- Department of Organ Transplantation, Shanghai Changhai Hospital, Naval Medical University, Shanghai, China
Background: Kidney transplantation is recognized as the optimal treatment for end-stage kidney disease (ESKD) in children, which significantly improves growth delay, pubertal development, and social prognosis in pediatric patients. This study analyzed the long-term prognosis and influencing factors following pediatric kidney transplantation at our center.
Methods: A total of 101 pediatric recipients who underwent kidney transplantation at our center were enrolled in this study. Post-transplant outcomes, including renal function, height development, pubertal progression, and social adaptation, were systematically analyzed.
Results: The height-for-age Z-score (HAZ) significantly improved from −2.27 ± 1.64 at transplantation to −0.76 ± 1.13 after achieving post-transplant stability. The graft survival rates were 100% and 93.4% at 5 and 10 years post-transplantation, respectively, while patient survival rates remained 100% at both 5 and 10 years. At the last follow-up, the mean serum creatinine level was 83.85 ± 38.34 μmol/L, with an estimated glomerular filtration rate (eGFR) of 79.49 ± 27.25 ml/min/1.73 m2. Among school-aged recipients, 93.75% successfully returned to school, while only 33.3% of those who completed their education achieved employment. Among male adolescents (13 years, n = 43), 37 cases (86.0%) experienced spermarche, with a mean age of 14.5 years in prepubertal transplant recipients. In the female cohort (n = 45), 42 patients (12 years) reached puberty, demonstrating a mean menarche age of 12.5 years in prepubertal recipients vs. 13.2 years in postpubertal transplants (P > 0.05). Menstrual irregularities were observed in 8 cases, accounting for 19.51% of menstruating females.
Conclusion: This study demonstrates significant improvements in height development, pubertal progression, and social adaptation following kidney transplantation in pediatric recipients. While recipient gender, pre-transplant dialysis modality, and dialysis duration showed no significant impact on near-final height (NFH), both transplantation age and height at transplantation significantly influenced NFH attainment. These findings emphasize that early transplantation and maintaining optimal graft function are crucial for ameliorating growth delay and pubertal development, while also positively influencing long-term social outcomes in pediatric transplant recipients.
1 Introduction
Poor long-term prognosis remains a significant concern in pediatric patients with chronic kidney disease (CKD). Kidney transplantation, recognized as the optimal treatment for end-stage kidney disease (ESKD) in children, has been shown to significantly improve growth and developmental, enhance both physical and psychological development, and promote social-psychological well-being, ultimately leading to improved quality of life and survival rates in this population (1). Prolonged dialysis in children with renal failure not only adversely affects growth and pubertal development but also significantly impacts long-term social outcomes, substantially increasing the risk of negative social and occupational consequences (2). In the past, the development of pediatric kidney transplantation in China has been hindered by the persistent shortage of pediatric donor organs. Our center pioneered the pediatric-to-pediatric (PTP) allocation model and has systematically validated its feasibility and clinical efficacy (3). Following the 2018 revision of China's kidney allocation policy, which prioritized pediatric recipients for pediatric donor kidneys, the field of pediatric kidney transplantation has developed rapidly in China.
Currently, significant improvements in transplantation success rates have been achieved through advancements in surgical techniques, optimization of immunosuppressive therapies, and enhanced comprehensive pre-transplant evaluation and perioperative management (4). While short-term prognosis of pediatric kidney transplantation has shown significant improvement, there remains substantial room for enhancing long-term graft survival. Studies have identified that all pediatric recipients undergo a high-risk period for graft failure during adolescence. This high-risk period is associated with multiple contributing factors, including enhanced immune reactivity, decreased medication adherence, and the transition from parental supervision to self-management, all of which collectively contribute to suboptimal long-term graft survival rates in pediatric recipients (1, 5, 6).
Given the relatively late initiation of pediatric kidney transplantation in China, there is a notable scarcity of research and data on long-term outcomes among pediatric recipients. As the pioneering transplant center in China to implement pediatric-to-pediatric kidney transplantation, our institution conducted a retrospective analysis of 101 pediatric recipients who received transplants from young pediatric donors. This study comprehensively evaluated post-transplant growth and development, pubertal progression, educational and occupational status. The detailed findings are presented below.
2 Methods
This retrospective study analyzed pediatric recipients who underwent kidney transplantation from 2013 to 2021 at Shanghai Changhai Hospital, Shanghai, China. Study data were systematically collected using a dual approach: (1) Targeted queries for patient basic information in our center's electronic medical record system. (2) The follow-up information of patients was collected by means of structured questionnaires. The research information includes donor information, recipient demographic characteristics, transplantation modality, primary disease, pre-transplant dialysis method and duration, graft function, recipient/graft survival rates, complication profiles, and the age of spermatorrhea in male patients and the age of menarche in female patients. Social outcome assessments included educational attainment and employment status.
