- 1College of Medical Technology, Chongqing Medical and Pharmaceutical College, Chongqing, China
- 2Intensive Care Unit, The First Affiliated Hospital of Chongqing Medical and Pharmaceutical College, Chongqing, China
- 3Department of Pharmacy, The First Affiliated Hospital of Chongqing Medical and Pharmaceutical College, Chongqing, China
Acute osteofascial compartment syndrome (AOCS) is rarely seen in patients with acute herbicide poisoning, and no cases have been reported in children. The correct treatment approach is of vital importance. We report a case of a 13-year-old girl who was admitted to our hospital 17 h after oral ingestion of “diquat” (a herbicide), and developed clinical symptoms of AOCS 21 h later. The girl was treated with measures such as removing the toxins, using mannitol for dehydration, and elevating the affected limb. The patient's AOCS progressed relentlessly; on hospital day 4, after failure of conservative management, she underwent decompressive surgical fasciotomy (DSF) of the lower legs. The girl responded well to continuous renal replacement therapy (CRRT) and continuous fascial decompression treatment. On hospital day 25, she underwent debridement and suture surgery on both lower legs. At discharge, the girl's lower legs bilateral suture incisions were healing well, and the affected legs could stand. Two months later, the girl was able to walk independently.
1 Introduction
AOCS is a serious complication of traumatic orthopedic injuries, and is rarely observed in patients with acute poisoning, especially children (1). Children and adults exhibit markedly distinct diagnostic and therapeutic approaches to AOCS. This is the first case of AOCS in children caused by acute herbicide poisoning reported (2). There are no statistical results on the incidence of pediatric acute osteofascial compartment syndrome (PAOCS), and the main cause is trauma, such as fractures (3). Research statistics indicate that the annual incidence of AOCS in women is 0.7 per 100,000, which is one-tenth that in men, and the rate of those requiring DSF is less than 1% (4, 5). DSF is critical and challenging to determine, with leg circumference serving as an important indicator of AOCS tissue pressure. Muscle necrosis in AOCS induces to rhabdomyolysis, even acute renal failure in severe patients (6). In the case report, the diagnosis and treatment of a pediatric patient with AOCS, rhabdomyolysis and even acute renal failure as a result of acute herbicide poisoning. Unlike AOCS of traumatic origin, toxicant-induced AOCS mandates concurrent aggressive detoxification alongside standard limb-salvage measures. Additionally, special attention needs to be paid to the psychological issues of children. We report an exceptional instance of diquat-associated AOCS in a child and chronicle the temporal evolution of disease, decision points at every therapeutic juncture, the urgency and continuity of toxin elimination, and the timing for selecting DSF. These data may serve as a reference for treating herbicide-related AOCS in the pediatric population.
2 Case report
The case presents a 13-year-old girl who consumed approximately 60 ml (12 g) of diquat, underwent gastric lavage 10 min post-ingestion, subsequently being transferred to our hospital 17 h later. On arrival the girl was alert and reported that she had initially been asymptomatic immediately after ingestion, but subsequently developed persistent nausea, more than ten episodes of emesis, and profuse diarrhoea. Physical examination revealed a cooperative child with erythematous, oedematous pharyngeal mucosa; no oedema was present in the lower extremities, and the remainder of the systemic survey was unremarkable. Whole-blood qualitative screening confirmed diquat, and quantitative urine diquat concentration was 15,000 ng·ml−1. Laboratory data demonstrated hepatic and renal dysfunction. Myoglobin (MB) and creatine kinase-MB (CK-MB) were markedly elevated, indicating acute myocytolysis. Blood indicators were increased, such as C-reactive protein, interleukin 6, white blood cell count and neutrophil granulocyte; peripheral blood smear showed a mild left shift with toxic granulation of neutrophils. Etailed indicators are shown in Table 1. Chest CT revealed bilateral inflammatory infiltrates; ultrasonography and other imaging studies were otherwise normal. Based on the history of massive diquat ingestion, the diagnosis of severe acute diquat poisoning was established. Immediate management included comprehensive gastrointestinal decontamination with concurrent administration of activated charcoal plus mannitol and montmorillonite plus mannitol for adsorption and catharsis; intravenous omeprazole for gastric protection; glutathione plus vitamin C for antioxidant therapy; and high-dose dexamethasone pulse therapy (30 mg daily for 3 days, then tapered by 10 mg daily until discontinuation). On the day of admission extracorporeal toxin removal was initiated: alternating cycles of haemoperfusion and plasma exchange were performed for four consecutive days, complemented by daily CRRT. On hospital days 11 and 19, CRRT was terminated after blood return because the blood pump could not maintain continuous flow. Consequently, the patient received no dialysis sessions on days 11, 20, or 21 of admission.
