- 1Department of Oncology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 2Department of Surgery, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
Objective: The aim of the present study was to determine the prognosis of different types of acute infection in pediatric leukemia patients.
Methods: A retrospective study was carried out on pediatric leukemia patients with acute infections admitted to the Second Affiliated Hospital of Harbin Medical University between 1 September 2004 and 31 August 2022. Clinical characteristics, diagnostic findings, and prognostic outcomes were extracted from the eligible cases and analyzed.
Results: There were 36 cases of acute myeloid leukemia (AML) and 72 cases of acute lymphoblastic leukemia (ALL) that met the inclusion criteria. There were significant differences in the incidence of pneumonia (47.2% vs. 27.8%, p = 0.045) and sepsis (19.4% vs. 2.8%, p = 0.006) between the AML and ALL groups. There were 10 cases with a poor prognosis and 26 cases with a favorable prognosis in the AML group. There were no significant differences between the poor prognosis and the favorable prognosis groups except for age (14.2 ± 1.2 years vs. 9.6 ± 4.3 years, p = 0.003). There were 14 cases with a poor prognosis and 58 cases with a favorable prognosis in the ALL group. There were no significant differences between the poor prognosis and favorable prognosis groups except for age (13.4 ± 2.7 years vs. 9.2 ± 4.7 years, p = 0.002).
Conclusions: There were significantly more incidence of pneumonia and sepsis in children with AML. Younger AML and ALL children with acute infections have more favorable prognoses than older children.
1 Introduction
Acute pediatric leukemia is a serious condition in childhood. In 2020, there were 67,008 new reported cases of pediatric leukemia globally, with male patients accounting for 57.85% of all cases (1). The survival rates of pediatric acute lymphoblastic leukemia (ALL) have been reported to be in the range of 83%–94% (2), compared to 65%–70% for pediatric acute myeloid leukemia (AML) (3). Infections may increase morbidity and mortality in patients with acute pediatric leukemia who have a much higher risk of infection, potentially due to their malfunctioning immune system, as well as the burden of their therapies, which could lead to repeated, prolonged, and complicated deterioration of immune cells (4). Infections in acute pediatric leukemia patients, besides causing a higher mortality, also lead to prolonged hospitalizations, disrupt chemotherapy schedules, negatively affect patients’ quality of life, and escalate the demand for extra healthcare resources and costs (5). This multifaceted impact underscores the need for vigilant infection management to optimize treatment outcomes and patient wellbeing.
In this study, we analyzed the prognosis of different types of acute infection in patients with pediatric leukemia and showed the most relevant factors that could affect the prognosis.
2 Methods
This retrospective study was conducted in pediatric leukemia patients with acute infections admitted to the Second Affiliated Hospital of Harbin Medical University between 1 September 2004 and 31 August 2022. The inclusion criteria were as follows: (1) patients aged under 18 years; (2) patients diagnosed with acute leukemia according to the established criteria (6, 7); (3) patients experiencing a first episode of leukemia with no prior treatment; (4) patients diagnosed with acute infection based on clinical symptoms and signs, supported by laboratory test results (8); (5) patients with signed informed consent to participate in medical research upon admission. The exclusion criteria were as follows: (1) patients with chronic infectious diseases; (2) patients with other types of cancer; (3) patients with COVID-19 infection, confirmed by nucleic acid amplification tests (NAATs) of nasal swab samples (9, 10); (4) patients with acute conditions needing surgical treatments; and (5) incomplete data.
Clinical characteristics such as age, gender, hospital stay, and different types of acute infections, including upper respiratory tract infection, pneumonia, pleurisy, sepsis, bronchitis, urinary tract infection, oral infection, perianal infection, acute enteritis, herpetic angina, skin infection, and soft tissue infection, were abstracted and analyzed.
Poor prognoses referred to uncontrolled infection or death before discharge from hospital. Favorable prognoses referred to controlled infection or cure of infection before discharge from the hospital.
2.1 Statistical analysis
Clinical data were extracted and summarized in an Excel file, then analyzed using SPSS 25.0. The t-test was used for continuous data and the chi-square test was used for categorical data. Fisher’s exact test was used when the number in the group was less than five.
The level of significance was set at p < 0.05 (two sided).