The inclusion criteria were: (1) age <18 years at the time of transplantation; (2) minimum follow-up duration of 3 years; (3) no recombinant human growth hormone (rhGH) treatment during the follow-up period.
The final follow-up data included height measurements (in centimeters) and serum creatinine levels (Scr) for all patients. The estimated glomerular filtration rate (eGFR) was calculated using the creatinine-based Schwartz formula (7), this equation is applicable for children and adolescents aged 2 to 18 years. Growth evaluation methodology: The height-for-age z-score (HAZ) was utilized for assessment, calculated as (measured height—standard height for age and gender)/standard deviation. Height standards were referenced to the WHO 2007 Growth curve (8). Growth status was determined based on z-score values: normal growth was defined as |Z| ≤ 2, while growth delay was identified when Z < −2. As bone age assessment was not performed, near-final height (NFH) was defined as annual height gain < 0.5 cm (9). The assessment of pubertal development followed international standards, defining adolescence as 13–15 years for males, and 12–13 years for females (10).
Immunosuppressive therapy: The immunosuppressive protocol consisted of induction therapy with either anti-CD25 monoclonal antibody or rabbit anti-thymocyte globulin, followed by corticosteroid withdrawal within the first postoperative week. The maintenance treatment was tacrolimus combined with mycophenol sodium enteric-coated tablets combined with immunosuppressive therapy. Target tacrolimus trough levels were maintained at 10–15 ng/ml during the first month, 8–10 ng/ml for the initial three months, and 5–8 ng/ml thereafter. Mycophenolate sodium was administered at a dose of 540 mg·1.73 m−²·d−¹.
Delayed graft function (DGF) was defined as the requirement for dialysis within the first week post-transplantation (11). Rejection episodes were characterized by clinical manifestations including elevated serum creatinine levels or proteinuria, confirmed through allograft biopsy as either antibody-mediated rejection (AMR) or T cell-mediated rejection (TCMR) (12).
Statistical analysis: Statistical analyses were performed using SPSS software (version 22.0). Normally distributed continuous variables were expressed as mean ± standard deviation, while non-normally distributed data were presented as median. Comparisons between groups were conducted using Student's t-test for continuous variables and χ2 test for categorical variables, with P < 0.05 considered statistically significant. Graft and recipient survival rates were calculated using the Kaplan–Meier method.
3 Results
3.1 Patient characteristics
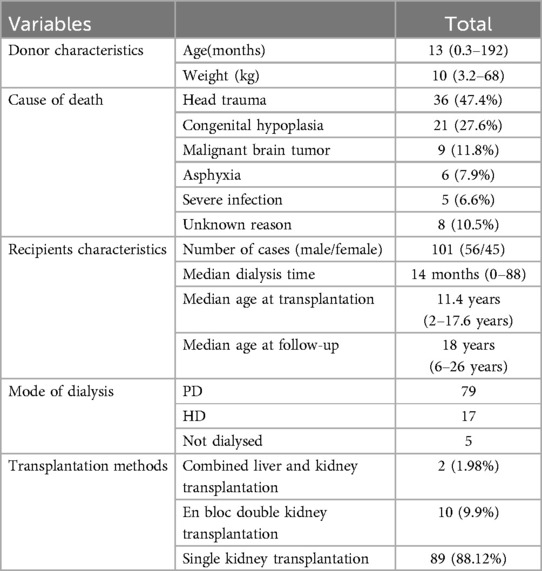
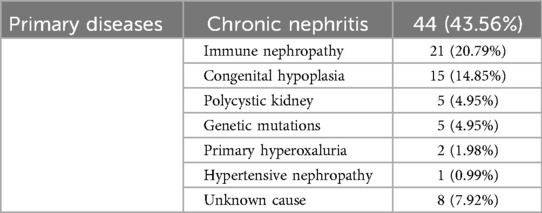
All kidney transplants in our center were from deceased donors. There were 85 donors in this survey (Table 1), with an average age of 37.8 months, a median age of 13 months (0.3–192), an average weight of 14.1 kg, and a median weight of 10 kg (3.2–68). The most common cause of death was head trauma; 36 cases (accounting for 47.4%), followed by congenital hypoplasia; 21 cases (accounting for 27.6%), then malignant brain tumor; 9 cases (accounting for 11.8%), asphyxia: 6 cases (accounting for 7.9%), severe infection: 5 cases (accounting for 6.6%), and 8 cases of unknown reason of death (accounting for 10.5%). This study enrolled 101 patients, comprising 56 males and 45 females. Preoperatively (Table 1), 79 patients (78.22%) underwent peritoneal dialysis, 17 patients (16.83%) received hemodialysis, and 5 patients (4.95%) had no dialysis history. The median dialysis duration was 14 months (range: 0–88 months). At transplantation, the median age was 11.4 years (range: 2–17.6 years), with a median follow-up age of 18 years (range: 6–26 years) and a median post-transplant follow-up duration of 80 months (range: 30–138 months). The transplantation modalities included 2 cases of combined liver-kidney transplantation and 10 cases of en bloc kidney transplantation (Postoperative thrombosis occurred in 3 cases and secondary single kidney transplantation was performed after resection), along with 89 cases of single kidney transplantation. The most prevalent etiology of ESKD (Table 2) was chronic glomerulonephritis (44 cases, 43.56%), followed by immune-mediated nephropathy (21 cases, 20.79%), and congenital anomalies of the kidney and urinary tract (15 cases, 14.85%). Other causes included polycystic kidney disease (5 cases, 4.95%), genetic mutations (5 cases, 4.95%), primary hyperoxaluria (2 cases, 1.98%), hypertensive nephropathy (1 case, 0.99%), and unknown etiologies (8 cases, 7.92%). All donor-recipient pairs were ABO blood type identical or compatible, with negative panel reactive antibodies in recipients and negative lymphocyte crossmatch results in all cases.
