- 1Department of Otolaryngology and Head and Neck Surgery, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Clinical Sciences and Community Health, Dipartimento di Eccellenza 2023-2027, University of Milan, Milan, Italy
- 3Neonatal Intensive Care Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 4Pediatric Anesthesia and Resuscitation Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 5Department of Obstetrics and Gynecology, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 6Prenatal Diagnosis and Fetal Surgery Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy
- 7Neonatal Intensive Care Unit, Clínica Universidad de Navarra, Madrid, Spain
- 8Division of Neonatology, MosaKids Children’s Hospital, Maastricht University Medical Center (MUMC+), Research Institute for Oncology and Reproduction (GROW), Maastricht University, Maastricht, Netherlands
The “Ex Utero Intrapartum Treatment” (EXIT) procedure is a specialized surgical technique used during cesarean delivery to perform life-saving fetal interventions while maintaining placental circulation. By preserving feto-placental gas exchange, EXIT enables the treatment of severe conditions such as predictable severe breathing difficulties at birth. EXIT's origins date back to removing tracheal occlusion devices used for congenital diaphragmatic hernias. It has since expanded to treat conditions such as congenital high airway obstruction syndrome and airway compression by masses. Despite the risks of adverse maternal and fetal events, it shows high perinatal survival rates. The success of EXIT depends on an accurate prenatal diagnosis through fetal ultrasound and magnetic resonance imaging. Anesthetic management differs from standard cesarean sections, balancing the need for uterine relaxation and avoiding maternal-fetal risks. Inhaled anesthetics are preferred, although recent studies suggest the potential of neuraxial anesthesia combined with tocolytics. Although the EXIT procedure can be performed safely in specialized centers, it does carry risks for both the mother and the fetus. Neonatal mortality and complications vary depending on indications and postnatal management. Research and clinical practice must advance to improve safety and efficacy.
Key points
• Ex utero intrapartum treatment (EXIT) is a specialized surgical procedure performed during a cesarean section that facilitates fetal interventions. At the same time, the fetus remains connected to the placenta, allowing for gas exchange through the fetoplacental circulation.
• Maintaining fetoplacental circulation enables effective management of specific medical situations in which the fetus may experience significant respiratory difficulties at birth.
• Indications for EXIT include conditions such as high airway obstruction, compressive cervical or thoracic masses requiring removal, and other scenarios where postnatal respiratory compromise is anticipated, especially when intubation is difficult or normal cardiorespiratory function is altered.
• The success of the EXIT procedure relies on the collaboration of a highly specialized interdisciplinary team, which should include obstetricians, pediatric surgeons, anesthetists, neonatologists, otolaryngologists, specialized nurses, and, when necessary, cardiac surgeons and perfusionists for extracorporeal membrane oxygenation (ECMO).
1 Introduction
“Ex utero intrapartum treatment” (EXIT) is a highly specialized surgical approach used in obstetrics and neonatology (1). This procedure, performed during cesarean delivery, enables interventions on the fetus while still connected to the placenta, utilizing feto-placental circulation for gas exchange (1). This approach is valuable in cases where the fetus experiences significant respiratory distress at birth (2–4).
Norris et al. first described the operation on placental support (OOPS) in 1989, while the term “EXIT procedure” was later coined by Mychaliska et al. in 1997, outlining a systematic approach for managing complex fetal airways (5, 6). Since then, EXIT has become a revolutionary method adopted worldwide for managing congenital airway anomalies that would otherwise be incompatible with life (7, 8). This procedure was initially employed to remove tracheal occlusion devices (clips, plugs, or balloons) to decrease pulmonary hypoplasia in congenital diaphragmatic hernias. Since then, its application has expanded to include other conditions, such as congenital high airway obstruction syndrome (CHAOS) or airway compression caused by intra- or extra-thoracic masses (9–11).
These obstructions can be critically life-threatening or result in prolonged hypoxia with long-term neurodevelopmental consequences (9, 11–14).
In the contemporary clinical practice, the EXIT procedure is indicated in three main scenarios:
1. Upper Airway Obstruction: When an obstruction prevents the newborn from establishing a clear airway.
2. Cervical or Thoracic Masses: To remove masses that compress vital neck or chest structures.
3. Postnatal Respiratory Distress: Cases where immediate respiratory support is needed.
These are further reclassified, and in this context, we will explore specific variations of the EXIT procedure (Table 1) (12, 15):
1. “EXIT to Airways”: Securing the airway in obstruction cases.
2. “EXIT to Resection”: Removing compressive masses.
3. “EXIT to ECMO”: Providing ECMO support for severe respiratory or cardiac failure.
4. “EXIT to Separation”: Facilitating surgical separation of conjoined twins or complex anomalies.
This narrative review aims to provide an overview of the indications, techniques, potential complications, and outcomes associated with the EXIT airway management procedure, focusing on treating airway obstruction and compressive masses.
1.1 Upper airway obstructions – “EXIT to airways” and “EXIT to resection”
Obstructive lesions can be extrinsic, such as compressions by a cervical, pharyngeal, or thoracic mass; intrinsic, such as CHAOS; or iatrogenic, such as occurs with tracheal obstruction through the insertion of a tracheal plug/balloon during fetal life to treat severe congenital diaphragmatic hernia (8, 9, 15–18).
1.1.1 Lymphatic malformations
Lymphatic malformations are the second most common cause of neonatal soft tissue tumors, following hemangiomas, and are often linked to the EXIT procedure (19, 20). These malformations develop during the second and third trimesters of pregnancy due to abnormal embryological processes affecting the lymphatic system, such as isolation of the original lymph sac, disturbances in lymphatic and venous flow, or abnormal hyperplasia of lymphatic tissue (20, 21).
Lymphatic malformations are classified into macrocystic (>2 cm), microcystic (<2 cm), or mixed types. Macrocystic lesions tend to compress surrounding tissues, while microcystic lesions infiltrate them. Postnatally, macrocystic lymphatic malformations appear as soft masses, whereas microcystic ones are more complex and infiltrative (22).
The severity and prognosis of lymphatic malformations in the head and neck depend on their position relative to the hyoid bone (supra- or sub-hyoid) and laterality (unilateral or bilateral). Bilateral forms in both positions are linked to a higher risk of complications (23).
In addition, growth in the oral or pharyngolaryngeal cavities can lead to issues with swallowing, speech, and temporomandibular joint disorders (22).
1.1.2 Teratomas
Teratomas are germ cell tumors that usually develop during the fourth or fifth week of gestation and arise from the three embryonic layers: ectoderm, mesoderm, and endoderm (24). These ectopic germ cells proliferate and differentiate into either mature tissue (mature teratoma) or fetal tissue (immature teratoma). The incidence of these congenital tumors is approximately 1 in 20,000 to 40,000 live births. Although they can originate in various anatomical regions, such as the sacrococcygeal area, reproductive organs, anterior mediastinum, and retroperitoneum, 6% of these tumors are in the head and neck region, often developing from the thyrocervical area of the palate or nasopharynx (25). Although primarily solid, they typically contain cysts and calcifications (26–28).
Epignathus, a rare oropharyngeal teratoma from Rathke's pouch, occurs in about 1 in 35,000 to 200,000 live births. While usually benign, its size and location can cause airway obstruction and difficulty swallowing (29).
1.1.3 Congenital giant ranula
Congenital giant ranula is a rare condition in neonates, presenting as a large cyst in the floor of the mouth due to atresia or failure to channel the salivary ducts of the sublingual or submandibular gland. It affects about 0.7% of infants (30, 31). The condition arises from the rupture of the salivary gland's excretory duct, leading to an accumulation of mucinous secretion and local inflammation, resulting in pseudocyst formation. Treatment options include observation, aspiration, marsupialization, and surgical excision, with the latter being the most effective for preventing recurrence (32). In rare cases, the cyst can obstruct the airway, necessitating the EXIT procedure, which involves decompressing the ranula for safe intubation (4, 12, 15). Literature on this condition and the EXIT procedure is limited (30, 32).
1.1.4 Severe micrognathia
Severe micrognathia, caused by mandibular hypoplasia, can cause glossoptosis that obstructs the upper aero-digestive tract, complicating endotracheal intubation (33). Although it could be isolated, it often accompanies conditions like otocephaly, dysgnathia, Pierre Robin sequence, and Treacher-Collins, Nager, or velocardiofacial syndromes (34–36). Although many infants with micrognathia do not experience airway obstruction, severe cases have a high risk of respiratory failure, with a survival rate of 10%–20% (37). Less severe micrognathia presents with obstruction in 54%–88% of patients, with 42%–57% requiring intubation or tracheostomy (38, 39). Recent findings suggest intubation is only necessary in 25% of patients, with a mortality rate of 0%–6%. Consequently, the EXIT procedure is reserved for severe cases of mandibular hypoplasia, where difficult intubation or emergency tracheostomy is anticipated (39–41).
Diagnosing the degree of airway obstruction in severe micrognathia is challenging. A 2021 study by Tay et al. demonstrated that a mandibular index below the 5th percentile or abnormal amniotic fluid index accurately predicts the severity of airway obstruction (40). Therefore, if the mandibular index, which is calculated by dividing the anteroposterior diameter of the mandible by the biparietal diameter and then multiplying by 100, falls below the 5th percentile and there are signs of obstruction, such as an absent ultrasound-visible stomach, polyhydramnios, or glossoptosis, an EXIT procedure should be considered (39, 42).
1.1.5 Congenital high airway obstruction syndrome (CHAOS)
Congenital high airway obstruction syndrome is a rare condition resulting from the failed canalization of the upper airways during fetal development, typically around the 10th week of gestation. This condition includes anomalies such as laryngeal atresia, laryngeal cysts, tracheal agenesis, and laryngeal webs, with laryngeal atresia being the most common cause.
