 Study population and outcomes
This single-center cross-sectional study was conducted from January 2018 through December 2022 at the Maternal-Fetal Medicine and Neonatology Divisions of the Instituto Nacional de Perinatologia in Mexico City, a tertiary-level national maternal and neonatal care referral center. The study population consisted of all consecutive extremely preterm newborns (i.e., gestational age 22.0–27.6 weeks) born from a singleton pregnancy. Gestational age was primarily determined using the best obstetric estimation, defined as a reliable last menstrual period confirmed by a first-trimester crown-rump length measurement or early second-trimester biometry (i.e., biparietal diameter) (13). In cases where this information was unavailable, gestational age was estimated using standardized neonatal assessment tools (14). Births with unknown gestational age were excluded from the analysis. Stillbirths occurring outside the hospital, neonates with major structural anomalies (i.e., those expected to cause mortality or severe morbidity identifiable at birth) or genetic syndromes, cases with incomplete medical records, and neonates receiving perinatal palliative care were excluded from the study.
For the present analysis, fetal death was defined as the delivery of a neonate without signs of life (i.e., absence of breathing, heartbeat, umbilical cord pulsation, or voluntary muscle movement) after complete expulsion or extraction from the mother, occurring either before labor (i.e., antepartum mortality) or during labor and delivery (i.e., intrapartum mortality) (15). Live birth was defined as the delivery of a neonate exhibiting any sign of life following complete expulsion or extraction (15). Obstetric and neonatal management were at the discretion of the attending obstetrician and neonatologist. Notably, all live-born neonates included in this analysis received active neonatal care at birth, including cardiopulmonary resuscitation, intubation, and invasive or non-invasive mechanical ventilation (16). The Neonatal Intensive Care Unit (NICU) level at our institution is classified as level III, according to the American Academy of Pediatrics (17).
