- Department of Critical Care Medicine, Children’s Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatric Metabolism and Inflammatory Diseases, Chongqing, China
Objective: Timely liberation from invasive mechanical ventilation (IMV) is important. We aimed to determine the feasibility of our study protocol for the conduction of a larger prospective trial to examine the utility of a computer-driven liberation protocol in pediatric patients.
Design: Single-center pilot randomized controlled trial.
Setting: Single, tertiary care, 52-bed, academic pediatric intensive care unit (PICU).
Patients: Patients aged from 28 days to 18 years undergoing IMV for more than 24 h.
Interventions: Patients were randomly assigned to test and control groups in a ratio of 1:1. The test group underwent ventilator liberation driven by a computerized algorithm combining protocolized screening, air leak testing, and spontaneous breathing testing. The control group underwent ventilator liberation driven by the attending physician according to standard care.
Measurements and main results: A total of 40 patients (20 in each group) were randomized. Baseline characteristics of the two groups were similar. Durations of IMV were 95.3 h (95%CI, 9.07–181.53) in the test group and 113.3 h (95%CI, 85.90–140.70) in the control group and were similar (p = 0.62). PICU length of stay [6.9 days [95%CI, 5.00–8.86] vs. 7.0 days [95%CI, 5.58–8.40]; p = 0.74] and hospital length of stay [22.9 days [95%CI, 11.48–34.24] vs. 26.9 days [95%CI, 17.86–35.94]; p = 0.31] were similar between the test and control groups, respectively.
Conclusions: Our pilot study suggests that the conduction of a larger prospective trial of a computer-driven ventilator liberation protocol is feasible in our PICU. And a larger trial is needed to further explore the utility of a computer-driven ventilator liberation protocol.
Clinical Trial Registration: https://www.chictr.org.cn/showproj.html?proj=168024, Chinese Clinical Trial Registry ChiCTR2200060033.
1 Introduction
Invasive mechanical ventilation (IMV) is an important life support measure in critical care units (1–3), but can be a double-edged sword. Prolonged IMV is significantly correlated with ventilator-associated pneumonia, ventilator-associated lung injury, and airway injury; and is an independent risk factor of poor clinical outcomes (1–7). Thus, timely liberation from IMV after resolution of the primary indication for ventilation is important. However, the identification of a feasible timepoint for IMV liberation is difficult. Up to 50% of patients experiencing unplanned extubation do not require re-intubation, which suggests that physician-driven standard care can often miss the optimal timepoint of liberation (7).
A review of 17 trials comparing protocolized to non-protocolized liberation from IMV disclosed that most studies were centered on spontaneous breathing testing (SBT), and that the use of protocols significantly reduced the duration of ventilation, medical costs, and iatrogenic complications (8). The use of IMV liberation protocols has been recommended in guidelines for adult patients (9, 10). A recent international clinical practice guideline for pediatric IMV liberation has also recommended the use of a protocolized approach (11). Unfortunately, the certainty of evidence was low in the guideline. Although observational and interventional studies exist, most of the pediatric literature consists of narrative reviews and meta-analyses, underscoring the need for high-quality research in this field (11).
Most studies reported that protocolized liberation shortened IMV duration and reduced extubation failure rates (12, 13). Potential drawbacks, however, include increased staff burden and screening fatigue, which may contribute to low protocol compliance (14). Computer-driven liberation protocols can screen vital signs, ventilator parameters, and laboratory data automatically; provide a reliable measure to inform IMV liberation; and can overcome screening fatigue that may lower compliance rates and reduce IMV duration ultimately. The objectives of this pilot randomized controlled clinical trial were to determine the feasibility of applying our computer-driven decision support tool to pediatric patients; the feasibility of conducting a larger prospective trial of our study protocol to explore the efficacy of the intervention; and to train pediatric intensive care unit (PICU) personnel.
2 Materials and methods
2.1 Study design
We conducted a single-center pilot randomized controlled pilot study in the 52-bed PICU of the Children's Hospital of Chongqing Medical University, China.
The study protocol was approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University (file no. 2022-75; approval date: April 2, 2022). The study was conducted according to CONSORT guidelines, was performed in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and was registered in Chinese Clinical Trial Registry (ChiCTR2200060033). Written informed consent for participation was obtained from children's parents or legal guardians.
