- 1Department of Prenatal Diagnosis, Reproductive Medicine Center, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China
- 2Department of Neurosurgery, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, China
- 3Department of Computer Science and Information Technologies, Elviña Campus, University of A Coruña, A Coruña, Spain
- 4Center for Reproductive Medicine, First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
Background: Congenital heart anomalies (CHAs) are the most prevalent birth defects, significantly impacting pediatric populations and healthcare systems worldwide. This study provides a comprehensive analysis of the global burden of CHAs, focusing on sex-stratified trends across Socio-Demographic Index (SDI) quintiles from 1990 to 2021.
Methods: Utilizing data from the Global Burden of Disease (GBD) study, we assessed sex-disaggregated metrics including prevalence, incidence, mortality, disability-adjusted life years (DALYs), years of life lost (YLLs), and years lived with disability (YLDs) for CHAs in 21 GBD regions and 204 countries.
Results: CHA prevalence and incidence remained stable, with higher incidence in lower SDI regions. Mortality rates, age-standardized DALYs, and YLLs consistently decreased, with males showing higher rates, particularly in low SDI areas. YLDs increased in non-high SDI regions, indicating a growing disability burden. Globally, age-standardized prevalence rates showed a downward trend in countries like China and Ethiopia, while an upward trend was seen in Turkmenistan and Argentina. Incidence rates generally decreased, except in Spain and France. Age-standardized death rates nearly universally declined. YLDs increased in over half of the nations, including Belgium and Spain. Correlation analysis revealed increasing trends of prevalence and YLDs with rising SDI, while incidence, deaths, DALYs, and YLLs demonstrated a declining trend with SDI elevation. From 2022 to 2050, the prevalence of congenital heart anomalies is projected to rise significantly, especially in the 0−4 age group, while mortality rates are expected to continue a slow downward trend, particularly in the 20−69 age group.
Conclusions: The burden of CHAs is influenced by sex and SDI, with a projected increase in prevalence and a continued decline in mortality. The increasing trend of YLDs highlights the need for targeted public health strategies to address the growing disability burden and ensure global health equity.
Introduction
Globally, congenital heart anomalies (CHA) represent a significant burden of disease, contributing to substantial morbidity and mortality, particularly among pediatric populations (1–3). In 2019, there were 8.52 million cases and 0.55 million fatalities attributed to congenital birth defects, with CHAs constituting the predominant group globally (4). The impact of CHA extends beyond individual health, affecting families, healthcare systems, and society at large (5–7). The burden of CHA is not uniformly distributed, it exhibits sex disparities, age variations, and significant regional and national differences (8). Understanding the uneven distribution of CHA across different Socio-demographic Index (SDI) regions, sex, and age groups is crucial for developing targeted interventions to address these disparities (9).
CHAs are the most common type of birth defect, and their management is a critical component of global child health initiatives (10). International policies, such as the Sustainable Development Goals (SDG) set by the World Health Organization (WHO), aim to reduce neonatal and under-five mortality rates (11), which are directly influenced by the prevalence and outcomes of CHA. Efforts to improve prenatal care, screening, and access to pediatric cardiac care are essential in mitigating the disease burden (12, 13). The prevention of congenital birth defects necessitates a multifaceted approach that encompasses prenatal screening, nutritional support, vaccination programs, and the avoidance of detrimental exposures (14). Prenatal screening, which includes ultrasound and blood testing, is instrumental in identifying potential fetal abnormalities early in gestation, allowing for early decision-making and possible corrective measures. Low-resource settings often lack access to advanced diagnostics (e.g., fetal echocardiography, genetic screening), leading to underreporting of mild/asymptomatic cases and delayed interventions. In high-SDI regions, improved diagnostics increase detected prevalence but reduce mortality through early management (15).
Nutritional supplementation is also paramount, with a particular emphasis on folic acid and iodine (16). The study suggests that maternal folic acid supplementation before pregnancy is associated with a reduced risk of congenital heart defects, and lower dietary folate intake during pregnancy is linked to an increased risk of CHDs (17). Preconception folic acid supplementation and adequate dietary folate intake are crucial for the prevention of congenital heart defects (18).
Pregnant women should abstain from alcohol, tobacco, and illicit drugs, as these substances can elevate the risk of birth defects (19). Maintaining a healthy lifestyle, replete with a balanced diet and regular physical activity, is supportive of maternal health and avoiding CHA (20).
The significance of our study lies in its ability to provide insights into the changing landscape of CHA and its impact on global health. Our findings reveal that incidence and years lived with disability (YLDs) increase with higher SDI, indicating a complex relationship between economic development and health outcomes. Additionally, the higher disability-adjusted life years (DALYs) and years of life lost (YLLs) among males compared to females underscore the need for sex-specific health policies. By examining these trends, we can better allocate resources and develop strategies to reduce the global burden of CHA.
The study builds on this foundation by examining long-term trends and the impact of policy changes on the incidence and mortality of CHA. Additionally, the study provides insights into the potential future trends of disease burdens attributable to CHA, emphasizing the need for continued vigilance and intervention strategies to mitigate the impact of these conditions. Through this comprehensive analysis, we aim to contribute to the body of knowledge on CHA and inform future policy and research directions.
Methods
Data source
The Global Burden of Disease (GBD) 2021 study quantified the impact of 371 diseases and injuries across 204 nations from 1990 to 2021, encompassing metrics such as prevalence, incidence, mortality, and DALYs, including YLDs and YLLs (21). For CHA, the study utilized ICD−11 codes BE14, LA8Z, PK80.10–13, and corresponding ICD−10 codes Q20.8–21.9, 22.3, 24.5, 24.8, and 24.9. The Socio-demographic Index (SDI), a key development indicator linked to health outcomes, was also utilized. Data for this research are accessible via the Institute for Health Metrics and Evaluation (IHME) (https://www.healthdata.org/) and (https://www.healthdata.org/data-tools-practices/data-sources).
In studies on Years Lived with Disability (YLD) and Disability - Adjusted Life Years (DALYs) for congenital heart disease (CHD), “disability” covers all health - related functional limitations, symptoms, and impacts on quality of life from birth to adulthood. It includes: (1) pre - treatment disability like cyanosis, exercise intolerance, poor growth, and developmental delays in untreated CHD. (2) Residual disability after surgery or catheter interventions, such as cardiac dysfunction, activity limits, and medication side - effects. (3) Treatment - related disability from post - surgical complications, arrhythmias, prosthetic device issues, and surgical sequelae. (4) Long - term disability from chronic problems like heart failure, sudden cardiac death risk, neurodevelopmental issues, and psychosocial impacts.
Disability is measured by using severity - stratified disability weights (from 0.033 for mild asymptomatic CHD to 0.324 for severe symptomatic cases) throughout the patient's life, regardless of treatment. This method assesses the overall health impact of CHD, without differentiating between “natural” and “treatment - related” disability (22–24).
Statistical analysis
The Estimated Annual Percentage Change (EAPC) is a key metric for assessing trends in Age-Standardized Rates (ASR) over time. Within a regression model formatted as (y = α + β x + ε), y represents the annual rate change per 100,000 people, α is the intercept, x denotes the calendar year, and ε signifies the error term. The EAPC is calculated using the formula 100 × [exp(β) - 1], with its 95% confidence interval (CI) derived from the linear regression model parameters. Statistical significance was set at a two-sided p-value of less than 0.05. These analyses were performed using R software, version 4.4.1. Concurrently, we employed the Pearson correlation coefficient to assess the correlation between ASR and the SDI, with statistical significance defined by a p-value of less than 0.001.
Projection analysis
The Bayesian Age-Period-Cohort (BAPC) R package, a statistical tool for projecting future disease burden within a Bayesian framework, was not applied to GBD 2021 due to the availability of less than five years of incidence data. Utilizing age-specific population data from 1990 to 2021 and projected figures for 2022 to 2050, we analyzed trends in prevalence and mortality across various age groups. These data were also used to forecast future prevalence, deaths, DALYs, YLDs, and YLLs for congenital anomalies from 2022 to 2050. The BAPC package's default settings were employed for this analysis.
Results
Sex-stratified analysis of disease burden in global and SDI quintile from 1990 to 2021
It presents the sex-disaggregated trends and variations in congenital heart anomalies across different SDI quintiles over time. The prevalence and incidence of congenital heart anomalies, depicted in panels (Figures 1A,B), respectively, have remained relatively stable, with incidence rates being higher in regions with lower SDI. Our results indicate a statistically significant but modest global increase in prevalence, with an overall EAPC of 0.04 (95% CI: 0.03–0.05). This corresponds to an approximate cumulative increase of 1.24% over the 31-year period. Notably, the upward trend was more pronounced in females (EAPC: 0.08, 95% CI: 0.06–0.10) compared to males (EAPC: 0, 95% CI: −0.01–0.01). In contrast, there has been a consistent decrease in mortality rates, age-standardized DALYs (Figures 1C,D), and YLLs, with males consistently showing higher rates, particularly in low SDI areas. Notably, age-standardized YLDs have shown an increasing trend in non-high SDI regions, indicating a growing burden of disability (Figures 1E,F). Concurrently, age-standardized YLLs are disproportionately higher in males.

