- 1Department of Pediatrics, University of Oklahoma Health College of Medicine, Oklahoma City, OK, United States
- 2Department of Pediatrics, McGill University Health Centre, Montreal, QC, Canada
- 3Department of Pediatrics, Wayne State University, Detroit, MI, United States
- 4Warren Alpert Medical School, Brown University, Providence, RI, United States
Background: Among late preterm (LPT) infants, there is significant variability in reaching milestones for safe discharge. We examined the associations of early measures of heart rate variability (HRV) and amplitude-integrated electroencephalogram (aEEG) with time to wean to an open-air cot and to achieve full oral feeds.
Methods: This is a prospective, multicenter observational cohort study that enrolled infants between 340/7 and 346/7 weeks gestational age (GA). Infants with growth restriction and major congenital anomalies were excluded. Electrocardiogram (ECG) for 1 h and cross-cerebral aEEG for 6 h were recorded within 96 h after birth. Correlations of HRV and aEEG parameters with outcomes were evaluated using stepwise linear regression.
Results: Of the 26 infants from three centers, 23 were included for analysis for time to an open-air cot. The analysis for time to full oral feeds was limited to 19 infants from two centers with similar feeding policies. Including HRV parameters (time domain, median and standard deviation of R-wave to R-wave interval; frequency domain, ratio of the low frequency to high frequency power and their interaction) and aEEG parameters (total and immature cycles/hour) strengthened associations with time to open-air cot (adjusted R2 = 0.72) and time to full oral feeds (adjusted R2 = 0.53) compared with each parameter alone.
Conclusions: Early measurements of HRV and aEEG parameters correlate with time to an open-air cot and to achieve full oral feeds in LPT infants born between 340/7 and 346/7 weeks GA.
Summary highlights
• Among late preterm (LPT) infants, there is large variability in the time to reach milestones needed for discharge that cannot be explained by their gestational age (GA) alone.
• Early measurements of heart rate variability (HRV) and amplitude-integrated electroencephalogram (aEEG) parameters correlated with time to an open-air cot and to achieve full oral feeds in this prospective cohort of LPT infants born between 340/7 and 346/7 weeks GA.
• Differences in autonomic and central nervous system function may contribute to the large variability in the time to reach milestones for safe discharge among infants at the same GA.
• Both physiological measures of HRV and aEEG may serve as potential biomarkers of clinical trajectories and assessment of neurophysiological maturation.
Introduction
Over the last 25 years, the overall rate of preterm birth has progressively risen. Much of this increase is due to late preterm (LPT) births, including infants born at a gestational age (GA) between 340/7 and 346/7 weeks (1, 2). Safe hospital discharge of preterm infants typically requires that respiratory morbidities, including apnea of prematurity, are resolved, core body temperature can be maintained at ambient temperature, and adequate feedings are taken orally (3). However, among LPT infants, there is large variability in the time to reach such milestones that can neither be explained by their GA alone nor by morbidities of prematurity (4, 5).
Previous research in LPT infants has demonstrated a relationship between length of hospitalization and amplitude-integrated electroencephalogram (aEEG) findings (4). Neurological function and maturation can also be evaluated by assessment of the autonomic nervous system (ANS) with measures such as heart rate variability (HRV) (6–10). In fact, changes in HRV have been demonstrated to reflect neurophysiological maturation in the neonatal population (11). Advancing GA is accompanied by increasing HRV values, with a power increase most marked in the high frequency (HF) band. As the HF component reflects parasympathetic influence on HR control, this finding suggests a greater maturation of the vagal component during maturation of the ANS (12–15). Therefore, we hypothesized that early postnatal measurement of HRV and aEEG in LPT infants could be a physiological biomarker of their variable clinical trajectories. For that, we examined the associations of HRV and aEEG measured shortly after birth with time to wean to an open-air cot and to time to achieve full oral feeds.
