- Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Department of Pediatrics, Indiana University School of Medicine, Indianapolis, IN, United States
Gastrointestinal (GI) endoscopy is a valuable tool to diagnose and treat GI conditions. Traditional pediatric GI endoscopy uses sedation or general anesthesia, with associated risks of cardiopulmonary compromise and social and economic costs like school or work absence. Unsedated, transnasal endoscopy is an approach that mitigates these disadvantages but provides similar diagnostic benefit to conventional endoscopy. Ongoing advances in the field of pediatric transnasal endoscopy will be driven by an enhanced understanding of current indications, available equipment, procedural comfort strategies, and recent developments in new diagnostic and therapeutic uses.
Introduction
In pediatrics, gastrointestinal (GI) endoscopy is a valuable tool to assess GI symptoms, diagnose mucosal diseases, and provide therapeutic interventions. Pediatric endoscopy is often done with sedation or general anesthesia for patient comfort and procedural safety (1). Cardiopulmonary compromise is the most common adverse event (AE) in pediatric endoscopy with a higher risk with intravenous sedations compared to general anesthesia (2). Additionally, anesthesia may lead to activity limitations and delayed return to work or school. Unsedated (“u”) GI endoscopy, more specifically upper endoscopy or esophagogastroduodenoscopy (EGD), is an alternative method to sedated (“s”) endoscopy that is safe, well-tolerated, and with expanding clinical uses. Though conventional pediatric EGD uses a peroral (“P”) approach, transnasal (“TN”) endoscopy is gaining popularity. In this article, we aim to review the history of TN endoscopy, scopes available for use and their features, anesthetic regimens, current applications in children and adults, and future directions.
History of unsedated and transnasal GI endoscopy
The earliest report of successful unsedated GI endoscopy documented completion of uP-EGD in 187 of 200 adult patients seen in a one-visit clinic for dyspepsia in the 1970s (3). Esophagitis, gastritis, duodenitis, hiatal hernia, and other abnormalities were identified with this approach. 60% of patients found the procedure mildly unpleasant, and 5.5% of procedures were aborted due to inability to tolerate the procedure (3). A larger prospective study from the 1980s reported 2,000 consecutive successful uP-EGD in patients (8–86 years) with only 1.6% of patients requiring IV sedation in order to complete the procedure (4). Conventional P-EGD typically uses endoscopes of 9.2–9.9 mm diameter.
The volume of publications about unsedated endoscopy increased in the 1990s with emergence of the TN approach. Esophageal intubation with a bronchoscope (5.3 mm diameter) transnasally was first introduced in 1991 in adult patients undergoing evaluation for cervical dysphagia (5). The investigator then performed successful uTN-EGD on 20 healthy adult volunteers with topical nasal xylocaine gel and pharyngeal cetacaine spray (5). A 1995 study (6) compared uTN-EGD to P-EGD with or without general anesthesia within the same patient in 24 adults. Eighteen required IV conscious sedation for sP-EGD, while 6 did not. These patients reported significantly higher acceptability of uTN-EGD compared to uP-EGD and sP-EGD, with fewer symptoms of choking sensation, sore throat, and discomfort. There was overall agreement between endoscopic findings between the uTN-EGD and P-EGD (6). A 1998 study (7) showed similar efficacy and tolerance of uTN-EGD vs. sP-EGD and estimated that 12 TN-EGDs could be performed in the same time as 9 P-EGDs with 65% decrease in consumable and medication costs and 92% decrease in time in procedure and recovery area (7).
Though a majority of historical studies of unsedated endoscopies are adult studies, a 2002 study (8) specifically explored feasibility of unsedated endoscopy in children. Twenty-seven sP-EGDs (mean 12.2 years ± 2.7) were compared to 21 uP-EGDs (mean 13.5 years ± 2.7) using a 9.8 mm diameter endoscope with similar success rate between the two groups at 96.3% and 95.2%, respectively. There was no significant change in anxiety scores before and after uP-EGD. Anxiety scores were higher in the sP-EGD group but reduced post procedure, and so authors suspected that anxious children were more likely to request sedation. Children undergoing sP-EGD spent twice as long in the endoscopy suite compared to uP-EGD (8).
With growing interest in the TN approach came the development of ultra-thin scopes for GI use. Early studies in healthy adults using endoscopes with 5.9–6.0 mm diameter demonstrated equivalence of the quality of GI assessment between uTN-EGD and uP-EGD (9). Both routes of insertion were generally well-tolerated, but with variable results on pain/discomfort and willingness to repeat the procedure (9–11). Using an ultra-slim scope for uP-EGD significantly reduced direct and indirect costs compared to sP-EGD (12).
Decreasing scope size to improve comfort with TN approach has also been explored, and procedure success is higher with a smaller scope. For example, a large study of 1,100 adults compared uTN-EGD using scopes with diameters of 5.9–5.3 mm (13). Overall, procedure success was 93.9%. The larger scope diameter was a predictor of procedure failure and was also more associated with epistaxis or nasal pain. 95.2% (982/1,100) said they would elect to do uTN-EGD again (13).