2.2 Study participants
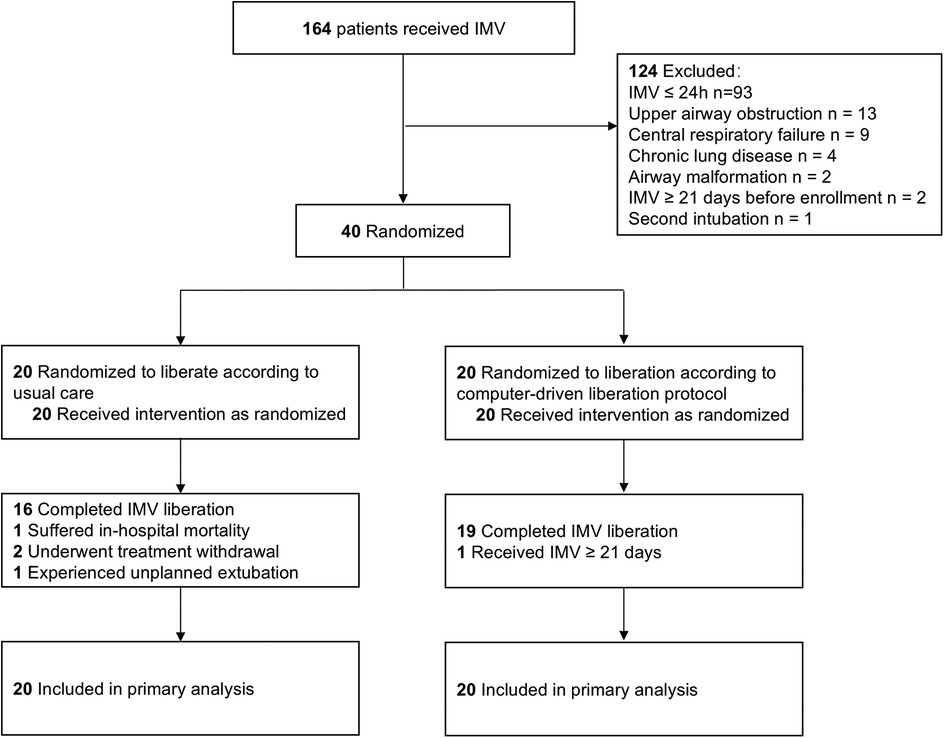
Patients aged from 28 days to 18 years receiving IMV for more than 24 h were included. Exclusion criteria were: intubation indicated for upper airway obstruction; or the presence of either airway malformation, diaphragmatic hernia or paralysis, cyanotic congenital heart disease, primary pulmonary hypertension, neuromuscular disease, central respiratory failure, chronic lung disease, tracheostomy, or an imminently fatal prognosis. Chronic lung disease was defined as a condition requiring chronic oxygen therapy before hospitalization, such as bronchopulmonary dysplasia. Patients were enrolled only after the first intubation during their hospitalization. Patients were excluded from the final analysis if they had experienced either IMV for more than 21 days; tracheostomy; interhospital transfer; withdrawal of treatment; in-hospital mortality; or if parents or guardians opted out of the study.
2.3 Randomization and allocation masking
Patients were randomly assigned to undergo IMV liberation according to either a computer-driven liberation protocol or usual care in a ratio of 1:1. The allocation sequence, presented in prenumbered and sealed envelopes, was randomly designated as either “test” or “control” by a computer-generated random number table. The computer-generated allocation sequence was completed by a third-party researcher who was not involved in this study. Once a patient entered the study, investigators immediately opened the envelope in order.
2.4 Intervention masking
The two study groups were treated by the same medical team. However, medical team members were blinded to patient group assignment until the computer-driven IMV liberation protocol group patients completed protocolized screening.
2.5 Intervention
Our study design is illustrated in Figure 1. Usual (control) care was driven by the attending physician. Synchronized intermittent mandatory ventilation with pressure support was used during standard care. When the patient was recovering from the disease for which intubation was indicated, ventilator parameters were decreased gradually to enable spontaneous breathing.