3 Results
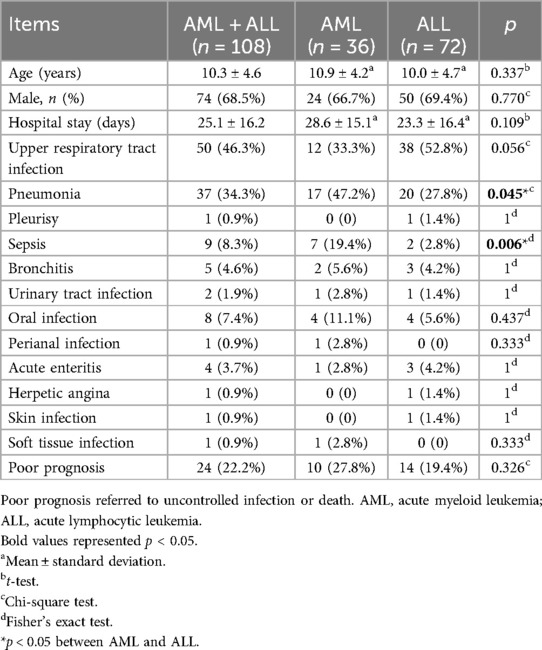
There were 36 cases of AML and 72 cases of ALL that met the inclusion criteria. There were no differences between the AML and ALL groups in terms of patient age, gender, hospital stay, upper respiratory tract infection, pleurisy, bronchitis, urinary tract infection, oral infection, perianal infection, acute enteritis, herpetic angina, skin infection, soft tissue infection, or poor prognosis (p > 0.05 for all comparisons). There were significant differences in the incidence of pneumonia (47.2% vs. 27.8%, p = 0.045) and sepsis (19.4% vs. 2.8%, p = 0.006) between the AML and ALL groups (Table 1).
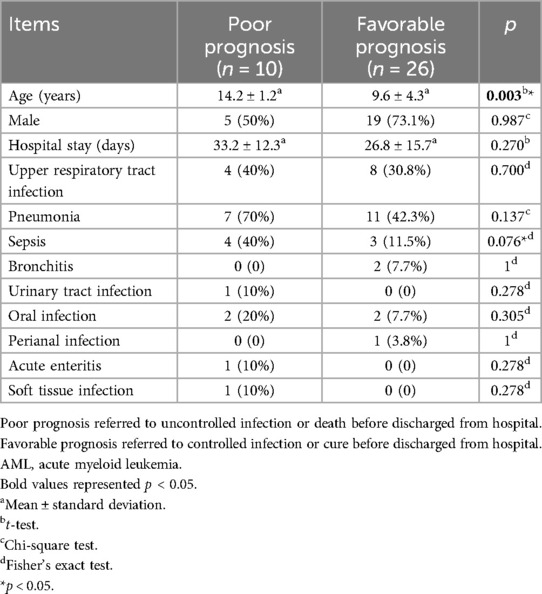
Patients in the AML or ALL groups were further analyzed based on their prognosis at discharge. There were 10 cases with a poor prognosis and 26 cases with a favorable prognosis in the AML group. There were no significant differences between the poor prognosis and favorable prognosis groups except for age (14.2 ± 1.2 years vs. 9.6 ± 4.3 years, p = 0.003) (Table 2).
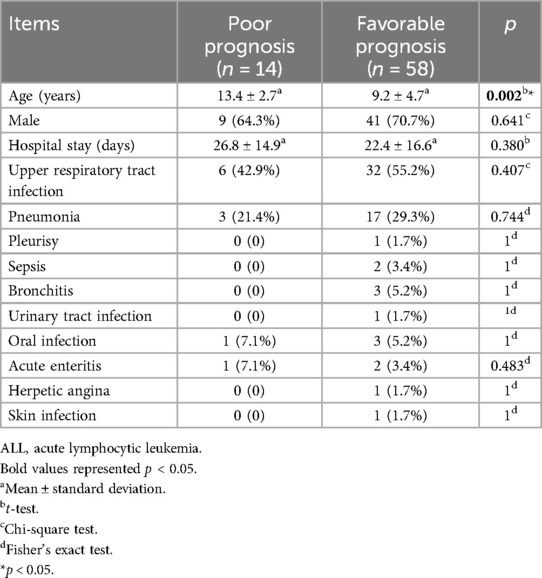
In the same token, there were 14 cases with a poor prognosis and 58 cases with a favorable prognosis in the ALL group. There were no significant differences between the poor prognosis and favorable prognosis groups except for age (13.4 ± 2.7 years vs. 9.2 ± 4.7 years, p = 0.002) (Table 3).
