- 1Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
- 2Division of Dermatology, Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada
Benzoyl peroxide is a widely used and effective topical treatment for acne vulgaris, particularly in pediatric and adolescent populations. Despite its established safety profile, recent concerns emerged regarding its potential to decompose into benzene, a known carcinogen, under specific environmental conditions, like elevated temperatures and exposure to ultraviolet radiation. In this paper we review the management of acne vulgaris in pediatric patients, examine the evidence supporting benzoyl peroxide use and explore the recent studies evaluating the association between benzoyl peroxide use and malignancy risk. While initial reports raised alarm over benzene formation, subsequent investigations have not demonstrated an increased risk of hematologic malignancies. Here, we assess the strengths and limitations of existing evidence and identify future research priorities. Additionally, we provide recommendations for the safe and evidence-based use of benzoyl peroxide in pediatric acne treatment.
Introduction
Acne vulgaris is a common inflammatory disorder of the pilosebaceous unit with an estimated prevalence ranging from 26.8% to 96% globally (1). Although acne vulgaris can present in all age groups, the peak incidence occurs during the teen years, affecting up to 85% of adolescents and young adults (2, 3). The inflammatory manifestations of acne can also damage skin, leading to disfiguring scars and pigmentation changes. Consequently, acne vulgaris is often frustrating for pediatric patients and has been linked to mood disorders, social isolation, and impaired health-related quality of life (HRQoL) (4, 5). Given the high burden worldwide, it is necessary to encourage awareness around therapeutics used to address acne in younger populations.
One of the mainstay treatments for acne vulgaris is benzoyl peroxide (BPO). Known for its safety, minimal irritation at low concentrations (2.5%–5.0%), and efficacy in reducing Cutibacterium acnes, BPO is widely available in over the counter (OTC) and prescribed formulations. However, recent studies raised safety concerns, suggesting BPO in skin care products can decompensate into benzene, a recognized carcinogen (6). Given the significance of BPO in acne treatment, we sought to investigate this topic further. This perspective synthesizes current evidence on the safety of BPO, including concerns about benzene formation and studies that challenge this risk, and outlines future research directions alongside recommendations for pediatric acne management.
Acne vulgaris in the pediatric population
Acne lesions can be divided into “non-inflammatory” open and closed comedones, or “inflammatory” papules, pustules, nodules, and cysts. The pathogenesis of acne is multifactorial and traditionally involves a complex interplay of four main factors: (1) excessive sebum production, (2) hyperkeratinization of pilosebaceous follicles, (3) bacterial colonization of sebaceous follicles with C. acnes and (4) inflammation. In the initial stages of comedone formation, it is suggested that a hyperkeratotic plug forms and acts as a bottleneck for the accumulation of shed keratin and sebum, which clogs follicles. Androgen hormones and insulin-like growth factor 1 are thought to increase sebum secretion, which may explain why acne emerges during the pre-adolescence phase (7). C. acnes colonization can upregulate innate immune responses and prime a pro-inflammatory milieu, that worsens with comedo rupture (8, 9). In nodular or cystic acne formation, there is pronounced inflammation, which can lead to more painful, slow healing lesions.
Acne vulgaris typically first appears between the ages of 12 and 24. When acne develops before puberty, it is categorized into different age groups. Neonatal acne arises within the first 2–3 weeks of life and is relatively common, affecting 20% of newborns (10). It resolves on its own by 3 months of age and is believed to result from an inflammatory response to Malassezia, a yeast that is a component of the normal skin microbiome. Infantile acne onsets between 1 and 12 months of age. It tends to be less prevalent, and includes comedomes, papulopustules and nodules seen in classic acne. Interestingly, infantile acne may reflect physiologic elevations of androgen levels in infants and these patients often have a family history of severe acne (10). Mid-childhood acne, occurring between 1 and 7 years of age, is quite rare. Since this age group lacks a physiologic source of androgens, hyperandrogenism should be ruled out as a cause (11). Pre-adolescent acne emerges between 7 and 12 years of age and is suggested to be more common now due to the trend for earlier onset of puberty (12).
Current therapeutics for acne vulgaris
Mild to moderate acne is commonly managed with topical therapies. First-line treatment is a topical retinoid to reduce sebum production and regulate ductal keratinocyte growth (13). BPO is strongly recommended as it was shown to reduce both inflammatory and noninflammatory acne lesions (14, 15). Topical antibiotics, like erythromycin and clindamycin, are utilized to reduce inflammation and colonization with C. acnes (16). However, rising antibiotic resistance has limited the standalone effectiveness and poses broader public health risks (17). To mitigate resistance, BPO is suggested to be used with antibiotics concomitantly (18). Overall, multimodal regimens remain the standard of care (16).