Figure 1. Sex-disaggregated global disease burden rates of congenital heart anomalies by SDI quintile from 1990 to 2021. (A) Prevalence. (B) Incidence. (C) Deaths. (D) DALYS. (E) YLDs. (F) YLLs.
There is no significant sex-based difference in the number of people with congenital heart disease prevalence and incidence across GBD regions (Supplementary Figures S1A,B). However, the number of deaths and DALYs are significantly higher among males, indicating a substantial burden of mortality (Supplementary Figures S1C,D). YLDs exhibit an increasing trend across all SDI regions, suggesting a rising burden of disability. In contrast, YLLs are substantially higher in males compared to females (Supplementary Figures S1E,F).
Sex-stratified disease burden in 21 distinct GBD regions in 1990 and 2021
The sex-specific number of prevalence and prevalence percentages across 21 GBD regions for the years 1990 and 2021 are presented in (Figures 2A,B). The amount of prevalence in 1990, with South Asia exhibiting the highest counts at approximately 1,191,604 for females and 1,128,855 for males. By 2021, these numbers have escalated to 1,747,665 for females and 1,583,144 for males in South Asia, marking the most substantial increase in the number of prevalence (Table 1).
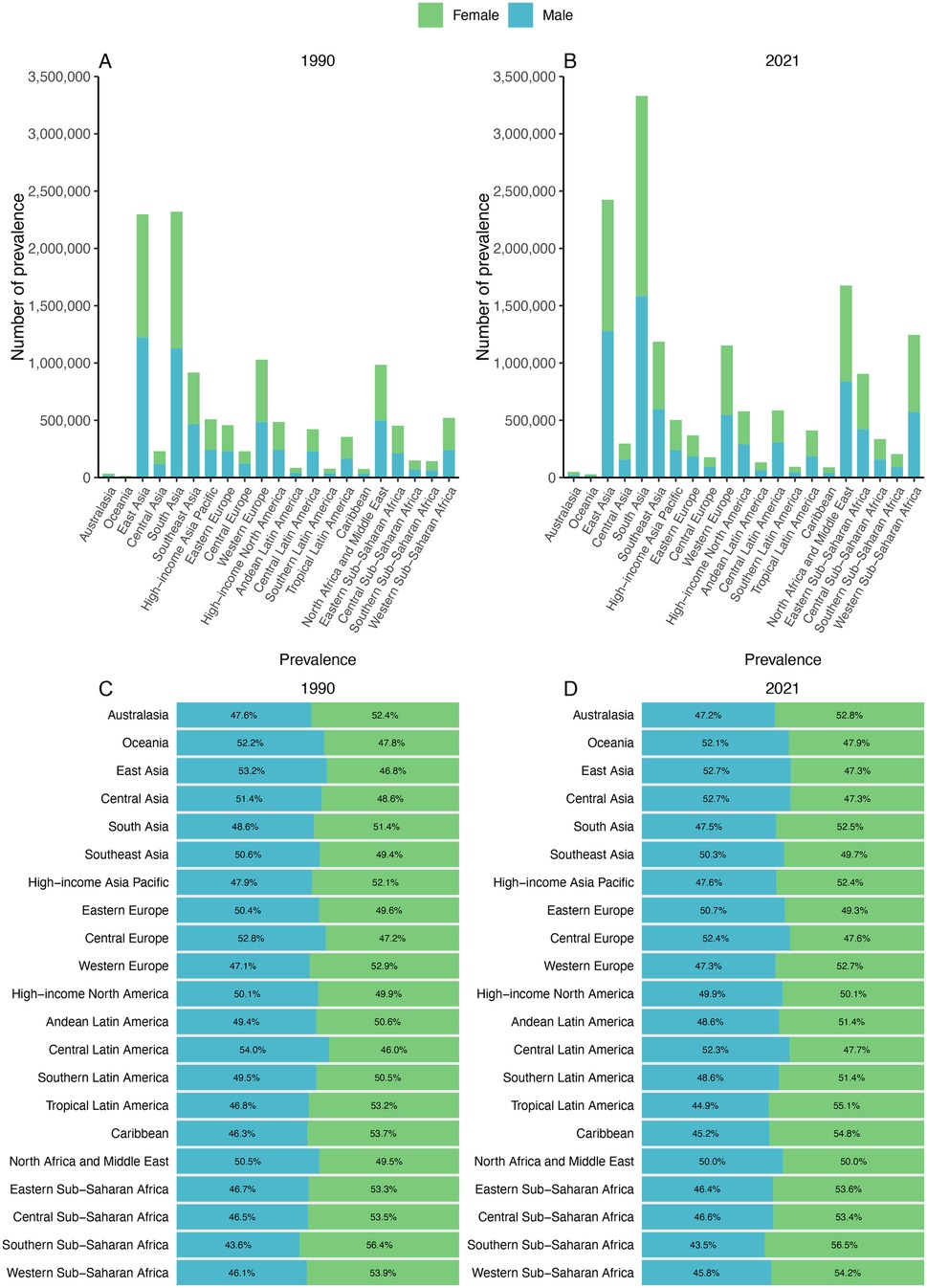
Figure 2. Contribution of prevalence cases by the 21 GBD regions to congenital heart anomalies numbers and proportions, for the years 1990 (A) and 2021 (B) Additionally, the proportion of prevalence cases attributed to each congenital heart anomalies is depicted for 1990 (C) and 2021 (D).
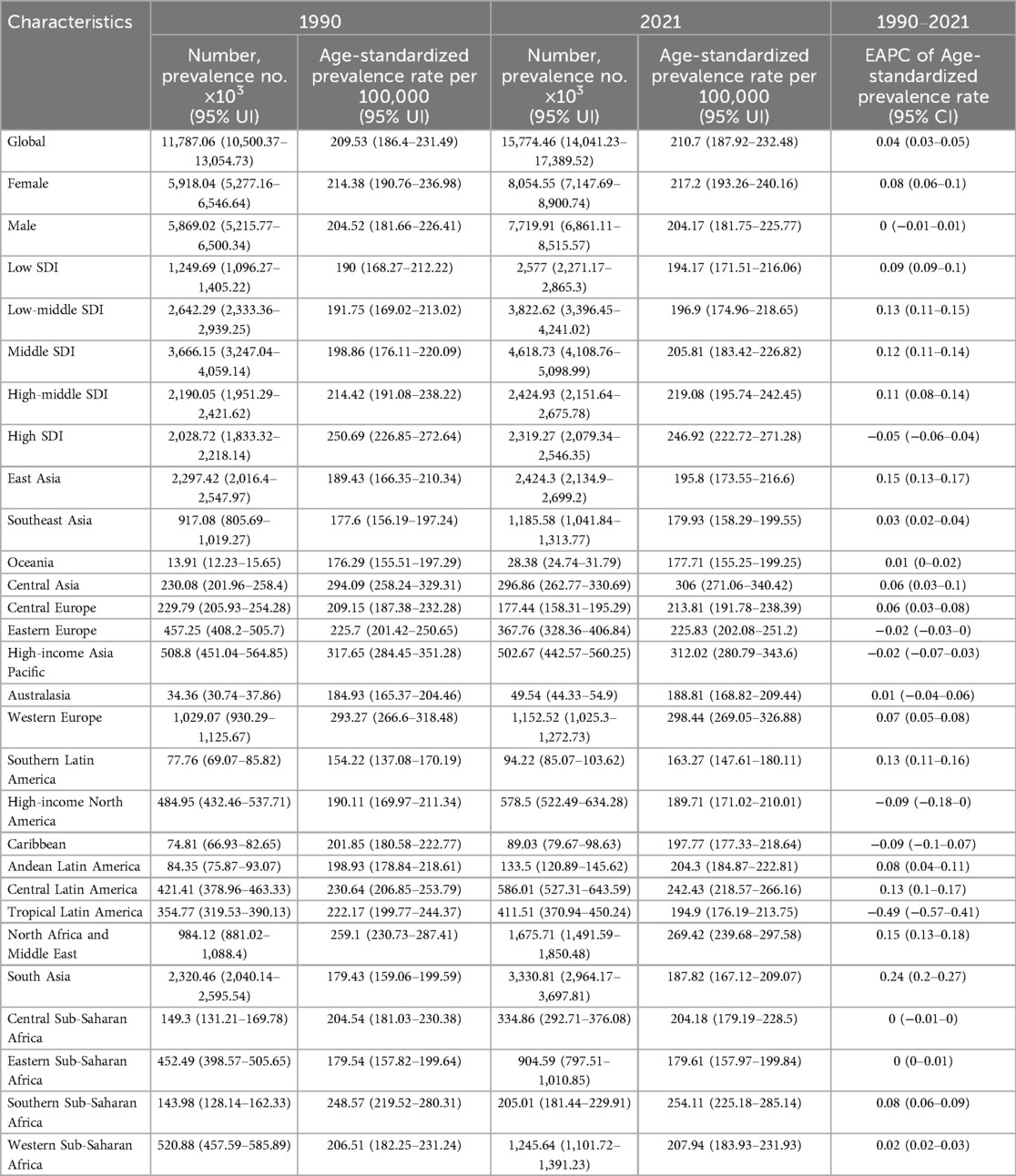
Table 1. EAPC of age-standardized prevalence rates of congenital heart anomalies and the temporal trends from 1990 to 2021.