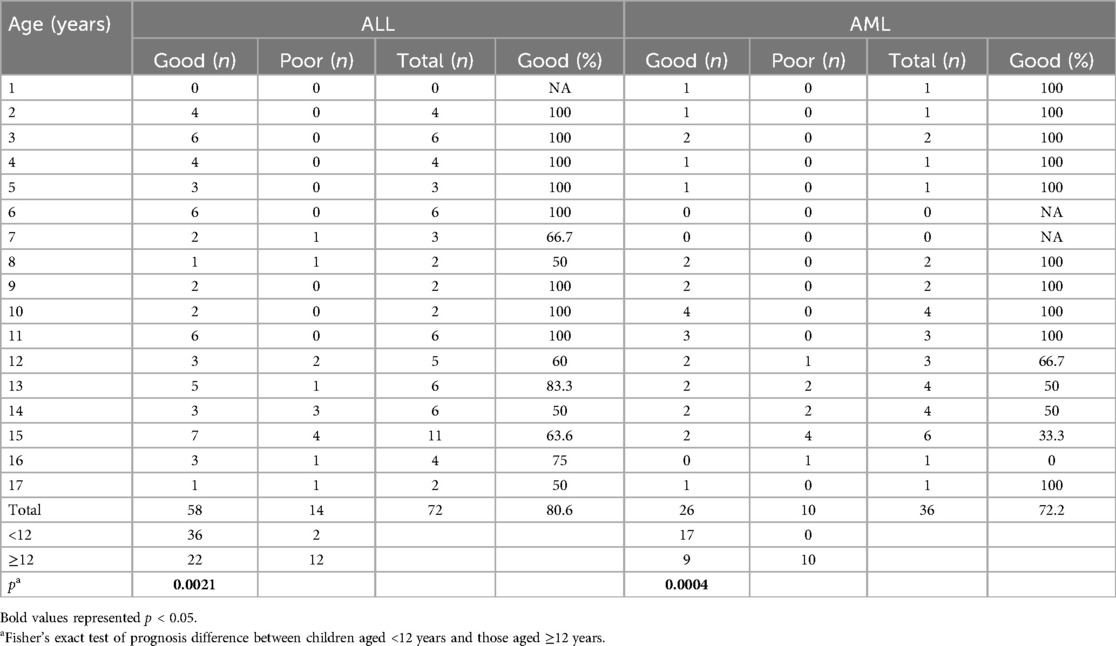
The age distribution of prognosis is shown in Table 4. A decreasing trend in favorable prognosis was observed with increasing age in participants in both the ALL and AML groups. There was a significant difference in prognosis between participants aged <12 years and those aged ≥12 years in both cohorts (ALL: p = 0.0021; AML: p = 0.0004).
4 Discussion
In another study, ALL was reported as the most common type of pediatric acute leukemia, accounting for approximately 75%–80% of cases (11). In our study, 66.7% of pediatric leukemia patients were diagnosed with ALL. This discrepancy might be due to differences in the inclusion and exclusion criteria, as well as variations related to environmental factors and racial demographics.
The most common acute infection observed among the enrolled acute leukemia patients in our study were upper respiratory tract infection (46.3%), followed by pneumonia (34.3%), sepsis (8.3%), and oral infections (7.4%). Neutropenia emerged as the leading risk factor for infection development (12). In addition, several other factors contribute to increased infection risk, including compromised cellular or humoral immunity, disruption of natural barriers (such as skin and mucous membranes), and the use of medical devices, such as vascular access catheters (13). Often, patients are affected by a combination of these risk factors, which can further elevate their susceptibility to infections and the risk of adverse outcomes.
We found that pneumonia (47.2% vs. 27.8%, p = 0.045) and sepsis (19.4% vs. 2.8%, p = 0.006) were significantly more common in AML than in ALL cases. This can be explained by the differing immunological deficits associated with each leukemia type. In AML, deficits in neutrophilic granulocytes lead to a higher incidence of bacterial and fungal infections, whereas in ALL, deficits in lymphocytes result in hypogammaglobulinemia, leading to reduced cell-mediated immunity (14).
During treatment for ALL and AML, different agents and schedules might be employed, leading to differences in prognosis (14, 15). Both the disease and its therapies place a heavy burden on the developing immune system. In acute leukemia, normal production of blood cells in the bone marrow is disrupted (16, 17) and leukemia cells crowd out healthy white blood cells, including lymphocytes and granulocytes, which are both essential for immune defense. This results in children experiencing frequent infections and fevers, as the body struggles to mount an effective response against pathogens.