Methods
Population
The Neurophysiologic Maturation Index (NEMO) Project (ClinicalTrials.gov Identifier: NCT02156817) was a pilot, international study in stable infants born with GA between 340/7 and 346/7 weeks at three neonatal intensive care units (NICU), namely, McGill University Health Center (Montreal, QC, CA), Hutzel Women's Hospital (Detroit, MI, USA), and Women and Infants Hospital (Providence, RI, USA). GA was assessed by obstetric criteria [presence of a reliable last menstrual period (LMP) date or sonogram performed in the first trimester or agreement between LMP and a sonogram performed between the first trimester and 20 weeks]. Infants were excluded if they had major congenital or genetic anomalies, growth restriction (birth weight <10%, Fenton growth curves), exposure to medications within the preceding 12 h which may affect CNS function (e.g., fentanyl, morphine, and midazolam), seizures, neonatal opioid withdrawal syndrome (NOWS) secondary to in utero exposure to opioids or at high risk for development of NOWS, and encephalopathy or were expected to be on mechanical ventilation for the first 96 h after birth. The research ethics board of each institution approved the study, and written informed consent was obtained from parents or legal guardians.
Study design and data acquisition
Three-lead electrocardiogram (ECG) and cross-cerebral aEEG were recorded simultaneously within the first 96 h of life. ECG was recorded for the first hour, whereas aEEG recordings continued up to 6 h. This was timed in between hands-on or kangaroo care typically after the feeding. ECG leads were placed on the infant's chest or limbs, positioned at least 1 cm apart from the pre-existing leads used for clinical care, and connected to a bioamplifier and PowerLab data acquisition system (ADInstruments, Colorado Springs, CO, USA). The aEEG was obtained using the BRM3 BrainZ Monitor with the Neonatal Hydrogel Sensors (Natus Medical Inc., Pleasanton, CA, USA). Sensors were placed at the C3-P3 and C4-P4 locations on the respective left and right sides of the head with an extra ground sensor placed on the shoulder or between the scapulae.
Heart rate variability (HRV)
HRV analysis was performed offline using the HRV module of the LabChart (version 2.0, ADInstruments). The last 5 min segment of the ECG that had ≥500 regular intervals (100–200 bpm) was selected independent of the wake/sleep cycle. Time domain, frequency domain, and non-linear HRV parameters were calculated from the segment. The following time domain parameters were calculated: standard deviation of the RR intervals (SDRR), standard deviation of heart rate (SD heart rate), coefficient of variation of RR intervals (CVRR), standard deviation of the successive differences between RR intervals (SDSD), and percentage of adjacent RR intervals that differ by greater than 50 ms (pRR50). The following non-linear parameters were obtained: standard deviation of intervals perpendicular to the identity line of the Poincaré plot (SD1) and standard deviation of intervals along the identity line (SD2). For the frequency domain the following parameters were obtained: TP, total power (<0.4 Hz); VLF, very low frequency (LF) power (<0.04 Hz); LF, low frequency power (0.04–0.15 Hz); HF, high frequency power (>0.15 to <0.4 Hz); and LF/HF ratio, ratio of the low frequency to high frequency power.
Amplitude-integrated electroencephalography (aEEG)
The scalp was systematically prepared with Nuprep gel to achieve a low impedance. Recordings were stored as digital files for offline analysis. The cross-cerebral aEEG was derived from P3-P4 EEG channels after amplification, filtering, smoothing, and time compression to yield a band of activity which is plotted on a semilogarithmic scale (µV) on the vertical axis and time on the horizontal axis. The voltage values were determined by the BRM3 and analyzed by two investigators (BAS and ARL) using a combined approach of visual and offline digital analysis (BrainZ Analyze software program). Initial visual inspection identified intermittent widening of the band, which was evaluated for the criteria of cycles. These potential cycles allowed preliminary separation of the aEEG tracing into cycles and inter-cycle periods. The Analyze program provided voltages for the upper and lower borders at 1 min intervals throughout the tracing and was exported to a Windows Excel spreadsheet. Voltages were used to determine if visually assessed cycles fulfilled predefined voltage criteria of a cycle and subtypes (mature, immature, and interrupted) based on prior work (4). Cycle frequency was calculated as the number of cycles in the tracing divided by the length of the recording. The percent distribution of the type of cycles was derived for each infant. The inter-cycle period was characterized by an upper and lower border voltage and the difference representing the span, and the percent discontinuity was defined as the percentage time when the lower border voltage is <5 µV.