Moderate-to-severe acne can be treated systemically. This involves the use of oral tetracyclines, primarily doxycycline or minocycline. Hormonal agents, like combined oral contraceptives and anti-androgen medications, can be used in individuals that have hormonally responsive acne, but have a multitude of side effects to take into consideration due to its systemic nature. Oral isotretinoin is known for its excellent efficacy in treating severe, treatment refractory nodulocystic acne (19, 20).
For infantile or pre-adolescent acne vulgaris, these conditions are managed with topical antibiotics, BPO and retinoids. Oral tetracyclines may be prescribed for acne in pediatric patients aged 9 years and older. However, caution is warranted as tetracyclines can bind to calcium in developing teeth, causing permanent discoloration (21, 22). If severe, isotretinoin can be used, but it would be off-label as it is not Food and Drug Administration (FDA)-approved for treatment of acne in children under 12 years of age. Moreover, hormonal therapies are not indicated for preadolescent acne.
Benzoyl peroxide in treating pediatric acne
BPO reduces bacterial colonization of the skin and possesses mild keratolytic effects. Its antimicrobial properties stem from the release of free oxygen radicals that disrupt protein function in cellular membranes (23), reducing the survival of common cutaneous bacteria and yeasts, including C. acnes, Staphylococcus epidermidis, and Malassezia spp (24). As emphasized earlier, antibiotic resistance in C. acnes continues to rise with antibiotic use for acne. In contrast, BPO remains effective without reported resistance. Side effects of BPO include burning sensation, dryness, erythema, peeling, irritation, and bleaching of hair and clothes. However, BPO is a dose-dependent skin irritant; therefore, lower-concentration formulations and wash-off product options are better tolerated as observed in patients 12 years and older (14). It is important to note that BPO's bactericidal properties are not concentration dependent (14).
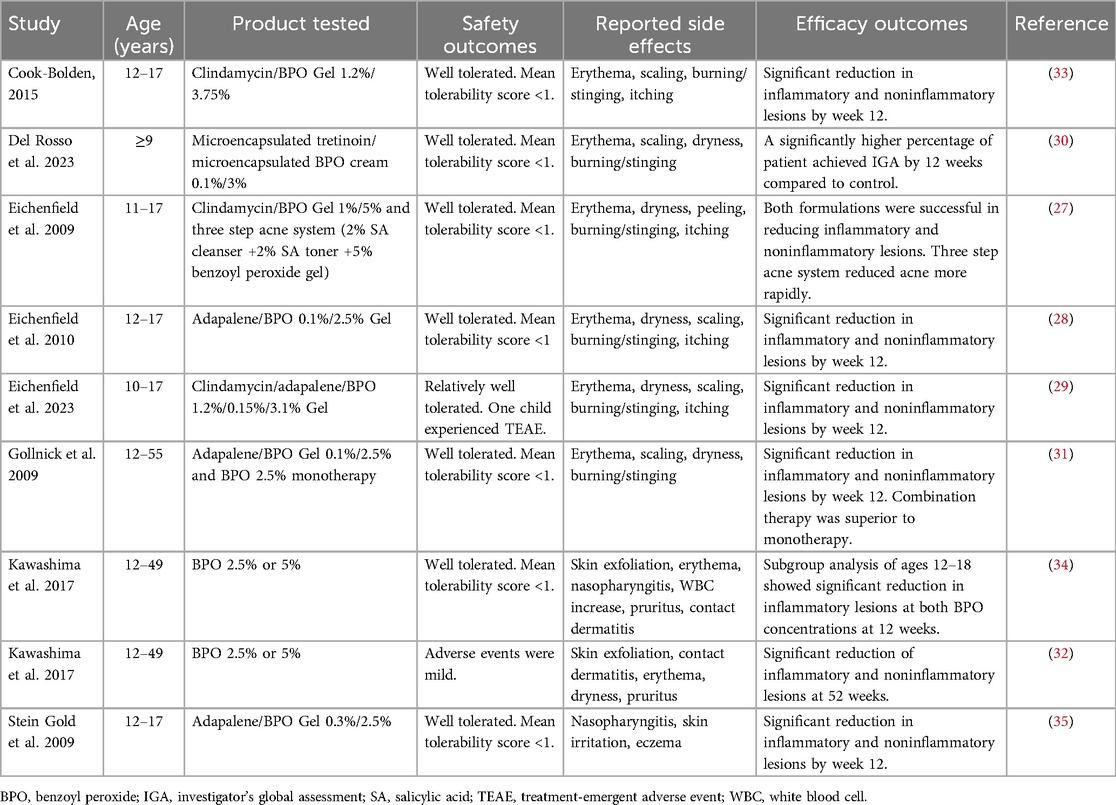
While BPO is widely recommended by both North American and European guidelines (16, 25, 26), the majority of literature focuses on adults and data on pediatric populations are limited (Table 1). Eichenfield and colleagues conducted multiple studies in patients with ages ranging from 10 to 17 years old with acne vulgaris, evaluating the safety and efficacy of BPO in combination with topical retinoids or antibiotics (27–29). Their findings demonstrate that these regimens are generally well tolerated, with minimal adverse events. Consistent results have been observed across additional studies in both preadolescent and adolescent populations, supporting BPO, whether in combination therapy or as monotherapy, as a safe and effective treatment modality in this age group (30–35). Additionally, the skin microbiome of preadolescent females (7–12 years) was profiled following daily 4% benzoyl peroxide wash for 4–8 weeks (36). BPO use was reported to be associated with decreased acne lesions without significant changes in skin microbial diversity (36).
