- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, China
Introduction: Lead is a naturally occurring metal with highly toxic effects on humans, particularly children, who are particularly vulnerable to its long-lasting adverse impacts.
Patient concerns: This report presents the case of a 10-year-old boy with a 10-month history of recurrent vomiting. No organic lesions were identified. However, the symptoms were inconsistent with cyclic vomiting syndrome (CVS), given the presence of growth retardation, vitamin D deficiency, bilateral cervical lymphadenopathy, academic difficulties, and impaired concentration.
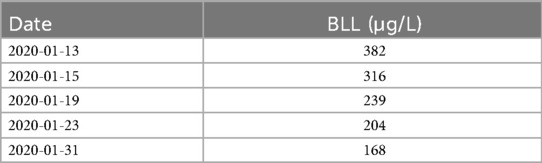
Diagnosis: Detailed history-taking revealed proximity to a lead mining facility. Blood lead level (BLL) was significantly elevated (382 μg/L), confirming chronic lead poisoning.
Interventions: Chelation therapy with dimercaptosuccinic acid (DMSA) was administered for 19 days at a 350 mg/m2 dose per administration.
Outcomes: Following treatment, BLL decreased to 168 µg/L, accompanied by significant improvement in vomiting and abdominal pain.
Conclusion: Pediatric lead poisoning should be considered in the differential diagnosis of children presenting with cyclic vomiting and neurological symptoms, due to its diagnostic complexity.
Introduction
Lead poisoning remains a significant global public health concern due to its potential for irreversible damage in children. Clinical manifestations include acute encephalopathy, peripheral neuropathy, hearing loss, neurobehavioral dysfunction, abdominal pain, constipation, growth retardation, vitamin D deficiency, anemia, and kidney disease (1). However, cyclic vomiting as an initial symptom is rarely reported in pediatric cases. This study describes a child with lead poisoning presenting primarily with cyclic vomiting; the clinical features were retrospectively analyzed. This case provides valuable insights into the association between lead exposure and atypical gastrointestinal symptoms in children.
Case presentation
A 10-year-old boy was admitted to our hospital with a 10-month history of recurrent vomiting. Episodes occurred without obvious triggers. Although non-projectile, the vomiting was severe, occurring more than 20 times per day and lasting 3–7 days, followed by symptom-free intervals of approximately 20 days. As episodes progressed, bilious and coffee-ground emesis developed. During severe episodes, the child experienced lethargy and epigastric pain, without radiation or referred pain. Notably, the child had no symptoms of cough, bloating, diarrhea, constipation, or hematochezia. Between episodes, his mental status and appetite returned to normal after appropriate treatment.
On admission, a comprehensive physical examination was performed. The child appeared alert but undernourished. His height (130 cm) and weight (24 kg) were 1–2 standard deviations below the mean for his age. No skin rash was observed. However, multiple enlarged, soft, mobile, and non-tender lymph nodes were palpable bilaterally, with the largest measuring approximately 3.0 cm × 1.5 cm. The tonsils were grade II enlarged without purulent discharge. Abdominal examination revealed epigastric tenderness without rebound pain or guarding. The liver and spleen were not palpable, and bowel sounds were normal. Cardiopulmonary and neurological examinations were unremarkable.
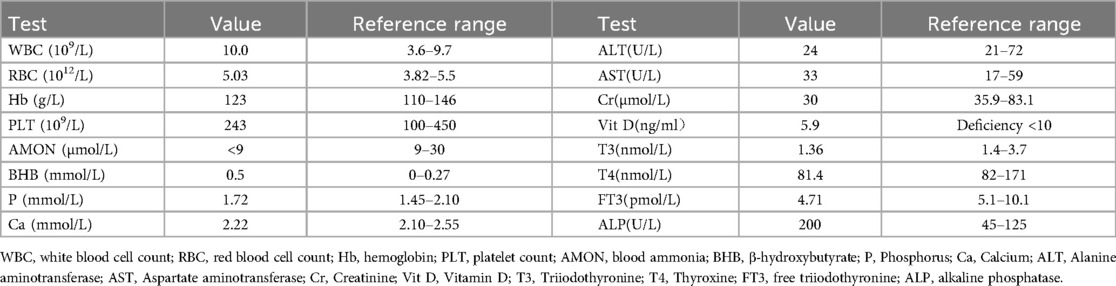
Gastroscopy at a local hospital revealed chronic non-atrophic gastritis with bile reflux. Upon admission to our hospital, routine blood, stool, and urine tests showed no significant abnormalities. Liver and kidney function, serum electrolytes, blood ammonia, and β-hydroxybutyrate were all within normal limits (Table 1). However, serum vitamin D was deficient at 5.9 ng/ml (deficiency < 10 ng/ml). Thyroid function tests revealed mildly reduced T3, T4, and free T3 levels. Cervical ultrasonography showed enlarged bilateral lymphadenopathy, with the largest nodes measuring 3.3 cm × 1.0 cm on the left and 3.4 cm × 1.0 cm on the right. Non-contrast abdominal computed tomography (CT) revealed no abnormalities.