Transnasal endoscopy equipment
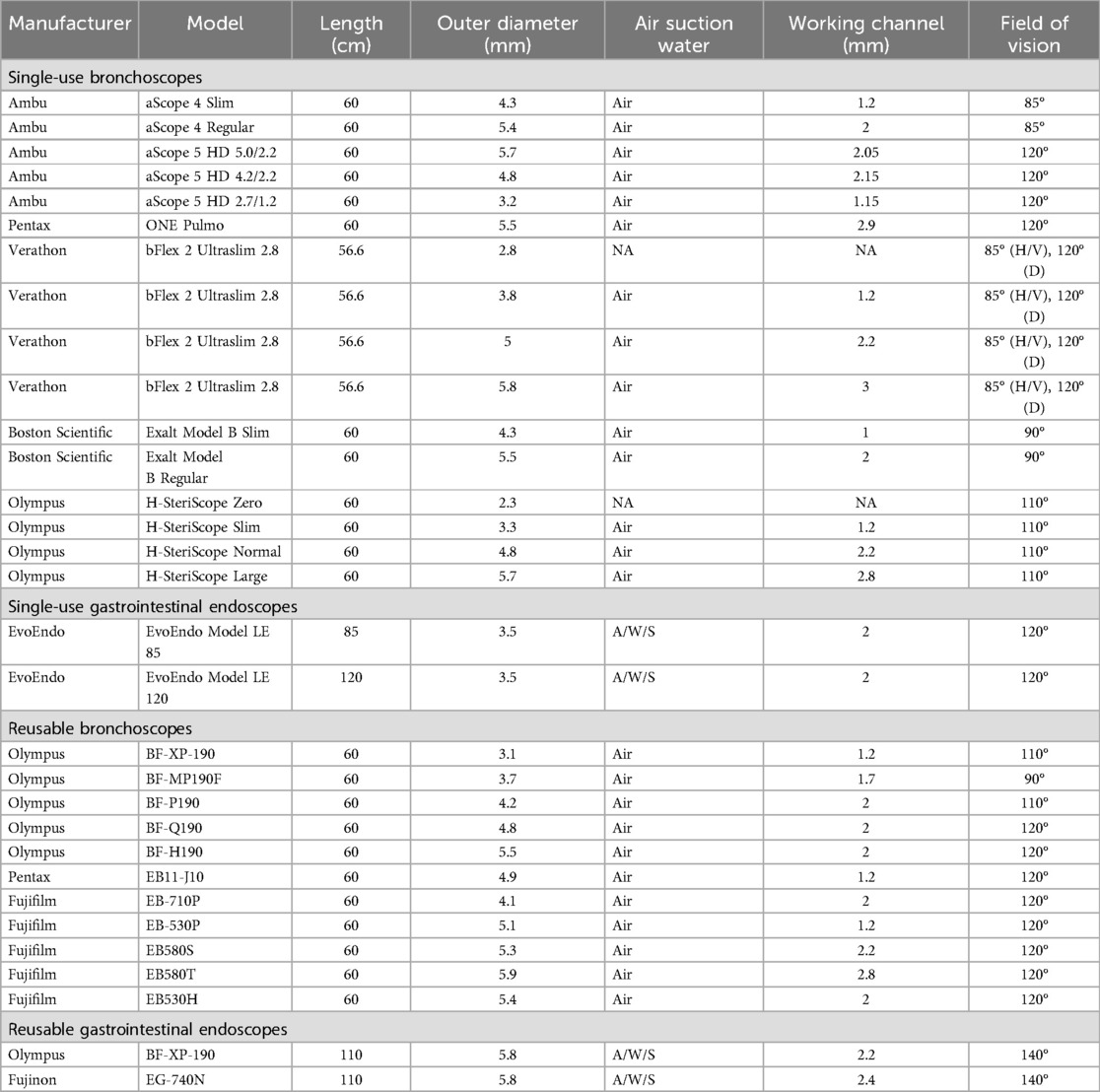
Recent advances in ultra-thin endoscopes have driven the interest in TN endoscopy in children. Early reports of uTN-EGD in adults using “ultra-thin” adult and pediatric endoscopes (5.3–6.0 mm diameter) (5, 6) had limited application to children due to their smaller nasopharyngeal anatomy (14). Thus, the first reports of pediatric uTN esophagoscopy (uTN-E) utilized even smaller diameter reusable pediatric bronchoscopes (4.1 mm) (14–16). Flexible pediatric bronchoscopes are an attractive option for uTN-E with a small external diameter (3.7–4.2 mm) and a single channel (1.2–2.0 mm) for insufflation of air and biopsy forceps for tissue sampling. Adequacy of biopsies samples has been established despite the small working channel of current pediatric bronchoscopes (14, 15, 17). Further, care of reusable pediatric bronchoscopes match established facility protocols for endoscopy processing and maintenance. The utility of pediatric bronchoscopes for TN-EGD is limited by their short length (60 cm), 2-way tip deflection (instead of 4-way deflection), and a single channel for air/water/suction and biopsy instruments. Reusable pediatric bronchoscopes are also limited due to their fragile nature and potential need for repair. Recently, single-use ultra-thin bronchoscopes were introduced to streamline endoscopy efficiency and minimize risk of infectious complications of multi-use endoscopy equipment. While there are no current reports of the use of single-use bronchoscopes for pediatric uTN-E, these may be good options for uTN-E at some institutions.
Gastroscopes are superior to bronchoscopes for uTN-E/EGD due to longer length, 4-way tip deflection, and buttons for insufflating the upper GI lumen, suctioning secretions, and instilling water. The larger working channel (2.2 mm) ensures adequate biopsies specimens and may allow use of interventional equipment. However, the smallest neonatal pediatric gastroscope has an external diameter of 5.8 mm, limiting its use in pediatric uTN-EGD. Dedicated single-use endoscopes have been developed to address the limitations of gastroscopes and bronchoscopes (18). The EvoEndo endoscopy system has multiple length options to perform uTN-E or uTN-EGD (85 or 110 cm) with a small external diameter (3.5 mm) and adequate internal working channel (2 mm) (18). A wide variety of endoscopes are available for use in TN endoscopy (Table 1). Further advancements in reusable and single-use endoscopes will enhance patient tolerance, endoscopic visibility, and facilitate interventional techniques in pediatric TN endoscopy.