South Asia exhibited the highest increasing trend with an EAPC of 0.24 (95% CI: 0.20–0.27), while some regions showed stable or declining trends—for example, certain low-SDI regions had negative EAPCs as low as −0.49 (95% CI: −0.57 to −0.41).
Shifts in sex ratio are most notable in Tropical Latin America, where it shows a 1990 prevalence percentage of 53.2% for females and 46.8% for males. By 2021, this ratio has increased to 55.1% for females and 44.9% for males. In most GBD regions, females are overrepresented compared with males in prevalence.
The most significant increase in incidence rates was observed in Western Sub-Saharan Africa. In Andean Latin America, the incidence rate among men notably surpassed that of women, rising from 60.6% in 1990 to 61.8% in 2021 (Supplementary Figure S2 and Table 2). A decline in mortality was recorded across all 21 GBD regions, with particularly pronounced reductions noted in East Asia, South Asia, and North Africa and the Middle East. Consistently across GBD regions, male mortality rates exceeded those of females (Supplementary Figure S3 and Table 3). DALYs and YLLs exhibited a downward trend in the majority of GBD regions; however, an increase was observed in Western Sub-Saharan Africa. As of 2021, the burden of DALYs and YLLs remained greater for men than for women across all 21 GBD regions (Supplementary Figures S4, S6). YLD increased across all 21 distinct GBD, with South Asia experiencing the most pronounced rise. With exceptions for Sub-Saharan Africa, the Caribbean, Tropical Latin America, and High-Income Asia Pacific regions where this pattern did not hold true, the proportion of YLDs among men consistently outstripped that among women (Supplementary Figure S5 and Supplementary Tables S1–S3).
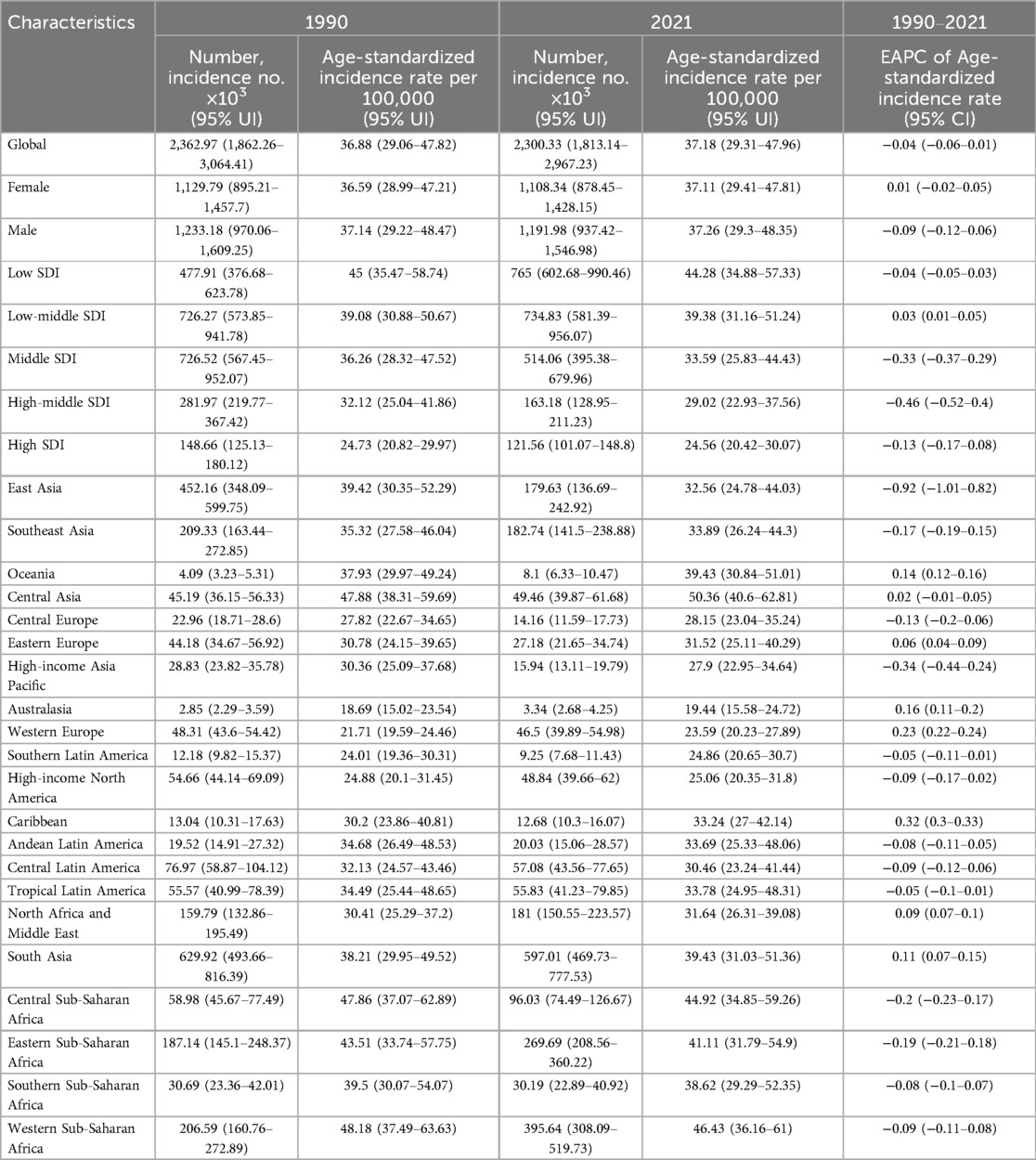
Table 2. EAPC of age-standardized incidence rates of congenital heart anomalies and the temporal trends from 1990 to 2021.
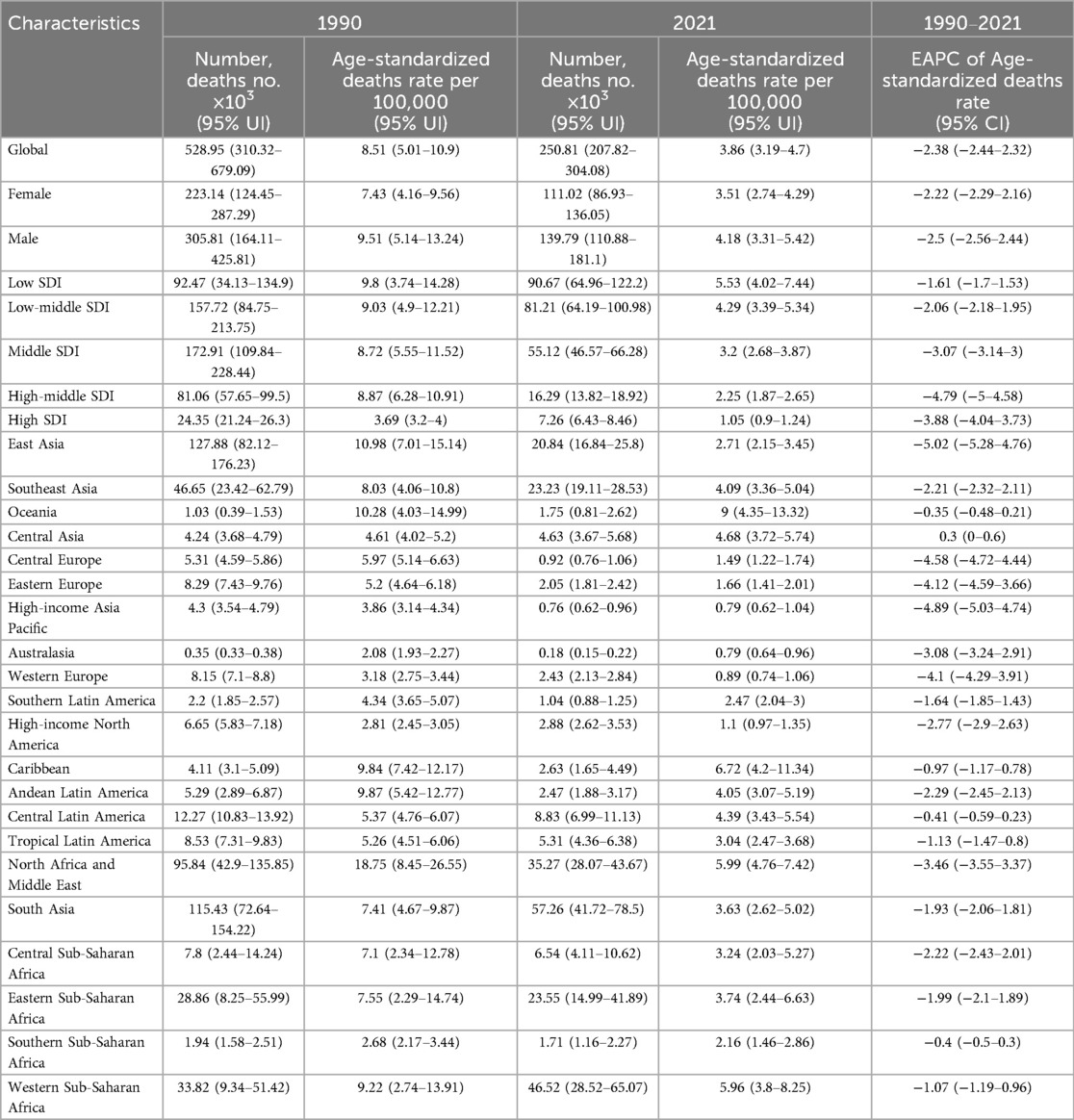
Table 3. EAPC of age-standardized deaths rates of congenital heart anomalies and the temporal trends from 1990 to 2021.