However, the FDA labelling for various BPO products state the safety and effectiveness in children below the age of 12 have not been established. Definitive evidence to guide optimal BPO management in this age group remains lacking. In practice, clinicians often extrapolate from studies in older populations, leading to the off-label use of BPO in children under 12 years of age. In addition to topical retinoid or antibiotics, there are no absolute contraindications to the use BPO in this population. However, the main concerns include a heightened risk of local irritation. When using off-label treatments in younger children, clinical judgment and close monitoring are essential. A prudent approach includes initiating therapy with the lowest effective concentration and avoiding more irritating formulations, like BPO and retinoid combinations. Strategies to minimize irritation may involve alternate-day or short-contact application during the initiation phase, with gradual titration based on tolerability. Earlier follow up at 2–3 weeks may be appropriate to assess response and tolerance. It is critical to provide the patient and their caregiver education regarding potential side effects and appropriate product use.
When comparing to other treatment regimes, BPO has demonstrated its effectiveness with, typically mild and dose-dependent side effects. Systemic absorption is minimal (37) and allergic reactions are rare. In contrast, while topical retinoids are also first-line agents for comedonal and mixed acne, they may cause more frequent irritation and require additional counseling on sun protection. Topical antibiotics are less preferred as monotherapy due to resistance concerns and should always be combined with BPO. Oral antibiotics are reserved for more severe acne but should be used judiciously due to the systemic impacts.
Current developments regarding benzoyl peroxide for acne vulgaris
BPO can thermally decompose into benzene, which is a known carcinogen (6). Valisure, an independent testing laboratory based in Connecticut, found that some common OTC BPO products may contain alarmingly high levels of benzene when incubated for days to weeks at elevated temperatures. This was found to be a problem with the intrinsic stability of BPO during delivery or storage of the product rather than an issue with contamination (6, 25). These findings were the reason behind the Citizen Petition filed to the U.S. FDA on March 5th, 2024 (38). In the petition, they called for the recall and suspension of sale of BPO-containing products, along with further investigations and newly updated guidelines around these products (38).
This is particularly relevant since in 2011 the FDA identified BPO as a tumor promoter in animals when combined with a chemical initiator (50). Although animal studies may not reflect human use conditions. Soon after Valisure filed their Citizen Petition, organizations such as the American Academy of Dermatology and the American Acne & Rosacea Society published response statements emphasizing the importance of public health and safety while waiting for further guidance from the FDA (39, 40). Many leading dermatologists published commentaries of their own, calling for more data on BPO testing and transparent independent laboratory verification (41). There were also concerns raised around Valisure's methodology. Although in their study an incubation temperature of 37°C was used to simulate standard body temperature, 50°C to stimulate shelf-life performance at an accelerated stability testing temperature, and 70°C to stimulate transportation/passenger vehicle excursion, BPO products are often stored at room-temperature or are refrigerated.
