- Department of Critical Care Medicine, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatric Metabolism and Inflammatory Diseases, Chongqing, China
Background: A lung abscess is a thick-walled cavity containing purulent material that results from pulmonary infection. It is an uncommon condition that can occur at any age. Bronchopleural fistula (BPF) is a severe complication with a poor prognosis that may arise with the progression of the condition or as a result of treatment.
Case presentation: We describe a case of lung abscess complicated by a BPF and septic shock in a 7-year-old girl. A chest tube was inserted and venoarterial extracorporeal membrane oxygenation (ECMO) was emergently used. To selectively block the BPF, an endobronchial blocker was placed in the right intermediate bronchus under bronchoscopic guidance. This approach allowed the BPF to heal, enabled the recruitment of the other lung, and obstructed the purulent fluid. The patient recovered and was discharged after 70 days of treatment.
Conclusions: This case demonstrates that combined endobronchial blocker and ECMO can be an effective approach for patients with lung abscesses and BPFs (especially those aged < 8 years) when the adjustment of conventional therapy is unsuccessful.
1 Introduction
A lung abscess is a thick-walled cavity containing purulent material that results from pulmonary infection. It is an uncommon condition that can occur at any age (1, 2). Based on the presence of pre-existing conditions, lung abscesses are classified as primary (occurring in previously healthy individuals) and secondary (occurring in individuals with underlying lung abnormalities) (1). These abscesses cause significant morbidity and have a mortality rate of 10%–20% in adults; in children, the mortality rate is significantly lower (1–3).
Conservative management with systemic antibiotics is the standard treatment for lung abscess. Chest tube drainage and surgery are options in cases of treatment failure or large (diameter > 6 cm) abscesses (4–6). Bronchopleural fistula (BPF) is a severe complication that may arise with the progression of the condition or as a result of treatment (drainage tube insertion) (7–9). Defined as a connection between the pleural space and bronchus causing > 24 h air leakage, BPF occurs primarily in patients who undergo lung resection (7, 10). Its prognosis is poor, with mortality rates of 67% in patients requiring mechanical ventilation and 94% in cases of major air leakage (>5 ml/kg) (7, 8).
Here, we report a case of lung abscess in a previously healthy girl, which was complicated by BPF and septic shock. It was treated successfully with combined endobronchial blocker administration and extracorporeal membrane oxygenation (ECMO).
2 Case presentation
A 7-year-old girl (weighing 22 kg) presented to the Emergency Department (ED) with a 20-day history of fever and non-productive cough. Twenty days previously, she had been diagnosed with Mycoplasma pneumoniae pneumonia, which was treated sequentially with azithromycin for 4 days, doxycycline for 3 days, and levofloxacin for 9 days. With this treatment, the patient's fever had subsided and her cough had been briefly alleviated, but her fever had returned with no worsening of the cough 3 days prior to presentation. Blood testing performed in the ED showed a white blood cell count of 20.53 × 109/L, neutrophil count of 14.29 × 109/L, and C-reactive protein level of 196.76 mg/L. The patient was admitted to the hospital.
A chest computed tomography (CT) examination performed after admission showed a large cavity in the right inferior lobe of the lung with a pronounced gas-fluid level (Figure 1A), leading to the diagnosis of lung abscess. Polymerase chain reaction (PCR) analysis of the patient's sputum revealed M. pneumoniae (2.28 × 107) and A2063G/A2064G positivity. As the most common pathogens causing lung abscess are Streptococcus pneumoniae and Staphylococcus aureus, a combination of levofloxacin (10 mg/kg/day) and vancomycin (60 mg/kg/day) was administered.
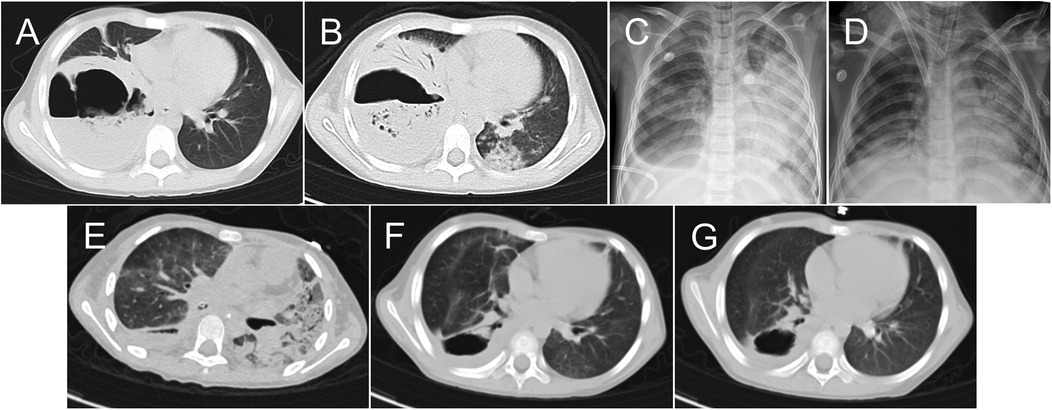
Figure 1. Chest images obtained during and after the patient's hospital stay. Chest computed tomography (CT) images obtained on days 1 (A) and 6 (B) of hospitalization. Chest x-rays obtained before extracorporeal membrane oxygenation initiation (C) and weaning (D) Chest CT images obtained on days 36 (E) and 65 (F) of hospitalization and 1 month after discharge (G).