At 20:54 on the day of admission—21 h post-ingestion-the patient developed cold, cyanotic extremities with mild bilateral lower-limb swelling, increased calf tension and feeble dorsalis pedis pulses. She reported severe bilateral leg pain; the legs were elevated and analgesia was provided with sufentanil. 28 h after ingestion she became delirious and uncooperative, with transient tachycardia that resolved within 10 min. On hospital day 2, physical examination revealed progressive soft-tissue swelling of both lower legs and increased muscular compartment tension; plantar reflexes were absent. All laboratory indices related to AOCS were markedly abnormal (Table 1). Serum CK exceeded 8,000 U·L−1, indicating severe muscle necrosis. Ultrasonography of the extremities disclosed circumferential subcutaneous oedema and soft-tissue thickening of both thighs. In conjunction with the patient's clinical presentation and all ancillary investigations-including the triad of agitation, anxiety and inadequate analgesia (“3A” sign)—the multidisciplinary team concluded that severe acute diquat poisoning had precipitated AOCS and rhabdomyolysis, with concomitant multi-organ failure involving the liver, kidneys, myocardium and central nervous system, complicated by systemic inflammatory response syndrome and toxic encephalopathy. Orthopaedic surgery recommended immediate DSF. Although counselled that ongoing compartment hypertension would inexorably worsen the injury, the girl's mother, anxious about possible postoperative sequelae and the long incisional scar that DSF would leave, declined the procedure. Conservative measures were therefore instituted: intravenous mannitol for osmotic diuresis and limb elevation to attenuate oedema. Hospital day 3 was characterised by episodic restlessness and high-pitched screaming that were refractory to incremental doses of analgesics and sedatives.
On hospital day 4 the patient became obtunded (Glasgow Coma Scale E3V4M5); both lower extremities were massively swollen, the distal toes were pale, dorsalis pedis pulses were absent, and a violaceous marbling of the overlying skin was evident. Faced with relentless clinical deterioration, the mother consented to emergency bilateral lower-limb DSF. The procedure involved making incisions approximately 20 cm in length, starting from the lower margin of the fibular head on both sides and extending to 6 cm above the tip of the lateral malleolus. The subcutaneous and fascial layers were carefully dissected using a stopover technique. Following DSF surgery, continuous decompression was applied to both lower limbs, with simultaneous monitoring of incisional exudate volume and bilateral lower-limb tension. On hospital day 6, copious serosanguinous drainage persisted from the calf incisions while thigh pressures remained elevated. Dressings were changed and the deep posterior fascial compartments were further released to ensure complete decompression. The main monitoring indicators and leg circumference measurements are detailed in Tables 1, 2, including MB, CK, creatinine, blood urea nitrogen, urine volume and blood indicators, as well as liver enzymes et al. Leucocytosis worsened throughout hospitalisation, attributed to infection and a probable leukemoid reaction. Concurrent critical care included management of respiratory failure, circulatory collapse, toxic encephalopathy, toxic shock syndrome and severe pneumonia caused by carbapenem-resistant Acinetobacter baumannii, complicated by recurrent sepsis and septic shock. Broad-spectrum antimicrobials and supportive therapies were administered accordingly. On hospital day 25, necrotic skin and subcutaneous tissues adjacent to the fasciotomy wounds were debrided and the wounds were closed primarily (Figure 1B). By hospital day 32 renal function had normalised, the fasciotomy wounds had healed satisfactorily and lower-limb motor function had recovered sufficiently to allow unassisted standing (Figure 1C). At two-month follow-up the patient had regained independent ambulation and renal indices remained within reference limits.