Clinical data
Maternal and pregnancy demographics (multiple gestation, gestational hypertension, chronic hypertension, hypothyroidism, preterm labor, length of rupture of membranes, placental abnormality, cord accident, cesarean section, gestational diabetes, antenatal antibiotics, and betamethasone), patient demographics [birth weight, GA, sex, Apgar scores, transient tachypnea of the newborn, respiratory distress syndrome, use of surfactant, delivery room respiratory support, hypoglycemia (<40 mg/dl beyond 4 h of age), highest respiratory support received], and outcome variables (time to wean to an open-air cot and time to achieve full oral feeds) were prospectively collected until discharge or transfer from the units. Local practices were followed for the time to wean to an open-air cot when the infant's temperature remained stable (≥36.5°C/97.7°F) in an ambient temperature of 28°C or less in an incubator for at least 12–24 h, which typically occurred at a weight of approximately 1,800 g, and time to achieve full feeds at 140 cc/kg/day orally at the enrolling centers as per institutional guidelines (16, 17).
Statistical analysis
Values are presented as median [IQR] or n (%). Correlations were evaluated using stepwise linear regression testing with the following model building procedure: first, a subset of physiological variables were preselected by excluding one of variable of pairs with obvious collinearity (i.e., SDRR and SD heart rate); second, univariate linear regressions were assessed between each preselected variable and each outcome; third, variables with regressions at p ≤ 0.25 were chosen; fourth, collinearity was assessed within those chosen variables with removal of one of the two variables if strongly correlated (R ≥ 0.7); the final variables were tested using stepwise linear regression. Stepwise regression model settings included a p-value entry of <0.05 and a p-value removal of >0.10 automatically tested toward minimizing the sum of squared errors (MATLAB R2018b, MathWorks, Natick, MA, USA).
Results
A total of 26 late preterm infants were studied, of which 23 were included for analysis for the outcome of time to an open-air cot (Table 1); three patients were not included due to poor quality or lack of aEEG recordings. The analysis for time to full oral feeds was limited to 19 infants from two centers with similar feeding policies. Infants included for analysis had a median birth weight of 2,140 g (2,045–2,238 g) and GA 34.3 weeks (34.0–34.4 weeks); all patient demographics, maternal and pregnancy demographics, and final outcomes are presented in Table 1. All HRV and aEEG values are summarized in Table 2.
The details of the regression models for both outcomes are provided in Tables 3 and 4. For both outcomes, the best model was found with both HRV and aEEG variables (Table 4). The model for time to an open-air cot included HRV parameters SDRR and LF/HF ratio, an interaction term between SDRR and LF/HF ratio, and the aEEG parameter total cycles/hour. The time to an open-air cot model is strong, with an adjusted R2 of 0.720 (Table 4; Figure 1a). Similarly, for the outcome of time to full oral feeds, the best model included median RR and LF/HF ratio HRV parameters, with the immature cycles/hour aEEG parameter (Table 4). This model has modest strength, with an adjusted R2 of 0.527 (Table 4; Figure 1b).