Treatment for acute leukemia, primarily via chemotherapy, further exacerbates immune dysfunction (18–21). Chemotherapy targets rapidly dividing cells, affecting not only cancer cells but also healthy immune cells, leading to suppression of the immune system. This increases children’s susceptibility to infections both during and after treatment. The effects of chemotherapy on the immune system can persist even after treatment completion, as evidenced by persistent abnormalities in immune parameters such as lymphocyte subsets and natural killer cell function. In the context of AML, children often experience multiple episodes of infection during intensive treatment, with sepsis being the most common (15). Infection-related mortality rates are in the range of 5.4%–7.3%. In ALL, the induction and consolidation phases pose significant risks for infections due to severe neutropenia. Infection-related mortality in ALL is generally lower, in the range of 2%–4%; however, infections remain a primary cause of treatment-related mortality. Understanding these risks is crucial for developing effective strategies to manage and prevent infections during leukemia treatment.
It is also interesting to note that a previous study in children and adolescents with ALL showed there were no significant associations between sex, race, age, and the development of acute respiratory infections (22). Another study investigating childhood AML with infections showed that age above 16 years was a factor associated with infection-related mortality (23). In general, independent of acute infections, the survival rate of ALL is highest when children diagnosed at 1–4 years of age, with a decline observed in older age groups. Infants aged under 1 year have the lowest survival rate in both ALL and AML (24). In our study, children aged under 12 years demonstrated a more favorable prognosis in both ALL and AML populations. The underlying pathophysiological mechanisms need further investigation and might be related to deficiencies of key factors in metabolism (25, 26).
The present study has some limitations. Notably, the sample sizes in certain subgroups—particularly when comparing infection type and age—were relatively small. Therefore, the conclusions drawn need to be validated in more robust studies, such as meta-analyses or large-scale studies.
5 Conclusion
In this study, by analyzing clinical data collected over an 18-year period at our hospital, we found a significantly higher incidence of pneumonia and sepsis in children diagnosed with AML compared to those with ALL. Younger children with AML or ALL who developed acute infections tended to have a better prognosis than older children.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical Committee of the Second Affiliated Hospital of Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SsL: Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. SnL: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. YC: Data curation, Writing – original draft, Writing – review & editing. SJ: Data curation, Writing – original draft, Writing – review & editing. KL: Data curation, Writing – original draft, Writing – review & editing. FC: Data curation, Writing – original draft, Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mohammadian0-Hafshejani A, Farber IM, Kheiri S. Global incidence and mortality of childhood leukemia and its relationship with the human development Index. PLoS One. (2024) 19(7):e0304354. doi: 10.1371/journal.pone.0304354
2. Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. (2012) 30(14):1663–9. doi: 10.1200/JCO.2011.37.8018
3. Creutzig U, Zimmermann M, Lehrnbecher T, Graf N, Hermann J, Niemeyer CM, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: results of AML-BFM 98. J Clin Oncol. (2006) 24(27):4499–506. doi: 10.1200/JCO.2006.06.5037
4. Nair A, Elballushi R, Joshi R, Anjanappa S, Akter M, Arif S, et al. Assessment of the prevalence of infections in pediatric patients with acute lymphoblastic leukemia. Cureus. (2023) 15(10):e46837. doi: 10.7759/cureus.46837
5. Patel PA, DeGroote NP, Jackson K, Cash T, Castellino SM, Jaggi P, et al. Infectious events in pediatric patients with acute lymphoblastic leukemia/lymphoma undergoing evaluation for fever without severe neutropenia. Cancer. (2022) 128(23):4129–38. doi: 10.1002/cncr.34476
6. PDQ Pediatric Treatment Editorial Board. Childhood Acute Lymphoblastic Leukemia Treatment (PDQ®): Health Professional Version. 2024 Dec 5. in: PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute (US) (2002).
7. Mitchell C, Hall G, Clarke RT. Acute leukaemia in children: diagnosis and management. Br Med J. (2009) 338:b2285. doi: 10.1136/bmj.b2285
8. Venge P, Xu S. Diagnosis and monitoring of acute infections with emphasis on the novel biomarker human neutrophil lipocalin. J Appl Lab Med. (2019) 3(4):664–74. doi: 10.1373/jalm.2018.026369
9. Caliendo AM, Hanson KE. COVID-19: diagnosis. In: RF C, editor. UpToDate. Wolters Kluwer. (2024). Available at: https://www.uptodate.com/contents/covid-19-diagnosis (accessed on March 23, 2025).
10. CDC. Overview of Testing for SARS-CoV-2. Available online at: https://www.cdc.gov/covid/hcp/clinical-care/overview-testing-sars-cov-2.html (accessed on March 23, 2025).