In 1994, Hedrick et al. introduced the term “CHAOS” to describe ultrasound findings in four fetuses with upper airway obstruction considered incompatible with survival (43). The exact incidence of CHAOS remains unknown due to its rarity (44). In some cases, spontaneous reductions of airway obstruction can occur in the third trimester of pregnancy. Complete obstruction is often associated with tracheoesophageal fistula (37, 45). Without intervention, CHAOS usually leads to intrauterine or neonatal death (43, 46).
During fetal life, this condition prevents normal lung fluid outflow, leading to increased intrathoracic pressure, pulmonary hyperplasia, heart failure, and fetal hydrops (45, 47, 48). Hyperechogenic lungs, flattening or inversion of the diaphragmatic domes, and dilation of the distal airway represent the triad of radiological diagnostic signs of the syndrome (49). CHAOS may be linked to various congenital anomalies, such as esophageal atresia, imperforate anus, renal agenesis, ambiguous genitalia, hydrocephalus, anophthalmia, spinal abnormalities, and syndactyly. Additionally, it can be associated with genetic syndromes like Fraser syndrome and Fragile X syndrome, which may increase the risk of fetal death (4, 50). The EXIT procedure has significantly reduced morbidity and mortality by enabling the safe placement of a tracheostomy while maintaining fetoplacental circulation. This approach has transformed the management of CHAOS, offering a lifeline for affected fetuses (51–54).
1.1.6 Iatrogenic tracheal stenosis
Historically, the EXIT procedure was used to remove tracheal clips placed in fetuses with congenital diaphragmatic hernia (CDH). Today, clips have been replaced by the fetoscopic insertion of a tracheal balloon between the 27th and 29th weeks of gestation, with fetoscopic removal occurring around 34 weeks (55). However, the EXIT procedure can still be utilized in complex cases, such as when spontaneous labor occurs before the second fetoscopy or when the fetal position makes the fetoscopic approach impractical. Recently, researchers developed a magnetic-controlled tracheal occlusion balloon that can be deflated using a magnetic field. This eliminates the need for a second fetoscopy or an emergency EXIT procedure (56–58).
1.2 Cervical or thoracic compressive mass - “EXIT to resection”
Cervical and thoracic compressive masses, such as teratomas, lymphatic malformations, congenital pulmonary airway malformation, bronchogenic cysts, and bronchopulmonary sequestration, can profoundly influence fetal development. These conditions may result in serious complications, including tracheal obstruction, mediastinal shift, pulmonary hypoplasia, hydrops, and hypoxia at birth (59). While there is potential for many thoracic masses to regress spontaneously during the third trimester, some may continue to expand, necessitating medical intervention to avert perinatal mortality (60, 61).
The EXIT procedure represents a crucial intervention for neonates with large thoracic masses who are at high risk of airway obstruction and hypoxia at birth. In this particular scenario, the primary goal of the EXIT procedure is to remove the mass, taking advantage of placental oxygenation surgically (11, 61). Therefore, EXIT reduces the need for emergency postnatal surgery and enhances long-term pulmonary function and survival, even though definitive postnatal resection of thoracic masses may still be necessary later (59, 60, 62).
1.3 Other indications for EXIT - “EXIT to ECMO” and “EXIT to separation”
In cases of severe cardiothoracic malformations, securing the airway through an EXIT procedure may not always be feasible. In such scenarios, implementing ECMO effectively during the procedure can facilitate a more adaptable and gradual postnatal treatment plan (63). Although this approach has shown success, cases involving significant cervical masses remain rare and are associated with high mortality due to the need for anticoagulation and central cannulation via sternotomy (59–67, 61). Therefore, combining EXIT with ECMO should only be considered a last resort after evaluating all other possible measures.
The use of EXIT and ECMO for severe CDH has been described, but the literature remains controversial (65). Studies indicate that survival rates, as well as pulmonary, cardiac, and psychomotor development, in neonates with severe CDH who received ECMO via EXIT were comparable to those who received ECMO postnatally (65).
The EXIT procedure has also been described for the separation of conjoined twins, particularly thoracopagus twins with congenital heart defects (68, 69). However, the literature is divided on this approach, as many authors suggest that postnatal delivery, imaging studies, and controlled tissue expansion yield better outcomes than separation during EXIT (4).
1.4 Prenatal diagnosis and EXIT timing
The success of the EXIT procedure is closely tied to accurate and timely prenatal diagnosis. As such, prenatal evaluation plays a critical role in this process. When a malformation involving the fetal high airway is detected or suspected, the patient must be promptly referred to a specialized center with expertise in fetal ultrasound and MRI (70–79). Additionally, collaboration with a medical genetics center experienced in conducting specific genetic investigations is essential to ensure a comprehensive diagnosis (76).
The optimal timing for conducting an EXIT procedure is generally established between 35 and 37 weeks of gestation. This timeframe permits adequate fetal lung maturation while minimizing potential risks for both the mother and the infant (12, 62). A systematic review of EXIT procedures demonstrated an average gestational age of 35.1 weeks, underscoring the necessity of balancing neonatal survival and maternal safety (12).
In severe airway obstruction or polyhydramnios, it may be necessary to consider an earlier intervention, potentially as early as 30–34 weeks. However, such early procedures are associated with an increased risk of neonatal morbidity (80). Consequently, performing EXIT procedures at gestational ages greater than 35 weeks is advisable, as this approach mitigates the risk of preterm complications while facilitating safe airway management (62).
1.5 Anesthesiologic considerations
Anesthesia management during the EXIT procedure presents unique challenges compared to a conventional cesarean section (81). A primary objective during EXIT is to achieve optimal uterine relaxation, intending to reduce the risk of placental abruption while ensuring the maintenance of placental circulation (1, 82).
Goals for the EXIT procedure include providing adequate general anesthesia to the mother, maximizing uterine relaxation for fetal head expulsion, ensuring uterine blood flow for fetal oxygenation, and minimizing fetal movement during surgery in EXIT to resection (82).
General anesthesia is typically preferred as it allows for better control of inhalation agents, although maternal factors can influence anesthesia choice (81–92).
Achieving uterine relaxation involves not just tocolytics, such as beta-mimetic drugs, oxytocin receptor antagonists, and cyclooxygenase inhibitors. It may also include high concentrations of inhalational anesthetics, which can cause maternal hypotension and uteroplacental hypoperfusion. Alterations in maternal cardiorespiratory function may further complicate the situation, potentially leading to hypoxia (82, 93–98).
In addition, inhalational anesthetics can negatively impact fetal cardiovascular homeostasis (94, 99–101). Recent studies suggest combining neuraxial anesthesia with tocolytics could improve conditions during EXIT, but this approach requires careful patient selection since the mother remains alert (81, 88, 93). Neuraxial anesthesia can reduce bleeding and transfusion needs, but it is not suitable for long-term placental bypass cases (81, 88).
Inhalational agents like desflurane, sevoflurane, and isoflurane are commonly chosen to promote uterine relaxation (91, 102–105). Intravenous agents such as propofol and remifentanil have also shown potential as alternatives (91). Fetal anesthesia should be considered when a mother undergoes neuraxial anesthesia. This entails the intramuscular administration of a combination of medications, specifically fentanyl, atropine, and a drug for neuromuscular blockage (106).
Monitoring is crucial during the procedure. Maternal blood pressure, oxygen saturation, end-expiratory carbon dioxide levels, and fetal preductal oxygen saturation help detect potential complications (1, 4).
1.6 Obstetrical considerations
Both maternal health and uteroplacental stability must be systematically evaluated and prioritized throughout the EXIT procedure (107, 108). Effective management of polyhydramnios is critical during the planning phase of the EXIT procedure to reduce maternal-fetal complications. In cases of severe polyhydramnios, preoperative amnioreduction is recommended to alleviate uterine overdistension, which is a recognized risk factor for placental abruption and intraoperative uterine rupture (6, 8, 109–111). Furthermore, it is essential to assess both placental location and fetal head position, as these factors are crucial for guiding the uterine incision and minimizing the risk of hemorrhagic complications (112–114). Patients are typically positioned with a left lateral tilt to prevent inferior vena cava compression (115). The uterine incision is generally executed using a Pfannenstiel or low midline approach. Accurate and precise execution of the incision is paramount in order to reduce myometrial bleeding and minimize associated risks (83). In urgent situations where amnioreduction is either contraindicated or not feasible, the risk of placental abruption can be effectively reduced through controlled amniotic drainage (116, 117). Following the myometrial incision, the procedure necessitates meticulous preparation and exposure of an intact amniotic sac. Subsequently, multiple small punctures are performed in the sac, facilitating gradual uterine decompression before the delivery of the fetal head (118). The hysterotomy is strategically positioned slightly above the lower uterine segment to optimize space for fetal head extraction while avoiding highly vascularized areas (107). To prevent uterine wall bleeding, the free uterine margin can be manually sutured or stapled before the incision of the amniotic sac (4, 87, 96, 119). Incisions may be extended to ensure safe fetal exposure when addressing large neck masses (85, 87, 96). The umbilical cord may be clamped upon establishing the neonatal airway and initiating ventilation. The moment of birth for the newborn is specifically defined as the time of umbilical cord clamping, rather than at the point of head emergence (120). Fetal malpresentation poses a significant challenge during an EXIT procedure. In cases of a breech or transverse lie, the uterine incision must be tailored to ensure optimal exposure of the presenting part while safeguarding placental integrity. A high transverse or classical vertical incision may be appropriate, provided the placenta is not previa and its location is favorable (10). In cases of breech presentation, an alternative approach is the internal cephalic version, which aims to facilitate the delivery of the fetal head. While the execution of this maneuver can be complex, coexisting polyhydramnios may provide adequate space, thus simplifying the process. Conversely, significant challenges arise when the EXIT procedure is further complicated by placenta previa, necessitating careful management to ensure that the uterine incision does not transect the placenta, as this could result in catastrophic hemorrhage. One strategy that has been proposed for addressing cases of placenta accreta spectrum involves the exteriorization of the gravid uterus, followed by a fundal hysterotomy. Each step of this intricate procedure must be verified through real-time intraoperative ultrasound to accurately assess the placenta's position (121). Attention must be paid to the potential resultant iatrogenic angulation of the uterine arteries, as this condition can compromise placental perfusion, thereby necessitating more prompt management of the fetal airway. It is crucial to anticipate intraoperative complications, such as uterine hemorrhage, and it is recommended to ensure the availability of blood transfusion capabilities throughout the procedure (122). To mitigate the risk of postpartum hemorrhage, especially following prolonged uterine manipulation with sustained placental perfusion, the administration of prophylactic uterotonics, along with readiness for advanced interventions, is a critical component of care (120). Continuous instillation of lactated Ringer's solution aids in managing fluid loss throughout the procedure (123). Among secondary therapeutic strategies, the Bakri intrauterine balloon tamponade has demonstrated significant efficacy in controlling severe postpartum hemorrhage, achieving success rates of up to 84.5%, even in placenta accreta cases (124). Moreover, uterine devascularization techniques, such as bilateral uterine artery ligation, continue to represent a cost-effective and fertility-preserving option when bleeding persists or when balloon tamponade proves unsuccessful (125). Post-EXIT care repairs the uterus and ensures hemostasis, thereby minimizing the risk of future uterine rupture (87).