Topical anesthesia
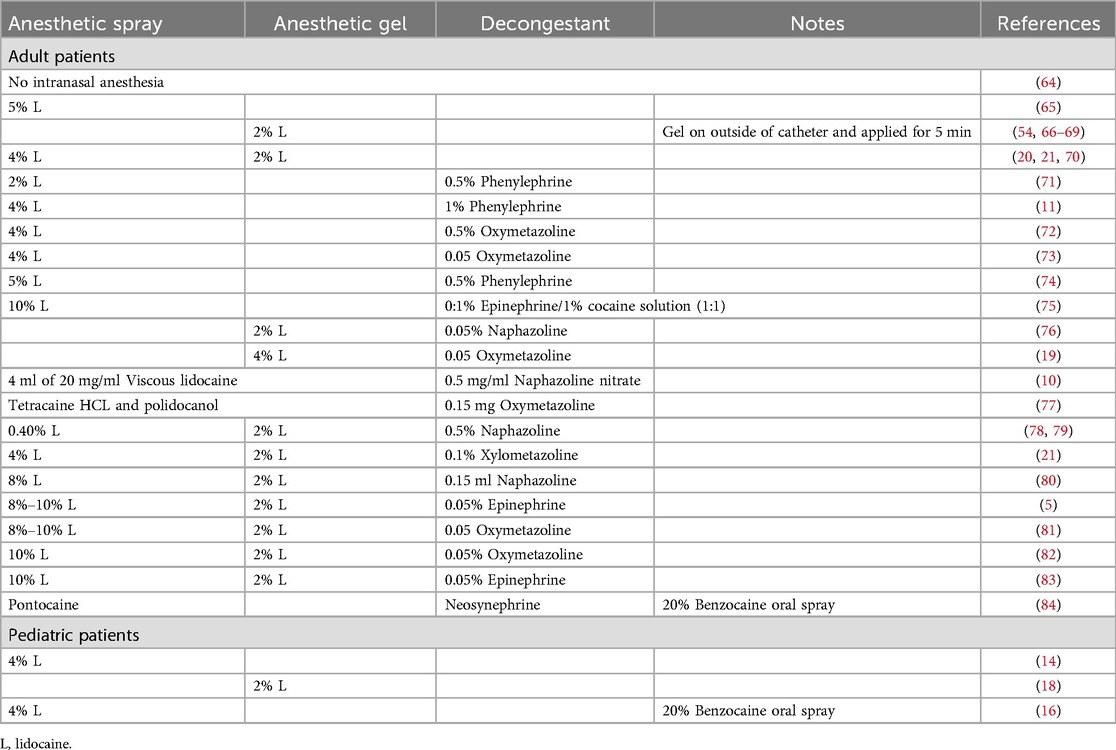
While general anesthesia is not used during uTN endoscopy, topical anesthesia of the nasopharynx and oropharynx is commonly used to decrease the gag reflex and minimize discomfort (14). A wide range of topical anesthetics have been described with most regimens including lidocaine applied to the nasopharynx/posterior oropharynx by spray or transcatheter application, as well as lidocaine jelly applied to the anterior nares and nasopharynx (19). Unfortunately, there are no prospective comparison studies of topical anesthetic regimens during uTN endoscopy. Vasoconstricting medications including epinephrine, oxymetazoline, naphazoline, phenylephrine as nasal decongestants are variably reported in the uTN endoscopy literature, and some authors suggest that nasal decongestants enhance uTN endoscopy performance and tolerability (20, 21). Prospective evaluations of anesthetic regimens in pediatric flexible laryngoscopy have not demonstrated significant improvement in patient tolerance with topical nasal anesthetics, while nasal decongestant improves both tolerance and visibility of the nasal turbinates (22, 23). Table 2 summarizes the anesthetic regimens reported in both adult and pediatric uTN endoscopy. Topical anesthesia is distasteful, stimulating, and may contribute to discomfort during TN endoscopy (24). Some centers add flavoring to the lidocaine preparation or allow patients to lick a lollipop during anesthesia administration. Anxiolytic medications are not commonly used and may cause disinhibition, compromising the ability to complete the procedure safely. Many efforts have been made to improve patient experience and cooperation and decrease anxiety. A child life specialist can enhance the patient and family experience with calming techniques or visual distraction including access to visual media. Virtual reality systems have been utilized as an effective distraction tool in pediatric uTN endoscopy, however, not all patients elect to utilize virtual reality goggles (25). Prospective studies investigating anesthetic protocols, including medication regimens and routes of administration, are needed to advance the broader adoption of uTN endoscopy technology.
Clinical uses of transnasal GI endoscopy
There are many clinical uses of uTN GI endoscopy in adults including pre-operative assessment for bariatric surgery candidates and screening and monitoring of Barrett's esophagus or esophageal varices. In pediatrics, uTN-E is primarily used to assess esophageal conditions such as eosinophilic esophagitis and surveillance of esophageal atresia. uTN-E is well tolerated in children, and tolerance is not impacted by age, gender, diagnosis of anxiety, ADHD, or autism (26). We discuss advances in TN endoscopy in adults and children below, with particular relevance to pediatric gastroenterology.
Eosinophilic esophagitis
Eosinophilic esophagitis (EoE) is a chronic esophageal disorder that requires endoscopy for diagnosis and monitoring, thus an ideal condition for TN endoscopy (27). A 2016 pediatric study (14) showed successful uTN-E using a 4.0 mm bronchoscope on 21 patients with EoE ages 8–17 years with no AE. 90.5% of parents and 81% of children were highly satisfied with the procedure, and 100% and 76.2% would elect to do uTN-E again, respectively. Endoscopic findings had high correlation with histologic findings in 85.7% of cases. Total charges for each patient undergoing uTN-E were 60.1% ± 10.7% less than those of their previous sP-EGD (14).
A larger 2019 pediatric study (25) reviewed 294 uTN-Es (including 1 uTN-EGD) in 190 patients ages 3–22 years, with many subjects undergoing multiple uTN-Es to assess EoE treatment changes. While the most common AEs were epistaxis (3.7%) and vomiting (2.7%), a majority (89.8%) experienced no AE. Full thickness epithelium was present in 88% of biopsies obtained with 1.2 mm biopsy forceps compared to 94% in 2.0 mm biopsy forceps. There was an estimated 53.4% reduction in total charges for uTN-E compared to sP-EGD at the time of the study (25).
Since TN-E does not require sedation, the procedure can be repeated in the same patient in succession quickly. In a small study of 6 children ages 11–18 years with EoE treated with diet elimination, uTN-E was repeated 2–4 weeks after food reintroductions. Four patients had active EoE at these early intervals and also had active EoE at a traditional 6-week TN-E (28). TN-E is feasible, effective, and saves cost for children and may allow for more precise management of diet elimination in EoE.
Esophageal atresia
Esophageal atresia (EA) is a congenital malformation of the esophagus requiring surgical treatment. Children born with EA are at increased risk of gastroesophageal reflux disease, esophagitis, and esophageal strictures and are recommended to have surveillance endoscopies periodically through childhood and into adulthood (29). A case series described four patients with EA who underwent successful uTN-E for surveillance or monitoring of reflux esophagitis and EoE (30). uTN-E demonstrated a variety of findings including normal screening endoscopy, identification of new esophagitis, and visual and histologic remission of esophagitis and EoE after treatment (30).
Barrett’s esophagus
Barrett's esophagus (BE), which is a transformation of esophageal squamous epithelium to intestinal columnar epithelium, is a risk factor for esophageal adenocarcinoma. BE is associated with gastroesophageal reflux disease (GERD), occurring in 5%–15% of patients with longstanding symptoms (31). Esophageal endoscopic evaluation is the gold standard to assess for BE, traditionally with P-EGD (32, 33). uTN-E or uTN-EGD may serve as a well-tolerated and effective screening for Barrett's esophagus and dysplasia. A 2006 study (34) evaluating detection of Barrett's esophagus and dysplasia in adult patients with chronic GERD demonstrated no difference in BE between uTN-EGD or sP-EGD among 116 patients who completed both procedures. Nine cases of BE were identified with uTN-EGD but missed on sP-EGD, while 5 cases of BE were identified with sP-EGD and missed on uTN-EGD. Biopsy specimens from sP-EGD were significantly larger than those obtained via uTN-EGD (2.60 vs. 1.39 mm2, p < 0.001). Though anxiety, pain, gagging and choking were more frequent in the uTN-EGD group, discomfort overall was reported as “mild”. Seventy-one percent preferred to have uTN-EGD again over sP-EGD (34).