3.2 Recipient and graft survival
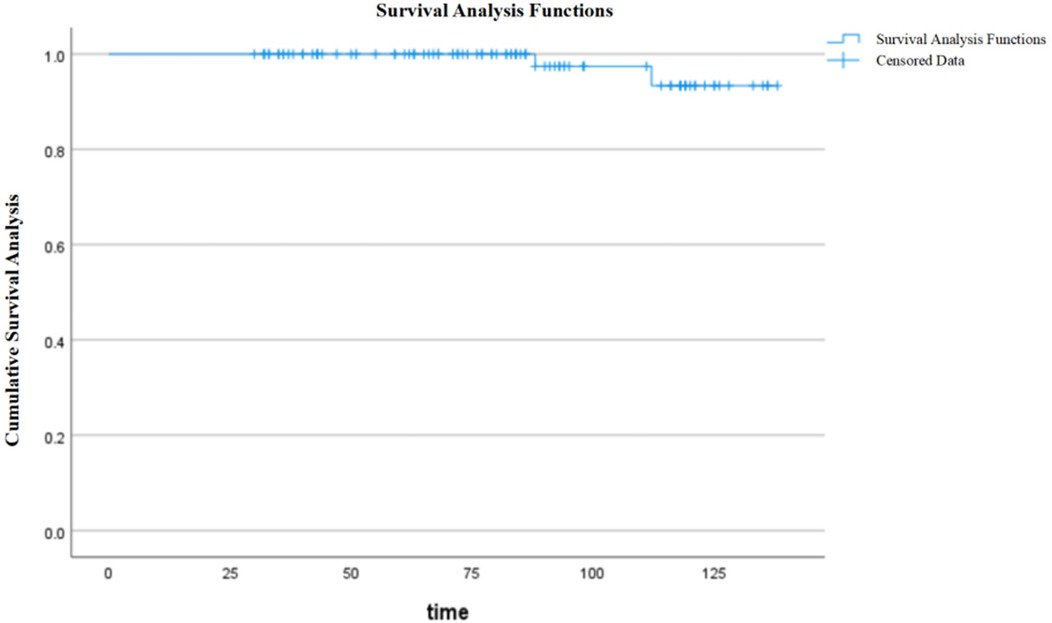
During follow-up, graft function remained stable, with a mean serum creatinine level of 83.85 ± 38.34 μmol/L and an eGFR of 79.49 ± 27.25 ml/min/1.73 m2 at the last follow-up. Among the study cohort, 74 patients had a transplant duration exceeding 5 years. The graft survival rates were 100% and 93.4% at 5 and 10 years, respectively (Figure 1), while patient survival rates remained 100% at both time points. Among the study cohort, 91 patients received single kidney transplants while 10 underwent en bloc kidney transplantation. Notably, two recipients in the en bloc kidney transplantation group developed graft thrombosis postoperatively, necessitating transplant nephrectomy, but both patients subsequently underwent successful single kidney retransplantation. Post-transplant complications were observed in 45 cases, including infections (pulmonary and urinary tract): 43 cases; acute rejection: 24 cases; post-transplant lymphoproliferative disorder (PTLD): 2 cases; delayed graft function (DGF): 17 cases; renal artery stenosis: 7 cases; cytomegalovirus (CMV) infection: 3 cases; parvovirus B19 infection: 3 cases; and ureteral stenosis: 1 case. During the follow-up period, two cases of graft loss and blood dialysis recovery were observed.