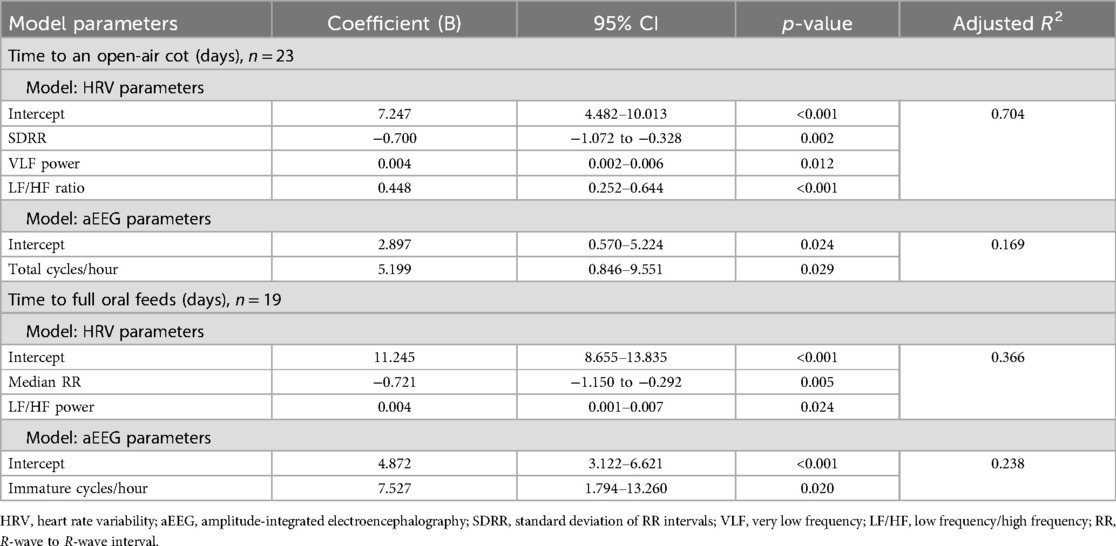
Table 3. Multivariate regression analysis of outcomes influencing length of hospital stay with HRV and aEEG parameters separately.
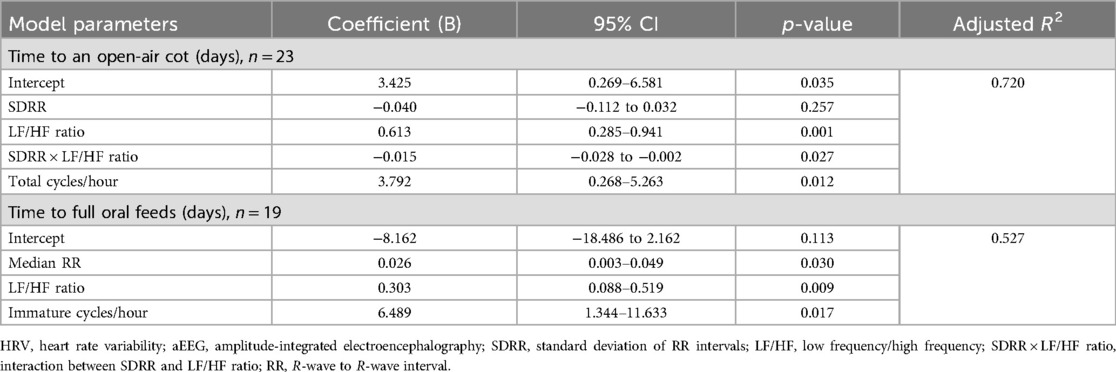
Table 4. Stepwise regression models for clinically important outcomes integrating physiological variables of HRV and aEEG.