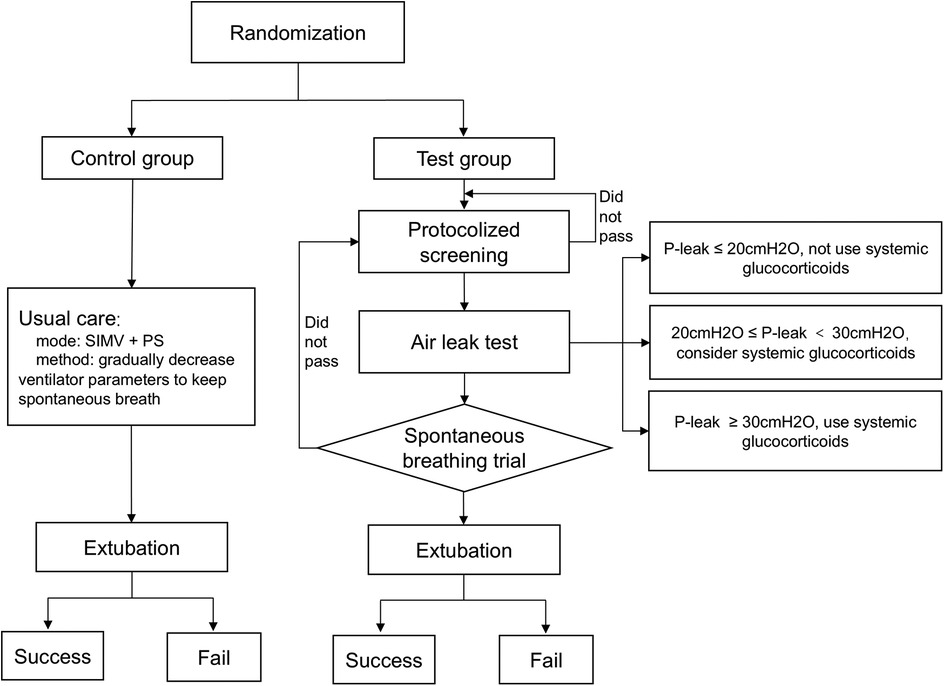
Figure 1. Study design. SIMV, synchronized intermittent mandatory ventilation; PS, pressure support; P-leak, the pressure causing endotracheal peritubal leak.
The test group was driven by a computerized algorithm. The IMV liberation protocol, which combined protocolized screening, air leak testing, and SBT was based on the IntelliSpace Critical Care and Anesthesia decision support tool (ICCA, Koninklijke Philips N.V., The Netherlands). ICCA captured vital signs, ventilator parameters, laboratory data, and treatment measures. Based on those data, ICCA screened patients fit for SBT automatically, and drove the liberation protocol. If a patient had passed the protocol, ICCA would advise the attending physician, who would make final patient management decisions.
ICCA screened all patients at 8:00 AM daily. Investigators were alerted to patients who had met the following criteria: fraction of inspired oxygen (FiO2) ≤50%; positive end-expiratory pressure (PEEP) ≤8 cmH2O; peak inspiratory pressure (PIP) ≤25 cmH2O; state behavioral scale (SBS) = −1 or 0 (15); no continuous use of neuromuscular blockers in the preceding 24 h; stable hemodynamic status (vasoactive-inotropic score [VIS] ≤20; VIS = epinephrine (μg/kg/min) × 100 + norepinephrine (μg/kg/min) × 100 + milrinone (μg/kg/min) × 10 + vasopressin (U/kg/min) × 10,000 + dopamine (μg/kg/min) + dobutamine (μg/kg/min)) (16, 17); and presence of respiratory drive. All the above data were recorded automatically in ICCA or manually by the nursing staff every 2 h.
Screened patients underwent immediate air leak testing. The air leak test, as a predictor of postextubation stridor, was used to guide systemic glucocorticoid therapy (9, 11, 18, 19). Its application has been described in detail (20–22). The air leak test was performed with the patient supine and head in the midline position. After complete deflation of the endotracheal tube cuff, the patient was manually ventilated to a maximal pressure of 30 cmH2O. The pressure at which an audible air leak heard was recorded. If air leak pressure was ≤20 cmH2O, systemic glucocorticoid therapy was not to be considered; if air leak pressure was >20 cmH2O and ≤30 cmH2O, systemic glucocorticoid therapy could be considered in the presence of other factors that might cause postextubation stridor; if air leak pressure was ≥30 cmH2O, systemic glucocorticoid therapy given 4–6 h prior to extubation was considered (9, 11, 18).
The air leak test was followed by SBT, which simulates postextubation conditions (23, 24). Patients were placed on FiO2 of 40%, PEEP of 5cmH2O, and pressure support of 5–8 cmH2O. The SBT was conducted for 0.5–2 h. The test was interrupted if patients exhibited either SpO2 < 90%; a heart rate increases of more than 20% compared to baseline; signs of tachypnea, paradoxical respiration, or increased respiratory work; or tidal volume ≤5 ml/kg (weight ≥60 kg, tidal volume ≤300 ml) (12, 25–27). An arterial blood gas analysis was performed after 30 min of SBT. If the patient passed the SBT and exhibited body temperature <38.5°C, pH > 7.3, lactate <2 mmol/L, and PaCO2 < 55 mmHg, ICCA would advise IMV liberation. The attending physician made final management decision according to patient's clinical status. If the attending physician declined the ICCA recommendation, previous MV parameters would be rebuilt, and the entire process would be repeated the next day.
Both groups received the same sedation protocol. SBS was adopted to assess sedation level. Analgesia with sufentanil (0.04–0.12 ug/kg/h) and sedation with midazolam (1–5 ug/kg/min) were routinely administered to all intubated patients.