1.7 Neonatal and ENT considerations
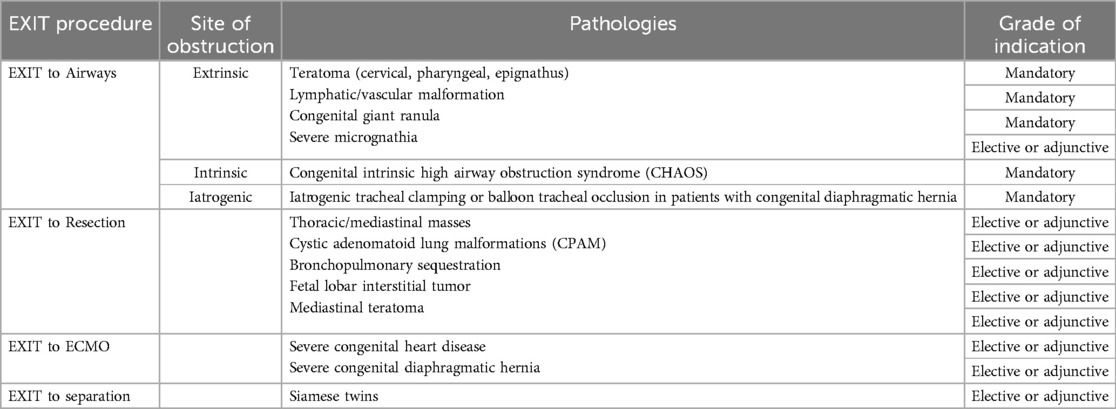
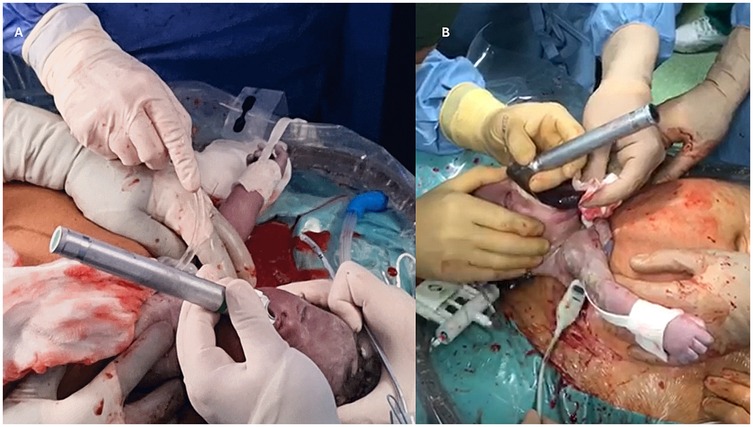
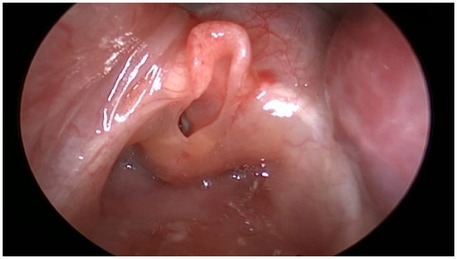
During the EXIT procedure, neonatologists and/or ear, nose, and throat (ENT) surgeons focus on securing and maintaining a clear fetal airway while utilizing uteroplacental circulation to ensure optimal oxygenation (90). These teams must address possible airway obstructions that may arise from masses, congenital anomalies, or structural abnormalities. To manage these obstructions, a range of strategies may be employed, including direct laryngoscopy (Figure 1), rigid or flexible video laryngeal tracheoscopy (Figure 2), surgical tracheostomy (Figure 3), resection of cervical-facial or chest masses, and cannulation for ECMO. The approach will depend on the severity of the obstruction, starting with intubation attempts and escalating to more invasive measures, such as establishing a surgical airway as needed (126). Careful management of the fetal head and neck positions is crucial to optimize airway visualization and facilitate intervention. In cases where large cervical or thoracic masses are present, the ENT team plays a key role in planning for resection. The uterine incision may be extended to allow for adequate mass exposure while ensuring that placental gas exchange remains uninterrupted throughout the procedure. Special attention is given to avoiding pressure on critical structures, including the trachea, esophagus, and major blood vessels (119).
The degree of fetal exposure during the EXIT procedure varies based on intervention needs. Minimizing exposure is crucial to reduce risks like heat loss and umbilical cord issues (90). Continuous fetal monitoring is essential, including pulse oximetry and ultrasounds for blood flow and heart rate (10, 90, 120).
Fetal arterial saturation is monitored with a pulse oximeter on the right hand, typically within a normal range of 60%–70%, where values above 40% indicate adequate oxygenation. Intraoperative fetal echocardiography helps assess cardiovascular function and identify distress signs, necessitating timely interventions. In cases of fetal distress, blood gas samples can be drawn from umbilical vessels to guide treatment. Establishing intravenous access is also essential for fluids and medications (10, 90, 120). Additionally, capnography is used to rapidly confirm neonatal intubation by detecting exhaled carbon dioxide, proving faster and more reliable than colorimetric methods (127, 128). After delivery, the neonatology team transfers the infant to a nearby stabilization unit, prioritizing respiratory support and hemodynamic monitoring. Moreover, additional neonatal surgical interventions are evaluated at this time, and an operating block is prepared adjacent to the relevant team. Postnatal evaluation thoroughly assesses residual airway abnormalities, potential feeding difficulties, and long-term respiratory function (129).
1.8 Length of EXIT
The first documented EXIT procedure lasted 5–20 min and had high fetal morbidity (5). Recent advancements have increased uterine relaxation and prolonged uterine-placental circulation times, typically between 45 and 60 min, although the literature has reported uterine-placental circulation times as long as 150 min (6, 82, 86, 130, 131). A study by Bouchard et al. found an average of approximately 30.3 min for uteroplacental circulation in 31 EXIT cases (120).
The EXIT procedure is complex and requires careful consideration of maternal and fetal factors, appropriate anesthetic techniques, and monitoring for successful outcomes.
1.9 Maternal and fetal complications
The EXIT procedure carries more significant risks than conventional cesarean deliveries, particularly concerning bleeding, procedure duration, and scar-related complications that increase the risk of uterine rupture in future pregnancies. Approximately 6% of mothers may need a blood transfusion during the procedure. There is an estimated 11% risk of uterine rupture in subsequent pregnancies, which is similar to the risk associated with prenatal spina bifida surgery (132–134).
Fetal and neonatal mortality rates for the EXIT procedure range from 5% to 25%, while fetal complications occur in about 13% of cases (12) (135). Common causes of neonatal death include cardiopulmonary arrest, pulmonary hypoplasia, and hypoxia due to failed intubation or tracheostomy (80, 136, 137). Complications arising from the procedure are frequently associated with cardiovascular problems resulting from compression of the chest, neck, or umbilical cord, as well as the effects of anesthesia. Additionally, there have been documented cases of umbilical cord spasms in conjunction with temperature fluctuations (12, 109, 138).
Neurodevelopmental outcomes following EXIT procedures were generally positive, with most children demonstrating age-appropriate cognitive, language, and motor development. However, mild deficits were observed in 31% of language-related cases and 23% of motor skills cases, with no severe impairments reported (139). Improving resource allocation and implementing evidence-based protocols to enhance neurodevelopmental outcomes and reduce associated risks is crucial (129).