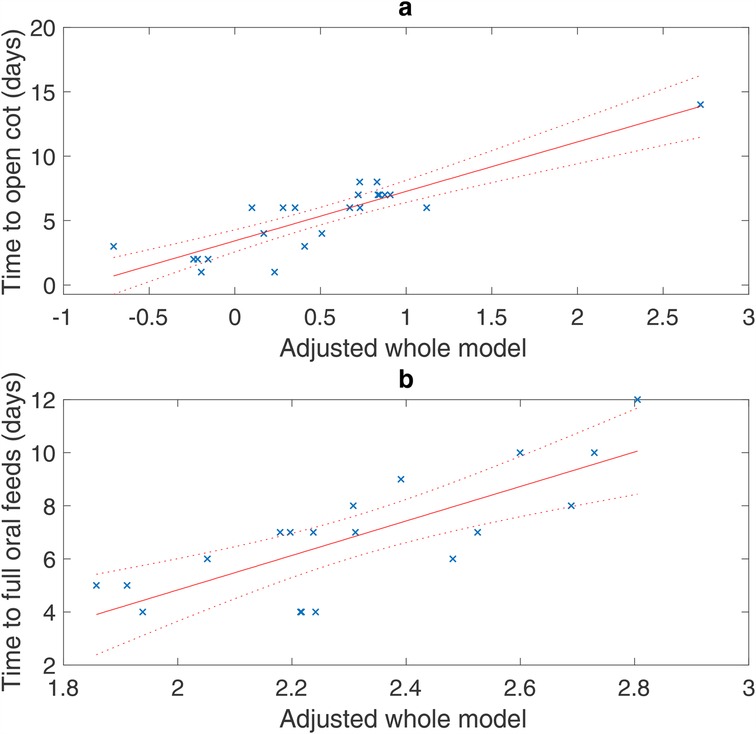
Figure 1. Regression models of all adjusted data (×) with the 95% confidence bounds (dotted line). X-axis: adjusted model is a single-value representation of the multiple variables and their coefficients for each patient included in the model, e.g., adjusted model = [b1 × 1 + b2 × 2 + b3 × 3 +…]. (a) Time to an open-air cot: R2 = 0.720 adjusted for SDRR, LF/HF ratio, interaction between SDRR and LF/HF ratio, and total cycles/hour (p < 0.001). (b) Time to full oral feeds: R2 = 0.527 adjusted for median RR interval, LF/HF ratio, immature cycles/hour (p = 0.002).
Discussion
This pilot prospective observational study of LPT infants explored possible associations between HRV and aEEG measured shortly after birth, and subsequent time to reach important clinical milestones required for safe discharge from the NICU. The combination of the HRV and aEEG variables has a stronger effect on the subsequent time to achieve full oral feeds given a substantial increase in R2 when both variables are included. In contrast, HRV alone has an R2 of 0.70 for time to an open-air cot and goes up by a small amount when combined with aEEG.
HRV
Increases in HRV are described with increasing GA and post-conceptional age (18–21). Specifically, increases in LF and HF power have been observed (18–21) with slight decreases in the LF/HF ratio (18), indicating an unequal rise, and increases in time domain parameters (21). With these changes, higher values in time domain and frequency domain parameters (more mature) would be associated with shorter times of incubator weaning times and to achieve full oral feeds; thus, negative coefficients should be observed. Our results are in line with these maturational changes, as a negative correlation was observed with the time domain parameter SDRR. Moreover, a positive correlation with both outcomes and the LF/HF ratio was noted, as the LF/HF ratio decreases with maturation (18).
A positive correlation was also observed with the median RR interval for the time to full oral feeds outcome; this, however, was not expected given that the RR interval should typically increase, equivalent to heart rate decreasing, with maturation (18, 21). However, this finding may suggest an indication of better adaptability within this narrow range of GA. A maturational lag in vagal function may contribute to feeding difficulties in LPT infants (22). Moreover, preterm infants often have an underdeveloped parasympathetic nervous system, which could impair their response to feeding (23). This impaired parasympathetic response could potentially correlate with longer median RR intervals and delayed feeding progression. Since this was a pilot study with limited power, this hypothesis-generating unexpected observation warrants further study to advance our understanding of the neurophysiological maturation of preterm infants.
aEEG
Distinct changes in aEEG have been demonstrated across GAs, characterized by increasing presence of continuous activity and the development of sleep–wake cyclic changes with maturity (24, 25). Furthermore, Sommers et al. (4) examined the aEEG sleep–wake cycles in a cohort of LPT infants within a narrow GA of 340/7–346/7 weeks as in this study with variations in cycling, which was inversely correlated with the length of hospital stay. Our results are consistent with a previous study as inclusion of aEEG variables improved the correlation between HRV and clinical determinants of length of hospital stay.