2.6 Outcomes
Extubation was defined as successful if re-intubation was not indicated within 48 h. The primary outcome was the duration of IMV from initiation until the first extubation. Secondary outcomes were successful extubation frequency, noninvasive respiratory support usage after extubation, PICU length of stay (PICU LOS), and hospital LOS.
2.7 Power and sample size calculations
A comparison of a ventilator liberation protocol that combined daily evaluation and SBT to standard care disclosed a median IMV duration of 3.5 days [95% confidence interval (CI), 3.0–4.0] in the test group and 4.7 days (95%CI, 4.1–5. 3) in the control group (p = 0.0127) (12). The aforementioned study suggests that a sample size of 375 patients (187 patients for the control group, 188 patients for the test group) would confer adequate statistical power (1-β = 80%, two-side α = 0.05, accrual time = 20 months, and proportion lost = 5% for each group). Our pilot study included 40 patients (20 in each group), or 10% of the sample size calculated above.
2.8 Data analysis
Statistical analysis was performed with SPSS version 26 (IBM Corp, Armonk, NY, USA). Continuous variables were presented as median (IQR). Intergroup differences were evaluated with the Mann–Whitney U test. Categorical variables were presented as counts (percentages). Kaplan–Meier survival curves were used to analyze the probability of remaining on IMV, with comparisons based on the log-rank test. A p-value < 0.05 was considered statistically significant.
3 Results
3.1 Patient characteristics
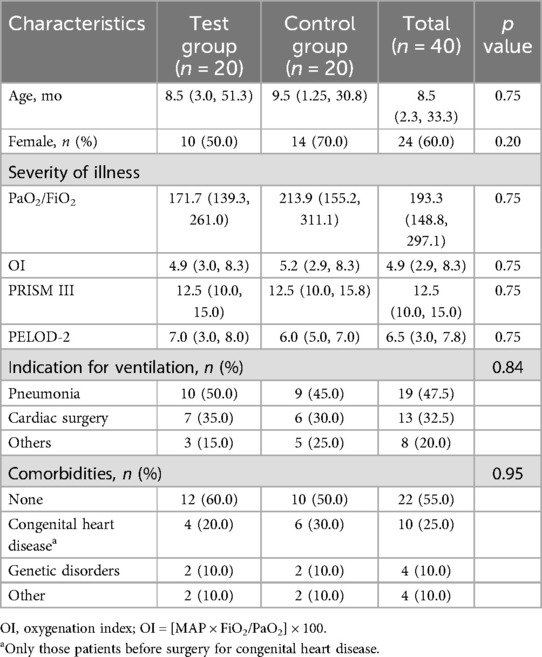
A total of 164 patients underwent IMV from April 17, 2023 to June 16, 2023. Seventy-one patients received IMV for more than 24 h. Thirty-one patients were excluded. A total of 40 patients (20 patients for each group) were recruitment, enrolled and randomized (Figure 2) and followed to hospital discharge. Baseline patient characteristics of the study groups were similar (Table 1). Pneumonia and cardiac surgery were the most common indications for IMV. Most patients did not have comorbidities.
3.2 Outcomes
Four control patients did not undergo standard IMV liberation. One patient suffered in-hospital mortality, 2 underwent treatment withdrawal, and 1 experienced unplanned extubation. One test group patient received IMV for more than 21 days and did not undergo extubation during the follow-up period. A total of 35 patients completed IMV liberation [16 (80%) control patients, 19 (95%) test patients]. No patient liberated by the attending physician before the advice by ICCA in the test group. Two patients in the test group were not liberated from IMV after the first ICAA recommendation because the attending physician assessed that their clinical status precluded liberation. One patient in the test group experienced extubation failure caused by postextubation upper airway obstruction within 24 h.
Three investigators were fully proficient in all aspects of the research protocol. All chief nurses were competent in the correct use of ICCA and could coordinate investigators to complete the process during routine clinical practice. All predetermined parameters were fully recorded.
Kaplan–Meier curves of the probability of continued IMV are shown in Figure 3. Median durations of IMV were 95.3 h (95%CI, 9.07–181.53) in the test group and 113.3 h (95%CI, 85.90–140.70) in the control group, with inconclusive effect [hazard ratio [HR]= 1.18 [95%CI, 0.61–2.31]; p = 0.62]. There were inconclusive effect in use of systemic glucocorticoid therapy [75.0% vs. 50.0%; relative risk [RR] = 1.50 [95%CI, 0.90–2.49]; p = 0.10]; noninvasive respiratory support [35.0% vs. 25.0%; RR = 1.40 (95%CI, 0.53–3.68); p = 0.49]; PICU LOS [6.9 days [95%CI, 5.00–8.86] vs. 7.0 days [95%CI, 5.58–8.40]; HR = 1.12 [95%CI, 0.57–2.21]; p = 0.74]; and hospital LOS [22.9 days [95%CI, 11.48–34.24] vs. 26.9 days [95%CI, 17.86–35.94]; HR = 0.69 [95%CI, 0.34–1.41]; p = 0.31] between the control and test groups, respectively.