In a 2023 systematic review and metanalysis of 623 patients who each underwent both uTN-EGD and sP-EGD, pooled sensitivity and specificity were high for detecting intestinal epithelium and intestinal metaplasia with uTN-EGD (35). Patient tolerance was higher with uTN-EGD compared to P-EGD in 3 studies, but no different in 3 other studies. Procedure completion rate was the same between both procedure types, and overall AE rate was low (2.0%). The most common AEs with uTN-EGD were epistaxis and vasovagal symptoms (35).
BE is rare in children compared to adults with prevalence of 0.25%–4.8% (36, 37). The mean duration of reflux symptoms prior to BE diagnosis is 5.3 years, and additional risk factors include neurodevelopmental disorders, congenital esophageal atresia, hiatal hernia, high BMI, and older age (36–39). Due to its low prevalence, there are no consensus guidelines on screening for BE in children with GERD, though endoscopy and biopsy remain the primary tool for detection. These adult studies suggest uTN-EGD may be an effective tool to detect complications of GERD, which could be extrapolated to pediatrics.
Varices
Adult patients with liver cirrhosis undergo screening endoscopy to evaluate for esophageal varices, and those with large varices are prescribed prophylactic treatment given the high risk of morbidity and mortality from variceal bleeding (40). Sedation in this population poses risk of encephalopathy (41), thus making unsedated endoscopy appealing. A 2002 study (42) of uTN-EGD followed immediately by sP-EGD in 15 adult patients with cirrhosis and no history of variceal bleeding found esophageal varices in 66.7% in both procedures. Identification of gastric varices, large esophageal varices, small esophageal varices, and esophageal rings was concordant between the two procedures. Portal hypertensive gastropathy was identified in one additional patient by sP-EGD compared to uTN-EGD, and Barrett's esophagus was identified in one additional patient by uTN-EGD compared to sP-EGD. No interventions were performed, and procedural tolerance was similar between procedure types. A larger study of 40 adult patients with compensated cirrhosis without active varices prophylaxis underwent successful endoscopic surveillance with uP-EGD (43). Grade 1 varices (17.5%), grade 2 varices (20%), and portal hypertensive gastropathy (22.5%) were identified. Two patients underwent subsequent variceal band ligation with identical findings on sP-EGD to uP-EGD (43). Literature on transnasal pediatric varices assessment is limited. In a 2023 report, a 13-year-old with end-stage renal disease and an 18-year-old with autoimmune hemolytic anemia and autoimmune hepatitis with hepatosplenomegaly required screening for esophageal varices but were deemed high-risk for anesthesia. These patients successfully underwent uTN-EG and uTN-E (respectively) that did not show esophageal varices (44). Unsedated endoscopy may be a safe way to evaluate for varices in populations at high risk of complications from sedation. uTN endoscopy may be better tolerated than uP-EGD and is just as effective at detecting esophageal varices, though endoscopic interventions are limited.
Obesity
Endoscopic evaluation of the upper GI tract is recommended prior to bariatric surgery in adults to evaluate for conditions that may inhibit surgery success (45). Patients with overweight and obese status, and comorbid complications like sleep apnea, may have higher risk of cardiopulmonary events related to sedation (46), thus unsedated endoscopy is a reasonable approach to pre-operative evaluation. In a small study of 25 adult patients undergoing pre-operative endoscopy for bariatric surgery, all patients tolerated uTN-EGD, and 56% had abnormalities identified including hiatal hernia, gastritis, esophagitis, BE, gastric polyps, gastric ulcer, and esophageal varices (47). In a 2020 study (48) of 94 adult patients who underwent uTN-EGD at a bariatric center, the procedure was successful in 98.9% of patients with endoscopic abnormalities in 88.3% and actionable abnormalities in 23.4%, including hiatal hernia, peptic ulcers, esophagitis, gastritis, varices, and subepithelial lesions. Biopsies obtained in 80.8% were adequate for pathology evaluation. A majority of patients tolerated the procedure well with 84% reporting minimal discomfort, and all patients agreed to repeat uTN-EGD if needed (48).
Preoperative endoscopic evaluation may be of value in children as well. In a 2021 study, 80 children ages 12–18.1 years underwent sP-EGD before bariatric surgery, and 54% had abnormalities identified as esophagitis, gastritis including helicobacter pylori, and duodenitis. Seventy-seven percent were prescribed medical therapy for these findings prior to bariatric surgery (49). A 2024 study reviewed 244 patients aged 9–25 years who had sP-EGD before bariatric surgery; the findings of which affected medical or surgical management in 24.6% (60 cases) (50). Adults and children with overweight or obese status may benefit from GI evaluation with uTN-EGD to optimize preoperative care while reducing risk of complications from sedation.
Advanced unsedated endoscopy and future directions
In addition to diagnostic uTN-E and uTN-EGD as described above (18), the ultra-thin endoscopes can be used for a broad range of other diagnostic and therapeutic approaches to unsedated endoscopy. Trans-gastrostomy EGD to evaluate the upper GI mucosa in children with gastrostomy can be performed with an ultra-thin endoscope (51) with adequate visualization and biopsy sampling. Ultra-thin endoscopes can also be used to identify aspiration by pediatric flexible endoscopic evaluation of swallowing (FEES) in children with dysphagia (52). Further, uTN-E can be used for esophageal varices screening in children with cirrhosis and portal hypertension (44). While sedated EGD has a limited role in the assessment of gastrointestinal motility, uTN-esophagogastroscopy (EG) may be able to help identify patients with delayed gastric emptying by repeating uTN-EG at defined time intervals after ingestion of a standard meal (53) or by assessing esophageal motor function with swallowing during uTN-E (54). Placement of esophageal Bravo pH probe can also be guided by uTN-E for monitoring of esophageal acid exposure (55).
Additional uTN-EGD interventional procedures have been described in the adult literature including percutaneous gastrostomy placement (56), nasoenteral feeding tube placement (57), lower esophageal sphincter botox injection (58), balloon dilation of esophageal strictures (59), and polypectomy of gastric polyposis (60). uTN-EGD has been proposed as a screening method to identify patients with upper GI bleeds amenable to treatment during sP-EGD (61, 62). As the number of skilled providers performing pediatric uTN-EGD increases, the opportunity for interventional unsedated endoscopy will increase, particularly in children who are at increased risk of complications related to anesthesia.