This association was robust for time to an open-air cot compared with time to achieve full oral feeds. Successful oral feeding is a complex neurologic milestone as it requires the integration and coordination of oropharyngeal, respiratory, and gastrointestinal systems, as well as maturation of sensory systems (visual, auditory, somatosensory, gustatory, and olfactory) and hypothalamic pathways which encode satiety and hunger (26). Additionally, unlike the stabilization of suck and swallow rhythms, which occur before 36 weeks' GA, improvement in coordination of respiration and swallow begins later (27, 28). These biological complexities may contribute to the limited strength of the correlation for time to achieve full oral feeds. Further research is warranted to advance our understanding of the differential developmental maturation of the parasympathetic and sympathetic nervous systems in LPT infants.
Limitations
This study has some limitations. First, there are variations in clinical practice across sites (e.g., timing of cot weaning and feeding advancement) as none of the involved sites had established policies on when to transfer LPT infants from a closed incubator to an open-air cot and advancing oral feeds. Weaning often took place subjectively, based on general thermoregulatory stability as established by minimal fluctuations in incubator temperature, the level of respiratory support, and infants' weight of typically ≥1,800g. It is unclear what kind of effect this may have had on the data. We suggest standardization of the protocol for these clinical milestones in future studies to control for any potential bias. Second, the study was stopped due to a change in the affiliation of the investigator resulting in a small sample size, which may limit the generalizability and the robustness of regression models, and a larger sample size would permit the introduction of clinical variables. Third, 23 infants had usable aEEG data, and 3 infants were excluded due to lack or suboptimal quality signals with high impedance (>5 kΩ). Additionally, four infants from one site could not have their aEEGs analyzed beyond the total cycles/hour due to incompatibility of file formats. Finally, the median age at time of physiological recording was 71 h [IQR, 48–78 h], and the median time to open-air cot was 6 days [IQR, 3–7 days]. Hence, in some cases, recordings may have been done after cot transition. We therefore advise caution in interpretations regarding the predictive ability of this regression.
Conclusion
HRV and aEEG parameters measured shortly after birth correlated with subsequent time to an open-air cot and to achieve full oral feeds in infants born between 340/7 and 346/7 weeks GA. Both parameters provided complementary information regarding the neurophysiological maturation. These results support the hypothesis that differences in ANS function may contribute to the variability in the time to reach milestones for safe discharge among LPT infants at the same GA. Future studies with larger sample sizes are needed to confirm these preliminary observations, which would further allow the addition of other variables and better understand the potential of these physiological measurements as biomarkers of clinical trajectories.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Women and Infants Hospital of Rhode Island, McGill University Health Center, and Hutzel Women's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