1.10 Multidisciplinary team and simulation
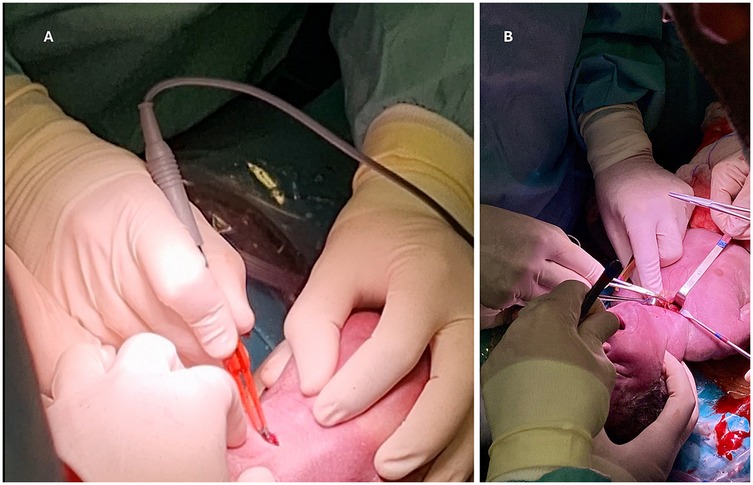
The efficacy of the EXIT procedure is significantly influenced by meticulous multidisciplinary coordination and comprehensive preparation of the operating theater (OT) (140). These guarantee comprehensive patient care from preoperative planning through postoperative management (112). Essential team members include obstetricians, pediatric surgeons, anesthesiologists, neonatologists, otolaryngologists, specialized nursing staff, and, when necessary, cardiac surgeons and perfusionists to support ECMO (Figure 4) (10, 37, 70). Conducting a detailed preoperative briefing that includes a simulation of procedural steps enhances clarity of roles and preparedness for potential emergencies (120). The OT must be equipped with essential tools for neonatal resuscitation and intubation, capnography, sterile surgical instruments, uterine relaxants, and blood products to manage maternal hemorrhage effectively (6). Establishing parallel surgical fields for both neonatal and maternal care is necessary, and a secondary operating room or adjacent resuscitation area must be prepared to address any complications that may arise in either patient (141). Adequate team preparation through simulations and training is essential for ensuring safe and efficient procedures, consequently leading to improved outcomes for both the infant and the mother (78, 142–145). Neonatologists and otolaryngologists should receive training in advanced airway management techniques (Figure 5). Regular rehearsals and tailored strategies advance team synchronization, enhance communication, and mitigate risks, thus optimizing patient care (146, 147). This integrative approach emphasizes the critical importance of collaboration and simulation within the EXIT context, particularly for neonates necessitating complex, high-stakes interventions (123). Furthermore, three-dimensional modeling has proven instrumental in airway planning, family counseling, and perinatal management, especially in intricate clinical scenarios (148).
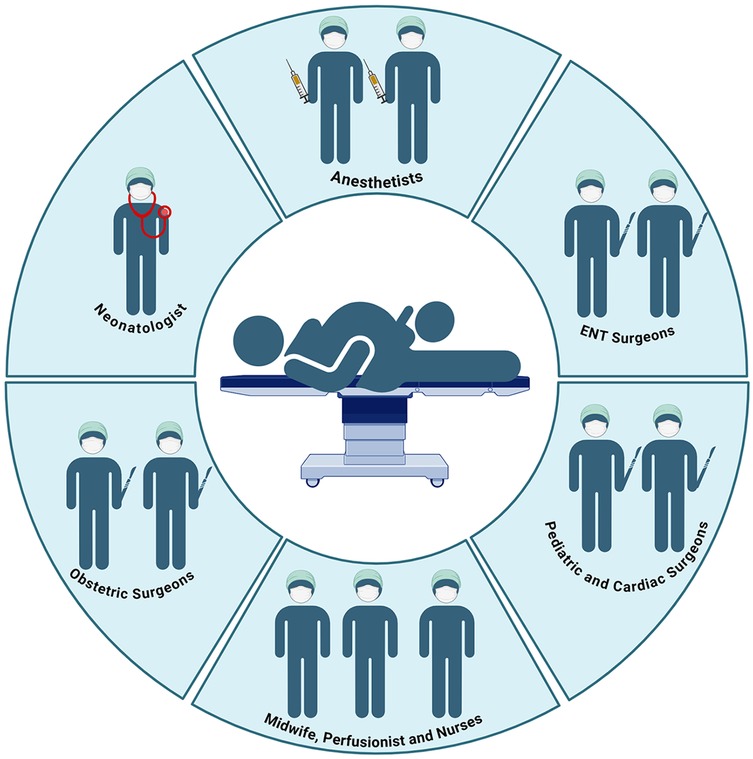
Figure 4. The complexity of the operating room during the EXIT procedure and the professional roles involved. Created in https://BioRender.com.
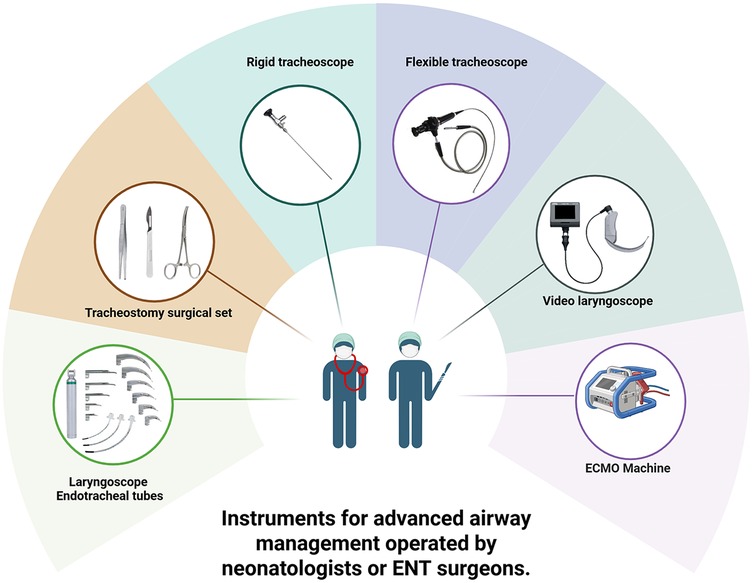
Figure 5. Instruments for advanced airway management operated by neonatologists or ENT surgeons. Created in https://BioRender.com.
2 Conclusions
The EXIT procedure can be considered safe when conducted in specialized centers by a skilled multidisciplinary team. Despite inherent risks, it is crucial to address complex fetal anomalies. Maternal and fetal risks include bleeding, procedural complications, and neonatal mortality, emphasizing the need for careful case selection and expert execution. Ongoing research is essential to improve techniques, enhance safety, and broaden the procedure's applications for better outcomes.
Author contributions
MG: Investigation, Project administration, Methodology, Conceptualization, Writing – review & editing, Formal analysis, Visualization, Data curation, Writing – original draft, Validation. GR: Conceptualization, Investigation, Writing – original draft, Writing – review & editing, Validation, Visualization, Data curation, Project administration, Formal analysis, Methodology. EB: Validation, Investigation, Writing – review & editing, Conceptualization, Writing – original draft, Formal analysis, Project administration, Data curation, Visualization, Methodology. GP: Writing – original draft, Writing – review & editing, Visualization, Conceptualization, Validation, Methodology, Investigation. OC: Visualization, Writing – review & editing, Investigation, Methodology, Writing – original draft. NP: Data curation, Visualization, Writing – original draft, Investigation, Writing – review & editing, Methodology. MC: Conceptualization, Writing – review & editing, Writing – original draft, Methodology, Investigation, Visualization. FG: Writing – original draft, Supervision, Data curation, Methodology, Investigation, Conceptualization, Validation, Visualization, Writing – review & editing. EV: Investigation, Writing – original draft, Conceptualization, Supervision, Visualization, Data curation, Writing – review & editing, Validation, Methodology. IC: Writing – review & editing, Writing – original draft. MF: Writing – original draft, Writing – review & editing. LP: Writing – original draft, Writing – review & editing. GC: Project administration, Writing – review & editing, Visualization, Supervision, Formal analysis, Writing – original draft, Funding acquisition, Validation, Methodology, Investigation, Resources, Conceptualization, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was (partially) supported by the Italian Ministry of Health (Ricerca Corrente).