Data regarding the pregnancy, neonatal period, and infant outcomes were retrieved from electronic medical records. Definitions for neonatal morbidities are presented in Supplementary Table S1. According to institutional regulations, retrospective analyses of anonymized data are exempt from formal ethics committee review and approval. Additionally, all women provided written consent at the time of prenatal care enrollment to use their routinely collected hospital data in retrospective studies, ensuring no patient identifiers were included.
2.2 Statistical analysis
Baseline characteristics and morbidity are reported using n (%) for categorical variables, mean (standard deviation) for normally distributed continuous variables, and median (interquartile range) for non-normally distributed variables. For this descriptive analysis, the neonatal survival rate was estimated using three different denominators and expressed as percentages: (1) the total number of extremely preterm births, including intrapartum fetal deaths; (2) the total number of all live births, including neonatal deaths in the delivery room, and (3) the total number of extremely preterm neonates admitted to the NICU. A 95% confidence interval (CI) for proportions using the method proposed by Clopper-Pearson was calculated for the outcomes of interest. Statistical analyses were performed in Stata (Version 18.0, StataCorp LLC, Texas, USA).
3 Results
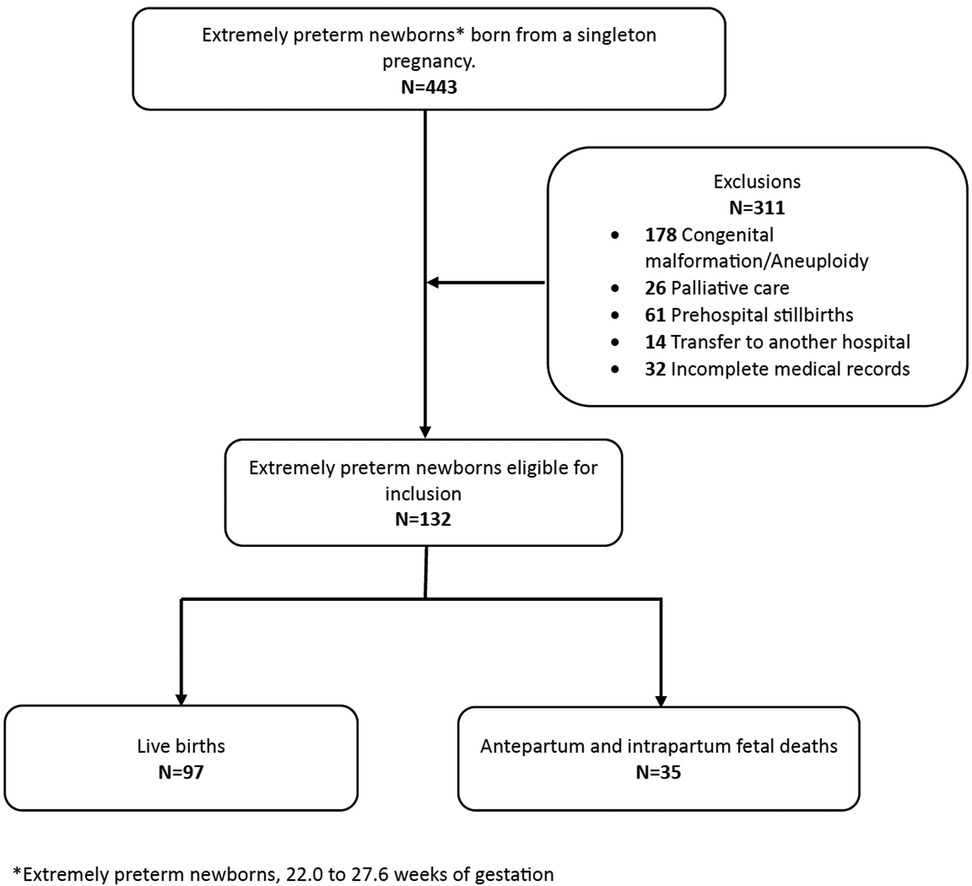
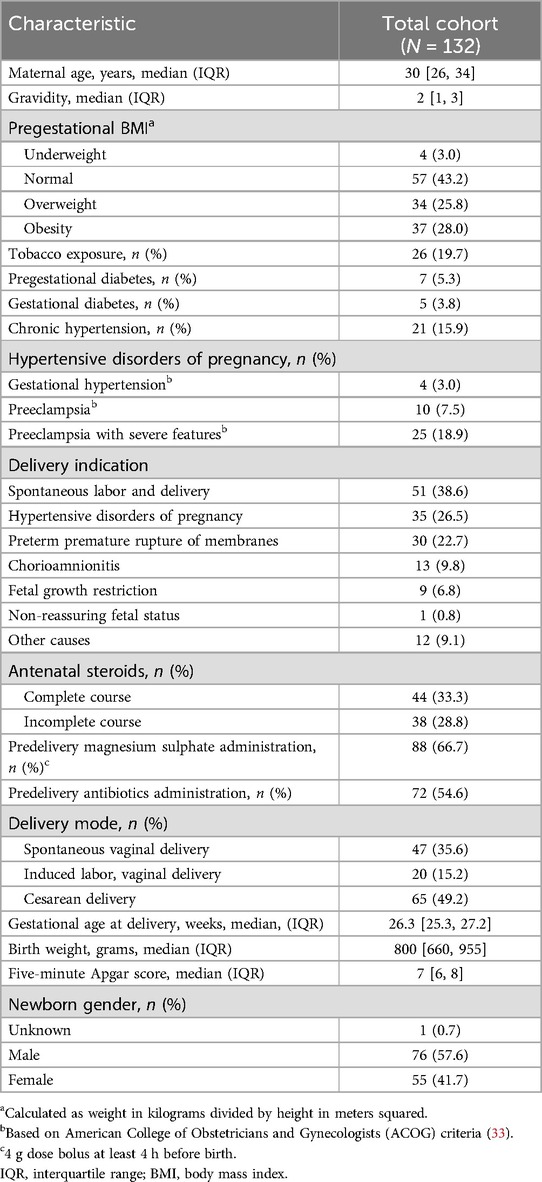
During the study period, 12,426 births were registered at the institution, with an extremely preterm birth prevalence of 3.5% (443/12,426). Data from 132 extremely preterm newborns who met the inclusion criteria were available for analysis (Figure 1). Relevant maternal demographic, obstetric, and neonatal characteristics are presented in Table 1.