3.3 Post-transplant growth
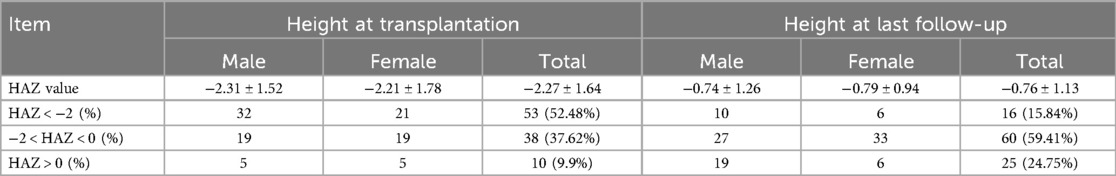
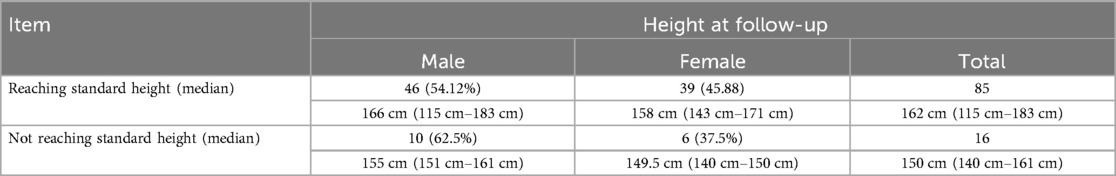
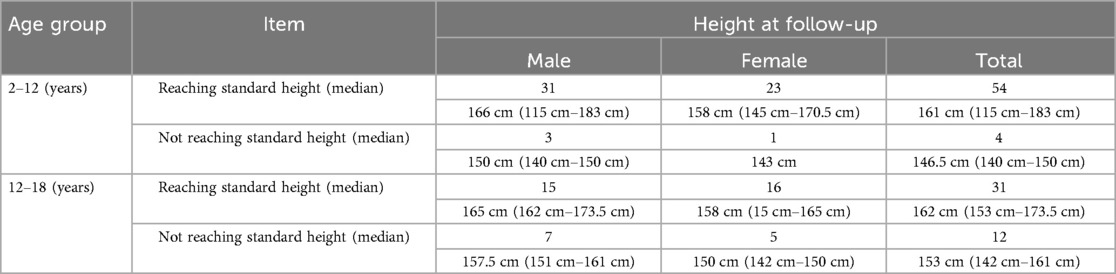
Post-transplant growth outcomes are presented in (Table 3). Significant improvement in growth parameters was observed, with the proportion of children exhibiting growth delay (HAZ < −2) decreasing from 52.48% pre-transplantation to 15.84% at final follow-up (P < 0.001). Among all pediatric recipients, 85 cases (84.16%) achieved near-final height (NFH) within the normal range for their age group (Table 4a), including 46 males (54.12%) with a median NFH of 166 cm (range: 115–183 cm) and 39 females (45.88%) with a median NFH of 158 cm (range: 143–171 cm). Sixteen recipients (15.84%) demonstrated significantly lower NFH compared to their age-matched peers, comprising 10 males (62.5%) with a median NFH of 155 cm (range: 151–161 cm) and 6 females (37.5%) with a median NFH of 149.5 cm (range: 140–150 cm) (P > 0.05). All 16 recipients with subnormal NFH had pre-transplant heights below the normal range, and except for 4 recipients aged <12 years, the remaining 12 were >12 years old. The younger the age at transplantation, the higher the proportion of patients reaching the standard height (|Z|≤2) at follow-up (Table 4b), while there was no difference in those that achieved standard height by sex. Statistical analysis revealed that NFH in pediatric transplant recipients correlated significantly with age at transplantation and height at transplantation (P < 0.05), but showed no association with gender, dialysis modality, or dialysis duration (P > 0.05).
3.4 Pubertal development status
The study cohort comprised 56 male patients, including 43 (76.8%) who had attained pubertal onset (age >13 years). Among these pubertal patients, 37 (86.0%) achieved spermarche, with 4 cases occurring prior to kidney transplantation and 33 cases developing postoperatively. The mean age at spermarche was 14.5 years in prepubertal transplant recipients and 15 years in postpubertal recipients (P > 0.05). The cohort included 45 female patients, with 42 (93.3%) having reached puberty (age >12 years). Among pubertal patients, those who underwent kidney transplantation before puberty had a mean menarche age of 12.5 years, whereas those transplanted after puberty showed a mean menarche age of 13.2 years (P > 0.05). Of these, 7 patients experienced menarche prior to kidney transplantation surgery, while 35 cases occurred postoperatively. Menstrual irregularities were observed in 8 patients (19.5% of pubertal individuals).