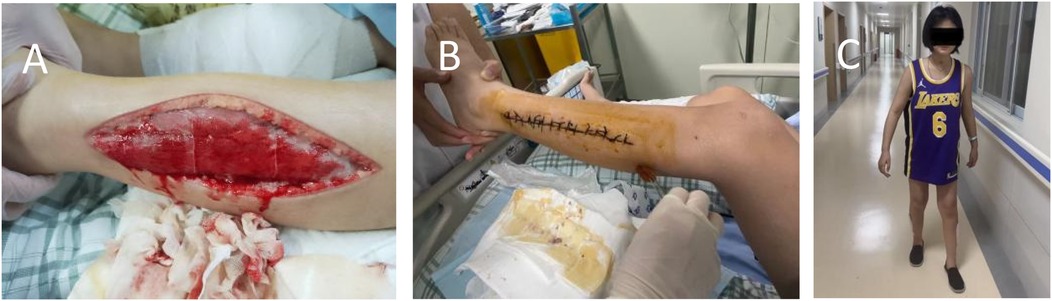
Figure 1. (A) Decompressive surgical fasciotomy; (B) incisional suture; (C) standing photograph of child.
3 Discussion
AOCS is a series of symptoms and signs caused by acute ischemia of muscles and nerves in osteofascial compartment. If it is not treated in time, it can lead to tissue necrosis. Rhabdomyolysis occurs secondary in 12%–44% of patients with compartment syndrome, and acute kidney injury occurs in 10%–55% of patients with rhabdomyolysis (7). Children are more vulnerable to reperfusion injury, acute kidney injury and recurrent compartmental re-stricture; therefore, they require more precise volume management and multimodal analgesia (8). Such patients are often difficult to diagnose and treat early and have poor prognosis. Reports of rhabdomyolysis caused by herbicides are not uncommon. Feng et al. (9) first documented diquat-induced rhabdomyolysis in a 36-year-old woman who ingested 30 ml of diquat; after aggressive extracorporeal elimination therapy, the patient achieved complete clinical recovery and was discharged in stable condition. However, AOCS is rare that it is caused by herbicide poisoning, and no pediatric case. In a systematic review and meta-analysis of PAOCS, fasciotomy was performed in 227 of 233 cases (97.4%). Pain was the most common presenting symptom (88%), followed by paresthesias (32%), and the mean interval from injury to fasciotomy was 25.4 h. Among the documented aetiologies, toxic causes included snake or insect envenomation, drug overdose producing vasotoxic effects (10). A previously reported 2-year-old toddler ingested diquat. During the first 18 h, manifestations were confined to localized signs of intoxication. Systemic toxicity subsequently developed, yet AOCS did not occur. The child succumbed to progressive multi-organ failure 77 h post-exposure (11). We report a case of acute herbicide (diquat) poisoning induced AOCS in a girl with secondary rhabdomyolysis and acute renal failure. The girl underwent necessary DSF due to AOCS and eventually recovered.
PAOCS remains a condition without evidence-based guidelines. Although the underlying pathophysiology mirrors that in adults, developmental differences in anatomy, haemodynamics and pain perception render adult protocols only partially applicable. The classic “5P” signs-pain (or its progression to painless), pallor, pulselessness, paralysis and paresthesia-are frequently appear late in children and are therefore unreliable for early diagnosis. Noonan and McCarthy have proposed the “3A” signs-anxiety, agitation and escalating analgesia-as a more sensitive bedside screen for impending compartmental ischaemia in the pediatric population (12). Clinical history and evolving symptoms remain the cornerstone of diagnosis; indeed, DSF itself is often the de facto confirmatory test (13). Objective confirmation relies on intracompartmental pressure (ICP) monitoring. In adults, an ICP ≥ 30 mmHg is widely accepted as an operative threshold, but normal resting pressures are higher in children and vary anatomically, rendering an adult cut-off inappropriate (14). Consequently, pressure criteria for PAOCS remain controversial. Several laboratory markers correlate with AOCS in adult cohorts: CK > 1,000 U·L−1 and elevated myoglobin are consistently associated with muscle necrosis, and serial CK measurements have been proposed as an early diagnostic adjunct. Comparable pediatric data are lacking. Elevated hepatic transaminases and cardiac troponins further reflect global myocyte injury. Inflammatory indices—C-reactive protein, interleukin 6, white blood cell count and neutrophil granulocyte—quantify the systemic inflammatory burden (15, 16). When rhabdomyolysis is present, a neutrophil-to-lymphocyte ratio >3.64 has been validated as an independent predictor of disease severity (17).