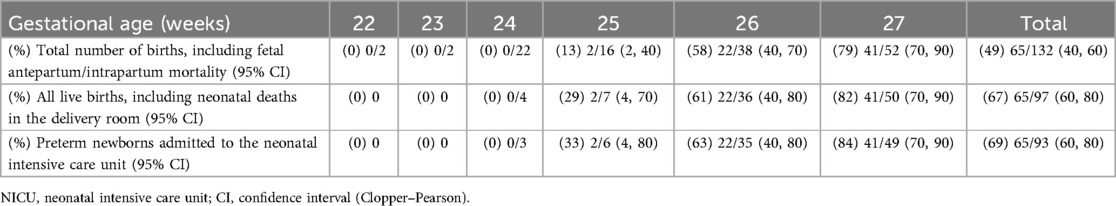
A total of 97 (73%) live births and 35 (27%) fetal deaths were identified in the study population. Table 2 presents data on fetal mortality, live births, and mortality in the delivery room and NICU by gestational age. No live births were recorded between 22.0 and 23.6 weeks of gestation. Fetal mortality decreased from 100% (4/4) at <23.0 weeks to 4% (2/52) at 27 weeks, while live birth rates increased from 18.2% (4/22) at 24 weeks to 96% (50/52) at 27 weeks. Moreover, overall mortality declined with increasing gestational age, from 100% (22/22) at <24.0 weeks to 87% (14/16) at 25 weeks, 42% (16/38) at 26 weeks, and 21% (11/52) at 27 weeks. Notably, 27% (35/132) of overall mortality occurred before delivery, with 6% (2/35) classified as antepartum deaths and 94% (33/35) as intrapartum deaths. An additional 3% (4/132) of deaths occurred in the delivery room, while 21% (28/132) occurred in the NICU.
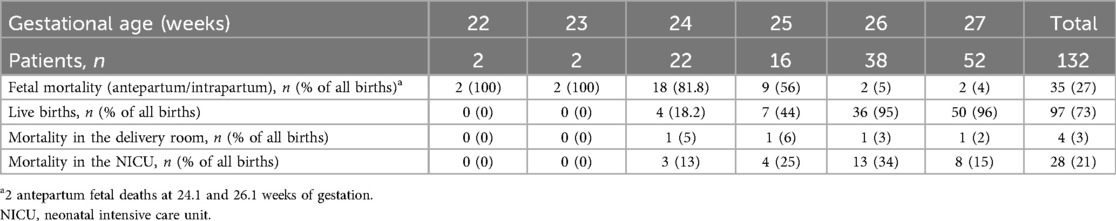
Table 2. Fetal mortality, live births, and mortality in the delivery room, and NICU by gestational age.
3.1 Survival rate to NICU discharge
Table 3 presents survival rates to NICU discharge calculated using three different denominators: (1) all births (including antepartum and intrapartum fetal deaths), (2) all live births, and (3) neonates admitted to the NICU. Additionally, Figure 2 provides a visual representation of survival estimates across gestational ages for each denominator.

Figure 2. Visual representation of estimated survival rates across gestational ages for each denominator.
The overall survival rate to NICU discharge among extremely preterm infants was 49% (65/132) when considering all births, 67% (65/97) when considering all live births, and 69% (65/93) when considering NICU admissions. No neonates born before 24.6 weeks of gestation survived to discharge. At all gestational ages, the survival rate calculated based on all births was lower than the estimated when only live births or NICU admissions were considered. However, the difference in survival rates between denominators diminished with increasing gestational age, as shown in Table 3.