Six days after admission, the patient's condition declined, with tachypnea, moaning, and pallor. She was transferred to the pediatric intensive care unit (PICU), where invasive mechanical ventilation was initiated. A contrast-enhanced chest CT examination showed no significant change to the large cavity, but additional lesions in other parts of the lungs (Figure 1B). Bronchoscopy revealed large amounts of yellowish-white secretion in the bronchus, suggesting that the lung abscess had ruptured.
Seven days after admission, the patient experienced worsening hypoxemia [partial pressure of oxygen (PaO2) = 53.7 mmHg, fraction of inspired oxygen (FiO2) = 1.0] and hypercarbia [partial pressure of carbon dioxide = 68.7 mmHg]. A chest x-ray demonstrated significantly increased opacities, accompanied by consolidation in the left lung (Figure 1C). Bronchoalveolar lavage was urgently performed, but the patient's oxygenation continued to worsen (PaO2 = 36.4 mmHg, FiO2 = 1.0) and she exhibited hypotension (blood pressure = 58/34 mmHg with 0.1 ug/kg/min noradrenaline). We decided to emergently perform venoarterial extracorporeal membrane oxygenation (VA-ECMO). Considering the size of the lung abscess and the risk of bleeding with the performance of percutaneous transthoracic tube drainage after ECMO initiation, a chest tube was inserted into the right chest before ECMO administration, producing a gush of air followed by continuous air leakage and purulent fluid drainage. Fourteen- and 19-Fr heparin-coated cannulas (Bio-Medicus; Medtronic Inc., Minneapolis, MN, USA) were implanted surgically via the right common carotid artery and internal jugular vein. The patient's antibiotic treatment was adjusted (imipenem/cilastatin, 15 mg/kg every 6 h; linezolid, 10 mg/kg every 8 h; voriconazole, two loading doses of 9 mg/kg every 12 h, maintenance doses of 8 mg/kg every 12 h; and doxycycline, 2 mg/kg every 12 h).
Lung-protective ventilation was considered [Initiation ventilator settings: Tidal volumes 4–6 ml/kg, FiO2 30%, post end-expiratory pressure (PEEP) 5 cmH2O, Respiratory rate 10 breaths per minute], as most of the tidal volume was leaking continuously through the chest tube and gas exchange could not be maintained in either lung. On bronchoscopy, yellowish-white secretions could be aspirated continuously from the inferior lobe of the right lung (RB9), indicating that a BPF had developed in that location. An endobronchial blocker (auto-inflation tube, external diameter = 2.3 mm; Tappa Medical Technology Co., Hangzhou, China) was guided into the right intermediate bronchus through the outside of the endotracheal tube by bronchoscopy (Figure 2). The air leakage decreased significantly as soon as the endobronchial balloon occupied the right intermediate bronchus. We adjusted the balloon filling degree to allow a small amount of air to leak from the fistula, discharging the purulent fluid, and maintained PEEP at 8–12 cmH2O with the help of the endobronchial blocker. Bronchoalveolar lavage was performed repeatedly over the subsequent days to eliminate the purulent fluid from the bronchus.