3.5 Educational attainment and employment status
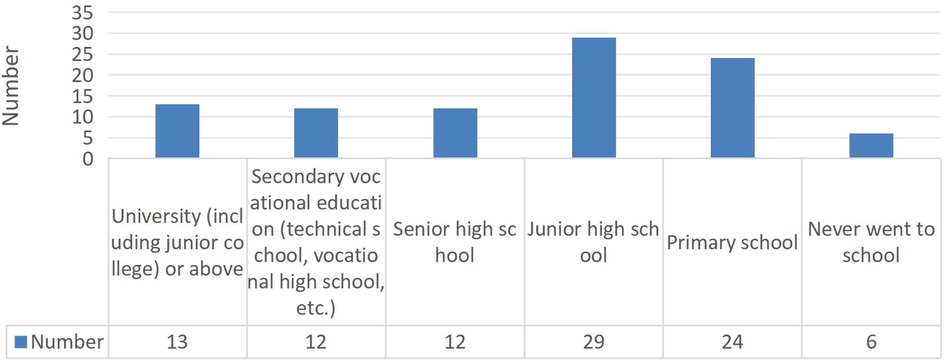
Among 96 school-aged patients in the follow-up cohort (Figure 2), 90 (93.75%) were actively pursuing their education. Employment outcomes were suboptimal, with only 33.3% of recipients who had completed their education being employed, though this analysis was limited by the small number of patients who had finished their academic pursuits.
4 Discussion
Kidney transplantation represents the optimal treatment for children with end-stage renal disease, offering benefits beyond renal function improvement. For pediatric recipients, transplantation not only promotes physical growth and pubertal development but also significantly contributes to psychological well-being and cognitive development, enabling better engagement in daily activities and social participation (13, 14). Prolonged dialysis in children with CKD can lead to endocrine dysfunction and metabolic disturbances, resulting in adverse outcomes including growth delay and delayed pubertal development (15). Prolonged dialysis in children with CKD may also lead to psychological complications and psychosocial challenges, potentially causing emotional disturbances and significantly impacting educational attainment and social interactions (16). Although these children with CKD are well cared for by their families and society, long-term personal and social problems still exist (17, 18).
The severity of growth delay in pediatric patients shows a strong inverse correlation with the age at CKD onset, with earlier disease manifestation associated with more pronounced growth impairment (17). Japanese studies evaluating long-term outcomes of pediatric kidney transplantation recipients have identified persistent short stature as a significant concern, with approximately 30% of patients achieving a final height below −2.5 standard deviation scores (SDS) 2. Consistent with our findings, most pediatric patients exhibited short stature prior to kidney transplantation, falling below the normal range for their age group (19, 20). In this study, 52.48% of recipients demonstrated growth delay (HAZ < −2) pre-transplantation, which significantly decreased to 15.84% at final follow-up (P < 0.001). Height at the start of dialysis and duration of dialysis are important determinants of baseline height at renal transplant (21). Our study revealed that longer pre-transplant dialysis duration correlated with lower height Z-scores, with approximately 15.84% of recipients achieving near-final height (NFH) below that of their healthy peers. Height at dialysis initiation and age at dialysis onset were identified as significant determinants of NFH, highlighting the importance of early transplantation, including pre-emptive transplantation, to minimize dialysis duration and its detrimental effects on growth (22–24). However, neither dialysis modality nor dialysis duration showed significant impact on final NFH (P > 0.05).
Another significant complication in children with CKD involves pubertal development and sexual maturation disorders. For pre-dialysis patients, management primarily focuses on preserving renal function and implementing growth hormone therapy, while therapeutic options for improving pubertal development remain limited (15). Research indicates that maintaining optimal pubertal development and normal sexual maturation presents a significant clinical challenge in managing children with CKD: approximately 50% of patients requiring renal replacement therapy (RRT) before age 13 exhibit delayed pubertal development, while early kidney transplantation represents the most effective intervention for improving pubertal progression and sexual maturation in pediatric CKD patients (25). Ferraris et al. (26) demonstrated that following successful kidney transplantation, nearly all patients demonstrated serum testosterone levels within the normal range for their pubertal stage, and follicle-stimulating hormone (FSH) levels were normal in two-thirds of the recipients. Winkler et al. (27) observed that following successful kidney transplantation female recipients exhibited normal cyclic fluctuations of gonadotropins and estradiol (E2) corresponding to ovulatory cycles. Our findings suggest that most pediatric patients can achieve normal pubertal development and sexual maturation following successful kidney transplantation. However, studies indicate that pubertal growth (particularly in pre-pubertal kidney transplantation recipients) and sexual maturation are associated with post-transplant immunosuppressive regimens, highlighting the need for more effective immunosuppressive protocols to facilitate normal physical and sexual development in children with growth and maturation delays (28).