Therapeutic approaches to pediatric vs. adult AOCS differ markedly. Current recommendations for fasciotomy in children-encompassing incision technique, underlying pathophysiological rationale, and timing of wound closure-are almost exclusively extrapolated from adult literature. Accumulating evidence, however, demonstrates age-dependent variations in fascial thickness, wound-healing capacity, baseline compartment pressures and post-operative outcomes, underscoring the need for pediatric-specific treatment algorithms rather than direct adoption of adult protocols (18). The American Academy of Orthopaedic Surgeons' guideline on the management of AOCS advises against late fasciotomy in adults when muscle necrosis is established or diagnosis has been delayed >8–12 h (19). By contrast, children possess more robust muscle and skin viability, a wider therapeutic window, and an enhanced reparative capacity; decompression remains indicated irrespective of elapsed time from injury (20). Even in delayed presentations with evident neuromuscular compromise, pediatric patients derive net benefit from fasciotomy, as permanent neurological sequelae are uncommon (21). A recent meta-analysis by Lin et al. (10) found no statistically significant difference in functional outcome between pediatric patients undergoing early vs. late decompression; 88% of children treated >48 h after symptom onset achieved complete recovery. Compared with the extensive incisions typically employed in adults, pediatric practice must also consider psychological sequelae. Multiple small-incision decompressions have been successfully utilised in early AOCS without neurovascular compromise, although their restricted exposure limits application to select cases (22, 23). Adjunctive strategies—including fluorescence-based microangiography for real-time perfusion monitoring, hyperbaric oxygen therapy, and negative-pressure wound therapy prior to delayed primary closure—further enhance wound healing and functional recovery in PAOCS (24).
Diquat is dipyridine herbicide which is some of the most toxic pesticides available, it distribute to most tissues, causing acute renal injury and ultimately, multiorgan failure. Diquat poisoning has no specific antidote, and the mechanism by which diquat causes AOCS remains unclear (25). There is a paucity of pediatric literature on diquat poisoning, and case fatality rates as high as 43% have been reported (11). Chen et al. reported the first successful management of pediatric diquat poisoning complicated by rhabdomyolysis and shock (26). Two additional cases of diquat-induced rhabdomyolysis in adolescents resulted in fatal outcomes (27). The underlying mechanisms of rhabdomyolysis likely involve mitochondrial damage from oxyradical generation and cell membrane disruption via lipid peroxidation, culminating in cellular injury or death. Diquat compromises myocyte membrane integrity through oxidative stress, triggering calcium influx and cellular necrosis. Subsequent systemic inflammatory responses and shock may exacerbate muscular ischemia-hypoxia, establishing a “second-hit” injury model (9, 26–28). Notably, none of these three cases developed AOCS. Rhabdomyolysis and AOCS exhibit a bidirectional causal relationship; when severe, both conditions converge on acute kidney injury as a complication. Other toxic aetiologies of AOCS include snake envenomation, hymenopteran stings and insect bites. A recent systematic review (29) identified 31 such cases, the majority occurring in the pediatric population. Various drugs may also cause AOCS in the patient through local mechanisms including direct cellular toxicity, vasotoxicity or non-traumatic rhabdomyolysis. De Nobili et al. reported three cases of compartment syndrome following intramuscular hydrocarbon injection. When toxic substances injected in a tissue, hydrocarbons can damage microvasculature, leading to liquefactive necrosis. These organic compounds, due to their fat-dissolving properties, can expand and destroy tissue planes, mimicking necrotizing fasciitis. This condition can increase pressure within the tissue fascia, leading to venous hypertension, tissue ischemia, and necrosis (30). In addition, acute compartment syndrome has been reported following opioid epidemic (31), anticoagulant therapy (32), and intramuscular injection of rodenticides (33). AOCS is a surgical emergency, which requires emergent fasciotomy to avoid potentially life—or limb-threatening sequelae. Regardless of the toxic aetiology, the cornerstone of managing PAOCS centres on prompt toxin elimination, targeted supportive care, and emergent fasciotomy. We discuss a pediatric case of acute diquat poisoning resulting in AOCS, emphasizing the seriousness of that highlights the severity of diquat toxicity and the importance of DSF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
XS: Formal analysis, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. XW: Conceptualization, Data curation, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. PG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Wei S, Bowei W, Xinbao W, Changqing Z. Guidelines on early diagnosis and treatment of acute compartment syndrome in China. Chin J Orthop Trauma. (2020) 22(8):645–54.