Conflict of interest
The author(s) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Goh S, Peled C, Kuo M. A review of EXIT: interventions for neonatal airway rescue. Curr Otorhinolaryngol Rep. (2023) 11(1):27–36. doi: 10.1007/s40136-023-00442-9
2. Sawyer T, Yamada N, Umoren R. The difficult neonatal airway. Seminars in Fetal and Neonatal Medicine. (2023) 28(5):101484. doi: 10.1016/j.siny.2023.101484
3. Ferschl MB, Rollins MD. Fetal Surgery and the EXIT procedure. In: Lerman J, editor. Neonatal Anesthesia. Cham: Springer International Publishing (2023). p. 485–503. doi: 10.1007/978-3-031-25358-4_14
4. Spiers A, Legendre G, Biquard F, Descamps P, Corroenne R. Ex utero intrapartum technique (EXIT): indications, procedure methods and materno-fetal complications—a literature review. J Gynecol Obstet Hum Reproduc. (2022) 51(1):102252. doi: 10.1016/j.jogoh.2021.102252
5. Norris MC, Joseph J, Leighton BL. Anesthesia for perinatal surgery. Am J Perinatol. (1989) 6(01):39–40. doi: 10.1055/s-2007-999541
6. Mychaliska GB, Bealer JF, Graf JL, Rosen MA, Adzick NS, Harrison MR. Operating on placental support: the ex utero intrapartum treatment procedure. J Pediatr Surg. (1997) 32(2):227–31. doi: 10.1016/S0022-3468(97)90184-6
7. Elliott R, Vallera C, Heitmiller ES, Isaac G, Lee M, Crino J, et al. Ex utero intrapartum treatment procedure for management of congenital high airway obstruction syndrome in a vertex/breech twin gestation. Int J Pediatr Otorhinolaryngol. (2013) 77(3):439–42. doi: 10.1016/j.ijporl.2012.11.023
8. García-Díaz L, Chimenea A, De Agustín JC, Pavón A, Antiñolo G. Ex-Utero intrapartum treatment (EXIT): indications and outcome in fetal cervical and oropharyngeal masses. BMC Pregnancy Childbirth. (2020) 20(1):598. doi: 10.1186/s12884-020-03304-0
9. Liechty KW. Ex-utero intrapartum therapy. Seminars in Fetal and Neonatal Medicine. (2010) 15(1):34–9. doi: 10.1016/j.siny.2009.05.007
10. Hirose S, Harrison MR. The ex utero intrapartum treatment (EXIT) procedure. Semin Neonatol. (2003) 8(3):207–14. doi: 10.1016/S1084-2756(03)00029-0
11. Hedrick HL. Ex utero intrapartum therapy. Semin Pediatr Surg. (2003) 12(3):190–5. doi: 10.1016/S1055-8586(03)00026-X
12. Novoa RH, Quintana W, Castillo-Urquiaga W, Ventura W. EXIT (Ex utero intrapartum treatment) surgery for the management of fetal airway obstruction: a systematic review of the literature. J Pediatr Surg. (2020) 55(7):1188–95. doi: 10.1016/j.jpedsurg.2020.02.011
13. Graham EM, Ruis KA, Hartman AL, Northington FJ, Fox HE. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am J Obstet Gynecol. (2008) 199(6):587–95. doi: 10.1016/j.ajog.2008.06.094
14. Perlman JM. Summary proceedings from the neurology group on hypoxic-ischemic encephalopathy. Pediatrics. (2006) 117(Suppl_1):S28–33. doi: 10.1542/peds.2005-0620E
15. Walz PC, Schroeder JW. Prenatal diagnosis of obstructive head and neck masses and perinatal airway management: the ex utero intrapartum treatment procedure. Otolaryngol Clin North Am. (2015) 48(1):191–207. doi: 10.1016/j.otc.2014.09.013
16. Chiu H-H, Hsu W-C, Shih J-C, Tsao P-N, Hsieh W-S, Chou H-C. The EXIT (ex utero intrapartum treatment) procedure. J Formos Med Assoc. (2008) 107(9):745–8. doi: 10.1016/S0929-6646(08)60121-7
17. Berrington J, Stafford F, Macphail S. Emergency EXIT for preterm labour after FETO. Arch Dis Childhood-Fetal Neonat Ed. (2010) 95(5):F376–F7. doi: 10.1136/adc.2009.177303
18. Ruano R, Yoshisaki CT, da Silva MM, Ceccon ME, Grasi MS, Tannuri U, et al. A randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound Obstet Gynecol. (2012) 39(1):20–7. doi: 10.1002/uog.10142
19. Johnson AB, Richter GT. Vascular anomalies. Clin Perinatol. (2018) 45(4):737–49. doi: 10.1016/j.clp.2018.07.010
20. Li J-l, Hai-Ying W, Liu J-r, He Q-m, Chen K-s, Yang J, et al. Fetal lymphangioma: prenatal diagnosis on ultrasound, treatment, and prognosis. Eur J Obstet Gynecol Reproduc Biol. (2018) 231:268–73. doi: 10.1016/j.ejogrb.2018.10.018
21. Scavelli C, Weber E, Aglianò M, Cirulli T, Nico B, Vacca A, et al. Lymphatics at the crossroads of angiogenesis and lymphangiogenesis. J Anat. (2004) 204(6):433–49. doi: 10.1111/j.0021-8782.2004.00293.x
22. Benazzou S, Boulaadas M, Essakalli L. Giant pediatric cervicofacial lymphatic malformations. J Craniofac Surg. (2013) 24(4):1307–9. doi: 10.1097/SCS.0b013e3182942b8f
23. de Serres LM, Sie KC, Richardson MA. Lymphatic malformations of the head and neck: a proposal for staging. Arch Otolaryngol Head Neck Surg. (1995) 121(5):577–82. doi: 10.1001/archotol.1995.01890050065012
24. Peterson CM, Buckley C, Holley S, Menias CO. Teratomas: a multimodality review. Curr Probl Diagn Radiol. (2012) 41(6):210–9. doi: 10.1067/j.cpradiol.2012.02.001
25. Tonni G, De Felice C, Centini G, Ginanneschi C. Cervical and oral teratoma in the fetus: a systematic review of etiology, pathology, diagnosis, treatment and prognosis. Arch Gynecol Obstet. (2010) 282(4):355–61. doi: 10.1007/s00404-010-1500-7
26. Alexander VRC, Manjaly JG, Pepper CM, Ifeacho SN, Hewitt RJ, Hartley BE. Head and neck teratomas in children—a series of 23 cases at great ormond street hospital. Int J Pediatr Otorhinolaryngol. (2015) 79(12):2008–14. doi: 10.1016/j.ijporl.2015.07.042
27. Kadlub N, Touma J, Leboulanger N, Garel C, Soupre V, Herminé L, et al. Head and neck teratoma: from diagnosis to treatment. J Cranio-Maxillofacial Sur. (2014) 42(8):1598–603. doi: 10.1016/j.jcms.2014.04.028
28. Puerto B, Eixarch E, Sanz-Cortés M. 71 - Neck teratoma. In: Copel JA, D'Alton ME, Feltovich H, Gratacós E, Krakow D, Odibo AO, et al., editors. Obstetric Imaging: Fetal Diagnosis and Care. 2nd edn. Amsterdam: Elsevier (2018). p. 334–38.e1. doi: 10.1016/B978-0-323-44548-1.00071-1
29. Dirican AÖ. Congenital epignathus and extra-uterine intrapartum treatment procedure. J Obstet Gynecol India. (2023) 74(6):547–9. doi: 10.1007/s13224-023-01901-5
30. Chan DFY, Lee CH, Fung TY, Chan DLW, Abdullah V, Ng PC. Ex utero intrapartum treatment (EXIT) for congenital giant ranula. Acta Paediatr. (2006) 95(10):1303–5. doi: 10.1080/08035250600580545
31. Iro H, Zenk J. Salivary gland diseases in children. GMS Curr Top Otorhinolaryngol Head Neck Surg. (2014) 13:Doc06. doi: 10.3205/cto000109
32. Kolker MT, Batti JS, Schoem SR. The ex utero intrapartum treatment procedure for congenital ranula in a jehovah’s witness. Otolaryngol Head Neck Surg. (2004) 130(4):508–10. doi: 10.1016/j.otohns.2003.09.010
33. Mohammad S, Olutoye OA. Airway management for neonates requiring ex utero intrapartum treatment (EXIT). Pediatric Anesthesia. (2020) 30(3):248–56. doi: 10.1111/pan.13818
34. Benacerraf BR, Bromley B, Jelin AC. Micrognathia. Am J Obstet Gynecol. (2019) 221(5):B13–B5. doi: 10.1016/j.ajog.2019.08.051
35. Fields CM, Poupore NS, Taniguchi AN, Smaily H, Nguyen SA, Cuff RD, et al. Evaluating prenatal diagnostic imaging for micrognathia: a systematic review and meta-analysis. The Cleft Palate Craniofacial J. (2024) 61(12):1957–68. doi: 10.1177/10556656231190525
36. Paladini D. Fetal micrognathia: almost always an ominous finding. Ultrasound Obstet Gynecol. (2010) 35(4):377–84. doi: 10.1002/uog.7639
37. Puricelli MD, Rahbar R, Allen GC, Balakrishnan K, Brigger MT, Daniel SJ, et al. International pediatric otolaryngology group (IPOG): consensus recommendations on the prenatal and perinatal management of anticipated airway obstruction. Int J Pediatr Otorhinolaryngol. (2020) 138:110281. doi: 10.1016/j.ijporl.2020.110281
38. Zhong C, Xie Z, Dong H, Chen T, Zhang X, Ran S. Prenatal diagnosis of pierre robin sequence and its prognosis: a retrospective cohort study. Am J Perinatol. (2024) 41(S01):e1639–46. doi: 10.1055/s-0043-1768233
39. Benjamin B, Walker P. Management of airway obstruction in the pierre robin sequence. Int J Pediatr Otorhinolaryngol. (1991) 22(1):29–37. doi: 10.1016/0165-5876(91)90094-R
40. Tay SY, Krishnasarma R, Mehta D, Mehollin-Ray A, Chandy B. Predictive factors for perinatal outcomes of infants diagnosed with micrognathia antenatally. Ear Nose Throat J. (2021) 100(1):NP16–20. doi: 10.1177/0145561319855641
41. Tonsager SC, Mader NS, Sidman JD, Scott AR. Determining risk factors for early airway intervention in newborns with micrognathia. Laryngoscope. (2012) 122(Suppl 4):S103–4. doi: 10.1002/lary.23788
42. Morris LM, Lim F-Y, Elluru RG, Hopkin RJ, Jaekle RK, Polzin WJ, et al. Severe micrognathia: indications for EXIT-to-airway. Fetal Diagn Ther. (2009) 26(3):162–6. doi: 10.1159/000240162