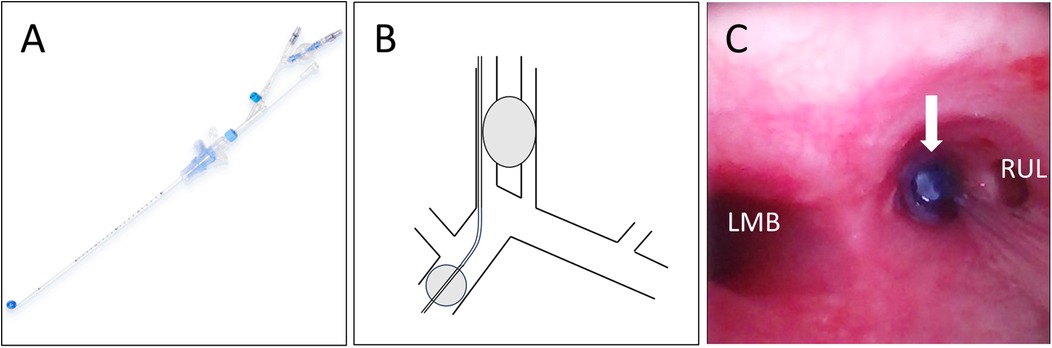
Figure 2. Endobronchial blocker placement. (A) An endobronchial blocker. Diagram (B) and bronchoscopic image (C) of the endobronchial blocker occluding the right intermediate bronchus, which resolved the air leakage through the bronchopleural fistula. LMB, left main-stem bronchus; RUL, right upper lobe.
Culture of the purulent fluid yielded Pseudomonas aeruginosa, and next-generation sequencing of the bronchoalveolar lavage fluid revealed P. aeruginosa and M. pneumoniae. According to these pathogen results, we stopped the voriconazole.
After 16 days of support, the patient's status improved; Figure 1D is a chest x-ray obtained before she was weaned from ECMO. On day 24 of hospitalization, the patient was successfully weaned from ECMO and decannulated. High-frequency oscillatory ventilation was used for 5 days, followed by conventional mechanical ventilation. A chest CT examination performed on day 36 showed extensive parenchyma and interstitial lesions in the bilateral lungs (Figure 1E). A systemic glucocorticoid (methylprednisolone sodium succinate, 1.5 mg/kg every 12 h) was administrated. An abdominal CT examination revealed liver and spleen abscesses. Considering the possibility of anaerobic infection, we added metronidazole (7.5 mg/kg every 8 h) to the patient's treatment regimen. As air still leaked continuously from the chest tube when the endobronchial blocker was released, blocker use was continued until day 41 of hospitalization, when we observed significantly decreased air leakage. At that time, we adjusted the chest drainage suction level from 0 to −10 cmH2O to aid purulent fluid drainage. On day 46 of hospitalization, the patient was weaned from ventilation. On day 56, no bubbling in the water seal of the chest drainage was seen and the chest tube was removed. Figure 1F is a chest CT image acquired on day 65 of hospitalization, when the patient was discharged from the PICU. The patient was discharged from the hospital without surgical intervention on day 70. Chest CT re-examination was performed 1 month after discharge (Figure 1G), and the girl returned to school with no symptom.
3 Discussion
In the case reported here, a lung abscess complicated by a BPF in a pediatric patient was treated successfully with combined endobronchial blocker and ECMO use. This report is the first to describe the use of this approach for lung isolation to manage these conditions in a patient aged < 8 years.
ECMO is an effective supportive method applied to patients with refractory respiratory failure and BPFs (7). It enables the control of infection with systemic antibiotics, ventilator-based management with reduced parameters to allow the lungs to rest, and BPF healing and closure. A few case reports and series describing the use of ECMO in BPF treatment have been published (11, 12). While most cases can be managed with venovenous ECMO (VV-ECMO), VA-ECMO was utilized in the current case. We selected VA-ECMO for two reasons: the unavailability of double-lumen cannulas in China limited VV access via the right internal jugular vein, and the patient's femoral vein was insufficiently sized to provide the target blood flow. Daoud et al. (8) reported the use of VA-ECMO for five patients with BPF and acute lung injury after thoracic surgery. Lung-protective ventilation was employed after ECMO initiation, and three of the patients recovered.
Ventilator management is crucial for patients with BPF. Conventional ventilation strategies include decreasing peak-inspiratory pressure by using lower tidal volumes, lowering PEEP, decreasing the inspiratory time, and decreasing the respiratory rate. High-frequency ventilation has also been evaluated for managing refractory air leak (7, 13). During ECMO support, ventilator pressure can be reduced or turned off altogether for hours or days until the leak seals. These approaches are suitable for patients with BPFs secondary to pneumonectomy or thoracic trauma which without severe infection. Grant et al. (13) shared successful utilization of ECMO with lung protective strategies to three traumatic BPF.