3.2 Neonatal morbidity
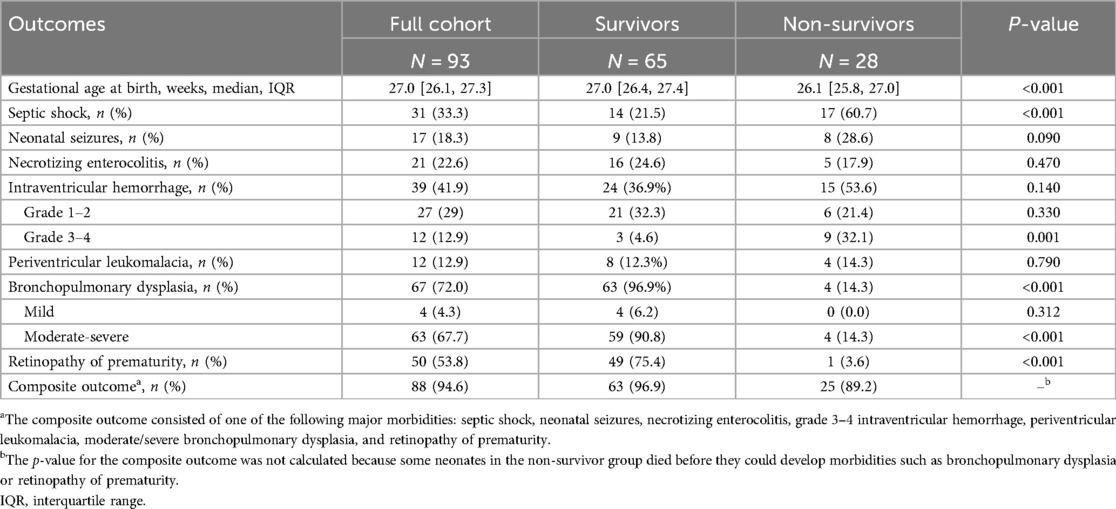
Among neonates who survived NICU discharge, 96.9% (63/65) developed at least one major morbidity, defined as the presence of one or more of the following: septic shock, neonatal seizures, necrotizing enterocolitis, grade 3–4 intraventricular hemorrhage, periventricular leukomalacia, moderate-to-severe bronchopulmonary dysplasia, and retinopathy of prematurity. The most common morbidity was moderate-to-severe bronchopulmonary dysplasia, occurring in 90.8% of cases, followed by retinopathy of prematurity (75.4%) and necrotizing enterocolitis (24.6%), as shown in Table 4. Therapeutic interventions administered in the NICU are detailed in Supplementary Table S2.
4 Discussion
In this study, we present a comprehensive assessment of survival in extremely preterm infants born at a tertiary-level center in a middle-income setting, using different denominators for survival estimations. Our analysis showed no survival to discharge before 24.6 weeks of gestation, regardless of the denominator used. At 25 weeks, survival to discharge rates varied widely (i.e., 13%–33%) depending on the denominator. Notably, from 26 weeks onwards, survival to discharge rates exceeded 50%, and differences between denominators were no longer clinically relevant.
The findings of this study provide a detailed perspective on the reality of neonatal care for extremely preterm infants in middle-income settings. Although the observed rate of extremely preterm births was higher than national and international data (18, 19)—likely due to the tertiary-level care provided at our institution (20)—our survival results are consistent with those reported in similar settings (18, 21–23). However, they differ substantially from findings in high-income countries, where survival rates are as high as 68%, 73%, and 94% for 22, 23, and 24 weeks of gestation, respectively (24–27). These discrepancies may be explained by significant variations in active obstetric and neonatal care policies among countries, institutions, and even cultures, as well as, substantial methodological heterogeneity across studies (e.g., inclusion/exclusion criteria, definitions of live birth, etc.). While survival outcomes in high-income nations serve as a reference for improvement (28), they also underscore the significant disparities in prenatal and neonatal care resources.
4.1 Impact of denominator selection on survival estimations
Our analysis highlights the impact of denominator selection when reporting neonatal survival. The choice of the denominator (i.e., all births, live births, NICU admissions) considerably affects reported survival rates, particularly at lower gestational ages. At 25 weeks, survival rates varied from 13 to 33%, depending on the denominator used. However, this variation diminished with increasing gestational age, and differences became clinically irrelevant from 26 weeks onwards. A decreasing fetal mortality rate may explain this trend as gestational age increases, as well as our institution's active neonatal care policy (i.e., ≥26.0 weeks of gestation or birth weight ≥700 g). Similar findings have been reported in previous studies (1, 26, 29, 30).
The denominator used in survival analysis can significantly influence the interpretation of neonatal outcomes. Estimations based on NICU admissions may exclude neonates who did not receive resuscitation. In contrast, reports based solely on live births may overestimate survival by ignoring pregnancies at the limits of viability, where active obstetric or neonatal care is not provided (1, 29). The WHO recommends considering fetal and delivery room mortality in survival analysis, yet many studies that estimate neonatal survival fail to include this critical information (31).