Global trends in the burden of congenital heart anomalies across 204 countries and territories
The EAPC data (Figure 3) reveals significant variations in health metrics across countries. For Age-Standardized Prevalence Rates (ASPR), a notable downward trend is observed in China, Ethiopia, and Canada, indicating a reduction in disease prevalence. In contrast, an upward trend is seen in Turkmenistan, Argentina, and Chile, suggesting an increase in disease burden in these regions (Supplementary Table S4). Incidence Rates (ASIR) generally show a downward trend, reflecting a global decrease in disease incidence, with the exception of Spain and France, where an upward trend may signal emerging health challenges (Supplementary Table S5). Age-Standardized Death Rates (ASDR) exhibit a nearly universal decline, with the exception of a few countries including the Northern Mariana Islands, American Samoa, Seychelles, and others (Supplementary Table S6).
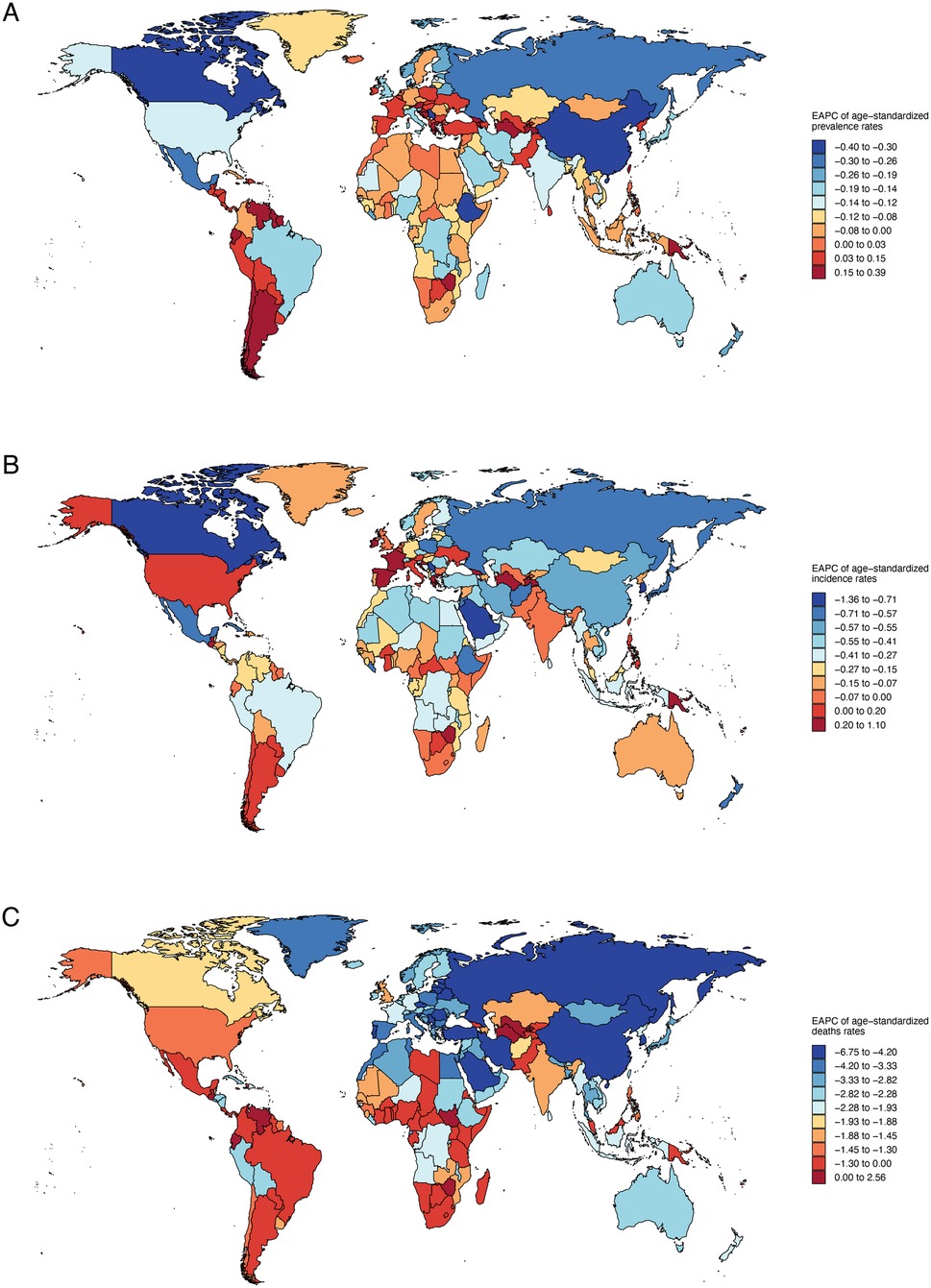
Figure 3. Annual percentage change in national age-standardized prevalence, incidence, death rates of congenital heart anomalies in 2021. (A) Prevalence. (B) Incidence. (C) Deaths.
It shows a general decline in age-standardized DALYs and YLLs, pointing to a reduced disease burden in many countries, yet an upward trend in YLDs affects over half of the nations, including Belgium, Spain, and Austria (Supplementary Table S8). Notably, Turkmenistan, Guatemala, Dominica, and Uzbekistan exhibit rising DALYs and YLLs, suggesting potential health challenges (Supplementary Tables S7, S9, Supplementary Figure S7).
Age-specific disease burden of congenital heart anomalies in various SDI regions
It succinctly captures the comparative dynamics of prevalence, incidence, and mortality rates for congenital heart anomalies across age groups and Socio-Demographic Index (SDI) regions from 1990 to 2021. The prevalence rate for children aged 0–4 is notably high in all SDI regions, with a decreasing trend in prevalence as age groups progress from 5 to 9 to 65–69 years. Incidence rates are high in all SDIs, particularly in low and low-middle SDI regions, with a decreasing trend observed in higher SDI regions over the study period. Mortality rates are particularly high in the 0–4 age group across all SDI regions and have shown a decline in recent years. While the 0–24 years age group has relatively high mortality, the 45–59 age group exhibits comparatively lower rates (Figure 4). DALYs and YLLs predominantly burden individuals aged 0–24 years, with a notable decrease in impact across all age groups from 1990 to 2021. YLDs, affecting primarily those aged 0–9 years, exhibit a lower impact in regions with Low SDI, indicating a reduced burden of Congenital Heart Anomalies in these areas (Supplementary Figure S8).