Over the next several months, various studies using different databases and surveys began publishing findings to help clarify the safety profile of BPO in the real world. Given benzene can induce oxidative damage and genetic and epigenetic modifications in hematopoietic stem cells, hematologic malignancies were a main focus in the BPO safety studies (39). The population studied were 12 years and older.
The first study was done by Veenstra et al. (42). They used Cosmos, which is a dataset created through a community collaboration of health systems on Epic. The study looked at over 2.3 million patients with acne and compared the prevalence of acute myeloid leukemia (AML) in patients with acne with a BPO prescription and those without. Since many patients use OTC BPO products that are not captured in electronic medical records, they also compared AML diagnosis in patients with acne vs. those without acne. The study observed decreased odds of AML in patients with acne with a BPO prescription or reported BPO use, or in patients with acne when compared to the general population to account for potential OTC use, stratified by age (42). The paper was criticized for its methodology due to the primary analysis not properly adjusting for confounders such as age, sex, race and ethnicity, smoking status, and other comorbidities, leading to significant bias. There was also criticism related to the degree of BPO exposure and follow up time, along with the poor methodology used to account for OTC BPO use (43). In response, Veenstra et al. reanalyzed the data after adjusting for age and sex, and did not find any significant differences in odds ratios, but did note that their analysis had several limitations (42).
Although there were issues regarding the Veenstra study, other more robust studies were published during the weeks following that all reported no evidence between BPO use and an increased risk of malignancy. Sadr et al. used the National Health and Nutrition Examination Survey to exact-match 79 individuals who reported BPO use with control individuals who did not use BPO and did not have acne (44). They found no association between BPO use and an increase in the detectable levels of benzene in the blood (44).
Afterwards, there were additional studies using the TriNetX US Collaborative Network, a database with over 132 million patients, to conduct more rigorous analyses. These studies found that patients with acne who received a BPO prescription, as well as patients with acne overall, did not have an increased risk of hematologic malignancies compared to a matched control group (45, 46).
Although these studies had robust statistical approaches, there were several limitations. The degree of exposure was not quantified, meaning there is a significant degree of unaccounted for variability that could have biased the results. Indeed, reported use of BPO among patients likely reflects inconsistencies and a broad range of exposures, from single applications to sustained, long-term use over several years. OTC BPO use was also not accounted for. These studies tried to control for it by comparing patients with acne to a comparator group, such as patients with melanocytic nevi or viral warts, but there are significant limitations with this approach. Firstly, not all patients with acne see a physician to get treatment for it, especially given the current barriers to access. In an online survey, only 25% of teenagers with acne have visited a doctor, with only 52% adhering to their acne treatment (47). This suggests most individuals with acne are categorized incorrectly within these databases. These patients are also more likely to use OTC products to manage their acne, including OTC BPO, meaning there is a significant degree of confounding variables which was not adjusted for. There was also no mention of excluding patients with both acne and the comparator characteristic, which is another potential confounding variable that likely did affect the results. These studies found there was a lower risk of hematologic malignancies with patients coded as having acne within these databases, which is contrary to the expected finding. Also, the risk with benzene depends on cumulative exposure, meaning the risk of adverse health events depends on dose and time. Longer-term follow up in these studies would allow for a better understanding of the risk of benzene exposure in BPO-containing products, along with exploring whether or not BPO increases the risk of other types of malignancies (48).
Valisure more recently published a paper where they tested 111 OTC BPO drug products at room temperature shortly after being acquired off the shelf. Of the 111 BPO products, 38 were above the conditionally restricted FDA limit of 2 ppm for benzene in drug products (49). Levels ranged from 0.16% ± 6% ppm to 35.30% ± 2% ppm, and product age did not seem to affect the levels of benzenes found in the product (49). Stability testing of a prescription encapsulated BPO product revealed no detectable benzene at 2°C (35.6°F), but a high level of formation at 50°C(122°F), suggesting encapsulation may not mitigate thermal degradation, although cold storage may prevent it. Ultraviolet exposure, even at one-third the intensity of peak sunlight, also induced substantial benzene formation (49).
On March 11th 2025, the FDA had announced that they indeed found benzene contamination in a number of BPO acne products, leading to voluntary recalls (46). Reassuringly, more than 90% of tested products had undetectable or extremely low levels of benzene (46). The full results of FDA testing is yet to be published.