Due to the absence of conclusive findings, a detailed environmental history was obtained. It was discovered that a lead mining facility had been established near the child's residence within the past two years. Considering his academic difficulties and impaired concentration, lead poisoning was suspected. Blood lead level (BLL) testing at an occupational disease hospital revealed a substantially elevated level of 382 μg/L (reference range: 0–100 μg/L), confirming a diagnosis of moderate lead poisoning. Chelation therapy with dimercaptosuccinic acid (DMSA) was initiated. For the first 5 days, DMSA was administered three times daily at a dose of 1,050 mg/m2/ day. This regimen was followed by 14 days of twice-daily dosing (700 mg/m2/day), totaling 19 days of treatment. Following therapy, BLL decreased to 168 μg/L (Table 2), significantly improving vomiting and abdominal pain. At one-year follow-up, after closure of the nearby lead mine, the child remained symptom-free, with no recurrence of vomiting, abdominal pain, or fatigue.
Discussion
In this case, the child's clinical presentation demonstrated cyclical vomiting patterns with symptom-free intervals. Based on physical examination and ancillary test results, common organic causes of vomiting were initially excluded. Among functional gastrointestinal disorders, cyclic vomiting syndrome (CVS) was first considered—a condition characterized by recurrent episodes of intense vomiting, with or without retching, occurring in a stereotypical pattern and followed by return to baseline health between episodes (2). Stress and infections are well-established triggers of CVS; additional factors such as certain foods (such as chocolate, cheese, and caffeine), fasting, fever, sleep deprivation, allergies, diet, and menstruation may precipitate episodes (3, 4).
CVS represents a final common manifestation of diverse pathophysiological mechanisms, rather than a single etiology (5). Its pathogenesis has been linked to gut–brain axis disturbances, mitochondrial enzyme dysfunction, gastrointestinal motility disorders, calcium channel abnormalities, and hypothalamic–pituitary–adrenal (HPA) axis hyperactivity (6). Among these, HPA axis overactivation is hypothesized to affect autonomic regulation (specifically sympathetic stimulation and vagal inhibition), leading to altered intestinal motility. Maternal lead exposure has been associated with long-term changes in HPA axis function, including hypercortisolism (7). Although the mechanisms remain unclear, lead may directly affect HPA axis regulation or exert indirect effects through neurochemical disruption, potentially triggering cyclic vomiting.
However, CVS alone could not fully account for the patient's other clinical findings—namely, vitamin D deficiency, growth retardation, and bilateral cervical lymphadenopathy. This discrepancy prompted a more detailed environmental history, revealing the presence of a lead mine plant near his residence. A considerably elevated BLL confirmed moderate lead poisoning. Although the child had no history of pica, he frequently played near the lead mine, and the hand-to-mouth behavior likely contributed to ingesting lead-contaminated dust or soil. The resolution of symptoms following chelation therapy strongly supports a causal link between lead exposure and his cyclic vomiting episodes. Lead, a naturally occurring heavy metal, is pervasive in the environment—found in air, dust, soil, and water—and widely used in mining, construction, paint, gasoline, ceramics, food, and traditional Chinese medicine (8). As a potential neurotoxin, lead causes irreversible harm in children, making lead poisoning a significant public health concern worldwide (9). Both the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO) recognize elevated blood lead levels in children as a critical marker of increased health risk (10, 11).
Lead poisoning presents in two distinct forms based on exposure duration. Acute poisoning results primarily from brief exposure to high lead concentrations, with chronic lead poisoning—more prevalent in children over 2–3 years of age—typically manifesting 3–6 months following continuous environmental exposure. The 10-month disease duration in this case aligns with a diagnosis of chronic lead poisoning. Neurological manifestations dominate in chronic exposure, especially concerning impaired cognitive development. As observed in this patient, potential outcomes include reduced intelligence quotient (IQ), attention deficits, antisocial behavioral tendencies, and poor academic performance (11). To the best of our knowledge, this represents the first reported case of chronic lead poisoning presenting with cyclic vomiting as the primary and predominant symptom. Initially, both lead poisoning and CVS were considered as differential diagnoses. In adults, CVS may coexist with various conditions, such as marijuana smoking, migraine, mental illness, diabetes, and irritable bowel syndrome. Besides managing cyclic vomiting episodes, treating coexisting conditions is also crucial. This strategy alleviates symptoms and leads to complete resolution within 1–2 years of treatment (12). While this conclusion is primarily based on adult data, it may also apply to children. However, in this case, the child's vomiting improved significantly following lead chelation therapy alone and did not recur during a 1-year follow-up without lead exposure. Hence, it is conclusive that lead poisoning was the primary cause of his cyclic vomiting, an unusual and distinctive feature of this case.