43. Hedrick MH, Ferro MM, Filly RA, Flake AW, Harrison MR, Scott Adzick N. Congenital high airway obstruction syndrome (CHAOS): a potential for perinatal intervention. J Pediatr Surg. (1994) 29(2):271–4. doi: 10.1016/0022-3468(94)90331-X
44. Roybal JL, Liechty KW, Hedrick HL, Bebbington MW, Johnson MP, Coleman BG, et al. Predicting the severity of congenital high airway obstruction syndrome. J Pediatr Surg. (2010) 45(8):1633–9. doi: 10.1016/j.jpedsurg.2010.01.022
45. Nolan HR, Gurria J, Peiro JL, Tabbah S, Diaz-Primera R, Polzin W, et al. Congenital high airway obstruction syndrome (CHAOS): natural history, prenatal management strategies, and outcomes at a single comprehensive fetal center. J Pediatr Surg. (2019) 54(6):1153–8. doi: 10.1016/j.jpedsurg.2019.02.034
46. Jaminet C, Leonard F, Devlieger R, Chantraine F. Diagnosis and management of a foetus with congenital high airway obstruction syndrome (CHAOS). Rev Med Liege. (2023) 78(2):74–8.36799323
47. Hamid-Sowinska A, Ropacka-Lesiak M, Breborowicz GH. Congenital high airway obstruction syndrome. Neuro Endocrinol Lett. (2011) 32(5):623–6.22167132
48. Lim F-Y, Crombleholme TM, Hedrick HL, Flake AW, Johnson MP, Howell LJ, et al. Congenital high airway obstruction syndrome: natural history and management. J Pediatr Surg. (2003) 38(6):940–5. doi: 10.1016/S0022-3468(03)00128-3
49. Saadai P, Jelin EB, Nijagal A, Schecter SC, Hirose S, MacKenzie TC, et al. Long-term outcomes after fetal therapy for congenital high airway obstructive syndrome. J Pediatr Surg. (2012) 47(6):1095–100. doi: 10.1016/j.jpedsurg.2012.03.015
50. Schauer GM, Dunn LK, Godmilow L, Eagle RC Jr, Knisely AS. Prenatal diagnosis of Fraser syndrome at 18.5 weeks gestation, with autopsy findings at 19 weeks. Am J Med Genet. (1990) 37(4):583–91. doi: 10.1002/ajmg.1320370433
51. Paek BW, Callen PW, Kitterman J, Feldstein VA, Farrell J, Harrison MR, et al. Successful fetal intervention for congenital high airway obstruction syndrome. Fetal Diagn Ther. (2002) 17(5):272–6. doi: 10.1159/000063179
52. Bui TH, Grunewald C, Frenckner B, Kuylenstierna R, Dahlgren G, Edner A, et al. Successful EXIT (ex utero intrapartum treatment) procedure in a fetus diagnosed prenatally with congenital high-airway obstruction syndrome due to laryngeal atresia. Eur J Pediatr Surg. (2000) 10(5):328–33. doi: 10.1055/s-2008-1072385
53. Crombleholme TM, Sylvester K, Flake AW, Adzick NS. Salvage of a Fetus with congenital high airway obstruction syndrome by ex utero intrapartum treatment (EXIT) procedure. Fetal Diagn Ther. (2000) 15(5):280–2. doi: 10.1159/000021022
54. Jeong SH, Lee MY, Kang OJ, Kim R, Chung JH, Won HS, et al. Perinatal outcome of fetuses with congenital high airway obstruction syndrome: a single-center experience. Obstet Gynecol Sci. (2021) 64(1):52–61. doi: 10.5468/ogs.20266
55. Deprest JA, Nicolaides KH, Benachi A, Gratacos E, Ryan G, Persico N, et al. Randomized trial of fetal surgery for severe left diaphragmatic hernia. N Engl J Med. (2021) 385(2):107–18. doi: 10.1056/NEJMoa2027030
56. Basurto D, Sananès N, Bleeser T, Valenzuela I, De Leon N, Joyeux L, et al. Safety and efficacy of smart tracheal occlusion device in diaphragmatic hernia lamb model. Ultrasound Obstet Gynecol. (2021) 57(1):105–12. doi: 10.1002/uog.23135
57. Basurto D, Sananès N, Verbeken E, Sharma D, Corno E, Valenzuela I, et al. New device permitting non-invasive reversal of fetal endoscopic tracheal occlusion: ex-vivo and in-vivo study. Ultrasound Obstet Gynecol. (2020) 56(4):522–31. doi: 10.1002/uog.22132
58. Sananès N, Basurto D, Cordier A-G, Elie C, Russo FM, Benachi A, et al. Fetoscopic endoluminal tracheal occlusion with smart-TO balloon: study protocol to evaluate effectiveness and safety of non-invasive removal. PLoS One. (2023) 18(3):e0273878. doi: 10.1371/journal.pone.0273878
59. Kunisaki SM, Fauza DO, Barnewolt CE, Estroff JA, Myers LB, Bulich LA, et al. Ex utero intrapartum treatment with placement on extracorporeal membrane oxygenation for fetal thoracic masses. J Pediatr Surg. (2007) 42(2):420–5. doi: 10.1016/j.jpedsurg.2006.10.035
60. Adzick NS. Management of fetal lung lesions. Clin Perinatol. (2003) 30(3):481–92. doi: 10.1016/S0095-5108(03)00047-2
61. Cass DL, Olutoye OO, Cassady CI, Zamora IJ, Ivey RT, Ayres NA, et al. EXIT-to-resection for fetuses with large lung masses and persistent mediastinal compression near birth. J Pediatr Surg. (2013) 48(1):138–44. doi: 10.1016/j.jpedsurg.2012.10.067
62. Bose SK, Stratigis JD, Ahn N, Pogoriler J, Hedrick HL, Rintoul NE, et al. Prenatally diagnosed large lung lesions: timing of resection and perinatal outcomes. J Pediatr Surg. (2023) 58(12):2384–90. doi: 10.1016/j.jpedsurg.2023.09.002
63. Cuestas J, Lohmann P, Hagan JL, Vogel AM, Fernandes CJ, Garcia-Prats JA. Mortality trends in neonatal ECMO for pulmonary hypoplasia: a review of the extracorporeal life support organization database from 1981 to 2016. J Pediatr Surg. (2021) 56(4):788–94. doi: 10.1016/j.jpedsurg.2020.09.005
64. Matte GS, Connor KR, Toutenel NA, Gottlieb D, Fynn-Thompson F. A modified EXIT-to-ECMO with optional reservoir circuit for use during an EXIT procedure requiring thoracic surgery. J ExtraCorporeal Technol. (2016) 48(1):35–8. doi: 10.1051/ject/201648035
65. Shieh HF, Wilson JM, Sheils CA, Smithers CJ, Kharasch VS, Becker RE, et al. Does the ex utero intrapartum treatment to extracorporeal membrane oxygenation procedure change morbidity outcomes for high-risk congenital diaphragmatic hernia survivors? J Pediatr Surg. (2017) 52(1):22–5. doi: 10.1016/j.jpedsurg.2016.10.010
66. Michel TC, Rosenberg AL, Polley LS. EXIT To ECMO. Anesthesiology. (2002) 97(1):267–8. doi: 10.1097/00000542-200207000-00036
67. Reeve N, Kahane J, Spinner A, O-Lee T. Ex utero intrapartum treatment to extracorporeal membrane oxygenation: lifesaving management of a giant cervical teratoma. J Laryngol Otol. (2020) 134(7):650–3. doi: 10.1017/S0022215120001206
68. Ossowski K, Suskind DL. Airway management in conjoined twins. Arch Otolaryngol Head Neck Surgery. (2005) 131(1):58. doi: 10.1001/archotol.131.1.58
69. Snoap A, Varadarajan VV, Mowitz ME, Islam S, Collins WO. Airway management protocol for conjoined twins delivery. Int J Pediatr Otorhinolaryngol. (2021) 140:110477. doi: 10.1016/j.ijporl.2020.110477
70. Moldenhauer JS. Ex utero intrapartum therapy. Semin Pediatr Surg. (2013) 22(1):44–9. doi: 10.1053/j.sempedsurg.2012.10.008
71. Saleem SN. Fetal MRI: an approach to practice: a review. J Adv Res. (2014) 5(5):507–23. doi: 10.1016/j.jare.2013.06.001
72. Dong S-Z, Zhu M, Ji H, Ren J-Y, Liu K. Fetal cardiac MRI: a single center experience over 14-years on the potential utility as an adjunct to fetal technically inadequate echocardiography. Sci Rep. (2020) 10(1):12373. doi: 10.1038/s41598-020-69375-3
73. Mota R, Ramalho C, Monteiro J, Correia-Pinto J, Rodrigues M, Guimarães H, et al. Evolving indications for the EXIT procedure: the usefulness of combining ultrasound and fetal MRI. Fetal Diagn Ther. (2007) 22(2):107–11. doi: 10.1159/000097106
74. Coleman AM, Merrow AC, Elluru RG, Polzin WJ, Lim F-Y. Tracheal agenesis with tracheoesophageal fistulae: fetal MRI diagnosis with confirmation by ultrasound during an ex utero intrapartum therapy (EXIT) delivery and postdelivery MRI. Pediatr Radiol. (2013) 43:1385–90. doi: 10.1007/s00247-013-2679-0
75. Kobayashi R, Sumiya W, Imanishi T, Kanno C, Kanno M, Unemoto J, et al. Fetal-onset malignant rhabdoid tumor: a case report. J Med Case Reports. (2022) 16(1):1–5. doi: 10.1186/s13256-022-03503-7
76. Sabra R, Gheorghe CP, Monson MA, Masri J, Chmait RH. In utero treatment of congenital high airway obstruction syndrome via fetal laryngoscopy and EXIT procedure. Fetal Diagn Ther. (2023) 49(9-10):385–93. doi: 10.1159/000526798
77. Papaioannou G, Caro-Domínguez P, Klein WM, Garel C, Cassart M. Indications for magnetic resonance imaging of the fetal body (extra-central nervous system): recommendations from the European society of paediatric radiology fetal task force. Pediatr Radiol. (2023) 53(2):297–312. doi: 10.1007/s00247-022-05495-4
78. Poutamo J, Vanninen R, Partanen K, Ryynänen M, Kirkinen P. Magnetic resonance imaging supplements ultrasonographic imaging of the posterior fossa, pharynx and neck in malformed fetuses. Ultrasound Obstet Gynecol. (1999) 13(5):327–34. doi: 10.1046/j.1469-0705.1999.13050327.x