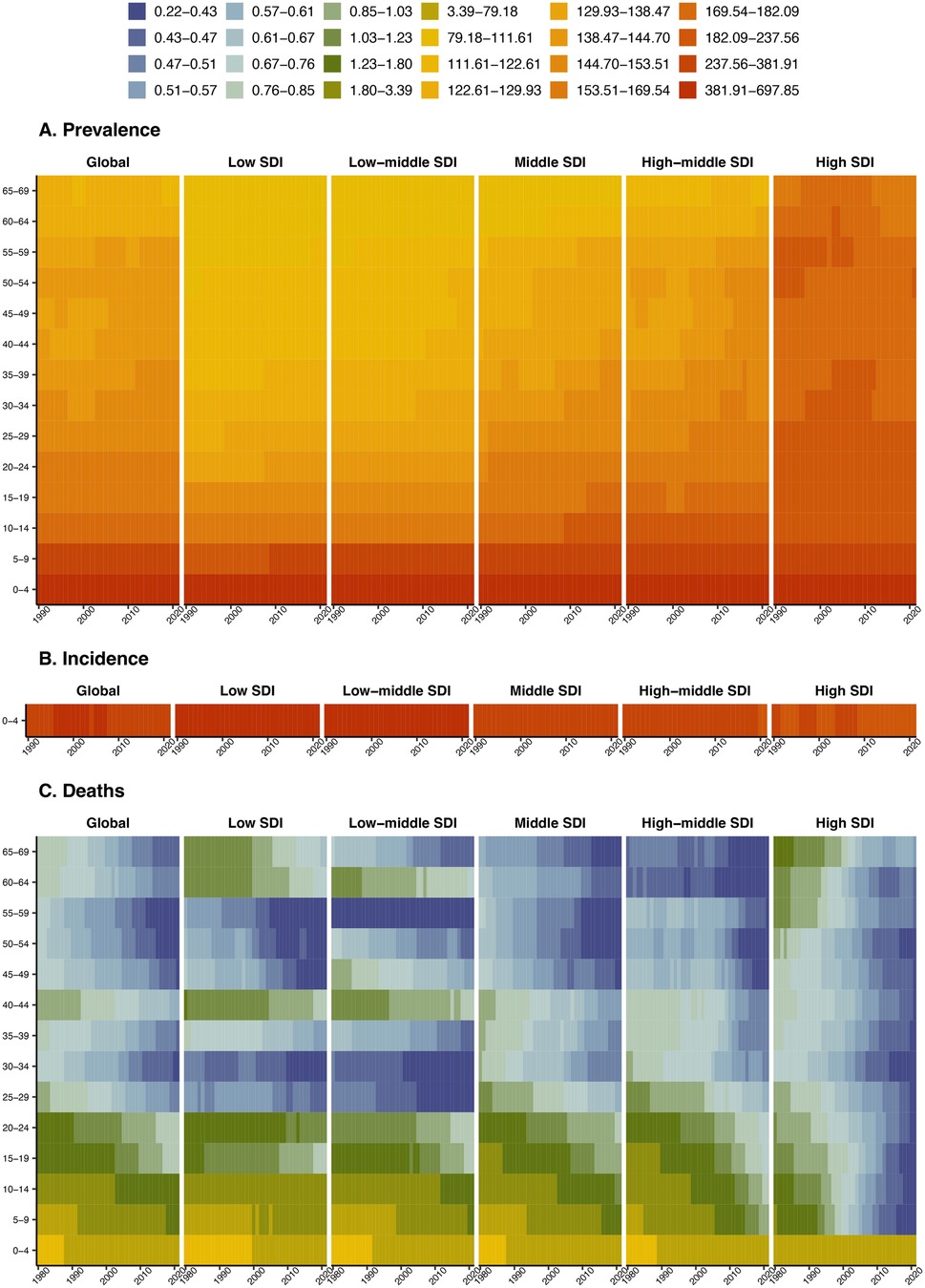
Figure 4. Age-specific disease burden of congenital heart anomalies in various SDI regions from 1990 to 2021. (A) Prevalence. (B) Incidence. (C) Deaths.
SDI correlations with congenital heart anomalies burden trends in 21 GBD regions and 204 countries and territories
Across the 21 GBD regions, both prevalence and YLDs exhibited an increasing trend in conjunction with rising SDI values, with correlation coefficients of 0.3661 and 0.6982, respectively, and a p-value below 0.001. Conversely, incidence, deaths, DALYs, and YLLs demonstrated a declining trend with the elevation of SDI, with correlation coefficients of −0.7660, −0.6713, −0.6658, and −0.6707, respectively. Their p-values were all less than 0.001 (Figure 5).
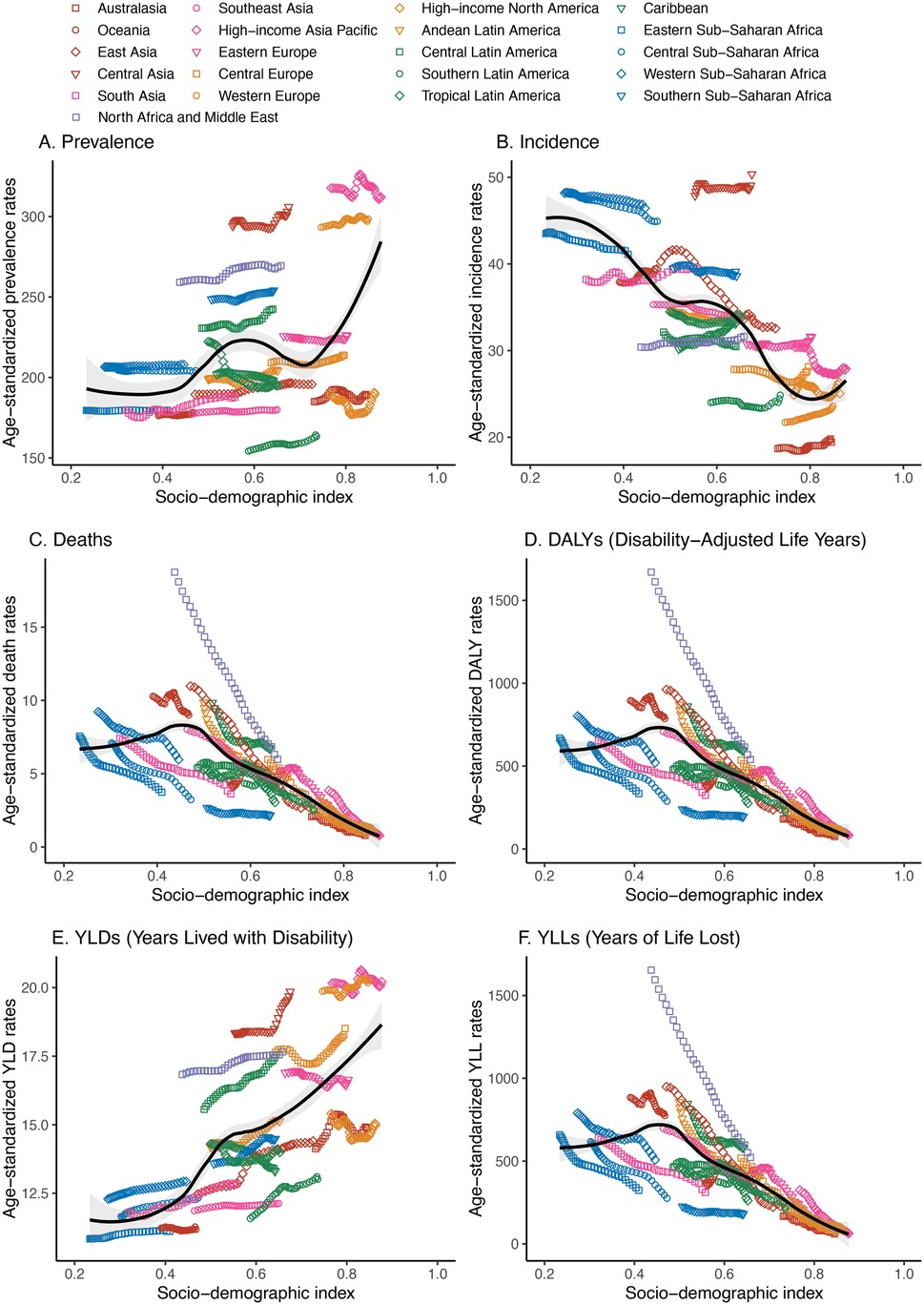
Figure 5. Association between the disease burden of congenital heart anomalies and sociodemographic index across 21 GBD regions from 1990 to 2021. (A) Prevalence. (B) Incidence. (C) Deaths. (D) DALYS. (E) YLDs. (F) YLLs.
In the 204 countries and regions, prevalence and YLDs rose with increasing SDI, with correlation coefficients of 0.4519 and 0.6456, respectively (both p < 0.001). The age-standardized incidence rate (ASIR) declined significantly with SDI, with a correlation coefficient of −0.7560 (p < 0.001). The age-standardized death rate (ASDR) also decreased with SDI, at −0.6039 (p < 0.001) (Figure 6 and Supplementary Figure S9). The trends in age-standardized DALY and YLL rates were closely aligned, though less markedly, with correlation coefficients of −0.5936 and −0.5999, respectively (both p < 0.001) (Supplementary Figure S9).