11. Tebbi CK. Etiology of acute leukemia: a review. Cancers (Basel). (2021) 13(9):2256. doi: 10.3390/cancers13092256
12. Rolston KVI. Infections in patients with acute leukemia. In: Maschmeyer G, Rolston K, editors. Infections in Hematology. Berlin and Heidelberg: Springer (2014). p. 3–23. doi: 10.1007/978-3-662-44000-1_1
13. Dropulic LK, Lederman HM. Overview of infections in the immunocompromised host. Microbiol Spectr. (2016) 4(4):DMIH2-0026-2016. doi: 10.1128/microbiolspec.DMIH2-0026-2016
14. Logan C, Koura D, Taplitz R. Updates in infection risk and management in acute leukemia. Hematology Am Soc Hematol Educ Program. (2020) 2020(1):135–9. doi: 10.1182/hematology.2020000098
15. Carlesse F, de Sousa AVL. Infections in children and adolescents with acute leukemia. EJC Paediatric Oncology. (2024) 3:100142. doi: 10.1016/j.ejcped.2024.100142
16. Sison EA, Brown P. The bone marrow microenvironment and leukemia: biology and therapeutic targeting. Expert Rev Hematol. (2011) 4(3):271–83. doi: 10.1586/ehm.11.30
17. Yao Y, Li F, Huang J, Jin J, Wang H. Leukemia stem cell-bone marrow microenvironment interplay in acute myeloid leukemia development. Exp Hematol Oncol. (2021) 10(1):39. doi: 10.1186/s40164-021-00233-2
18. Witkowski MT, Lasry A, Carroll WL, Aifantis I. Immune-based therapies in acute leukemia. Trends Cancer. (2019) 5(10):604–18. doi: 10.1016/j.trecan.2019.07.009
19. Khaldoyanidi S, Nagorsen D, Stein A, Ossenkoppele G, Subklewe M. Immune biology of acute myeloid leukemia: implications for immunotherapy. J Clin Oncol. (2021) 39(5):419–32. doi: 10.1200/JCO.20.00475
20. Serroukh Y, Hébert J, Busque L, Mercier F, Rudd CE, Assouline S, et al. Blasts in context: the impact of the immune environment on acute myeloid leukemia prognosis and treatment. Blood Rev. (2023) 57:100991. doi: 10.1016/j.blre.2022.100991
21. Senapati J, Kantarjian H, Habib D, Haddad FG, Jain N, Short NJ, et al. Frontline immunotherapeutic combination strategies in adult B-cell acute lymphoblastic leukemia: reducing chemotherapy intensity and toxicity and harnessing efficacy. Leuk Lymphoma. (2025):1–12. doi: 10.1080/10428194.2025.2449582
22. Hakim H, Dallas R, Zhou Y, Pei D, Cheng C, Flynn PM, et al. Acute respiratory infections in children and adolescents with acute lymphoblastic leukemia. Cancer. (2016) 122(5):798–805. doi: 10.1002/cncr.29833
23. Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. (2007) 110(10):3532–9. doi: 10.1182/blood-2007-05-091942
24. Wang Y, Huang J, Rong L, Wu P, Kang M, Zhang X, et al. Impact of age on the survival of pediatric leukemia: an analysis of 15083 children in the SEER database. Oncotarget. (2016) 7(50):83767–74. doi: 10.18632/oncotarget.11765
25. Werner ER, Werner-Felmayer G, Wachter H. Tetrahydrobiopterin and cytokines. Proc Soc Exp Biol Med. (1993) 203(1):1–12. doi: 10.3181/00379727-203-43566a
Keywords: prognosis, acute infection, acute pediatric leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, children
Citation: Li S, Li S, Chen Y, Jia S, Luan K and Cui F (2025) Prognosis of different types of acute infection in the first episode of childhood acute leukemia. Front. Pediatr. 13:1589770. doi: 10.3389/fped.2025.1589770
Received: 7 March 2025; Accepted: 10 April 2025;
Published: 9 May 2025.
Edited by:
Zhongjie Shi, Wayne State University, United StatesReviewed by:
Ying Meng, Chongqing Medical University, ChinaYuebo Yang, Third Affiliated Hospital of Sun Yat-sen University, China
Copyright: © 2025 Li, Li, Chen, Jia, Luan and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shasha Li, bGlzaGFzaGEyNzI3QDE2My5jb20=
†These authors share first authorship
 Shasha Li
Shasha Li Shanshan Li1,†
Shanshan Li1,†