Recommendations for benzoyl peroxide usage
Considering the findings, it is prudent to act cautiously. As research continues to be done regarding the safety of BPO drug products, patient care should continue to be the priority. Moreover, alternative options, such as oral antibiotics or isotretinoin in young patients, have their own risks associated with them (38). While the FDA continues their investigation, the focus should be to minimize any potential risks that might arise from the use of BPO in skin products. This includes educating young patients and their families about safe storage practices, such as keeping products refrigerated, or in an environment not exposed to direct sunlight (49). Instructing patients to move their BPO drug products to a cooler location while showering can also help reduce the risk of benzene formation. Cold chain storage and transportation to retailers or pharmacies needs to be standard practice, and when delivering BPO-containing products, the temperature of the cargo hold should be regularly monitored and upward fluctuations in temperature minimized (43). Batches of products should be regularly inspected throughout the supply chain to ensure benzene levels stay below recommended limits.
Healthcare providers can also counsel pediatric patients on reducing the amount of time BPO spends in contact with the skin and need for sun avoidance and sunscreen to minimize the amount of ultraviolet-initiated benzene formation. Unfortunately, there are no current studies that evaluate if the introduction to benzene-containing products early in life raises unique concerns relative to exposure later in life. With most BPO-containing drug products having acceptable levels of benzene and mounting evidence suggesting that BPO is not associated with increased malignancy risk, both patients and providers need to remain up-to-date regarding BPO's safety profile in acne management and treatment so decisions regarding its use can be made in an evidence-informed manner.
Conclusion
Acne is a pervasive and often difficult-to-treat condition that commonly affects pediatric and adolescent patients. Thus there exists an imperative to ensure that any future decisions regarding BPO are made in a well-informed and methodical manner. Although the recent findings raise significant concerns around the presence of benzenes in BPO drug products, more research and evidence are needed before any conclusions can be drawn. Research using a variety of different perspectives and methodologies, done in a transparent and rigorous manner, will be crucial to inform next steps regarding the future of BPO. Additionally, many BPO products are marketed to young audiences and prescribed “off-label” to pre-adolescent patients; however, their safety concerning benzene formation has not been studied. As the FDA continues to assess the situation, various measures can be enacted to ensure patient management and safety is prioritized. This includes patient education around proper storage practices, safer transportation and quality assurance measures, and a larger emphasis on risk reduction, such as by explaining to patients the importance of minimizing sunlight exposure when BPO is applied on the skin. It is crucial to remain pragmatic while minimizing any sensationalism that might arise as we wait to see what future steps may be.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SC: Writing – review & editing, Writing – original draft. KY: Writing – review & editing, Writing – original draft. FJ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BPO, benzoyl peroxide; OTC, over the counter; FDA, Food and Drug Administration; AML, acute myeloid leukemia.
References
1. Heng AHS, Chew FT. Systematic review of the epidemiology of acne vulgaris. Sci Rep. (2020) 10(1):5754. doi: 10.1038/s41598-020-62715-3
2. Wolkenstein P, Machovcova A, Szepietowski JC, Tennstedt D, Veraldi S, Delarue A. Acne prevalence and associations with lifestyle: a cross-sectional online survey of adolescents/young adults in 7 European countries. J Eur Acad Dermatol Venereol. (2018) 32(2):298–306. doi: 10.1111/jdv.14475