BS: Data curation, Formal analysis, Investigation, Software, Writing – original draft, Writing – review & editing. SL: Data curation, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. SC: Data curation, Project administration, Writing – review & editing. MK: Methodology, Resources, Validation, Writing – review & editing. RT: Formal analysis, Methodology, Resources, Validation, Writing – review & editing. AL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. GS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ramachandrappa A, Jain L. Health issues of the late preterm infant. Pediatr Clin North Am. (2009) 56:565–77. doi: 10.1016/j.pcl.2009.03.009
2. Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, Dolan S, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. (2006) 30:8–15. doi: 10.1053/j.semperi.2006.01.009
3. Jefferies AL. Going home: facilitating discharge of the preterm infant. Paediatr Child Health. (2014) 19:31–6. doi: 10.1093/pch/19.1.31
4. Sommers R, Tucker R, Harini C, Laptook AR. Neurological maturation of late preterm infants at 34 wk assessed by amplitude integrated electroencephalogram. Pediatr Res. (2013) 74:705–11. doi: 10.1038/pr.2013.157
5. Medoff Cooper B, Holditch-Davis D, Verklan MT, Fraser-Askin D, Lamp J, Santa-Donato A, et al. Newborn clinical outcomes of the AWHONN late preterm infant research-based practice project. J Obstet Gynecol Neonatal Nurs. (2012) 41:774–85. doi: 10.1111/j.1552-6909.2012.01401.x
6. Stock C, Teyssier G, Pichot V, Goffaux P, Barthelemy JC, Patural H. Autonomic dysfunction with early respiratory syncytial virus-related infection. Auton Neurosci. (2010) 156:90–5. doi: 10.1016/j.autneu.2010.03.012
7. Tirosh E, Ariov-Antebi N, Cohen A. Autonomic function, gastroesophageal reflux in apparent life threatening event. Clin Auton Res. (2010) 20:161–6. doi: 10.1007/s10286-010-0054-x
8. Lauer MS. Autonomic function and prognosis. Clevel Clin J Med. (2009) 76(Suppl 2):S18–22. doi: 10.3949/ccjm.76.s2.04
9. Schaffer L, Burkhardt T, Muller-Vizentini D, Rauh M, Tomaske M, Mieth RA, et al. Cardiac autonomic balance in small-for-gestational-age neonates. Am J Physiol Heart Circ Physiol. (2008) 294:H884–90. doi: 10.1152/ajpheart.00318.2007
10. McCain GC, Ludington-Hoe SM, Swinth JY, Hadeed AJ. Heart rate variability responses of a preterm infant to kangaroo care. J Obstet Gynecol Neonatal Nurs. (2005) 34:689–94. doi: 10.1177/0884217505281857
11. Iyer KK, Leitner U, Giordano V, Roberts JA, Vanhatalo S, Klebermass-Schrehof K, et al. Bedside tracking of functional autonomic age in preterm infants. Pediatr Res. (2023) 94:206–12. doi: 10.1038/s41390-022-02376-2
12. Longin E, Gerstner T, Schaible T, Lenz T, Konig S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. J Perinat.Med. (2006) 34:303–8. doi: 10.1515/JPM.2006.058
13. Curzi-Dascalova L, Christova-Gueorguieva L, Lebrun F, Firtion G. Respiratory pauses in very low risk prematurely born infants reaching normal term. A comparison to full-term newborns. Neuropediatrics. (1984) 15:13–7. doi: 10.1055/s-2008-1052333
14. Curzi-Dascalova L, Christova-Gueorguieva E. Respiratory pauses in normal prematurely born infants. A comparison with full-term newborns. Biol Neonate. (1983) 44:325–32. doi: 10.1159/000241747
15. Curzi-Dascalova L. Phase relationships between thoracic and abdominal respiratory movement during sleep in 31–38 weeks CA normal infants. Comparison with full-term (39–41 weeks) newborns. Neuropediatrics. (1982) 13 Suppl:15–20. doi: 10.1055/s-2008-1059629
16. Shankaran S, Bell EF, Laptook AR, Saha S, Newman NS, Kazzi SNJ, et al. Weaning of moderately preterm infants from the incubator to the crib: a randomized clinical trial. J Pediatr. (2019) 204:96–102.e4. doi: 10.1016/j.jpeds.2018.08.079