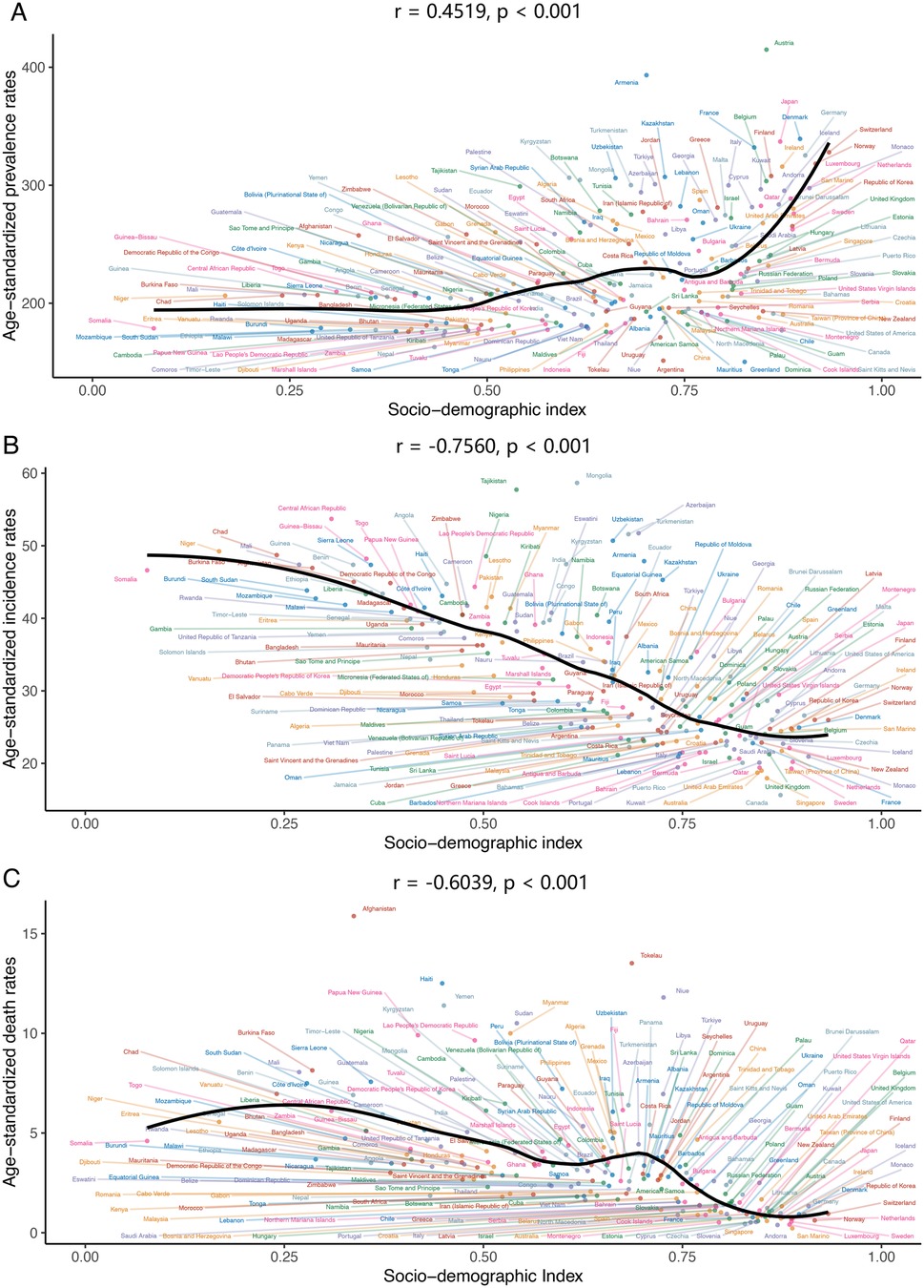
Figure 6. The association between age-standardized prevalence, incidence, deaths rate of congenital heart anomalies and sociodemographic index across 204 countries and territories in 2021. (A) Prevalence. (B) Incidence. (C) Deaths.
Projections of congenital heart anomalies from 2022 to 2050
From 2022 to 2050, the prevalence of congenital heart anomalies is projected to rise, particularly among the 0–29 age group, with minimal change anticipated in those aged 30 and above (Figure 7 and Supplementary Figure S10). Despite a significant decline in mortality from 1990 to 2021, future rates are expected to continue a slow downward trend, with the 20–69 age group showing a more pronounced reduction (Figure 7 and Supplementary Figure S11). Age-standardized DALY and YLL rates will slightly decrease, while YLD rates will markedly increase (Figure 7). By 2050, the number of prevalence is expected to rise significantly, with the 0–4 age group projected to reach 25,211,160 (95% UI, 6,556,715–43,865,606), a substantial increase from 4,183,259 (95% UI, 4,800,270–3,643,893) in 2021 (Supplementary Table S10). Conversely, the mortality rate for this age group is predicted to drop significantly, from 204,222 (95% UI, 255,409–165,238) to 178,084 (95% UI, 39,675–316,493) (Supplementary Table S11). While DALYs and YLLs are set to increase, YLDs are anticipated to decrease (Supplementary Tables S12–S14).
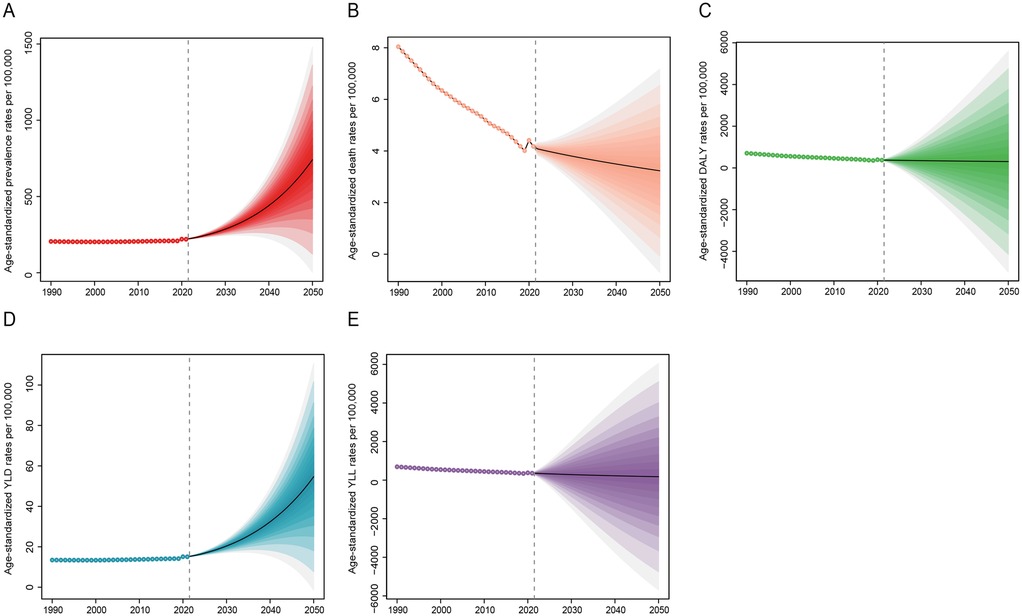
Figure 7. Projection of disease burden of congenital heart anomalies from 2022 to 2050. (A) Prevalence. (B) Deaths. (C) DALYS. (D) YLDs. (E) YLLs.
Discussion
The GBD 2021 analysis provides critical insights into the evolving burden of congenital heart anomalies. Global trends from 1990 to 2021 show that congenital heart anomalies had stable global prevalence and incidence but declining mortality, DALYs, and YLLs. Males had higher fatality rates, especially in low - SDI regions. YLDs increased significantly in non - high - SDI areas, indicating a shift to a disability - driven burden. Regionally, South Asia had the most cases in 2021 (1.75M females/1.58M males). Western Sub - Saharan Africa had the fastest incidence growth and rising DALYs/YLLs. Sex disparities grew in Latin America. Mortality fell in 97% of 204 countries, but disability (YLDs) worsened in 50%. SDI correlations: Higher SDI quintiles were associated with rising prevalence/YLDs (r > 0.64) but falling incidence/mortality (r < −0.6). Children aged 0–4 were the most vulnerable across all SDI levels. Projections to 2050 suggest a 500% increase in global cases among 0–4 - year - olds (to 25.2M), with slowly declining mortality and sharply rising YLDs. This shows an epidemiological transition from a lethal to a disabling disease. Low - SDI regions face high incidence, while middle/high - SDI regions deal with increasing disability. Targeted child - focused interventions are needed in poor areas, and disability management in developed economies.