3. White GM. Recent findings in the epidemiologic evidence, classification, and subtypes of acne vulgaris. J Am Acad Dermatol. (1998) 39(2):S34–S7. doi: 10.1016/S0190-9622(98)70442-6
4. Morshed ASM, Noor T, Uddin Ahmed MA, Mili FS, Ikram S, Rahman M, et al. Understanding the impact of acne vulgaris and associated psychological distress on self-esteem and quality of life via regression modeling with CADI, DLQI, and WHOQoL. Sci Rep. (2023) 13(1):21084. doi: 10.1038/s41598-023-48182-6
5. Fabbrocini G, Cacciapuoti S, Monfrecola G. A qualitative investigation of the impact of acne on health-related quality of life (HRQL): development of a conceptual model. Dermatol Ther (Heidelb). (2018) 8(1):85–99. doi: 10.1007/s13555-018-0224-7
6. Kucera K, Zenzola N, Hudspeth A, Dubnicka M, Hinz W, Bunick CG, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. (2024) 132(3):37702. doi: 10.1289/EHP13984
7. Stewart ME, Downing DT, Cook JS, Hansen JR, Strauss JS. Sebaceous gland activity and serum dehydroepiandrosterone sulfate levels in boys and girls. Arch Dermatol. (1992) 128(10):1345–8. doi: 10.1001/archderm.1992.01680200055006
8. Jahns AC, Lundskog B, Ganceviciene R, Palmer RH, Golovleva I, Zouboulis CC, et al. An increased incidence of propionibacterium acnes biofilms in acne vulgaris: a case-control study. Br J Dermatol. (2012) 167(1):50–8. doi: 10.1111/j.1365-2133.2012.10897.x
9. Mias C, Mengeaud V, Bessou-Touya S, Duplan H. Recent advances in understanding inflammatory acne: deciphering the relationship between cutibacterium acnes and Th17 inflammatory pathway. J Eur Acad Dermatol Venereol. (2023) 37(S2):3–11. doi: 10.1111/jdv.18794
10. Eichenfield LF, Krakowski AC, Piggott C, Del Rosso J, Baldwin H, Friedlander SF, et al. Evidence-based recommendations for the diagnosis and treatment of pediatric acne. Pediatrics. (2013) 131(S3):S163–S86. doi: 10.1542/peds.2013-0490B
11. Shope C, Ritter A, Matlock K, Wine Lee L. Pathologic acne in pre-pubertal children: a case series and review on when to refer to pediatric endocrinology. Pediatr Dermatol. (2023) 40(1):5–10. doi: 10.1111/pde.15166
12. Goldberg JL, Dabade TS, Davis SA, Feldman SR, Krowchuk DP, Fleischer AB. Changing age of acne vulgaris visits: another sign of earlier puberty? Pediatr Dermatol. (2011) 28(6):645–8. doi: 10.1111/j.1525-1470.2011.01643.x
13. Kolli SS, Pecone D, Pona A, Cline A, Feldman SR. Topical retinoids in acne vulgaris: a systematic review. Am J Clin Dermatol. (2019) 20(3):345–65. doi: 10.1007/s40257-019-00423-z
14. Mills OH Jr, Kligman AM, Pochi P, Comite H. Comparing 2.5%, 5%, and 10% benzoyl peroxide on inflammatory acne vulgaris. Int J Dermatol. (1986) 25(10):664–7. doi: 10.1111/j.1365-4362.1986.tb04534.x
15. Lookingbill DP, Chalker DK, Lindholm JS, Katz HI, Kempers SE, Huerter CJ, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. (1997) 37(4):590–5. doi: 10.1016/S0190-9622(97)70177-4
16. Reynolds RV, Yeung H, Cheng CE, Cook-Bolden F, Desai SR, Druby KM, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. (2024) 90(5):1006.e1–e30. doi: 10.1016/j.jaad.2023.12.017
17. Dessinioti C, Katsambas A. Propionibacterium acnes and antimicrobial resistance in acne. Clin Dermatol. (2017) 35(2):163–7. doi: 10.1016/j.clindermatol.2016.10.008
18. Leyden JJ, Wortzman M, Baldwin EK. Antibiotic-resistant propionibacterium acnes suppressed by a benzoyl peroxide cleanser 6%. Cutis. (2008) 82(6):417–21.19181031
19. Akman A, Durusoy C, Senturk M, Koc CK, Soyturk D, Alpsoy E. Treatment of acne with intermittent and conventional isotretinoin: a randomized, controlled multicenter study. Arch Dermatol Res. (2007) 299(10):467–73. doi: 10.1007/s00403-007-0777-2
20. Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. (2006) 54(4):644–6. doi: 10.1016/j.jaad.2005.11.1061
21. Enabulele JE, Chukwumah NM, Enabulele O. Tetracycline use in children and knowledge of its oral implications among nursing mothers. Pediatr Dent J. (2020) 30(3):224–30. doi: 10.1016/j.pdj.2020.08.003