17. Edwards L, Cotten CM, Smith PB, Goldberg R, Saha S, Das A, et al. Inadequate oral feeding as a barrier to discharge in moderately preterm infants. J Perinatol. (2019) 39:1219–28. doi: 10.1038/s41372-019-0422-x
18. Chatow U, Davidson S, Reichman BL, Akselrod S. Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations. Pediatr Res. (1995) 37:294. doi: 10.1203/00006450-199503000-00008
19. Khattak AZ, Padhye NS, Williams AL, Lasky RE, Moya FR, VERKLAN MT. Longitudinal assessment of heart rate variability in very low birth weight infants during their NICU stay. Early Hum Dev. (2007) 83:361–6. doi: 10.1016/j.earlhumdev.2006.07.007
20. Padhye NS, Williams AL, Khattak AZ, Lasky RE. Heart rate variability in response to pain stimulus in VLBW infants followed longitudinally during NICU stay. Dev Psychobiol. (2009) 51:638–49. doi: 10.1002/dev.20399
21. Sahni R, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, Myers MM. Maturational changes in heart rate and heart rate variability in low birth weight infants. Dev Psychobiol. (2000) 37:73–81. doi: 10.1002/1098-2302(200009)37:2%3C73::AID-DEV2%3E3.0.CO;2-C
22. Suess PE, Alpan G, Dulkerian SJ, Doussard-Roosevelt J, Porges SW, Gewolb IH. Respiratory sinus arrhythmia during feeding: a measure of vagal regulation of metabolism, ingestion, and digestion in preterm infants. Dev Med Child Neurol. (2000) 42:169–73. doi: 10.1111/j.1469-8749.2000.tb00065.x
23. Smith SL, Doig AK, Dudley WN. Impaired parasympathetic response to feeding in ventilated preterm babies. Arch Dis Child Fetal Neonatal Ed. (2005) 90:F505–8. doi: 10.1136/adc.2004.070334
24. Hellström-Westas L, De Vries LS, Rosén I. An Atlas of Amplitude-Integrated EEGs in the Newborn. Boca Raton, FL: CRC Press (2008).
25. Zhang D, Liu Y, Hou X, Zhou C, Luo Y, Ye D, et al. Reference values for amplitude-integrated EEGs in infants from preterm to 3.5 months of age. Pediatrics. (2011) 127:e1280–7. doi: 10.1542/peds.2010-2833
26. Barlow SM, Liao C, Lee J, Kim S, Maron JL, Song D, et al. Spectral features of non-nutritive suck dynamics in extremely preterm infants. Pediatr Med. (2023) 6:1–12. doi: 10.21037/pm-21-91
27. Gewolb IH, Vice FL. Abnormalities in the coordination of respiration and swallow in preterm infants with bronchopulmonary dysplasia. Dev Med Child Neurol. (2006) 48:595–9. doi: 10.1017/S0012162206001241
Keywords: biomarkers, neurophysiological assessment, length of hospital admission, developmental maturation, clinical milestones, neonatal morbidities, late preterm babies
Citation: Shah BA, Latremouille S, Chawla S, Keszler M, Tucker R, Laptook A and Sant'anna GM (2025) Heart rate variability and amplitude-integrated electroencephalography measured shortly after birth and time to reach clinical milestones: a pilot study in late preterm infants. Front. Pediatr. 13:1579197. doi: 10.3389/fped.2025.1579197
Received: 18 February 2025; Accepted: 15 May 2025;
Published: 5 June 2025.
Edited by:
Po-Yin Cheung, University of Alberta, CanadaReviewed by:
Jiang-Qin Liu, Shanghai First Maternity and Infant Hospital, ChinaVenkata Chaitanya Chirumamilla, Children’s National Hospital, United States
Copyright: © 2025 Shah, Latremouille, Chawla, Keszler, Tucker, Laptook and Sant'anna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birju A. Shah, YmlyanUtc2hhaEBvdWhzYy5lZHU=
 Birju A. Shah
Birju A. Shah Samantha Latremouille
Samantha Latremouille Sanjay Chawla
Sanjay Chawla Martin Keszler
Martin Keszler Richard Tucker4
Richard Tucker4 Abbot Laptook
Abbot Laptook Guilherme M. Sant'anna
Guilherme M. Sant'anna