The availability of ultra-thin TN endoscopy equipment may expand the use of TN endoscopy to initial in-office evaluations of the upper GI tract in children with abdominal complaints with low likelihood of needing endoscopic intervention. A comparison study of uTN-EGD to sP-EGD in 15 adolescents with undifferentiated abdominal pain demonstrated decreased procedure time, anesthesia use, and costs with similar diagnostic outcomes (63). Additional studies are required to determine the utility of in-office uTN-E as a primary method for evaluating a gastrointestinal complaint.
Conclusion
uTN endoscopy is a cutting-edge technique with capabilities of diagnosing and monitoring upper GI conditions without sedation or anesthesia. With the development of specific TN GI equipment, more pediatric GI physicians learning this technique, and more patients and families experiencing the procedure, it is likely that TN endoscopy will continue to gain popularity in pediatrics.
Author contributions
PB: Writing – original draft, Writing – review & editing. RP: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication was supported by the Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Department of Pediatrics, Indiana University School of Medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lightdale JR, Mahoney LB, Schwarz SM, Liacouras CA. Methods of sedation in pediatric endoscopy: a survey of NASPGHAN members. J Pediatr Gastroenterol Nutr. (2007) 45(4):500–2. doi: 10.1097/MPG.0b013e3180691168
2. Thakkar K, El-Serag HB, Mattek N, Gilger MA. Complications of pediatric EGD: a 4-year experience in PEDS-CORI. Gastrointest Endosc. (2007) 65(2):213–21. doi: 10.1016/j.gie.2006.03.015
3. Beavis AK, La Brooy S, Misiewicz JJ. Evaluation of one-visit endoscopic clinic for patients with dyspepsia. Br Med J. (1979) 1(6175):1387–9. doi: 10.1136/bmj.1.6175.1387
4. al-Atrakchi HA. Upper gastrointestinal endoscopy without sedation: a prospective study of 2000 examinations. Gastrointest Endosc. (1989) 35(2):79–81. doi: 10.1016/S0016-5107(89)72712-7
5. Shaker R. Unsedated trans-nasal pharyngoesophagogastroduodenoscopy (T-EGD): technique. Gastrointest Endosc. (1994) 40(3):346–8. doi: 10.1016/S0016-5107(94)70068-0
6. Dean R, Dua K, Massey B, Berger W, Hogan WJ, Shaker R. A comparative study of unsedated transnasal esophagogastroduodenoscopy and conventional EGD. Gastrointest Endosc. (1996) 44(4):422–4. doi: 10.1016/S0016-5107(96)70092-5
7. Bampton PA, Reid DP, Johnson RD, Fitch RJ, Dent J. A comparison of transnasal and transoral oesophagogastroduodenoscopy. J Gastroenterol Hepatol. (1998) 13(6):579–84. doi: 10.1111/j.1440-1746.1998.tb00693.x
8. Bishop PR, Nowicki MJ, May WL, Elkin D, Parker PH. Unsedated upper endoscopy in children. Gastrointest Endosc. (2002) 55(6):624–30. doi: 10.1067/mge.2002.123417
9. Roy JF, Duforest D, Marek TA. Prospective comparison of nasal versus oral insertion of a thin video endoscope in healthy volunteers. Endoscopy. (1996) 28(5):422–4. doi: 10.1055/s-2007-1005504
10. Murata A, Akahoshi K, Sumida Y, Yamamoto H, Nakamura K, Nawata H. Prospective randomized trial of transnasal versus peroral endoscopy using an ultrathin videoendoscope in unsedated patients. J Gastroenterol Hepatol. (2007) 22(4):482–5. doi: 10.1111/j.1440-1746.2006.04730.x
11. Zaman A, Hahn M, Hapke R, Knigge K, Fennerty MB, Katon RM. A randomized trial of peroral versus transnasal unsedated endoscopy using an ultrathin videoendoscope. Gastrointest Endosc. (1999) 49(3 Pt 1):279–84. doi: 10.1016/S0016-5107(99)70001-5
12. Gorelick AB, Inadomi JM, Barnett JL. Unsedated small-caliber esophagogastroduodenoscopy (EGD): less expensive and less time-consuming than conventional EGD. J Clin Gastroenterol. (2001) 33(3):210–4. doi: 10.1097/00004836-200109000-00008
13. Dumortier J, Napoleon B, Hedelius F, Pellissier PE, Leprince E, Pujol B, et al. Unsedated transnasal EGD in daily practice: results with 1100 consecutive patients. Gastrointest Endosc. (2003) 57(2):198–204. doi: 10.1067/mge.2003.59
14. Friedlander JA, DeBoer EM, Soden JS, Furuta GT, Menard-Katcher CD, Atkins D, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointest Endosc. (2016) 83(2):299–306.e1. doi: 10.1016/j.gie.2015.05.044
15. Shaul E, Kennedy KV, Spergel ZC, Daneshdoost S, Mahon M, Thanawala S, et al. Endoscopic and histologic utility of transnasal endoscopy in pediatric eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. (2024) 78(5):1155–60. doi: 10.1002/jpn3.12170
16. Sabe RMM, Elzayat A, Buckley A, Shah JR, Khalili AS, Sferra TJ. Transnasal endoscopy for children and adolescents with eosinophilic esophagitis: a single-center experience. Gastroenterology Res. (2022) 15(4):155–61. doi: 10.14740/gr1535
17. Lopez-Nunez O, Bernieh A, Kliewer KL, Kemtur P, Bolton SM, Mukkada VA, et al. Transnasal endoscopy acquires esophageal biopsies adequate for comprehensive pathology evaluation in patients with eosinophilic esophagitis. Pediatr Dev Pathol. (2024) 27(4):327–34. doi: 10.1177/10935266241255723
18. Smadi Y, Thomas J, Bittar K, Norton H, Friedlander JA, Bornstein J. Office-based sedation-free transnasal esophagogastroduodenoscopy with biopsies using single-use gastroscopes: a pediatric single-center experience. JPGN Rep. (2024) 5(1):29–34. doi: 10.1002/jpr3.12025
19. Hu CT. Endoscopic-guided versus cotton-tipped applicator methods of nasal anesthesia for transnasal esophagogastroduodenoscopy: a randomized, prospective, controlled study. Am J Gastroenterol. (2008) 103(5):1114–21. doi: 10.1111/j.1572-0241.2007.01769.x