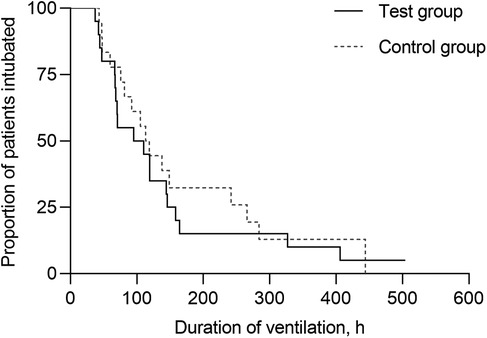
Figure 3. Kaplan–Meier curves of the probability remaining on invasive mechanical ventilation. The median duration of IMV was 95.3 h (95%CI, 9.07–181.53) in the test group and 113.3 h (95%CI, 85.90–140.70) in the control group, without significant difference (p = 0.62).
3.3 Ventilator parameters
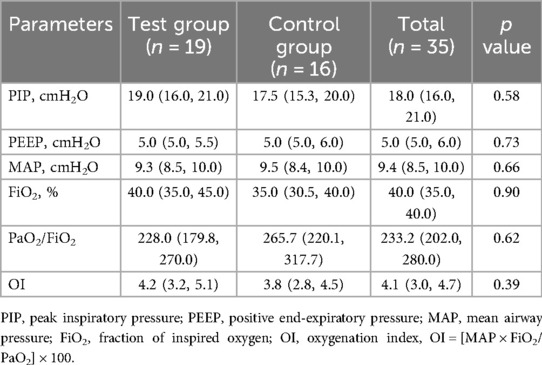
Pre-liberation values of PIP, PEEP, MAP, FiO2, PaO2/FiO2 and OI were similar between the two groups (Table 2).
4 Discussion
We conducted this 2-month pilot study validated the feasibility of applying our computer-driven decision support tool to pediatric patients; the feasibility of conducting a larger prospective trial of our study protocol with adequate statistical power to assess the efficacy of the intervention; and demonstrated successful training of PICU personnel. Our team of investigators mastered the application of the protocol. However, our statistical analysis predicted that an enrollment period of 20 months would be needed to enroll the calculated sample size.
We adopted screening criteria by combining those of previous studies (12, 28–30) and those learned from our clinical experiences. The first issue was whether ventilator criteria were too high. Previous study protocols adopted different criteria. Higher screening criteria may necessitate earlier patient assessments, which may increase staff workload, but might identify candidates for earlier liberation and thereby shorten the duration of IMV. Because of the potential benefits of reduced IMV duration, we adopted current criteria. The combining of our protocol with current clinical practice algorithms did not add an excessive work burden. Except for the patient who received IMV for more than 21 days, all patients in the test group completed our protocol and were liberated, and 84.21% (16/19) of patients passed the first SBT. In Fronda et al.'s study (12), 84.33% (113/134) of patients passed the first SBT, and only one patient required more than two SBTs. Most patients who met the screening criteria subsequently passed the SBT. Compared to clinician driven ventilator liberation protocols, computer-driven protocols reduce the need for repetitive screening. Therefore, implementing rational screening criteria alongside protocols tailored to daily clinical practice can reduce screening fatigue and increase compliance rates.
The next issue was whether the resolution of the indication for intubation should be considered before screening or after completion of the protocol. It was one of the criteria for weaning readiness in the study reported by Mehta et al. (30), but was not incorporated into our protocolized screening because this criterion was assessed subjectively, which may introduce bias. Participation of the attending physician in the test group after completion of the protocol might reduce bias. However, another possibility is that the attending physician might liberate patients before screening in the test group. This is an unavoidable challenge due to the inherent complexity of clinical practice. Both intention-to-treat and per-protocol analyses will be performed in the subsequent larger study.
Owing to the finely designed workflow, repeated program debugging and study simulation conducted prior to the pilot study, all 40 enrolled patients completed follow-up, all predetermined parameters were recorded, and relevant personnel were trained. These results demonstrate that a larger study is feasible in out unit. However, due to the small sample size of this pilot study, unforeseen issues may arise in subsequent research.