4.2 Neonatal morbidity and healthcare implications
Neonatal morbidity at discharge was high in our study population. Moderate-to-severe bronchopulmonary dysplasia (90.8%), retinopathy of prematurity (75.4%), and necrotizing enterocolitis (24.6%) were the most frequently observed complications. This pattern has been previously described in the literature (6); however, our observed morbidity rates were significantly higher than those reported in high-income countries (24, 32). While survival rates are critical in extremely preterm neonates, long-term morbidity remains a significant concern, posing challenges for healthcare systems and families. Our findings highlight the need for improved access to high-quality neonatal care and the potential optimization of existing management strategies.
4.3 Strengths and limitations
Our study has some strengths. It provides updated data on a topic with limited research in middle-income settings, addressing a critical knowledge gap. We also included an unselected population with reliable gestational age assessment and all neonatal care was overseen by experienced neonatologists. Moreover, the study performed a comprehensive survival analysis using different denominators, emphasizing their impact on reported outcomes and their importance when counseling families. Furthermore, we provide precision estimates of the reported results via 95% confidence intervals.
However, some limitations should be acknowledged. First, the retrospective design may introduce biases inherent to observational studies. Second, obstetric and neonatal management were at the discretion of the attending clinicians, potentially influencing neonatal outcomes. Third, the lack of active management at periviable gestational ages may have affected survival estimates. Additionally, long-term follow-up data (e.g., neurodevelopment outcomes) were unavailable, limiting a comprehensive assessment of health trajectories beyond NICU discharge.
Finally, as this was a single-center study conducted in a national tertiary-level referral hospital, the findings reflect local clinical practices and may not be generalizable to other institutions or healthcare systems. Further studies addressing causal relationships between outcomes and exposures are warranted beyond a detailed description. Moreover, the relatively small sample size, particularly at the lowest gestational ages, hampered the possibility of additional analyses. Future studies involving larger and diverse samples are needed to validate the reported results and to account for heterogeneity in care practices across regions and countries.
5 Conclusions
This study demonstrated that survival rates at NICU discharge exceed 50% from 26 weeks onwards in a middle-income setting. Importantly, survival estimates vary significantly depending on the denominator used, underscoring the need for careful selection of inclusion criteria in neonatal survival analyses. Furthermore, survival following extremely preterm birth was associated with significant morbidity, reinforcing the need for ongoing efforts to optimize neonatal care.
While survival rates and outcomes for extremely preterm infants continue to improve, substantial variability in clinical outcomes and reporting methods persists across different settings. Recognizing these discrepancies highlights the importance of research that provides standardized, up-to-date data to guide neonatal care decisions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics committee, Instituto Nacional de Perinatologia. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
MR-S: Conceptualization, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. OH-O: Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. ML-M: Conceptualization, Formal analysis, Investigation, Validation, Writing – review & editing. DM-B: Conceptualization, Validation, Writing – review & editing. SA-G: Conceptualization, Validation, Writing – review & editing. YC-M: Conceptualization, Validation, Writing – review & editing. DC-C: Conceptualization, Validation, Writing – review & editing. JG-G: Conceptualization, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to recognize the daily efforts and dedication of the Maternal-Fetal Medicine, Obstetrics, and Neonatology Division staff at the Instituto Nacional de Perinatologia. Additionally, we express our sincere gratitude to Brenda Frias-Madrid, MD, Barbara Delgadillo-Rojas-Corona, and Daniela M. Barajas-Roque for their detailed comments and expert advice. Finally, we thank the Instituto Nacional de Perinatologia for covering the publication charges associated with this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1574613/full#supplementary-material