Pediatric kidney transplantation enables timely reintegration into school and society, allowing school-aged children to pursue normal education and social development. Studies report that most kidney transplantation recipients successfully continue their education, and the majority of adult patients secure appropriate employment (2). Although some children experience grade-age mismatch or lower employment rates compared to the general population, they perceive their quality of life as comparable to their peers (14). In our study cohort, 96 school-aged children were identified, with 90 (93.75%) actively pursuing their education, demonstrating successful school reintegration. However, the employment rate was only 33.3% among the limited number of adult patients available for assessment. According to statistics released by China's National Bureau of Statistics, the employment rate among healthy individuals aged 16–24 years in 2024 was approximately 84.3%. The lower employment rate observed in kidney transplant recipients may be attributed to the following potential contributors: (1) this study's single-center retrospective design and limited sample size; (2) a majority of followed patients were still pursuing education; (3) patients' and families' concerns about employment interfering with medication adherence and follow-up care; (4) employers' reservations regarding hiring kidney transplant recipients; and (5) the limited availability of suitable job opportunities for this population at the societal level.
As pediatric kidney transplant recipients age and undergo physiological maturation, various factors encountered during this developmental process may potentially impact graft function, potentially leading to graft failure (6). During adolescence, the progressive maturation of the immune system may heighten immunological responses against the allograft, particularly increasing the risk of T cell-mediated rejection (TCMR) and antibody-mediated rejection (ABMR). This heightened HLA antibody production risk may be associated with suboptimal immunosuppression or poor medication adherence. Medication adherence is particularly crucial for adolescent kidney transplant recipients (29), but there can be difficulty with medication adherence in adolescent kidney transplant recipients due to psychosocial factors, limited disease awareness, social pressures, and mental health issues. Additionally, medication side effects such as acne and hirsutism that affect physical appearance may contribute to missed doses or self-initiated dose reduction or discontinuation of immunosuppressive therapy. Adolescent patients may experience interrupted follow-up and inadequate monitoring due to educational or occupational commitments, potentially leading to delayed recognition and management of rejection episodes or complications, particularly when facing changes in healthcare locations or transplant team transitions. We observed two patients with graft failure and resumed hemodialysis during follow-up. One patient always forgot to take medication for work reasons, and the other patient went to the hospital for examination and found that creatinine had irreversibly increased due to non-adherence to follow-up appointments. This study identified non-adherence as the primary contributor to rejection episodes and graft loss, manifested as self-discontinuation of immunosuppressive medications or prolonged loss to follow-up without medical consultation. Studies have shown that while many individuals successfully adapt to post-transplant life, achieving psychological and emotional growth with life satisfaction comparable to healthy peers—often demonstrating increased motivation and energy—a subset of patients continue to perceive themselves as chronically ill, experiencing difficulties with concentration, learning challenges, and episodes of anxiety and depression (14). Therefore, adolescent patients require comprehensive psychological support from both their families and healthcare teams to prevent treatment non-adherence resulting from depression, anxiety, or rebellious behaviors.
5 Conclusions
This study demonstrates significant improvements in height attainment, pubertal development, and social outcomes following pediatric kidney transplantation. While recipient gender, pre-transplant dialysis modality, and dialysis duration showed no significant impact on NFH, both age at transplantation and height at transplantation significantly influenced NFH. These findings emphasize that early transplantation and maintaining optimal graft function are crucial for ameliorating growth delay and pubertal development, while also positively influencing long-term social outcomes in pediatric transplant recipients.
6 Limitation
This single-center retrospective study has inherent limitations including a modest sample size and insufficient follow-up duration, with the majority of participants remaining school-aged at last assessment. While our analysis demonstrates that early kidney transplantation confers physical and psychological benefits for children with ESKD, crucial long-term social outcomes—particularly employment status, marital prospects, and reproductive health—remain unassessed due to current data constraints. To comprehensively evaluate the longitudinal impacts of pediatric renal transplantation, future multi-center collaborative studies with extended follow-up periods are imperative. Such investigations will better elucidate the lifelong benefits of transplantation in this vulnerable population while addressing critical knowledge gaps in post-transition care.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Ethics committee of Shanghai Changhai Hospital approved the project after review. Ethics approval code: CHEC2024-208. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because Has signed no informed consent. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JY: Data curation, Writing – original draft, Investigation, Formal analysis. XS: Investigation, Data curation, Project administration, Writing – original draft, Methodology. YL: Data curation, Writing – original draft, Formal analysis. MS: Methodology, Formal analysis, Investigation, Writing – review & editing. JF: Project administration, Writing – original draft, Formal analysis. LZ: Methodology, Writing – review & editing, Software, Formal analysis, Data curation. YL: Resources, Formal analysis, Project administration, Supervision, Writing – review & editing. WZ: Formal analysis, Data curation, Methodology, Conceptualization, Supervision, Software, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the “Deep Blue” Nursing Research Project of Naval Medical University (2022KYG24) and the Sixth Phase Nursing Research Project of the First Affiliated Hospital of Naval Medical University (2025HLMS-A06).