20. Cheung J, Bailey R, Veldhuyzen van Zanten S, McLean R, Fedorak RN, Morse J, et al. Early experience with unsedated ultrathin 4.9 mm transnasal gastroscopy: a pilot study. Can J Gastroenterol. (2008) 22(11):917–22. doi: 10.1155/2008/323027
21. Cheung J, Goodman K, Bailey R, Fedorak R, Morse J, Millan M, et al. A randomized trial of topical anesthesia comparing lidocaine versus lidocaine plus xylometazoline for unsedated transnasal upper gastrointestinal endoscopy. Can J Gastroenterol. (2010) 24(5):317–21. doi: 10.1155/2010/154791
22. Jonas NE, Visser MF, Oomen A, Albertyn R, van Dijk M, Prescott CA. Is topical local anaesthesia necessary when performing paediatric flexible nasendoscopy? A double-blind randomized controlled trial. Int J Pediatr Otorhinolaryngol. (2007) 71(11):1687–92. doi: 10.1016/j.ijporl.2007.07.001
23. Chadha NK, Lam GO, Ludemann JP, Kozak FK. Intranasal topical local anesthetic and decongestant for flexible nasendoscopy in children: a randomized, double-blind, placebo-controlled trial. JAMA Otolaryngol Head Neck Surg. (2013) 139(12):1301–5. doi: 10.1001/jamaoto.2013.5297
24. Friedlander JA, Leinwand K, Bhardwaj V, Nguyen N. A guide on transnasal endoscopy: setting up a pediatric unsedated endoscopy program. Front Pediatr. (2023) 11:1267148. doi: 10.3389/fped.2023.1267148
25. Nguyen N, Lavery WJ, Capocelli KE, Smith C, DeBoer EM, Deterding R, et al. Transnasal endoscopy in unsedated children with eosinophilic esophagitis using virtual reality video goggles. Clin Gastroenterol Hepatol. (2019) 17(12):2455–62. doi: 10.1016/j.cgh.2019.01.023
26. Nguyen N, Pan Z, Smith C, Friedlander JA. Transnasal endoscopy ease score “TNEase score” to evaluate patient tolerance of unsedated transnasal endoscopy. J Pediatr Gastroenterol Nutr. (2024) 78(2):381–5. doi: 10.1002/jpn3.12102
27. Dellon ES, Muir AB, Katzka DA, Shah SC, Sauer BG, Aceves SS, et al. ACG clinical guideline: diagnosis and management of eosinophilic esophagitis. Am J Gastroenterol. (2025) 120(1):31–59. doi: 10.14309/ajg.0000000000003194
28. Friedlander JA, Fleischer DM, Black JO, Levy M, Rothenberg ME, Smith C, et al. Unsedated transnasal esophagoscopy with virtual reality distraction enables earlier monitoring of dietary therapy in eosinophilic esophagitis. J Allergy Clin Immunol Pract. (2021) 9(9):3494–6. doi: 10.1016/j.jaip.2021.06.030
29. Krishnan U, Mousa H, Dall'Oglio L, Homaira N, Rosen R, Faure C, et al. ESPGHAN-NASPGHAN guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with esophageal atresia-tracheoesophageal fistula. J Pediatr Gastroenterol Nutr. (2016) 63(5):550–70. doi: 10.1097/MPG.0000000000001401
30. Joseph M, Corrado MM, Odiase E, Friedlander JA, Smith C, Nguyen N. Sedation-free transnasal esophagoscopy to evaluate and monitor esophageal diseases in children with esophageal atresia-tracheoesophageal fistula. JPGN Rep. (2024) 5(2):166–9. doi: 10.1002/jpr3.12063
31. Shaheen N, Ransohoff DF. Gastroesophageal reflux, Barrett esophagus, and esophageal cancer: scientific review. J Am Med Assoc. (2002) 287(15):1972–81. doi: 10.1001/jama.287.15.1972
32. Shaheen NJ, Falk GW, Iyer PG, Souza RF, Yadlapati RH, Sauer BG, et al. Diagnosis and management of Barrett’s esophagus: an updated ACG guideline. Am J Gastroenterol. (2022) 117(4):559–87. doi: 10.14309/ajg.0000000000001680
33. Katz PO, Dunbar KB, Schnoll-Sussman FH, Greer KB, Yadlapati R, Spechler SJ. ACG clinical guideline for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. (2022) 117(1):27–56. doi: 10.14309/ajg.0000000000001538
34. Jobe BA, Hunter JG, Chang EY, Kim CY, Eisen GM, Robinson JD, et al. Office-based unsedated small-caliber endoscopy is equivalent to conventional sedated endoscopy in screening and surveillance for Barrett’s esophagus: a randomized and blinded comparison. Am J Gastroenterol. (2006) 101(12):2693–703. doi: 10.1111/j.1572-0241.2006.00890.x
35. Huibertse LJ, Peters Y, Westendorp D, Siersema PD. Unsedated transnasal endoscopy for the detection of Barrett’s esophagus: systematic review and meta-analysis. Dis Esophagus. (2023) 36(2):1–11. doi: 10.1093/dote/doac045
36. Jeurnink SM, van Herwaarden-Lindeboom MY, Siersema PD, Fischer K, Houwen RH, van der Zee DC. Barrett’s esophagus in children: does it need more attention? Dig Liver Dis. (2011) 43(9):682–7. doi: 10.1016/j.dld.2011.02.003
37. El-Serag HB, Gilger MA, Shub MD, Richardson P, Bancroft J. The prevalence of suspected Barrett’s esophagus in children and adolescents: a multicenter endoscopic study. Gastrointest Endosc. (2006) 64(5):671–5. doi: 10.1016/j.gie.2006.03.010
38. Sullivan PB. Gastrointestinal disorders in children with neurodevelopmental disabilities. Dev Disabil Res Rev. (2008) 14(2):128–36. doi: 10.1002/ddrr.18
39. Hsieh H, Frenette A, Michaud L, Krishnan U, Dal-Soglio DB, Gottrand F, et al. Intestinal metaplasia of the esophagus in children with esophageal atresia. J Pediatr Gastroenterol Nutr. (2017) 65(1):e1–4. doi: 10.1097/MPG.0000000000001558
40. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Practice guidelines committee of the American association for the study of liver D, practice parameters committee of the American college of G. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. (2007) 46(3):922–38. doi: 10.1002/hep.21907
41. Assy N, Rosser BG, Grahame GR, Minuk GY. Risk of sedation for upper GI endoscopy exacerbating subclinical hepatic encephalopathy in patients with cirrhosis. Gastrointest Endosc. (1999) 49(6):690–4. doi: 10.1016/S0016-5107(99)70283-X