There was inconclusive effect of IMV duration, PICU LOS and hospital LOS between patients undergoing either a computer-driven liberation protocol or standard care. Given the limited sample size and inherent variability among the measured parameters, we opted not to perform multivariate adjustments in this preliminary analysis. In our subsequent larger study, we plan to conduct comprehensive multivariate analysis to better elucidate the factors influencing IMV duration. We compared pre-liberation ventilator parameters between the two groups to explore whether the computer-driven protocol could drive patients to liberate from higher ventilator parameters. Patients liberating from higher ventilator parameters may hypothetically undergo earlier liberation. However, we observed no significant difference in pre-liberation ventilator parameters between test and control groups.
Few studies of computer-driven ventilator liberation protocols have been completed among pediatric patients. Jouvet et al. (28) conducted a pilot study (n = 30) to assess whether a computer-driven protocol can decrease the duration of IMV compared to usual care, and found no significant differences in duration of IMV, PICU LOS, and hospital LOS between test and usual care groups, respectively.
Studies of protocols driven by physicians, nurses, or respiratory therapists have yielded varying results. The intergroup similarity of outcomes observed in our study might be explained by several factors. First, this was a pilot study with a small sample size that may have limited adjustments for suspected confounding factors. Second, indications for IMV were heterogenous. A multicenter study completed by Blackwood et al. (13) that featured high patient heterogeneity demonstrated that a ventilator liberation protocol significantly shortened the duration of IMV, but with small effect size. The PICU LOS was similar between groups. The hospital LOS was significantly longer in patients undergoing the ventilator liberation protocol. Meanwhile, Foronda et al. (12) demonstrated that a ventilator liberation protocol abbreviated the duration of IMV (from 4.7 days to 3.5 days) in a heterogenous cohort. Mehta et al.'s study (30) reported that a ventilator-weaning pathway decreased the duration of ventilation by a median of 3.6 days without different reintubation rates among pediatric patients with acute respiratory distress syndrome, although PICU LOS was not significantly reduced. Ferreiara et al. (25) compared SBT with physician-led weaning for predicting extubation success in pediatric patients following cardiac surgery. Extubation success was significantly higher (83% vs. 68%) and PICU LOS was significantly shorter (median 85 h vs. 367 h) in patients undergoing SBT compared to controls, respectively. However, durations of IMV and hospital LOS were similar between the test and control groups, respectively.
In summary, studies of patients receiving IMV for single or heterogenous indications have yielded discrepant results. Potential confounding factors should be minimized to the greatest possible extent. In addition, the protocolized screening interval may represent another confounding factor. Computer screened patients at 8:00 AM daily in our study. The time and interval we chosen were most fit for our routine. Most other studies also screened patients daily. Screening at shorter intervals may identify candidates for earlier liberation, but may burden investigators and clinicians. Although patients were screened every 3 h in Loberger et al.'s study (29), durations of IMV and PICU LOS did not differ between pre-intervention and intervention cohorts. In a study of adult patients that compared daily screening to at least twice daily screening led by respiratory therapists, more frequent screening was not associated with shorter duration of IMV or more successful extubation (31). Thus, the effect of screening intervals in ventilator liberation protocols needs further study. The most important limitations of the aforementioned studies were their unblinded study designs, which may have introduced bias but were inevitable given the role of the attending physician in final decision making.
5 Conclusions
This pilot study confirmed that the conduction of a large randomized controlled trial of a computer-driven IMV liberation protocol is feasible in our PICU. However, there was inconclusive effect in the durations of IMV between intervention and control groups. A larger prospective trial with adequate statistical power is needed to further explore the utility of a computer-driven IMV liberation protocol.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Children's Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
SC: Data curation, Validation, Formal analysis, Conceptualization, Methodology, Investigation, Writing – original draft. CXX: Methodology, Validation, Data curation, Writing – original draft, Conceptualization, Investigation, Formal analysis. XL: Conceptualization, Investigation, Validation, Writing – original draft, Formal analysis, Methodology, Data curation. ML: Methodology, Validation, Conceptualization, Writing – review & editing. CJL: Writing – review & editing, Supervision, Methodology, Conceptualization, Validation. FX: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. JL: Conceptualization, Writing – review & editing, Supervision, Resources, Funding acquisition, Validation, Project administration, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Program for Youth Innovation in Future Medicine of Chongqing Medical University (W0124 to JL).