Previous research on CHA has not consistently examined the disease burden from 1990 to 2021 through the lens of sex, age, and SDI (1, 8, 9). Our study addresses this omission, offering a comprehensive overview of CHA's global impact during this period, with detailed analysis across sex, age groups, SDI regions, and countries. It also projects prevalence, mortality, DALYs, YLDs, and YLLs for different age groups from 2022 to 2050, including specific predicted numerical values. This in-depth analysis offers fresh insights into CHA's global public health and informs future health policy and resource allocation.
The findings indicate that CHA prevalence and incidence remained largely stable from 1990 to 2021, with a minor increase. The ASPR rose slightly from 209.53 (186.4–231.49) to 210.7 (187.92–232.48), and the ASIR increased marginally from 36.88 (29.06–47.82) to 37.18 (29.31–47.96). In low SDI areas, age-standardized incidence rates slightly dipped from 45 (35.47–58.74) to 44.28 (34.88–57.33), but remained elevated, indicating a need for targeted interventions to reduce CHA incidence (25, 26). Age-standardized mortality and DALY rates saw a significant drop, from 8.51 (5.01–10.9) to 3.86 (3.19–4.7) and from 750.3 (440.94–960.11) to 345.24 (288.34–422.16), respectively. High SDI areas showed a more pronounced decline in death rates and age-standardized DALY rates, reflecting improved CHA treatment worldwide (5, 27, 28). In 2021, global female CHA prevalence rates were higher at 217.2 (193.26–240.16) compared to males at 204.17 (181.75–225.77), yet female death rates were lower at 3.51 (2.74–4.29) compared to males at 4.18 (3.31–5.42). This could suggest higher susceptibility in male, a protective role for estrogen (29, 30), or superior treatment adherence in female (31). From 1990 to 2021, CHA deaths in South Asia fell significantly, from 115.43 (72.64–154.22) thousand to 57.26 (41.72–78.5) thousand. East Asia is projected to experience the steepest decline in future mortality, with an EAPC of −5.02 (−5.28 to −4.76). The downward death rate trend across all SDI regions points to advancements in CHA treatment, improved public health policies, and better socioeconomic conditions (32, 33). Globally, the disease burden is highest in CHA patients aged 0–4, decreasing with age, except in high SDI regions where advanced medical facilities and treatments have led to improved survival rates, allowing patients to live longer and thus not showing a significant age-related prevalence decline (34). CHA prevalence and YLDs rise with SDI, while incidence, mortality, DALYs, and YLLs fall. Improved diagnostics have led to earlier detection of mild or asymptomatic cases, increasing prevalence (35). The increased survival rate of CHA patients has reduced mortality and DALYs, as many now survive to adulthood or old age.
Looking ahead to 2050, our predictions suggest a rise in the occurrence of CHA, particularly among younger individuals. The death rate is projected to decrease gradually, yet the DALYs are expected to see a substantial increase, maintaining an upward trajectory (Figure 7). While global pediatric populations are declining (UNPD 2022), CHA prevalence is rising disproportionately in low/middle-SDI regions due to: higher fertility rates and improving survival (reducing mortality but increasing lifelong disability burden) (36). With the anticipated growth in the global population, especially in regions with low and medium SDI, an increase in the number of CHA cases is likely, potentially raising prevalence rates. This necessitates targeted interventions. Despite the slow decline in mortality, individuals with CHA may still experience disabilities and a diminished quality of life, resulting in a rise in YLDs. Furthermore, as CHA patients age, they are likely to confront health issues associated with aging, further elevating DALYs (37).
Based on our GBD 2021 analysis, we acknowledge that adult congenital heart disease (ACHD) represents a growing clinical challenge as survival into adulthood has dramatically improved. While the GBD framework classifies congenital heart anomalies as a unified entity without explicit ACHD stratification, our findings likely capture this evolving burden. The sustained high DALYs and YLDs observed across older age groups probably reflect ACHD sequelae—including heart failure, arrhythmias, and complications from previous interventions that characterize this population. Geographic variations in burden may mirror disparities in pediatric cardiac surgical access and lifelong ACHD care infrastructure, which significantly influence outcomes. Recent registry data confirm the ACHD population is expanding and aging, with substantial numbers now surviving beyond 60 years. We agree that future studies should incorporate specific ACHD classifications and age-stratified analyses to better characterize this population's unique burden, as current global health metrics may underestimate the true impact of lifelong complications in ACHD patients who now routinely survive into their sixth and seventh decades.
This study utilizes the most recent GBD 2021 data, providing a more accurate reflection of CHA's current disease burden and enabling more reliable predictions of its future trajectory. We systematically analyzed how sex, age groups, and SDI levels impact CHA across various countries and regions. The EAPC was employed to forecast disease burden trends in different nations, while the BAPC model was used to project CHA disease burden from 2022 to 2050.
There are some limitations in our study: (1) a limitation of this study is its reliance on the BAPC model alone; it did not incorporate ARMIA or Nordped for a broader comparison of future CHA disease burden predictions (38–40). (2) Global diagnostic coverage disparities, (3) birth registration gaps, and (4) interconnected health conditions. As referenced (https://www.OurWorldinData.org), accurate neonatal diagnostics (ultrasound, genomic screening) remain highly inequitable—widely available in high-income countries but critically limited in LMICs, contributing to delayed diagnoses and higher mortality. Significant birth under-registration, particularly in sub-Saharan Africa and South Asia, compromises data accuracy and obscures true disease burden. Additionally, neonatal mortality is intrinsically linked to infections, congenital disorders, and maternal complications, with premature birth being the leading cause of child mortality globally. These diagnostic and registration gaps in LMICs hinder early detection of interconnected risks, preventing timely interventions and perpetuating preventable deaths.
To encapsulate, this study offers a thorough analysis of the global disease burden of CHA and its anticipated trends over the coming decades. The findings underscore the importance of devising health strategies tailored to different SDI regions, particularly those with low SDI, to alleviate the disease burden of CHA. With continued research and intervention, we anticipate a reduction in CHA's impact on global child health and an enhancement in the quality of life for patients in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The GBD adheres to the Guidelines for Accurate and Transparent Health Estimates Reporting statement. The Institute for Health Metrics and Evaluation, which is responsible for administering the GBD, provides only deidentified and aggregated data. All research adhered to the tenets of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
EY: Conceptualization, Methodology, Writing – original draft, Investigation, Visualization. EW: Conceptualization, Writing – original draft, Investigation. TT: Data curation, Writing – original draft, Formal analysis. XL: Writing – review & editing, Project administration, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Youth Medical Science and Technology Talents Research Foundation of Autonomous Regional Health Commission (Grant No: WJWY–202410), State Key Laboratory of Pathogenesis, Prevention and Treatment of High Incidence Diseases in Central Asia at Xinjiang Medical University (SKL-HIDCA-2024-GX2).