42. Saeian K, Staff D, Knox J, Binion D, Townsend W, Dua K, et al. Unsedated transnasal endoscopy: a new technique for accurately detecting and grading esophageal varices in cirrhotic patients. Am J Gastroenterol. (2002) 97(9):2246–9. doi: 10.1111/j.1572-0241.2002.05906.x
43. Eqbal A, Wickremeratne T, Turner S, Higgins SE, Sloss A, Mitchell J, et al. One-stop shop for variceal surveillance: integration of unsedated ultrathin endoscopy into the routine clinic visit. Frontline Gastroenterol. (2021) 12(7):545–9. doi: 10.1136/flgastro-2020-101680
44. Bhardwaj V, Hochstim C, Friedlander J, Yanni G. Or not needed! In-clinic unsedated transnasal esophagoscopy in high-risk pediatric patients for esophageal variceal evaluation using a single use pediatric transnasal gastroscope. Gastrointest Endosc. (2023) 97(6):AB1192–3. doi: 10.1016/j.gie.2023.04.1807
45. ASGE Standards of Practice Committee, Evans JA, Muthusamy VR, Acosta RD, Bruining DH, Chandrasekhara V, et al. The role of endoscopy in the bariatric surgery patient. Surg Obes Relat Dis. (2015) 11(3):507–17. doi: 10.1016/j.soard.2015.02.015
46. ASGE Standards of Practice Committee, Early DS, Lightdale JR, Vargo JJ 2nd, Acosta RD, Chandrasekhara V, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc. (2018) 87(2):327–37. doi: 10.1016/j.gie.2017.07.018
47. Alami RS, Schuster R, Friedland S, Curet MJ, Wren SM, Soetikno R, et al. Transnasal small-caliber esophagogastroduodenoscopy for preoperative evaluation of the high-risk morbidly obese patient. Surg Endosc. (2007) 21(5):758–60. doi: 10.1007/s00464-006-9101-z
48. Xavier AT, Alvares AV, Iyer PG, Arantes VN. Unsedated transnasal endoscopy for preoperative examination of bariatric patients: a prospective study. Obes Surg. (2020) 30(1):238–43. doi: 10.1007/s11695-019-04120-y
49. Sivan RF, Bar Lev MR, Silbermintz A, Mozer-Glassberg Y, Seguier-Lipzyc E, Shalitin S, et al. Clinical and esophagogastroduodenoscopy findings in pediatric patients with severe obesity evaluated before bariatric surgery. J Pediatr Gastroenterol Nutr. (2021) 72(6):854–8. doi: 10.1097/MPG.0000000000003109
50. Liman A, Koh L, Barakat M, Abu El Haija M. Preoperative esophagogastroduodenoscopy in pediatric bariatric surgery: a summary of the literature. JPGN Rep. (2024) 5(3):243–9. doi: 10.1002/jpr3.12095
51. Hall CHT, Nguyen N, Furuta GT, Prager J, Deboer E, Deterding R, et al. Unsedated in-office transgastrostomy esophagoscopy to monitor therapy in pediatric esophageal disease. J Pediatr Gastroenterol Nutr. (2018) 66(1):33–6. doi: 10.1097/MPG.0000000000001631
52. Friedlander JA, Smith C, Nguyen N, Mark J, Prager JD, DeBoer E, et al. Proof of concept functional endoscopic esophageal evaluation of swallowing (FEEES) using unsedated transnasal endoscopy (TNE). J Pediatr Gastroenterol Nutr. (2024) 78(3):749–51. doi: 10.1002/jpn3.12154
53. Attila T, Hellman RS, Krasnow AZ, Hofmann CL, Saeian K, Dua KS, et al. Feasibility and safety of endoscopic evaluation of gastric emptying. Endoscopy. (2005) 37(3):240–3. doi: 10.1055/s-2004-826196
54. Lim CH, Choi MG, Baeg MK, Moon SJ, Kim JS, Cho YK, et al. Novel disposable transnasal endoscopy for assessment of esophageal motor function. J Clin Gastroenterol. (2014) 48(5):402–6. doi: 10.1097/MCG.0b013e3182a8810a
55. Belafsky PC, Allen K, Castro-Del Rosario L, Roseman D. Wireless pH testing as an adjunct to unsedated transnasal esophagoscopy: the safety and efficacy of transnasal telemetry capsule placement. Otolaryngol Head Neck Surg. (2004) 131(1):26–8. doi: 10.1016/j.otohns.2004.01.019
56. Vitale MA, Villotti G, D'Alba L, De Cesare MA, Frontespezi S, Iacopini G. Unsedated transnasal percutaneous endoscopic gastrostomy placement in selected patients. Endoscopy. (2005) 37(1):48–51. doi: 10.1055/s-2004-826078
57. Kulling D, Bauerfeind P, Fried M. Transnasal versus transoral endoscopy for the placement of nasoenteral feeding tubes in critically ill patients. Gastrointest Endosc. (2000) 52(4):506–10. doi: 10.1067/mge.2000.107729
58. Rees CJ. In-office transnasal esophagoscope-guided botulinum toxin injection of the lower esophageal sphincter. Curr Opin Otolaryngol Head Neck Surg. (2007) 15(6):409–11. doi: 10.1097/MOO.0b013e3282f1bf39
59. Rees CJ, Fordham T, Belafsky PC. Transnasal balloon dilation of the esophagus. Arch Otolaryngol Head Neck Surg. (2009) 135(8):781–3. doi: 10.1001/archoto.2009.115
60. Lin J, Chen M, Lei W, Law W, Hu C. Eradication of diffuse gastric Peutz-Jeghers polyps by unsedated transnasal snare polypectomy and argon plasma coagulation. Endoscopy. (2009) 41(Suppl 2):E207–8. doi: 10.1055/s-0029-1215006
61. Rivory J, Lepilliez V, Gincul R, Guillaud O, Vallin M, Bouffard Y, et al. “First look” unsedated transnasal esogastroduodenoscopy in patients with upper gastrointestinal bleeding? A prospective evaluation. Clin Res Hepatol Gastroenterol. (2014) 38(2):209–18. doi: 10.1016/j.clinre.2013.10.010
62. Choe WH, Kim JH, Ko SY, Kwon SY, Kim BK, Rhee KH, et al. Comparison of transnasal small-caliber vs. peroral conventional esophagogastroduodenoscopy for evaluating varices in unsedated cirrhotic patients. Endoscopy. (2011) 43(8):649–56. doi: 10.1055/s-0030-1256474
63. Lee JW, Tai CS, Chang KC, Chiu YC, Ni YH, Wu JF. The benefits of transnasal endoscopy compared to conventional endoscopy in adolescents. J Formos Med Assoc. (2024):in press. doi: 10.1016/j.jfma.2024.11.007
64. Barberani F, Giovannone M, Boschetto S, Tosoni M, Fondi M, Diblasio L. A comparative study of unsedated trans-nasal esophagogastroduodenoscopy and conventional EGD feasibility, safety, cost effectivity evaluation. First Italian experience in 200 patients. Ital J Gastroenterol Hepatol. (1998) 30:A173.