Acknowledgments
We thank all the medical and nursing staff members of our department. We also appreciate the technical support provided by Philips Research China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ramnarayan P, Blackwood B, Khemani RG. What’s new in paediatric ventilator liberation? Intensive Care Med. (2022) 48(11):1635–37. doi: 10.1007/s00134-022-06865-0
2. Schults JA, Charles K, Harnischfeger J, Erikson S, Burren J, Waak M, et al. Ventilator weaning and extubation practices in critically ill children: an Australian and New Zealand survey of practice. Aust Crit Care. (2022) 36(4):509–14. doi: 10.1016/j.aucc.2022.06.004
3. van Dijk J, Blokpoel RGT, Abu-Sultaneh S, Newth CJL, Khemani RG, Kneyber MCJ. Clinical challenges in pediatric ventilation liberation: a meta-narrative review. Pediatr Crit Care Med. (2022) 23(12):999–1008. doi: 10.1097/PCC.0000000000003025
4. Elisa P, Francesca C, Marco P, Davide V, Laura Z, Fabrizio Z, et al. Ventilation weaning and extubation readiness in children in pediatric intensive care unit: a review. Front Pediatr. (2022) 10:867739. doi: 10.3389/fped.2022.867739
5. Burns KEA, Rizvi L, Cook DJ, Lebovic G, Dodek P, Villar J, et al. Ventilator weaning and discontinuation practices for critically ill patients. JAMA. (2021) 325(12):1173–84. doi: 10.1001/jama.2021.2384
6. van Dijk J, Koopman AA, de Langen LB, Dijkstra S, Burgerhof JGM, Blokpoel RGT, et al. Effect of pediatric ventilation weaning technique on work of breathing. Respir Res. (2022) 23(1):184. doi: 10.1186/s12931-022-02106-6
7. Newth CJ, Venkataraman S, Willson DF, Meert KL, Harrison R, Dean JM, et al. Weaning and extubation readiness in pediatric patients. Pediatr Crit Care Med. (2009) 10(1):1–11. doi: 10.1097/PCC.0b013e318193724d
8. Blackwood B, Burns KE, Cardwell CR, O’Halloran P. Protocolized versus non-protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database Syst Rev. (2014) 2014(11):Cd006904. doi: 10.1002/14651858.CD006904.pub3
9. Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. An official American thoracic society/American college of chest physicians clinical practice guideline: liberation from mechanical ventilation in critically ill adults. Rehabilitation protocols, ventilator liberation protocols, and cuff leak tests. Am J Respir Crit Care Med. (2017) 195(1):120–33. doi: 10.1164/rccm.201610-2075ST
10. Ouellette DR, Patel S, Girard TD, Morris PE, Schmidt GA, Truwit JD, et al. Liberation from mechanical ventilation in critically ill adults: an official American college of chest physicians/American thoracic society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. (2017) 151(1):166–80. doi: 10.1016/j.chest.2016.10.036
11. Abu-Sultaneh S, Iyer NP, Fernandez A, Gaies M, Gonzalez-Dambrauskas S, Hotz JC, et al. Executive summary: international clinical practice guidelines for pediatric ventilator liberation, a pediatric acute lung injury and sepsis investigators (PALISI) network document. Am J Respir Crit Care Med. (2023) 207(1):17–28. doi: 10.1164/rccm.202204-0795SO
12. Foronda FK, Troster EJ, Farias JA, Barbas CS, Ferraro AA, Faria LS, et al. The impact of daily evaluation and spontaneous breathing test on the duration of pediatric mechanical ventilation: a randomized controlled trial. Crit Care Med. (2011) 39(11):2526–33. doi: 10.1097/CCM.0b013e3182257520
13. Blackwood B, Tume LN, Morris KP, Clarke M, McDowell C, Hemming K, et al. Effect of a sedation and ventilator liberation protocol vs usual care on duration of invasive mechanical ventilation in pediatric intensive care units: a randomized clinical trial. JAMA. (2021) 326(5):401–10. doi: 10.1001/jama.2021.10296
14. Krawiec C, Carl D, Stetter C, Kong L, Ceneviva GD, Thomas NJ. Challenges with implementation of a respiratory therapist-driven protocol of spontaneous breathing trials in the pediatric ICU. Respir Care. (2017) 62(10):1233–40. doi: 10.4187/respcare.05477
15. Curley MA, Harris SK, Fraser KA, Johnson RA, Arnold JH. State behavioral scale: a sedation assessment instrument for infants and young children supported on mechanical ventilation. Pediatr Crit Care Med. (2006) 7(2):107–14. doi: 10.1097/01.PCC.0000200955.40962.38
16. Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. (2010) 11(2):234–8. doi: 10.1097/PCC.0b013e3181b806fc