3. Obey MR, Shlykov MA, Nickel KB, Keller M, Hosseinzadeh P. Incidence and risk factors for acute compartment syndrome in pediatric tibia fractures. J Pediatr Orthop B. (2023) 32(3):401–4. doi: 10.1097/BPB.0000000000000985
4. McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome. Who is at risk. J Bone Joint Surg (Br). (2000) 82(2):200–3.10755426
5. Zuchelli D, Divaris N, McCormack JE, Huang EC, Chaudhary ND, Vosswinkel JA, et al. Extremity compartment syndrome following blunt trauma: a level I trauma center’s 5-year experience. J Surg Res. (2017) 217:131–6. doi: 10.1016/j.jss.2017.05.012
6. Von Keudell AG, Weaver MJ, Appleton PT, Bae DS, Dyer GSM, Heng M, et al. Diagnosis and treatment of acute extremity compartment syndrome. Eur J Med Res. (2025) 30(10000):1299–310. doi: 10.1016/S0140-6736(15)00277-9
7. Zhaohui J, Zengru X. The dreaded osteofascial compartment syndrome. J Trauma Surg. (2022) 24(4):319–21.
8. Court-Brown CM, McQueen MM. Nonunions of the distal tibia: the role of fibular fixation. Arch Orthop Trauma Surg. (2010) 130(7):883–9. doi: 10.1007/s00402-009-1020-8
9. Feng D, Fu L, Du X, Yao L. Acute diquat poisoning causes rhabdomyolysis: a case report and literature review. Am J Med Sci. (2022) 364(2):472–80. doi: 10.1016/j.amjms.2022.04.032
10. Lin JS, Samora JB, Klingele KE. Pediatric acute compartment syndrome: a systematic review and meta-analysis. J Pediatr Orthop B. (2020) 29(6):90–6. doi: 10.1097/BPB.0000000000000593
11. Ness-Cochinwala M, Proaño JS, Bernstein JN, Martinez P, Ladd H, Totapally B. A case of a lethal diquat ingestion in a toddler. J Emerg Med. (2022) 62(2):e16–9. doi: 10.1016/j.jemermed.2021.10.007
12. Noonan KJ, McCarthy JJ. Compartment syndromes in the pediatric patient. J Pediatr Orthop. (2010) 30:S96–S101. doi: 10.1097/BPO.0b013e3181d07118
13. Osborn PM, Schmidt AH. Management of acute compartment syndrome. J Am Acad Orthop Surg. (2020) 28(3):e108–e114. doi: 10.5435/JAAOS-D-19-00270
14. Bussell HR, Aufdenblatten CA, Subotic U, Kalisch M, Staubli G, Weber DM, et al. Compartment pressures in children with normal and fractured lower extremities. Eur J Trauma Emerg Surg. (2019) 45(3):493–7. doi: 10.1007/s00068-019-01082-9
15. Nilsson A, Alkner B, Wetterlov P, Wetterstad S, Palm L, Schilcher J, et al. Low compartment pressure and myoglobin levels in tibial fractures with suspected acute com-partment syndrome. BMC Musculoskelet Disord. (2019) 20(1):15. doi: 10.1186/s12891-018-2394-y
16. Mcmillan TE, Gardner WT, Schmidt AH, Johnstone AJ. Diagnosing acute compartment syndrome- where have we got to? Int Orthop. (2019) 43(11):2429–35. doi: 10.1007/s00264-019-04386-y
17. Rademacher E, Miller PE, Jordan E, May CJ, Glotzbecker MP, Bae DS, et al. Management of fasciotomy incisions after acute compartment syndrome: is delayed primary closure more feasible in children compared with adults? J Pediatr Orthop. (2020) 40(4):e300–5. doi: 10.1097/BPO.0000000000001492
18. Bae JC, Sun KH, Park YJ. Role of the neutrophil-to-lymphocyte ratio at the time of arrival at the emergency room as a predictor of rhabdomyolysis in severe trauma patients. J Trauma Inj. (2020) 33(2):96–103. doi: 10.20408/jti.2020.018
19. Coe MP, Osborn PM, Schmidt AH. AAOS clinical practice guideline: management of acute compartment syndrome. J Am Acad Orthop Surg. (2021) 29(1):e1–4. doi: 10.5435/JAAOS-D-19-00326