Several reports support our findings. One study described a worker in a lead-manganese smelter who experienced nausea, vomiting, abdominal colic, dizziness, headache, and memory loss for over a year. Although intermittent abdominal pain was noted, the periodicity of vomiting episodes remained unspecified (13). Another investigation (unpublished) identified an association between persistent gastrointestinal symptoms and BLL ≥500 μg/L. Interestingly, the prevalence of intermittent gastrointestinal symptoms was twice as high in children with BLLs >200 μg/L compared to those with lower levels (14). In our case, the child's BLL was 382 μg/L—within the 200–500 μg/L range—and his recurrent symptoms aligned with previous observation.
The long-term consequences of lead poisoning in children encompass growth retardation, vitamin D deficiency, anemia, and renal impairment (15, 16). Although this child had no signs of anemia or nephropathy, he exhibited growth retardation and vitamin D deficiency. No skeletal deformities were noted, and serum calcium and phosphorus levels were normal; however, alkaline phosphatase (ALP) was mildly elevated (Table 1). His vitamin D deficiency was likely multifactorial, due to both chronic lead exposure and limited ultraviolet B radiation in a geographically sun-restricted basin (17, 18). Kersey et al. (19) found a negative but statistically insignificant association between BLLs and serum 25-hydroxyvitamin D [25(OH)D] levels in healthy toddlers and children under age 6. Lead may impair hepatic 25-hydroxylase activity, the enzyme responsible for converting vitamin D to its main circulating form, 25(OH)D. To address the deficiency, a two-phase supplementation regimen was implemented: an initial intensive phase of cholecalciferol (2,000 IU/day for 12 weeks), followed by a maintenance phase (600 IU/day) (18). Cervical lymphadenopathy and tonsillar hypertrophy were observed. Children residing in urban or industrialized areas are reported to have higher tonsillar and lymph node enlargement rates, possibly due to increased colonization of pathogenic bacteria on the tonsils' surface (20).
Given the irreversible neurocognitive and behavioral effects of lead exposure in children, primary prevention is paramount (9, 10). Recent studies indicate that children residing near lead mines, smelters, and chemical plants are at particularly high risk. These industries may leak substantial amounts of lead during production, contaminating regional air, soil, and water. The density of lead mining and lead smelting and chemical companies was positively correlated with the BLLs of children, higher BLLs indicated a more significant density of lead smelting and chemical companies in a specific area. The greater the volume and duration of lead leakage to a local area, the greater its effect on the BLLs of local children (21). Therefore, lead mining and smelting enterprises face significant challenges in strictly controlling emissions and enhancing environmental surveillance. Simultaneously, residential development in adjacent areas must be prohibited to prevent continued lead exposure among children. Furthermore, in confirmed cases of lead poisoning, children must remain in lead-free environments following chelation therapy to prevent re-exposure (1).
However, despite our repeated recommendations during the one-year telephone follow-up, the child's parents refused re-evaluation of the child's BLL. Remote mountainous residence, inadequate transportation, financial constraints, and the absence of recurring clinical symptoms over the past year influenced their decision. Consequently, we were unable to further assess whether BLL rebound indeed occurred in this case. Future research should focus on monitoring dynamic BLL changes following chelation therapy.
Conclusions
To the best of our knowledge, this is the first report of cyclic vomiting caused by lead poisoning. Given the declining incidence and atypical manifestations, lead poisoning is frequently misdiagnosed. This clinical case may be a valuable reference for future clinical diagnosis and treatment. Lead poisoning should be considered in pediatric patients presenting with cyclic vomiting and neurological symptoms.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the West China Second University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
LD: Conceptualization, Data curation, Writing – original draft. LW: Data curation, Writing – original draft. ZW: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors sincerely thank the patient and his parents for their cooperation and consent to share this case for educational and research purposes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BLL, blood lead level; DMSA, dimercaptosuccinic acid; CVS, cyclic vomiting syndrome; WHO, World Health Organization; HPA axis, hypothalamic–pituitary–adrenal axis; CT, computed tomography; CDC, Centers for Disease Control and Prevention; IQ, intelligence quotient; ALP, alkaline phosphatase; 25(OH)D, 25-hydroxyvitamin D.
References
1. Sample JA. Childhood lead poisoning: clinical manifestations and diagnosis. Available online at: https://www.uptodate.cn/contents/childhood-lead-poisoning-clinical-manifestations-and-diagnosis (Accessed July 15, 2025).