22. Sánchez AR, Rogers RS, Sheridan PJ. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity. Int J Dermatol. (2004) 43(10):709–15. doi: 10.1111/j.1365-4632.2004.02108.x
23. Nacht S, Yeung D, Beasley JN, Anjo MD, Maibach HI. Benzoyl peroxide: percutaneous penetration and metabolic disposition. J Am Acad Dermatol. (1981) 4(1):31–7. doi: 10.1016/S0190-9622(81)70004-5
24. Zhou L, Chen L, Liu X, Huang Y, Xu Y, Xiong X, et al. The influence of benzoyl peroxide on skin microbiota and the epidermal barrier for acne vulgaris. Dermatol Ther. (2022) 35(3):e15288. doi: 10.1111/dth.15288
25. Baldwin H, Elewski B, Hougeir F, Yamauchi P, Levy-Hacham O, Hamil K, et al. Sixty years of benzoyl peroxide use in dermatology. J Drugs Dermatol. (2023) 22(1):54–9. doi: 10.36849/JDD.7150
26. Nast A, Dréno B, Bettoli V, Bukvic Mokos Z, Degitz K, Dressler C, et al. European evidence-based (S3) guideline for the treatment of acne—update 2016—short version. J Eur Acad Dermatol Venereol. (2016) 30(8):1261–8. doi: 10.1111/jdv.13776
27. Eichenfield LF, Thiboutot D, Shalita A, Swinyer L, Tanghetti E, Tschen E, et al. A three-step acne system containing solubilized benzoyl peroxide versus benzoyl peroxide/clindamycin in pediatric patients with acne. J Clin Aesthet Dermatol. (2009) 2(11):21–6.20725576
28. Eichenfield LE, Jorizzo JL, Dirschka T, Taub AF, Lynde C, Graeber M, et al. Treatment of 2,453 acne vulgaris patients aged 12–17 years with the fixed-dose Adapalene-benzoyl peroxide combination topical gel: efficacy and safety. J Drugs Dermatol. (2010) 9(11):1395–402.21061762
29. Eichenfield LF, Stein Gold L, Kircik LH, Werschler WP, Beer K, Draelos ZD, et al. Triple-combination clindamycin phosphate 1.2%/benzoyl peroxide 3.1%/Adapalene 0.15% gel for moderate-to-severe acne in children and adolescents: randomized phase 2 study. Pediatr Dermatol. (2023) 40(3):452–9. doi: 10.1111/pde.15283
30. Del Rosso J, Sugarman J, Green L, Lain T, Levy-Hacham O, Mizrahi R, et al. Efficacy and safety of microencapsulated benzoyl peroxide and microencapsulated tretinoin for the treatment of acne vulgaris: results from two phase 3 double-blind, randomized, vehicle-controlled studies. J Am Acad Dermatol. (2023) 89(4):719–27. doi: 10.1016/j.jaad.2023.05.093
31. Gollnick HP, Draelos Z, Glenn MJ, Rosoph LA, Kaszuba A, Cornelison R, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. (2009) 161(5):1180–9. doi: 10.1111/j.1365-2133.2009.09209.x
32. Kawashima M, Nagare T, Katsuramaki T. Open-label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long-term use in patients with acne vulgaris: a secondary publication. J Dermatol. (2017) 44(6):635–43. doi: 10.1111/1346-8138.13741
33. Cook-Bolden FE. Efficacy and tolerability of a fixed combination of clindamycin phosphate (1.2%) and benzoyl peroxide (3.75%) aqueous gel in moderate or severe adolescent acne vulgaris. J Clin Aesthet Dermatol. (2015) 8(5):28–32.26029332
34. Kawashima M, Sato S, Furukawa F, Matsunaga K, Akamatsu H, Igarashi A, et al. Twelve-week, multicenter, placebo-controlled, randomized, double-blind, parallel-group, comparative phase II/III study of benzoyl peroxide gel in patients with acne vulgaris: a secondary publication. J Dermatol. (2017) 44(7):774–82. doi: 10.1111/1346-8138.13798
35. Gold LS, Werschler WP, Mohawk J. Adapalene/benzoyl peroxide gel 0.3%/2.5%: effective acne therapy regardless of age or gender. J Drugs Dermatol. (2017) 16(6):582–9.28686776
36. Ahluwalia J, Borok J, Haddock ES, Ahluwalia RS, Schwartz EW, Hosseini D, et al. The microbiome in preadolescent acne: assessment and prospective analysis of the influence of benzoyl peroxide. Pediatr Dermatol. (2019) 36(2):200–6. doi: 10.1111/pde.13741
37. Yeung D, Nacht S, Bucks D, Maibach HI. Benzoyl peroxide: percutaneous penetration and metabolic disposition. II. Effect of concentration. J Am Acad Dermatol. (1983) 9(6):920–4. doi: 10.1016/S0190-9622(83)70209-4
38. Valisure. Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. Connecticut: Valisure (2024). Available online at: https://www.regulations.gov/document/FDA-2024-P1130-0001 (Accessed March 24, 2025).