20. Yixin W, Zhiqun Z. Research progress of pediatric acute compartment syndrome. Orthopaedics. (2022) 13(6):573–6.
21. Flynn JM, Bashyal RK, Yeger-McKeever M, Garner MR, Launay F, Sponseller PD. Acute traumatic compartment syndrome of the leg in children: diagnosis and outcome. J Bone Joint Surg Am. (2011) 93(10):937–41. doi: 10.2106/JBJS.J.00285
22. Lee G, Murray PC, Hasegawa IG. Closed incision negative pressure wound therapy in the management of a complex fasciotomy wound in a pediatric patient. Cureus. (2020) 12(3):e7413. doi: 10.7759/cureus.7413
23. Yuan X, Wu J, Qu X, Li M, Jiang L, Liu X. Fasciotomy through multiple small skin incisions for the treatment of early acute osteofascial compartment syndrome in children. J Orthop Surg Res. (2020) 15:269. doi: 10.1186/s13018-020-01742-2
24. Bauerly NA, Bobbitt KL, Kvas S, Walter J. Utilization of fluorescence microangiography in pediatric acute compartment syndrome: a case report. J Foot Ankle Surg. (2020) 59(1):201–5. doi: 10.1053/j.jfas.2019.07.017
25. Meng F, Zhang Y, Cai H, Meng F, Liu B, Li Y. Lung transplant success in severe diquat poisoning: a case report. Am J Case Rep. (2025) 26:e40457604. doi: 10.12659/AJCR.947421
26. Chen Y, Ou Y, Zhang J, Long Y, Fu J, Tang L, et al. Case report: successful outcome of a young patient with rhabdomyolysis and shock caused by diquat poisoning. Front Pediatr. (2023) 11:1116295. doi: 10.3389/fped.2023.1116295
27. Yu G, Wang J, Jian T, Shi L, Zhao L, Li Y, et al. Case series: diquat poisoning with acute kidney failure, myocardial damage, and rhabdomyolysis. Front Public Health. (2022) 10:991587. doi: 10.3389/fpubh.2022.991587
28. Liu Y, Liu J, Wang Y, Zhang Y, Wang C, Liu X, et al. Autophagy-mediated FTH1 degradation activates gasdermin-E-dependent pyroptosis contributing to diquat-induced kidney injury. Front Pediatr. (2024) 12:1358472. doi: 10.3389/fped.2024.1358472
29. Steadman W, Wu R, Hamilton AT, Richardson MD, Wall CJ. Review article: a comprehensive review of unusual causes of acute limb compartment syndrome. Emerg Med Australas. (2022) 34(6):871–6. doi: 10.1111/1742-6723.14098
30. De Nobili G, Cericola S, Casolino V, Di Russo S, Peca E, Percario R, et al. Compartment syndrome following self-injection of hydrocarbon: a case series. Surg Case Rep Adv Tech. (2024) 3:100069. doi: 10.1016/j.sycrs.2024.100069
31. Parzych L, Jo J, Diwan A, Swart E. “Found down” compartment syndrome: experience from the front lines of the opioid epidemic. J Bone Joint Surg Am. (2019) 101(17):1569–74. doi: 10.2106/JBJS.18.01307
32. Ghassani DN, Suwanto D, Ardiana M. Atraumatic acute compartment syndrome in anticoagulated patient: a case report. Ann Med Surg. (2022) 82:104530. doi: 10.1016/j.amsu.2022.104530
Keywords: pediatric acute osteofascial compartment syndrome, herbicide poisoning, diquat, decompressive surgical fasciotomy, acute osteofascial compartment syndrome
Citation: Shi X, Wang X and Guo P (2025) Case Report: A rare case of acute herbicide poisoning induced pediatric acute osteofascial compartment syndrome. Front. Pediatr. 13:1603429. doi: 10.3389/fped.2025.1603429
Received: 31 March 2025; Accepted: 1 September 2025;
Published: 17 September 2025.
Edited by:
Manuela Berto Pucca, Sao Paulo State Universty, BrazilReviewed by:
Guangcai Yu, Shandong University, ChinaGiovanni De Nobili, Asl Lanciano Vasto Chieti, Italy
Copyright: © 2025 Shi, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Wang, OTMwMDI5MDg5QHFxLmNvbQ==; Pei Guo, MzQxNjU1OTQ2N0BxcS5jb20=
 Xueping Shi1
Xueping Shi1 Pei Guo
Pei Guo