65. Cho S, Arya N, Swan K, Cirocco M, Kandel G, Kortan P, et al. Unsedated transnasal endoscopy: a Canadian experience in daily practice. Can J Gastroenterol. (2008) 22(3):243–6. doi: 10.1155/2008/514297
66. Parker C, Alexandridis E, Plevris J, O'Hara J, Panter S. Transnasal endoscopy: no gagging no panic!. Frontline Gastroenterol. (2016) 7(4):246–56. doi: 10.1136/flgastro-2015-100589
67. Saeian K, Townsend WF, Rochling FA, Bardan E, Dua K, Phadnis S, et al. Unsedated transnasal EGD: an alternative approach to conventional esophagogastroduodenoscopy for documenting Helicobacter pylori eradication. Gastrointest Endosc. (1999) 49(3 Pt 1):297–301. doi: 10.1016/S0016-5107(99)70004-0
68. Atar M, Kadayifci A. Transnasal endoscopy: technical considerations, advantages and limitations. World J Gastrointest Endosc. (2014) 6(2):41–8. doi: 10.4253/wjge.v6.i2.41
69. Juhasz A, Mittal SK, Lee TH, Deng C, Chak A, Lynch HT. Prevalence of Barrett esophagus in first-degree relatives of patients with esophageal adenocarcinoma. J Clin Gastroenterol. (2011) 45(10):867–71. doi: 10.1097/MCG.0b013e31821f44a8
70. Siwiec RM, Dua K, Surapaneni SN, Hafeezullah M, Massey B, Shaker R. Unsedated transnasal endoscopy with ultrathin endoscope as a screening tool for research studies. Laryngoscope. (2012) 122(8):1719–23. doi: 10.1002/lary.23304
71. Thota PN, Zuccaro G Jr, Vargo JJ 2nd, Conwell DL, Dumot JA, Xu M. A randomized prospective trial comparing unsedated esophagoscopy via transnasal and transoral routes using a 4-mm video endoscope with conventional endoscopy with sedation. Endoscopy. (2005) 37(6):559–65. doi: 10.1055/s-2005-861476
72. Peery AF, Hoppo T, Garman KS, Dellon ES, Daugherty N, Bream S, et al. Feasibility, safety, acceptability, and yield of office-based, screening transnasal esophagoscopy (with video). Gastrointest Endosc. (2012) 75(5):945–53.e2. doi: 10.1016/j.gie.2012.01.021
73. Belafsky PC, Postma GN, Koufman JA. Normal transnasal esophagoscopy. Ear Nose Throat J. (2001) 80(7):438. doi: 10.1177/014556130108000706
74. Grant RK, Brindle WM, Robertson AR, Kalla R, Plevris JN. Unsedated transnasal endoscopy: a safe, well-tolerated and accurate alternative to standard diagnostic peroral endoscopy. Dig Dis Sci. (2022) 67(6):1937–47. doi: 10.1007/s10620-022-07432-9
75. Wang CP, Lee YC, Yang TL, Lou PJ, Ko JY. Application of unsedated transnasal esophagogastroduodenoscopy in the diagnosis of hypopharyngeal cancer. Head Neck. (2009) 31(2):153–7. doi: 10.1002/hed.20928
76. Watanabe H, Watanabe N, Ogura R, Nishino N, Saifuku Y, Hitomi G, et al. A randomized prospective trial comparing unsedated endoscopy via transnasal and transoral routes using 5.5-mm video endoscopy. Dig Dis Sci. (2009) 54(10):2155–60. doi: 10.1007/s10620-008-0614-2
77. Birkner B, Fritz N, Schatke W, Hasford J. A prospective randomized comparison of unsedated ultrathin versus standard esophagogastroduodenoscopy in routine outpatient gastroenterology practice: does it work better through the nose? Endoscopy. (2003) 35(8):647–51. doi: 10.1055/s-2003-41523
78. Yuki M, Amano Y, Komazawa Y, Fukuhara H, Shizuku T, Yamamoto S, et al. Unsedated transnasal small-caliber esophagogastroduodenoscopy in elderly and bedridden patients. World J Gastroenterol. (2009) 15(44):5586–91. doi: 10.3748/wjg.15.5586
79. Yagi J, Adachi K, Arima N, Tanaka S, Ose T, Azumi T, et al. A prospective randomized comparative study on the safety and tolerability of transnasal esophagogastroduodenoscopy. Endoscopy. (2005) 37(12):1226–31. doi: 10.1055/s-2005-921037
80. Tatsumi Y, Harada A, Matsumoto T, Tani T, Nishida H. Feasibility and tolerance of 2-way and 4-way angulation videoscopes for unsedated patients undergoing transnasal EGD in GI cancer screening. Gastrointest Endosc. (2008) 67(7):1021–7. doi: 10.1016/j.gie.2007.10.030
81. Hu CT. Gauze pledgetting versus endoscopic-guided aerosolized spray for nasal anesthesia before transnasal EGD: a prospective, randomized study. Gastrointest Endosc. (2010) 71(1):11–20. doi: 10.1016/j.gie.2009.06.016
82. Aedo MR, Zavala-Gonzalez MA, Meixueiro-Daza A, Remes-Troche JM. Accuracy of transnasal endoscopy with a disposable esophagoscope compared to conventional endoscopy. World J Gastrointest Endosc. (2014) 6(4):128–36. doi: 10.4253/wjge.v6.i4.128
83. Lin LF, Ma KZ, Tu HL. A prospective randomized study comparing transnasal and peroral 5-mm ultrathin endoscopy. J Formos Med Assoc. (2014) 113(6):371–6. doi: 10.1016/j.jfma.2012.06.003
Keywords: unsedated endoscopy, transnasal endoscopy, GERD (gastroesophageal reflux disease), EoE (eosinophilic esophagitis), EGD (esophagogastroduodenoscopy)
Citation: Bose P and Pitman R (2025) Pediatric unsedated transnasal endoscopy: applications, equipment, and future directions. Front. Pediatr. 13:1585705. doi: 10.3389/fped.2025.1585705
Received: 1 March 2025; Accepted: 25 April 2025;
Published: 9 May 2025.
Edited by:
Kenneth Ng, Johns Hopkins University, United StatesReviewed by:
Minna Rodrigo, University of Maryland, United StatesYolanda Rivas, Albert Einstein College of Medicine, United States
Copyright: © 2025 Bose and Pitman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ryan Pitman, cnRwaXRtYW5AaXUuZWR1
†These authors have contributed equally to this work
 Paroma Bose†
Paroma Bose† Ryan Pitman
Ryan Pitman