References
1. Zeballos-Sarrato S, Villar-Castro S, Zeballos-Sarrato G, Ramos-Navarro C, González-Pacheco N, Sánchez-Luna M. Survival estimations at the limit of viability. J Matern Fetal Neonatal Med. (2016) 29(22):3660–4. doi: 10.3109/14767058.2016.1140736
2. Youssefzadeh AC, Mazza GR, Mandelbaum RS, Ouzounian JG, Matsuo K. Trends of preterm delivery in the United States, 2016–2019. AJOG Glob Rep. (2023) 3(2):100181. doi: 10.1016/j.xagr.2023.100181
3. Simmons LVE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol. (2010) 34(6):408–15. doi: 10.1053/j.semperi.2010.09.005
4. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379(9832):2162–72. doi: 10.1016/S0140-6736(12)60820-4
5. Higgins BV, Baer RJ, Steurer MA, Karvonen KL, Oltman SP, Jelliffe-Pawlowski LL, et al. Resuscitation, survival and morbidity of extremely preterm infants in California 2011–2019. J Perinatol. (2024) 44(2):209–16. doi: 10.1038/s41372-023-01774-6
6. Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA Netw Open. (2021) 4(5):e219382. doi: 10.1001/jamanetworkopen.2021.9382
7. Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. N Engl J Med. (2015) 372(19):1801–11. doi: 10.1056/NEJMoa1410689
8. Pignotti MS. The definition of human viability: a historical perspective. Acta Paediatr. (2010) 99(1):33–6. doi: 10.1111/j.1651-2227.2009.01524.x
9. Stanojevic M. Limits of viability: should we play god? Psychiatr Danub. (2021) 33(2):280–91. doi: 10.5005/sar-1-1-2-46
10. El-Metwally T, Vohr B, Tucker R. Survival and neonatal morbidity at the limits of viability in the mid 1990s: 22–25 weeks. J Pediatr. (2000) 137(5):616–22. doi: 10.1067/mpd.2000.109143
11. Mercer BM. Periviable birth and the shifting limit of viability. Clin Perinatol. (2017) 44(2):283–6. doi: 10.1016/j.clp.2017.02.002
12. Arzuaga BH, Cummings CL. Deliveries at extreme prematurity: outcomes, approaches, institutional variation, and uncertainty. Curr Opin Pediatr. (2019) 31(2):182–7. doi: 10.1097/MOP.0000000000000731
13. Salomon LJ, Alfirevic Z, Berghella V, Bilardo CM, Chalouhi GE, Da Silva Costa F, et al. ISUOG Practice guidelines (updated): performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. (2022) 59(6):840–56. doi: 10.1002/uog.24888
14. Ballard JL, Khoury JC, Wang L, Eilers-Walsman BL, Lipp R, Wedig K. New ballard score, expanded to include extremely premature infants. J Pediatr. (1991) 119(3):417–23. doi: 10.1016/S0022-3476(05)82056-6
15. Kowalevski J. State Definitions and Reporting Requirements for Live Births, Fetal Deaths, and Induced Termination of Pregnancy. Hyattsville, Maryland, Hyattsville, Maryland: National Center for Health Statistic, U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics (1997). Available at: https://www.cdc.gov/nchs/data/misc/itop97.pdf (Accessed August 24, 2023).
16. Weiner GM, Zaichkin J. In Weiner G, editor. Textbook of Neonatal Resuscitation. 8th ed. Itasca, IL: American Academy of Pediatrics (2021). Vol. 8th Edition. p. 213–30.
17. Stark AR, Pursley DWM, Papile LA, Eichenwald EC, Hankins CT, Buck RK, et al. Standards for levels of neonatal care: II, III, and IV. Pediatrics. (2023) 151(6):e2023061957. doi: 10.1542/peds.2023-061957
18. Humberto Lozano-González C, Estela Flores-Tamez M, Castro-Mejía S, Alfredo Lozano-Flores J. Límites de la viabilidad neonatal. Perinatol Reprod Hum. (2013) 27(2):79–85. Available at: http://www.medigraphic.com/inper.
19. Behboudi-Gandevani S, Bidhendi-Yarandi R, Hossein Panahi M, Mardani A, Prinds C, Vaismoradi M, et al. Prevalence of preterm birth in scandinavian countries: a systematic review and meta-analysis. J Int Med Res. (2023) 51(10):1–19. doi: 10.1177/03000605231203843
20. Mulligan JA, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit Costs of Health Care Inputs in Low and Middle Income Regions. London (UK): Disease Control Priorities Project, London School of Hygiene and Tropical Medicine (2003). Available at: https://researchonline.lshtm.ac.uk/id/eprint/12952/1/Unit-costs-of-health-care-inputs-in-low-and-middle-income-regions.pdf (Accessed August 24, 2023).