79. Schmoke N, Nemeh C, Wu YS, Wilken T, Wang P, Kurlansky P, et al. Management of fetal head and neck masses: evaluation of prenatal factors associated with airway obstruction and decision for definitive airway and ex-utero intrapartum treatment at birth. Prenat Diagn. (2024) 44(10):1225–30. doi: 10.1002/pd.6586
80. Lazar DA, Olutoye OO, Moise KJ Jr, Ivey RT, Johnson A, Ayres N, et al. Ex-utero intrapartum treatment procedure for giant neck masses—fetal and maternal outcomes. J Pediatr Surg. (2011) 46(5):817–22. doi: 10.1016/j.jpedsurg.2011.02.006
81. Kumar K, Miron C, Singh SI. Maternal anesthesia for EXIT procedure: a systematic review of literature. J Anaesthesiol Clin Pharmacol. (2019) 35(1):19. doi: 10.4103/joacp.JOACP_302_17
82. Schwartz DA, Moriarty KP, Tashjian DB, Wool RS, Parker RK, Markenson GR, et al. Anesthetic management of the exit (ex utero intrapartum treatment) procedure. J Clin Anesth. (2001) 13(5):387–91. doi: 10.1016/S0952-8180(01)00287-2
83. Chinnappa V, Halpern SH. The ex utero intrapartum treatment (EXIT) procedure: maternal and fetal considerations. Can J Anesthesia J Canadien d'Anesthesie. (2007) 54(3):171–5. doi: 10.1007/BF03022636
84. Choleva AJ. Anesthetic management of a patient undergoing an ex utero intrapartum treatment (EXIT) procedure: a case report. AANA J. (2011) 79(6):497–503.22400417
85. Dahlgren G, Törnberg DC, Pregner K, Irestedt L. Four cases of the ex utero intrapartum treatment (EXIT) procedure: anesthetic implications. Int J Obstet Anesth. (2004) 13(3):178–82. doi: 10.1016/j.ijoa.2004.01.007
86. Gaiser RR, Cheek TG, Kurth CD. Anesthetic management of cesarean delivery complicated by ex utero intrapartum treatment of the fetus. Anesth Analg. (1997) 84(5):1150–3. doi: 10.1213/00000539-199705000-00039
87. Garcia PJ, Olutoye OO, Ivey RT, Olutoye OA, Riou B. Case scenario: anesthesia for maternal-fetal surgery. Anesthesiology. (2011) 114(6):1446–52. doi: 10.1097/ALN.0b013e31821b173e
88. Gitterman A, Reschke M, Berman DJ. Anesthesia for the EXIT procedure. In: Goudra BG, Singh PM, Green MS, editors. Anaesthesia for Uncommon and Emerging Procedures. Cham: Springer (2021) p. 333–42. doi: 10.1007/978-3-030-64739-1_33
89. Helfer DC, Clivatti J, Yamashita AM, Moron AF. Anestesia para tratamento intraparto extraútero (EXIT) em fetos com diagnóstico pré-natal de malformações cervical e oral: relato de casos. Rev Bras Anestesiol. (2012) 62(3):417–23. doi: 10.1590/S0034-70942012000300013
90. Marwan A, Crombleholme TM. The EXIT procedure: principles, pitfalls, and progress. Semin Pediatr Surg. (2006) 15(2):107–15. doi: 10.1053/j.sempedsurg.2006.02.008
91. Ngamprasertwong P, Vinks AA, Boat A. Update in fetal anesthesia for the ex utero intrapartum treatment (EXIT) procedure. Int Anesthesiol Clin. (2012) 50(4):26–40. doi: 10.1097/AIA.0b013e31826df966
92. Oliveira E, Pereira P, Retroz C, Mártires E. Anesthesia for EXIT procedure (ex utero intrapartum treatment) in congenital cervical malformation—a challenge to the anesthesiologist. Brazilian J Anesthesiol (English Edition). (2015) 65(6):529–33. doi: 10.1016/j.bjane.2013.07.020
93. Wood CL, Zuk J, Rollins MD, Silveira LJ, Feiner JR, Zaretsky M, et al. Anesthesia for maternal-fetal interventions: a survey of fetal therapy centers in the North American fetal therapy network. Fetal Diagn Ther. (2021) 48(5):361–71. doi: 10.1159/000514897
94. Subramanian R, Mishra P, Subramaniam R, Bansal S. Role of anesthesiologist in ex utero intrapartum treatment procedure: a case and review of anesthetic management. J Anaesthesiol Clin Pharmacol. (2018) 34(2):148–54. doi: 10.4103/joacp.JOACP_239_16
95. Butwick A, Aleshi P, Yamout I. Obstetric hemorrhage during an EXIT procedure for severe fetal airway obstruction. Can J Anesth. (2009) 56(6):437. doi: 10.1007/s12630-009-9092-z
96. Zadra N, Giusti F, Midrio P. Ex utero intrapartum surgery (EXIT): indications and anaesthetic management. Best Pract Res Clin Anaesthesiol. (2004) 18(2):259–71. doi: 10.1016/j.bpa.2003.11.001
97. Younger JD, Reitman E, Gallos G. Tocolysis: present and future treatment options. Semin Perinatol. (2017) 41(8):493–504. doi: 10.1053/j.semperi.2017.08.008
99. Varcoe TJ, Darby JRT, Holman SL, Bradshaw EL, Kuchel T, Vaughan L, et al. Fetal cardiovascular response to acute hypoxia during maternal anesthesia. Physiol Rep. (2020) 8(3):e14365. doi: 10.14814/phy2.14365
100. Sirianni J, Abro J, Gutman D. Delivery of an infant with airway compression due to cystic hygroma at 37 weeks’ gestation requiring a multidisciplinary decision to use a combination of ex utero intrapartum treatment (EXIT) and airway palliation at cesarean section. Am J Case Rep. (2021) 22:e927803. doi: 10.12659/AJCR.927803
101. Richard S. Maternal and fetal cardiovascular and acid–base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. (1974) 41(5):462–71. doi: 10.1097/00000542-197411000-00010
102. Boat A, Mahmoud M, Michelfelder EC, Lin E, Ngamprasertwong P, Schnell B, et al. Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Pediatric Anesthesia. (2010) 20(8):748–56. doi: 10.1111/j.1460-9592.2010.03350.x
103. Noguchi S, Tanaka M, Terui K. The first national survey of anesthesia techniques for fetal therapies in Japan. J Anesth. (2019) 33(6):665–9. doi: 10.1007/s00540-019-02690-w
104. Turner RJ, Lambros M, Holmes C, Katz SG, Downs CS, Collins DW, et al. The effects of sevoflurane on isolated gravid human myometrium. Anaesth Intensive Care. (2002) 30(5):591–6. doi: 10.1177/0310057X0203000508
105. Metodiev Y, Lucas DN. The role of total intravenous anaesthesia for caesarean delivery. Int J Obstet Anesth. (2022) 51:103548. doi: 10.1016/j.ijoa.2022.103548
106. Varghese R, Sebastian G, Thomas DE, Kumar L. Anaesthesia for foetal ex-utero intrapartum therapy (EXIT) surgery. Indian J Anaesth. (2024) 68(12):1103–5. doi: 10.4103/ija.ija_778_24
107. Abraham RJ, Sau A, Maxwell D. A review of the EXIT (ex utero intrapartum treatment) procedure. J Obstet Gynaecol. (2010) 30(1):1–5. doi: 10.3109/01443610903281656
108. Sangaletti M, Garzon S, Raffaelli R, D'Alessandro R, Bosco M, Casarin J, et al. The ex utero intrapartum treatment (EXIT) procedure: case report of a multidisciplinary team approach. Acta Biomed. (2021) 92(S1):e2021142. doi: 10.23750/abm.v92iS1.9964
109. Domínguez-Moreno M, Chimenea Á, García-Díaz L, Antiñolo G. Maternal and obstetric outcomes after ex-utero intrapartum treatment (EXIT): a single center experience. BMC Pregnancy Childbirth. (2023) 23(1):831. doi: 10.1186/s12884-023-06129-9
110. Hedrick HL, Flake AW, Crombleholme TM, Howell LJ, Johnson MP, Wilson RD, et al. The ex utero intrapartum therapy procedure for high-risk fetal lung lesions. J Pediatr Surg. (2005) 40(6):1038–44. doi: 10.1016/j.jpedsurg.2005.03.024
111. Domínguez-Moreno M, Chimenea Á, Viegas-González MR, Morales-Muñoz C, García-Díaz L, Antiñolo G. A detailed exploration of the ex utero intrapartum treatment procedure with center-specific advancements. Surg Techniq Dev. (2024) 13(1):76–86. doi: 10.3390/std13010005
112. Dadhwal V, Sahay N, Sharma KA. Standard operating procedures ex utero intrapartum therapy (EXIT). J Fetal Med. (2024) 11(03):167–71. doi: 10.1055/s-0044-1788283
113. Bence CM, Wagner AJ. Ex utero intrapartum treatment (EXIT) procedures. Semin Pediatr Surg. (2019) 28(4):150820. doi: 10.1053/j.sempedsurg.2019.07.003
114. Kloka JA, Jasny T, Jennewein L, Friedrichson B, Zacharowski K, Neef V. Maternal anemia and red blood cell requirements in 72 women undergoing ex-utero intrapartum treatment (EXIT) procedure. Front Med (Lausanne). (2024) 11:1353405. doi: 10.3389/fmed.2024.1353405
115. Dighe MK, Peterson SE, Dubinsky TJ, Perkins J, Cheng E. EXIT Procedure: technique and indications with prenatal imaging parameters for assessment of airway patency. Radiographics. (2011) 31(2):511–26. doi: 10.1148/rg.312105108
116. Coviello D, Bonati F, Montefusco SM, Mastromatteo C, Fabietti I, Rustico M. Amnioreduction. Acta Biomed. (2004) 75(Suppl 1):31–3.15301287
117. Hwang D, Jenkins S, Mahdy H. Polyhydramnios. Treasure Island (FL): StatPearls Publishing (2024). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK562140/
118. Olive AM, Kim AG, Flake AW. Fetal surgery. In: Puri P. editor. Pediatric Surgery. Berlin, Heidelberg: Springer (2020). p. 115–35. doi: 10.1007/978-3-662-43588-5_7
119. Stohl SM, Stohl HE, Weintraub AY, Tran KM. The EXIT procedure. In: Ginosar Y, Reynolds F, Halpern S, Weiner CP, editors. Anesthesia and the Fetus. 1st ed. Chichester, West Sussex, UK: Blackwell Publishing Ltd. (2013). p. 183–91.