Acknowledgments
We value the contributions made by the GBD 2021 Risk Factors Collaborators.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1601620/full#supplementary-material
References
1. GBD 2017 Congenital Heart Disease Collaborators. Global, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Child Adolesc Health. (2020) 4(3):185–200. doi: 10.1016/S2352-4642(19)30402-X
2. Stallings EB, Isenburg JL, Aggarwal D, Lupo PJ, Oster ME, Shephard H, et al. Prevalence of critical congenital heart defects and selected co-occurring congenital anomalies, 2014–2018: a U.S. Population-based study. Birth Defects Res. (2022) 114:45–56. doi: 10.1002/bdr2.1980
3. Chang CS, Hong SY, Kim SY, Kim YM, Sung JH, Choi SJ, et al. Prevalence of associated extracardiac anomalies in prenatally diagnosed congenital heart diseases. PLoS One. (2021) 16:e0248894. doi: 10.1371/journal.pone.0248894
4. Kang L, Cao G, Jing W, Liu J, Liu M. Global, regional, and national incidence and mortality of congenital birth defects from 1990 to 2019. Eur J Pediatr. (2023) 182(4):1781–92. doi: 10.1007/s00431-023-04865-w
5. Ma K, He Q, Dou Z, Hou X, Li X, Zhao J, et al. Current treatment outcomes of congenital heart disease and future perspectives. Lancet Child Adolesc Health. (2023) 7:490–501. doi: 10.1016/S2352-4642(23)00076-7
6. Mellion K, Uzark K, Cassedy A, Drotar D, Wernovsky G, Newburger JW, et al. Health-related quality of life outcomes in children and adolescents with congenital heart disease. J Pediatr. (2014) 164:781–8. doi: 10.1016/j.jpeds.2013.11.066
7. Westhoff-Bleck M, Briest J, Fraccarollo D, Hilfiker-Kleiner D, Winter L, Maske U, et al. Mental disorders in adults with congenital heart disease: unmet needs and impact on quality of life. J Affect Disord. (2016) 204:180–6. doi: 10.1016/j.jad.2016.06.047
8. Su Z, Zou Z, Hay SI, Liu Y, Li S, Chen H, et al. Global, regional, and national time trends in mortality for congenital heart disease, 1990–2019: an age-period-cohort analysis for the global burden of disease 2019 study. EClinicalMedicine. (2022) 43:101249. doi: 10.1016/j.eclinm.2021.101249
9. Bai Z, Han J, An J, Wang H, Du X, Yang Z, et al. The global, regional, and national patterns of change in the burden of congenital birth defects, 1990–2021: an analysis of the global burden of disease study 2021 and forecast to 2040. EClinicalMedicine. (2024) 77:102873. doi: 10.1016/j.eclinm.2024.102873
10. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. (2011) 58:2241–7. doi: 10.1016/j.jacc.2011.08.025
11. United Nations Economic and Social Commission for Asia and the Pacific (ESCAP). Sustainable Development Goal 3: Ensure Healthy Lives and Promote Well-being for all Ages. Bangkok: United Nations Economic and Social Commission for Asia and the Pacific (2016). Available online at: https://repository.unescap.org/items/c5df0c8f-e7c1-4136-bc66-1a0adfcd32cf
12. Chowdhury D, Elliott PA, Asaki SY, Amdani S, Nguyen QT, Ronai C, et al. Addressing disparities in pediatric congenital heart disease: a call for equitable health care. J Am Heart Assoc. (2024) 13:e032415. doi: 10.1161/JAHA.123.032415
13. Lopez KN, Allen KY, Baker-Smith CM, Bravo-Jaimes K, Burns J, Cherestal B, et al. Health equity and policy considerations for pediatric and adult congenital heart disease care among minoritized populations in the United States. J Cardiovasc Dev Dis. (2024) 11(2):36. doi: 10.3390/jcdd11020036
14. Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, et al. The national birth defects prevention study: a review of the methods. Birth Defects Res A Clin Mol Teratol. (2015) 103:656–69. doi: 10.1002/bdra.23384
15. Hjort L, Møller SL, Minja D, Msemo O, Nielsen BB, Christensen DL, et al. FOETAL For NCD-FOetal exposure and epidemiological transitions: the role of anaemia in early life for non-communicable diseases in later life: a prospective preconception study in rural Tanzania. BMJ Open. (2019) 9(5):e024861. doi: 10.1136/bmjopen-2018-024861
16. Lorente M, Azpiroz MJ, Guedes P, Burgos R, Lluch A, Dos L. Nutrition, dietary recommendations, and supplements for patients with congenital heart disease. Int J Cardiol Congenit Heart Dis. (2023) 12:100449. doi: 10.1016/j.ijcchd.2023.100449
17. Mao B, Qiu J, Zhao N, Shao Y, Dai W, He X, et al. Maternal folic acid supplementation and dietary folate intake and congenital heart defects. PLoS One. (2017) 12:e0187996. doi: 10.1371/journal.pone.0187996
18. Czeizel AE, Dudás I, Vereczkey A, Bánhidy F. Folate deficiency and folic acid supplementation: the prevention of neural-tube defects and congenital heart defects. Nutrients. (2013) 5(11):4760–75. doi: 10.3390/nu5114760
19. Scott-Goodwin AC, Puerto M, Moreno I. Toxic effects of prenatal exposure to alcohol, tobacco and other drugs. Reprod Toxicol. (2016) 61:120–30. doi: 10.1016/j.reprotox.2016.03.043
20. Feng Y, Yu D, Yang L, Da M, Wang Z, Lin Y, et al. Maternal lifestyle factors in pregnancy and congenital heart defects in offspring: review of the current evidence. Ital J Pediatr. (2014) 40:85. doi: 10.1186/s13052-014-0085-3
21. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2133–61. doi: 10.1016/S0140-6736(24)00757-8
22. Apers S, Kovacs AH, Luyckx K, Thomet C, Budts W, Enomoto J, et al. Quality of life of adults with congenital heart disease in 15 countries: evaluating country-specific characteristics. J Am Coll Cardiol. (2016) 67(19):2237–45. doi: 10.1016/j.jacc.2016.03.477
23. Best KE, Rankin J. Long-term survival of individuals born with congenital heart disease: a systematic review and meta-analysis. J Am Heart Assoc. (2016) 5(6):e002120. doi: 10.1161/JAHA.116.002120
24. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2021) 77(15):1958–9. doi: 10.1016/j.jacc.2021.02.039
25. Kenny DP, Hijazi ZM. Current status and future potential of transcatheter interventions in congenital heart disease. Circ Res. (2017) 120(6):1015–26. doi: 10.1161/CIRCRESAHA.116.309185
26. Zühlke L, Lawrenson J, Comitis G, De Decker R, Brooks A, Fourie B, et al. Congenital heart disease in low- and lower-middle-income countries: current status and new opportunities. Curr Cardiol Rep. (2019) 21(12):163. doi: 10.1007/s11886-019-1248-z
27. Louis JD S, Kurosawa H, Jonas RA, Sandoval N, Cervantes J, Tchervenkov CI, et al. The world database for pediatric and congenital heart surgery: the Dawn of a new era of global communication and quality improvement in congenital heart disease. World J Pediatr Congenit Heart Surg. (2017) 8(5):597–9. doi: 10.1177/2150135117725458
28. Su Z, Zhang Y, Cai X, Li Q, Gu H, Luan Y, et al. Improving long-term care and outcomes of congenital heart disease: fulfilling the promise of a healthy life. Lancet Child Adolesc Health. (2023) 7(7):502–18. doi: 10.1016/S2352-4642(23)00053-6
29. Salerni S, Di Francescomarino S, Cadeddu C, Acquistapace F, Maffei S, Gallina S. The different role of sex hormones on female cardiovascular physiology and function: not only oestrogens. Eur J Clin Invest. (2015) 45(6):634–45. doi: 10.1111/eci.12447
30. Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ. (2017) 8(1):33. doi: 10.1186/s13293-017-0152-8
31. de Marvao A, Alexander D, Bucciarelli-Ducci C, Price S. Heart disease in women: a narrative review. Anaesthesia. (2021) 76(Suppl 4):118–30. doi: 10.1111/anae.15376
32. Davey B, Sinha R, Lee JH, Gauthier M, Flores G. Social determinants of health and outcomes for children and adults with congenital heart disease: a systematic review. Pediatr Res. (2021) 89(2):275–94. doi: 10.1038/s41390-020-01196-6
33. Chowdhury D, Johnson JN, Baker-Smith CM, Jaquiss RDB, Mahendran AK, Curren V, et al. Health care policy and congenital heart disease: 2020 focus on our 2030 future. J Am Heart Assoc. (2021) 10(20):e020605. doi: 10.1161/JAHA.120.020605
34. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. (2021) 42(6):563–645. doi: 10.1093/eurheartj/ehaa554
35. Yoon SA, Hong WH, Cho HJ. Congenital heart disease diagnosed with echocardiogram in newborns with asymptomatic cardiac murmurs: a systematic review. BMC Pediatr. (2020) 20(1):322. doi: 10.1186/s12887-020-02212-8
36. United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2022: Summary of Results. UN DESA/POP/2022/TR/NO. 3. New York: United Nations (2022). Available online at: https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/wpp2022_summary_of_results.pdf
37. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. (2021) 20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0
38. Wafa HA, Marshall I, Wolfe CDA, Xie W, Johnson CO, Veltkamp R, et al. Burden of intracerebral haemorrhage in Europe: forecasting incidence and mortality between 2019 and 2050. Lancet Reg Health Eur. (2024) 38:100842. doi: 10.1016/j.lanepe.2024.100842
39. Foreman KJ, Lozano R, Lopez AD, Murray CJ. Modeling causes of death: an integrated approach using CODEm. Popul Health Metr. (2012) 10:1. doi: 10.1186/1478-7954-10-1
Keywords: congenital heart anomalies, global burden of disease, socio-demographic index, sex-stratified analysis, disability-adjusted life years, years lived with disability
Citation: Ye E, Wu E, Tang T and La X (2025) Global, regional, and national burdens of congenital heart anomalies from 1990 to 2021, and projections to 2050. Front. Pediatr. 13:1601620. doi: 10.3389/fped.2025.1601620
Received: 28 March 2025; Accepted: 25 July 2025;
Published: 18 August 2025.
Edited by:
Jing Su, Tsinghua University, ChinaReviewed by:
Estelle Naumburg, Umeå University, SwedenFabrizio De Rita, ARNAS Ospedali Civico Di Cristina Benfratelli, Italy
Copyright: © 2025 Ye, Wu, Tang and La. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin La, OTA5MjMyOTA1QHFxLmNvbQ==
 Erdengqieqieke Ye
Erdengqieqieke Ye Erman Wu
Erman Wu Tong Tang
Tong Tang Xiaolin La4*
Xiaolin La4*