17. Musick MA, Loftis LL, Kennedy CE. Comparing vasoactive-inotropic score reporting strategies in the PICU relative to mortality risk. Pediatr Crit Care Med. (2018) 19(12):1130–36. doi: 10.1097/pcc.0000000000001738
18. Iyer NP, López-Fernández YM, González-Dambrauskas S, Baranwal AK, Hotz JC, Zhu M, et al. A network meta-analysis of dexamethasone for preventing postextubation upper airway obstruction in children. Ann Am Thorac Soc. (2023) 20(1):118–30. doi: 10.1513/AnnalsATS.202203-212OC
19. Tanaka A, Uchiyama A, Horiguchi Y, Higeno R, Sakaguchi R, Koyama Y, et al. Predictors of post-extubation stridor in patients on mechanical ventilation: a prospective observational study. Sci Rep. (2021) 11(1):19993. doi: 10.1038/s41598-021-99501-8
20. Kriner EJ, Shafazand S, Colice GL. The endotracheal tube cuff-leak test as a predictor for postextubation stridor. Respir Care. (2005) 50(12):1632–8.16318644
21. Mhanna MJ, Zamel YB, Tichy CM, Super DM. The “air leak” test around the endotracheal tube, as a predictor of postextubation stridor, is age dependent in children. Crit Care Med. (2002) 30(12):2639–43. doi: 10.1097/00003246-200212000-00005
22. Wratney AT, Benjamin DK Jr, Slonim AD, He J, Hamel DS, Cheifetz IM. The endotracheal tube air leak test does not predict extubation outcome in critically ill pediatric patients. Pediatr Crit Care Med. (2008) 9(5):490–6. doi: 10.1097/PCC.0b013e3181849901
23. Sklar MC, Burns K, Rittayamai N, Lanys A, Rauseo M, Chen L, et al. Effort to breathe with various spontaneous breathing trial techniques. A physiologic meta-analysis. Am J Respir Crit Care Med. (2017) 195(11):1477–85. doi: 10.1164/rccm.201607-1338OC
24. Thille AW, Gacouin A, Coudroy R, Ehrmann S, Quenot JP, Nay MA, et al. Spontaneous-breathing trials with pressure-support ventilation or a T-piece. N Engl J Med. (2022) 387(20):1843–54. doi: 10.1056/NEJMoa2209041
25. Ferreira FV, Sugo EK, Aragon DC, Carmona F, Carlotti A. Spontaneous breathing trial for prediction of extubation success in pediatric patients following congenital heart surgery: a randomized controlled trial. Pediatr Crit Care Med. (2019) 20(10):940–46. doi: 10.1097/PCC.0000000000002006
26. Tan HL, Ma Y-J, Aguilan AB, Goh CY, Wong JCK, Ang LSL, et al. Respiratory therapist-driven extubation readiness testing in a single pediatric ICU. Respir Care. (2022) 67(7):833–41. doi: 10.4187/respcare.09680
27. Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, et al. Weaning from mechanical ventilation. Eur Respir J. (2007) 29(5):1033–56. doi: 10.1183/09031936.00010206
28. Jouvet PA, Payen V, Gauvin F, Emeriaud G, Lacroix J. Weaning children from mechanical ventilation with a computer-driven protocol: a pilot trial. Intensive Care Med. (2013) 39(5):919–25. doi: 10.1007/s00134-013-2837-8
29. Loberger JM, Jones RM, Prabhakaran P. A respiratory therapist-driven pathway improves timeliness of extubation readiness assessment in a single PICU. Pediatr Crit Care Med. (2020) 21(8):e513–21. doi: 10.1097/PCC.0000000000002326
30. Mehta SD, Martin K, McGowan N, Dominick CL, Madu C, Denkin BK, et al. Ventilator-weaning pathway associated with decreased ventilator days in pediatric acute respiratory distress syndrome. Crit Care Med. (2021) 49(2):302–10. doi: 10.1097/CCM.0000000000004704
Keywords: critical care, ventilation, pediatrics, liberation protocol, pilot study
Citation: Chen S, Xiao C, Lu X, Liao M, Liu C, Xu F and Li J (2025) A computer-driven ventilator liberation protocol in pediatric patients: a single-center pilot randomized controlled trial. Front. Pediatr. 13:1594160. doi: 10.3389/fped.2025.1594160
Received: 15 March 2025; Accepted: 7 July 2025;
Published: 18 July 2025.
Edited by:
Tanıl Kendirli, Ankara University, TürkiyeReviewed by:
Paulo André Freire Magalhães, Universidade de Pernambuco, BrazilEmanuele Rossetti, Bambino Gesù Children’s Hospital (IRCCS), Italy
Gokhan Ceylan, University of Health Sciences, Türkiye
Muhammed Üdürgücü, Ondokuz Mayıs University, Türkiye
Merve Nur Tekin, Ankara University, Türkiye
Copyright: © 2025 Chen, Xiao, Lu, Liao, Liu, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Li, bGlqaW5nd2FuZ3lpQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Song Chen
Song Chen Changxue Xiao†
Changxue Xiao† Chengjun Liu
Chengjun Liu Feng Xu
Feng Xu Jing Li
Jing Li