Acknowledgments
We thank the patients in this study and professor Qi Chen from the Department of Statistics, Naval Medical University for statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fadel FI, Bazaraa HM, Badawy H, Morsi HA, Saadi G, Abdel Mawla MA, et al. Pediatric kidney transplantation in Egypt: results of 10-year single-center experience. Pediatr Transplant. (2020) 24(6):e13724. doi: 10.1111/petr.13724
2. Hamasaki Y, Hashimoto J, Aoki Y, Kubota M, Muramatsu M, Kawamura T, et al. Long-term social outcome after pediatric kidney transplantation: a single-center experience. Clin Exp Nephrol. (2022) 26(4):368–75. doi: 10.1007/s10157-021-02158-w
3. Zhao W-Y, Zhang L, Zhu Y-H, Chen Y, Zhu F-Y, Shen Q, et al. En bloc kidneys transplanted from infant donors less than 5 kg into pediatric recipients. Transplantation. (2014) 97(5):555–8. doi: 10.1097/01.tp.0000437174.55798.0b
4. Francis A, Johnson DW, Melk A, Foster BJ, Blazek K, Craig JC, et al. Survival after kidney transplantation during childhood and adolescence. Clin J Am Soc Nephrol. (2020) 15(3):392–400. doi: 10.2215/CJN.07070619
5. Fernandez HE, Foster BJ. Long-term care of the pediatric kidney transplant recipient. Clin J Am Soc Nephrol. (2022) 17(2):296–304. doi: 10.2215/CJN.16891020
6. Larkins NG, Wong G, Alexander SI, McDonald S, Prestidge C, Francis A, et al. Survival and transplant outcomes among young children requiring kidney replacement therapy. Pediatr Nephrol. (2021) 36(8):2443–52. doi: 10.1007/s00467-021-04945-9
7. Schwartz GJ, Mun[Combining Tilde]oz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. (2009) 20(3):629–37. doi: 10.1681/ASN.2008030287
8. Dogan CS, Durmaz E, Comak E, Uslu Gokceoglu A, Koyun M, Akman S. Effect of renal graft on longitudinal growth in prepubertal children. Exp Clin Transplant. (2013) 11(4):315–9. doi: 10.6002/ect.2012.0226
9. Juan L, Huamei M, Zhe S, Yanhong L, Hongshan C, Qiuli C, et al. Near-final height in 82 Chinese patients with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency: a single-center study from China. J Pediatr Endocrinol Metab. (2016) 29(7):841–8. doi: 10.1515/jpem-2015-0406
10. Zhang YH, Cai S, Chen ZY, Zhang Y, Jiang JN, Liu YF, et al. Research on the association between the occurrence of spermarche and menarche and psychological distress among Chinese children and adolescents aged 9–18 years. Zhonghua Liu Xing Bing Xue Za Zhi. (2023) 44(10):1545–51. doi: 10.3760/cma.j.cn112338-20230514-00298
11. Leão-Reis FC, De Carvalho Silva BDP, De Morais JDP, Santos JFG, Dias-Sanches M. Delayed graft function duration in deceased donor kidney transplants. Transplant Proc. (2022) 54(5):1247–52. doi: 10.1016/j.transproceed.2022.02.062
12. Becker JU, Seron D, Rabant M, Roufosse C, Naesens M. Evolution of the definition of rejection in kidney transplantation and its use as an endpoint in clinical trials. Transpl Int. (2022) 35:10141. doi: 10.3389/ti.2022.10141
13. Shapiro R, Sarwal MM. Pediatric kidney transplantation. Pediatr Clin North Am. (2010) 57(2):393–400. table of contents. doi: 10.1016/j.pcl.2010.01.016
14. Fijo J, Sánchez-Moreno A. Life after a pediatric kidney transplant. Nefrologia. (2023) 43(5):606–15. doi: 10.1016/j.nefroe.2023.10.006
15. Haffner D, Zivicnjak M. Pubertal development in children with chronic kidney disease. Pediatr Nephrol. (2017) 32(6):949–64. doi: 10.1007/s00467-016-3432-3
16. Tong A, Samuel S, Zappitelli M, Dart A, Furth S, Eddy A, et al. Standardised outcomes in nephrology-children and adolescents (SONG-kids): a protocol for establishing a core outcome set for children with chronic kidney disease. Trials. (2016) 17:401. doi: 10.1186/s13063-016-1528-5