2. Raucci U, Borrelli O, Di Nardo G, Tambucci R, Pavone P, Salvatore S, et al. Cyclic vomiting syndrome in children. Front Neurol. (2020) 11:583425. doi: 10.3389/fneur.2020.583425
3. Kovacic K, Sood M, Venkatesan T. Cyclic vomiting syndrome in children and adults: what is new in 2018? Curr Gastroenterol Rep. (2018) 20(10):1–9. doi: 10.1007/s11894-018-0654-5
4. Moses J, Keilman A, Worley S, Radhakrishnan K, Rothner AD, Parikh S. Approach to the diagnosis and treatment of cyclic vomiting syndrome: a large single-center experience with 106 patients. Pediatr Neurol. (2014) 50(6):569–73. doi: 10.1016/j.pediatrneurol.2014.02.009
5. Li B. Managing cyclic vomiting syndrome in children: beyond the guidelines. Eur J Pediatr. (2018) 177(10):1435–42. doi: 10.1007/s00431-018-3218-7
6. Drossman DA, Hasler WL. Rome IV—functional GI disorders: disorders of gut-brain interaction. Gastroenterology. (2016) 150(6):1257–61. doi: 10.1053/j.gastro.2016.03.035
7. Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environ Health Perspect. (2004) 112(6):717–30. doi: 10.1289/ehp.6481
9. O’Connor D, Hou D, Ok YS, Lanphear BP. The effects of iniquitous lead exposure on health. Nat Sustain. (2020) 3(2):77–9. doi: 10.1038/s41893-020-0475-z
10. Centers for Disease Control and Prevention. Lead poisoning prevention. Available online at: https://www.cdc.gov/lead-prevention/prevention/?CDC_AAref_Val=https://www.cdc.gov/nceh/lead/prevention/default.htm (Accessed July 15, 2025).
11. World Health Organization. Lead poisoning and health. Available online at: https://www.who.int/news-room/fact-sheets/detail/lead-poisoning-and-health (Accessed July 15, 2025).
12. Herlihy JD, Reddy S, Shanker A, McCallum R. Cyclic vomiting syndrome: an overview for clinicians. Expert Rev Gastroenterol Hepatol. (2019) 13(12):1137–43. doi: 10.1080/17474124.2019.1691527
13. Du X, Zheng W, Ye Q. Rare cases of severe life-threatening lead poisoning due to accident or chronic occupational exposure to lead and manganese: diagnosis, treatment, and prognosis. Toxicol Ind Health. (2020) 36(12):951–9. doi: 10.1177/0748233720958969
14. Markowitz M. Lead poisoning: an update. Pediatr Rev. (2021) 42(6):302–15. doi: 10.1542/pir.2020-0026
15. Hanna-Attisha M, LaChance J, Sadler RC, Champney Schnepp A. Elevated blood lead levels in children associated with the flint drinking water crisis: a spatial analysis of risk and public health response. Am J Public Health. (2016) 106(2):283–90. doi: 10.2105/AJPH.2015.303003
16. McLaughlin TJ, Humphries O Jr, Nguyen T, Maljanian R, McCormack K. “Getting the lead out” in Hartford, Connecticut: a multifaceted lead-poisoning awareness campaign. Environ Health Persp. (2004) 112(1):1–5. doi: 10.1289/ehp.6391
17. Rahman A, Al-Awadi A, Khan K. Lead affects vitamin D metabolism in rats. Nutrients. (2018) 10(3):264. doi: 10.3390/nu10030264
18. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. (2017) 18(2):153–65. doi: 10.1007/s11154-017-9424-1
19. Kersey M, Chi M, Cutts DB. Anaemia, lead poisoning and vitamin D deficiency in low-income children: do current screening recommendations match the burden of illness? Public Health Nutr. (2011) 14(8):1424–8. doi: 10.1017/S1368980010003617
20. Schlipköter HW, Dolgner R. Luftverunreinigung und körpereigene abwehr [air pollution and biological defence (author’s transl)]. Zentralbl Bakteriol B. (1981) 172(4-5):299–311.
Keywords: lead poisoning, cyclic vomiting, cyclic vomiting syndrome, neurological involvement, pediatric
Citation: Ding L, Wang L and Wang Z (2025) Lead poisoning in a 10-year-old boy with cyclic vomiting as the first symptom: a case report. Front. Pediatr. 13:1580368. doi: 10.3389/fped.2025.1580368
Received: 20 February 2025; Accepted: 21 July 2025;
Published: 6 August 2025.
Edited by:
Lorenz S. Neuwirth, State University of New York at Old Westbury, United StatesReviewed by:
Morri Markowitz, Albert Einstein College of Medicine, United StatesTravis Hobart, Upstate Medical University, United States
Copyright: © 2025 Ding, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiling Wang, d2FuZ196aGlfbGluZ0AxMjYuY29t
 Liping Ding
Liping Ding Liyuan Wang
Liyuan Wang Zhiling Wang
Zhiling Wang