21. Mocking M, Adu-Bonsaffoh K, Osman KA, Tamma E, Ruiz AM, van Asperen R, et al. Causes, survival rates, and short-term outcomes of preterm births in a tertiary hospital in a low resource setting: an observational cohort study. Front Glob Womens Health. (2022) 3:989020. doi: 10.3389/fgwh.2022.989020
22. Vogel JP, Lee ACC, Souza JP. Maternal morbidity and preterm birth in 22 low- and middle-income countries: a secondary analysis of the WHO global survey dataset. BMC Pregnancy Childbirth. (2014) 14(1):56. doi: 10.1186/1471-2393-14-56
23. D’Apremont I, Marshall G, Musalem C, Mariani G, Musante G, Bancalari A, et al. Trends in perinatal practices and neonatal outcomes of very low birth weight infants during a 16-year period at NEOCOSUR centers. J Pediatr. (2020) 225:44–50.e1. doi: 10.1016/j.jpeds.2020.05.040
24. Miyazawa T, Arahori H, Ohnishi S, Shoji H, Matsumoto A, Wada YS, et al. Mortality and morbidity of extremely low birth weight infants in Japan, 2015. Pediatr Int. (2023) 65(1):e15493. doi: 10.1111/ped.15493
25. Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, Demauro SB, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA. (2022) 327(3):248–63. doi: 10.1001/jama.2021.23580
26. Smith LK, Morisaki N, Morken NH, Gissler M, Deb-Rinker P, Rouleau J, et al. An international comparison of death classification at 22–25 weeks’ gestational age. Pediatrics. (2018) 142(1):e20173324. doi: 10.1542/peds.2017-3324
27. Lundgren P, Morsing E, Hård AL, Rakow A, Hellström-Westas L, Jacobson L, et al. National cohort of infants born before 24 gestational weeks showed increased survival rates but no improvement in neonatal morbidity. Acta Paediatr. (2022) 111(8):1515–25. doi: 10.1111/apa.16354
28. Isayama T. The threshold of viability of extremely preterm infants in the current era in high-income countries. Pediatr Int. (2023) 65(1):e15577. doi: 10.1111/ped.15577
29. Evans DJ, Levene MI. Evidence of selection bias in preterm survival studies: a systematic review. Arch Dis Child Fetal Neonatal. (2001) 84(2):79–84. doi: 10.1136/fn.84.2.F79
30. Sharp M, French N, McMichael J, Campbell C. Survival and neurodevelopmental outcomes in extremely preterm infants 22–24 weeks of gestation born in Western Australia. J Paediatr Child Health. (2018) 54(2):188–93. doi: 10.1111/jpc.13678
31. World Health Organization. In World Health Organization, editor. The World Health Report 2005: Make Every Mother and Child Count. 1st ed. Geneva: World Health Organization (2005). p. 229.
32. Norman M, Hallberg B, Abrahamsson T, Björklund LJ, Domellöf M, Farooqi A, et al. Association between year of birth and 1-year survival among extremely preterm infants in Sweden during 2004–2007 and 2014–2016. JAMA. (2019) 321(12):1188–99. doi: 10.1001/jama.2019.2021
Keywords: extreme prematurity, neonatal survival, viability limit, grey zone, perinatal mortality
Citation: Rodriguez-Sibaja MJ, Herrera-Ortega O, Lumbreras-Marquez MI, Morales-Barquet D, Acevedo-Gallegos S, Copado-Mendoza Y, Camarena-Cabrera DM and Gallardo-Gaona JM (2025) Survival assessment in extremely preterm neonates in a middle-income setting. Front. Pediatr. 13:1574613. doi: 10.3389/fped.2025.1574613
Received: 11 February 2025; Accepted: 19 May 2025;
Published: 30 May 2025.
Edited by:
Qiuping Li, Bayi Children's Hospital, ChinaReviewed by:
Asaph Rolnitsky, University of Toronto, CanadaRufaida Mazahir, Teerthanker Mahaveer University, India
Copyright: © 2025 Rodriguez-Sibaja, Herrera-Ortega, Lumbreras-Marquez, Morales-Barquet, Acevedo-Gallegos, Copado-Mendoza, Camarena-Cabrera and Gallardo-Gaona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan M. Gallardo-Gaona, Z2FsbGFyZG9fZ21hbnVlbEBob3RtYWlsLmNvbQ==
 Maria J. Rodriguez-Sibaja
Maria J. Rodriguez-Sibaja Olivo Herrera-Ortega1
Olivo Herrera-Ortega1 Deneb Morales-Barquet
Deneb Morales-Barquet Sandra Acevedo-Gallegos
Sandra Acevedo-Gallegos Dulce M. Camarena-Cabrera
Dulce M. Camarena-Cabrera