39. Homiston S, Saunders R. Academy Response to Benzene Petition on Benzoyl Peroxide Products [Press Release]. Rosemont: American Academy of Dermatology (2024). Available online at: https://www.aad.org/news/benzene-benzoyl-peroxide2024 (Accessed March 24, 2025).
40. Response Statement from the American Acne & Rosacea Society to the Valisure Citizen Petition on Benzene in Benzoyl Peroxide Drug Products. New Jersey: American Acne Rosacea Society (2024). Available online at: https://acneandrosacea.org/uncategorized/american-acne-rosacea-society-aars-responds-to-citizen-petition-submitted-by-valisure-regarding-benzene-in-benzoyl-peroxide-drug-products/ (Accessed March 24, 2025).
41. Del Rosso JQ. Benzoyl peroxide, benzene, and lots of unanswered questions: where are we now? Cutis. (2024) 114(1):3–4. doi: 10.12788/cutis.1043
42. Veenstra J, Ozog D. Benzoyl peroxide use in acne therapy: evaluating the association with acute myeloid leukemia risk. J Am Acad Dermatol. (2024) 91(3):533–4. doi: 10.1016/j.jaad.2024.04.064
43. Barbieri JS. Response to veenstra et al’s “benzoyl peroxide use in acne therapy: evaluating the association with acute myeloid leukemia risk”. J Am Acad Dermatol. (2024) 91(4):e109–e10. doi: 10.1016/j.jaad.2024.05.090
44. Sadr N, Troger A, Chai PR, Barbieri JS. No evidence for an association between benzoyl peroxide use and increased blood benzene levels in the national health and nutrition examination survey. J Am Acad Dermatol. (2024) 91(4):763–5. doi: 10.1016/j.jaad.2024.06.050
45. Garate D, Thang CJ, Lai J, Golovko G, Wilkerson MG, Barbieri JS. Benzoyl peroxide for acne treatment is not associated with an increased risk of malignancy: a retrospective cohort study. J Am Acad Dermatol. (2024) 91(5):966–8. doi: 10.1016/j.jaad.2024.07.011
46. Chan R, Roster K, Neubauer Z, Hill RC, Lipner SR. No association of benzoyl peroxide use with acute myeloid leukemia and hematologic malignancies in a multi-center retrospective study. J Am Acad Dermatol. (2024) 91(6):1234–6. doi: 10.1016/j.jaad.2024.07.1504
47. Impact of acne on teens: results from harris interactive poll. J Am Acad Dermatol. (2009) 60(3):AB18.
48. Yang K, Czyz S, Zuberi R, Kreutz J, Bunick CG, Jafarian F. A disproportionality analysis on benzoyl peroxide and its risk of malignancy using the FDA adverse event reporting system. J Invest Dermatol. (2025) S0022–202X(25):00300–8. doi: 10.1016/j.jid.2025.02.139
49. Kucera K, Zenzola N, Hudspeth A, Dubnicka M, Hinz W, Bunick CG, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. (2024) 145(5):1147–1154.e11. doi: 10.1016/j.jid.2024.09.009
50. Food and Drug Administration, HHS. Classification of benzoyl peroxide as safe and effective and revision of labeling to drug facts format. Federal Register (2010). Available online at: https://www.federalregister.gov/documents/2010/03/04/2010-4424/classification-of-benzoyl-peroxide-as-safe-and-effective-and-revision-of-labeling-to-drug-facts (Accessed March 12, 2025).
Keywords: acne vulgaris, benzoyl peroxide, benzene, pediatric, safety, public health, dermatology, neoplasm
Citation: Czyz S, Yang K and Jafarian F (2025) Benzoyl peroxide in the treatment of acne: are there potential health concerns?. Front. Pediatr. 13:1599491. doi: 10.3389/fped.2025.1599491
Received: 25 March 2025; Accepted: 7 July 2025;
Published: 21 July 2025.
Edited by:
Florence Carrouel, Université Claude Bernard Lyon 1, FranceReviewed by:
Fifa Argentina, Sriwijaya University, IndonesiaCopyright: © 2025 Czyz, Yang and Jafarian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fatemeh Jafarian, RmF0ZW1laC5qYWZhcmlhbkB1Y2FsZ2FyeS5jYQ==
†These authors have contributed equally to this work
 Sonia Czyz1,†
Sonia Czyz1,† Kerry Yang
Kerry Yang Fatemeh Jafarian
Fatemeh Jafarian