17. Fine RN. Etiology and treatment of growth retardation in children with chronic kidney disease and end-stage renal disease: a historical perspective. Pediatr Nephrol. (2010) 25(4):725–32. doi: 10.1007/s00467-009-1409-1
18. Haffner D. Strategies for optimizing growth in children with chronic kidney disease. Front Pediatr. (2020) 8:399. doi: 10.3389/fped.2020.00399
19. Bonthuis M, Harambat J, Jager KJ, Vidal E. Growth in children on kidney replacement therapy: a review of data from patient registries. Pediatr Nephrol. (2021) 36(8):2563–74. doi: 10.1007/s00467-021-05099-4
20. Grohs J, Rebling R-M, Froede K, Hmeidi K, Pavičić L, Gellermann J, et al. Determinants of growth after kidney transplantation in prepubertal children. Pediatr Nephrol. (2021) 36(7):1871–80. doi: 10.1007/s00467-021-04922-2
21. Jung HW, Kim HY, Lee YA, Kang HG, Shin CH, Ha IS, et al. Factors affecting growth and final adult height after pediatric renal transplantation. Transplant Proc. (2013) 45(1):108–14. doi: 10.1016/j.transproceed.2012.07.146
22. Büyükkaragöz B, Bakkaloğlu SA, Tuncel AF, Kadıoğlu-Yılmaz B, Karcaaltıncaba D, Paşaoğlu H. Evaluation of growth in children and adolescents after renal transplantation. Turk J Pediatr. (2019) 61(2):217–27. doi: 10.24953/turkjped.2019.02.010
23. Rheda RGG, Pereira AML, Pestana JM, Koch Nogueira PC. Time from kidney failure onset to transplantation and its impact on growth in pediatric patients. Pediatr Transplant. (2023) 27(5):e14507. doi: 10.1111/petr.14507
24. Anand A, Malik TH, Dunson J, McDonald MF, Christmann CR, Galvan NTN, et al. Factors associated with long-term graft survival in pediatric kidney transplant recipients. Pediatr Transplant. (2021) 25(4):e13999. doi: 10.1111/petr.13999
25. Harambat J, Bonthuis M, van Stralen KJ, Ariceta G, Battelino N, Bjerre A, et al. Adult height in patients with advanced CKD requiring renal replacement therapy during childhood. Clin J Am Soc Nephrol. (2014) 9(1):92–9. doi: 10.2215/CJN.00890113
26. Ferraris J, Saenger P, Levine L, New M, Pang S, Saxena BB, et al. Delayed puberty in males with chronic renal failure. Kidney Int. (1980) 18(3):344–50. doi: 10.1038/ki.1980.144
27. Winkler L, Offner G, Krull F, Brodehl J. Growth and pubertal development in nephropathic cystinosis. Eur J Pediatr. (1993) 152(3):244–9. doi: 10.1007/BF01956154
28. Hebert SA, Swinford RD, Hall DR, Au JK, Bynon JS. Special considerations in pediatric kidney transplantation. Adv Chronic Kidney Dis. (2017) 24(6):398–404. doi: 10.1053/j.ackd.2017.09.009
Keywords: end-stage kidney disease, pediatric kidney transplantation, graft survival, long-term outcomes, near-final height
Citation: Yu J, Sheng X, Li Y, Sui M, Fu J, Zeng L, Li Y and Zhao W (2025) Long-term outcomes and influencing factors following pediatric kidney transplantation: a single-center cohort study from China. Front. Pediatr. 13:1599111. doi: 10.3389/fped.2025.1599111
Received: 24 March 2025; Accepted: 22 May 2025;
Published: 5 June 2025.
Edited by:
Pierluigi Marzuillo, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Sadaf Aba Umer Kodwavwala, Sindh Institute of Urology and Transplantation, PakistanAshton Chen, Wake Forest University, United States
Copyright: © 2025 Yu, Sheng, Li, Sui, Fu, Zeng, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanhua Li, eWFuaHVhMjAwNDAzQDE2My5jb20=; Wenyu Zhao, end5aXN5QGFsaXl1bi5jb20=
†These authors share first authorship
 Junhao Yu
Junhao Yu Xiaoju Sheng†
Xiaoju Sheng† Yuhong Li
Yuhong Li Mingxing Sui
Mingxing Sui Jiazhao Fu
Jiazhao Fu Li Zeng
Li Zeng Wenyu Zhao
Wenyu Zhao