120. Bouchard S, Johnson MP, Flake AW, Howell LJ, Myers LB, Adzick NS, et al. The EXIT procedure: experience and outcome in 31 cases. J Pediatr Surg. (2002) 37(3):418–26. doi: 10.1053/jpsu.2002.30839
121. Collins SL, Addley S, Weeks E, Chakravarti S, Halder S, Alazzam M. The modified radical peripartum cesarean hysterectomy (Soleymani-Alazzam-Collins technique): a systematic, safe procedure for the management of severe placenta accreta spectrum. Am J Obstet Gynecol. (2021) 225(2):175. e1–. e10. doi: 10.1016/j.ajog.2021.03.014
122. Otteson TD, Hackam DJ, Mandell DL. The ex utero intrapartum treatment (EXIT) procedure: new challenges. Archives of Otolaryngology–Head & Neck Surgery. (2006) 132(6):686–9. doi: 10.1001/archotol.132.6.686
123. Varela MF, Peiro JL. Ex-utero intrapartum treatment (EXIT). Revista Médica Clínica Las Condes. (2021) 32(6):690–8. doi: 10.1016/j.rmclc.2021.09.009
124. Hu Y, Cui L, Zhang C, Chen F. Timely use of bakri intrauterine balloon tamponade contributes to the effectiveness in controlling severe postpartum hemorrhage. Exp Ther Med. (2024) 27(5):177. doi: 10.3892/etm.2024.12465
125. Bonsen LR, van den Akker-van Marle ME, Caram-Deelder C, Ramler PI, de Groot CJ, Urlings T, et al. Cost analysis of intrauterine balloon tamponade versus uterine artery embolization in the management of persistent postpartum hemorrhage. Int J Gynaecol Obstet. (2025):1–9. doi: 10.1002/ijgo.70149
126. Schenone M, Thompson J, Thompson R, Giancarlo M. Exit procedure: an evidence-based practical approach. In: Thompson J, editor. Pediatric Otolaryngology: A Concise Guide to Pediatric Ear, Nose and Throat. Newcastle upon Tyne, UK: Cambridge Scholar Publishing (2020). p. 138–59.
127. Sankaran D, Zeinali L, Iqbal S, Chandrasekharan P, Lakshminrusimha S. Non-invasive carbon dioxide monitoring in neonates: methods, benefits, and pitfalls. J Perinatol. (2021) 41(11):2580–9. doi: 10.1038/s41372-021-01134-2
128. Williams E, Dassios T, Greenough A. Carbon dioxide monitoring in the newborn infant. Pediatr Pulmonol. (2021) 56(10):3148–56. doi: 10.1002/ppul.25605
129. Joshi D, Stellon M, Antony K, Beninati M, Luks FI, Puricelli M, et al. Indications, resource allocation, and outcomes associated with ex-utero intrapartum treatment procedures: a north American fetal therapy network survey. Fetal Diagn Ther. (2023) 50(5):376–86. doi: 10.1159/000531615
130. Catalano PJ, Urken ML, Alvarez M, Norton K, Wedgewood J, Biller HF. New approach to the management of airway obstruction in high risk neonates. Arch Otolaryngol Head Neck Surg. (1992) 118(3):306–9. doi: 10.1001/archotol.1992.01880030094019
131. Langer JC, Fitzgerald PG, Desa D, Filly RA, Golbus MS, Adzick NS, et al. Cervical cystic hygroma in the fetus: clinical spectrum and outcome. J Pediatr Surg. (1990) 25(1):58–62. doi: 10.1016/S0022-3468(05)80164-2
132. Moldenhauer JS, Soni S, Rintoul NE, Spinner SS, Khalek N, Martinez-Poyer J, et al. Fetal myelomeningocele repair: the post-MOMS experience at the children’s hospital of Philadelphia. Fetal Diagn Ther. (2015) 37(3):235–40. doi: 10.1159/000365353
133. Noah MMS, Norton ME, Sandberg P, Esakoff T, Farrell J, Albanese CT. Short-term maternal outcomes that are associated with the exit procedure, as compared with cesarean delivery. Am J Obstet Gynecol. (2002) 186(4):773–7. doi: 10.1067/mob.2002.112249
134. Zamora IJ, Ethun CG, Evans LM, Olutoye OO, Ivey RT, Haeri S, et al. Maternal morbidity and reproductive outcomes related to fetal surgery. J Pediatr Surg. (2013) 48(5):951–5. doi: 10.1016/j.jpedsurg.2013.02.010
135. Lin EE, Moldenhauer JS, Tran KM, Cohen DE, Adzick NS. Anesthetic management of 65 cases of ex utero intrapartum therapy: a 13-year single-center experience. Anesth Analg. (2016) 123(2):411–7. doi: 10.1213/ANE.0000000000001385
136. Barrette L-X, Morales CZ, Oliver ER, Gebb JS, Feygin T, Lioy J, et al. Risk factor analysis and outcomes of airway management in antenatally diagnosed cervical masses. Int J Pediatr Otorhinolaryngol. (2021) 149:110851. doi: 10.1016/j.ijporl.2021.110851
137. Masahata K, Soh H, Tachibana K, Sasahara J, Hirose M, Yamanishi T, et al. Clinical outcomes of ex utero intrapartum treatment for fetal airway obstruction. Pediatr Surg Int. (2019) 35(8):835–43. doi: 10.1007/s00383-019-04494-1
138. Dick JR, Wimalasundera R, Nandi R. Maternal and fetal anaesthesia for fetal surgery. Anaesthesia. (2021) 76(S4):63–8. doi: 10.1111/anae.15423
139. Danzer E, Siegle J, D’Agostino JA, Gerdes M, Hoffman C, Bernbaum J, et al. Early neurodevelopmental outcome of infants with high-risk fetal lung lesions. Fetal Diagn Ther. (2012) 31(4):210–5. doi: 10.1159/000336228
140. Vaishnav D, Gandhi S, Bansode S. Our experience with EXIT procedures: a case series. Indian J Otolaryngol Head Neck Surg. (2024) 76(6):5992–6. doi: 10.1007/s12070-024-05034-7
141. Olutoye O. Anaesthesia for the EXIT procedure: a review. Southern African J Anaesthesia Analgesia. (2009) 15(1):17–21. doi: 10.1080/22201173.2009.10872582
142. Auguste TC, Boswick JA, Loyd MK, Battista A. The simulation of an ex utero intrapartum procedure to extracorporeal membrane oxygenation. J Pediatr Surg. (2011) 46(2):395–8. doi: 10.1016/j.jpedsurg.2010.10.007
143. Daniels K, Auguste T. Moving forward in patient safety: multidisciplinary team training. Semin Perinatol. (2013) 37(3):146–50. doi: 10.1053/j.semperi.2013.02.004
144. Battista A, Nestel D. Simulation in medical education. In: Swanwick T, Forrest K, O'Brien BC, editors. Understanding Medical Education: Evidence, Theory and Practice. 3rd edn. The Association for the Study of Medical Education (ASME). Chichester: John Wiley & Sons Ltd (2018). p. 151–62. doi: 10.1002/9781119373780.ch11
145. Wild KT, Rintoul NE, Ades AM, Gebb JS, Moldenhauer JS, Mathew L, et al. The delivery room resuscitation of infants with congenital diaphragmatic hernia treated with fetoscopic endoluminal tracheal occlusion: beyond the balloon. Fetal Diagn Ther. (2024) 51(2):184–90. doi: 10.1159/000536209
146. Shalev S, Ben-Sira L, Wasserzug O, Shaylor R, Shiran SI, Ekstein M. Utility of three-dimensional modeling of the fetal airway for ex utero intrapartum treatment. J Anesth. (2021) 35(4):595–8. doi: 10.1007/s00540-021-02950-8
147. van Haren JS, van der Hout-van der Jagt MB, Meijer N, Monincx M, Delbressine FLM, Griffith XLG, et al. Simulation-based development: shaping clinical procedures for extra-uterine life support technology. Adv Simul. (2023) 8(1):29. doi: 10.1186/s41077-023-00267-y
Keywords: Ex utero intrapartum treatment, EXIT, newborn, airway obstruction, tracheal occlusion, congenital neck masses, vascular abnormalities, lymphatic malformations
Citation: Gaffuri M, Raffaeli G, Bullejos Garcia EE, Perugino G, Cassardo O, Persico N, Colnaghi M, Garrido F, Villamor E, Cetin I, Fumagalli M, Pignataro L and Cavallaro G (2025) The Ex-utero intrapartum treatment procedure: a narrative review. Front. Pediatr. 13:1601963. doi: 10.3389/fped.2025.1601963
Received: 28 March 2025; Accepted: 2 July 2025;
Published: 17 July 2025.
Edited by:
Simonetta Costa, Casilino General Hospital, ItalyReviewed by:
Luca Pierri, AORN Santobono Pausilipon, ItalyVatsla Dadhwal, University of Illinois Chicago, United States
Copyright: © 2025 Gaffuri, Raffaeli, Bullejos Garcia, Perugino, Cassardo, Persico, Colnaghi, Garrido, Villamor, Cetin, Fumagalli, Pignataro and Cavallaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Emilia Bullejos Garcia, ZWxlbmEuYnVsbGVqb3NnYXJjaWFAcG9saWNsaW5pY28ubWkuaXQ=
†These authors share first authorship
 Michele Gaffuri
Michele Gaffuri Genny Raffaeli3,†
Genny Raffaeli3,† Ottavio Cassardo
Ottavio Cassardo Mariarosa Colnaghi
Mariarosa Colnaghi Felipe Garrido
Felipe Garrido Eduardo Villamor
Eduardo Villamor Irene Cetin
Irene Cetin Monica Fumagalli
Monica Fumagalli Lorenzo Pignataro
Lorenzo Pignataro Giacomo Cavallaro
Giacomo Cavallaro