In the current case, the patient had a BPF secondary to a right lung abscess. Copious amounts of purulent fluid flooded into the left lung, resulting in the left lung consolidation. Sufficient drainage and protection of the rest lung from contamination was critical. Conventional ventilation strategies and high-frequency ventilation could not obstruct and drain the purulent fluid. Lung isolation can eliminate air leakage through a BPF, obstruct the purulent fluid, and improve gas exchange in the contralateral lung (7). It is most commonly performed using a double-lumen endotracheal tube. Garlick et al. (14) reported the successful treatment of trauma (lung contusion with prominent pneumatoceles that evolved into a BPF with persistent left-lung air leakage) in a 16-year-old boy with differential lung ventilation using a double-lumen tube and VV-ECMO. However, even the smallest double-lumen tube is too large for patients aged < 8 years. In addition, double-lumen tubes are not convenient for the performance of repeat bronchoscopy. Endobronchial intubation is the simplest lung isolation technique, but also impedes repeat bronchoscopy. Endobronchial blockers have been used in pediatric patients in the operating room (15). In the current case, the endobronchial blocker obstructed purulent fluid, preventing infection of the rest lung, and facilitated bronchoscopy performance. It also promoted collapse of the diseased lung and BPF healing. Simultaneously, with the blocker enabled adequate ventilation of the consolidated lung and recruitment.
The timing of ECMO weaning also deserving discussion. Maintaining ECMO support until BPF healing may represent a safer process. All three patients in Grant's report were weaned from ECMO after BPF resolved (11). In our case, we weaned ECMO and decannulated prior to BPF closure, with the BPF persisting for 30 more days. Our weaning decision was based on the following considerations. Unlike the patients reported by Grant et al, the fistula in this case was secondary to severe infection, requiring more time for healing. Prolonged duration of ECMO increases the risk of adverse effects. Before weaning, the patient was suffering cannulation site bleeding and right thumb ischemia. Concurrently, effect of the circuit was diminishing. Most importantly, the patient's left lung got significantly improved. Based on these factors, we performed a trial off, and it was successful. Consequently, we decided to wean and decannulate. With the endobronchial blocker in place, the patient tolerated higher ventilator settings post-ECMO.
4 Conclusions
With this case, we demonstrate that combined endobronchial blocker and ECMO use can be an effective approach for patients with lung abscesses and BPFs when the adjustment of conventional therapy is unsuccessful. It is especially suitable for patients aged < 8 years.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were approved by the Institutional Review Board of Children's Hospital of Chongqing Medical University (2024-KY-0192). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the minor's legal guardian/next of kin for the publication of this case report.
Author contributions
SC: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. SL: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. YS: Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft. WW: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. YC: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. JL: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing. CL: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Patradoon-Ho P, Fitzgerald DA. Lung abscess in children. Paediatr Respir Rev. (2007) 8(1):77–84. doi: 10.1016/j.prrv.2006.10.002
2. Sperling S, Dahl VN, Fløe A. Lung abscess: an update on the current knowledge and call for future investigations. Curr Opin Pulm Med. (2024) 30(3):229–34. doi: 10.1097/mcp.0000000000001058
3. Jin L, Li Y. Bronchoscopic interventions for bronchopleural fistulas. Ther Adv Respir Dis. (2023) 17:17534666231164541. doi: 10.1177/17534666231164541
4. Islam S, Calkins CM, Goldin AB, Chen C, Downard CD, Huang EY, et al. The diagnosis and management of empyema in children: a comprehensive review from the APSA outcomes and clinical trials committee. J Pediatr Surg. (2012) 47(11):2101–10. doi: 10.1016/j.jpedsurg.2012.07.047
5. Hadid W, Stella GM, Maskey AP, Bechara RI, Islam S. Lung abscess: the non-conservative management: a narrative review. J Thorac Dis. (2024) 16(5):3431–40. doi: 10.21037/jtd-23-1561
6. Lee JH, Hong H, Tamburrini M, Park CM. Percutaneous transthoracic catheter drainage for lung abscess: a systematic review and meta-analysis. Eur Radiol. (2021) 32(2):1184–94. doi: 10.1007/s00330-021-08149-5
7. Grotberg JC, Hyzy RC, De Cardenas J, Co IN. Bronchopleural fistula in the mechanically ventilated patient: a concise review. Crit Care Med. (2020) 49(2):292–301. doi: 10.1097/ccm.0000000000004771
8. Daoud O, Augustin P, Mordant P, Lasocki S, Al-Attar N, Maury J-M, et al. Extracorporeal membrane oxygenation in 5 patients with bronchial fistula with severe acute lung injury. Ann Thorac Surg. (2011) 92(1):327–30. doi: 10.1016/j.athoracsur.2011.01.060
9. de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. (2020) 396(10253):786–98. doi: 10.1016/s0140-6736(20)31550-6
10. Fariduddin MM, Wang K, Birjees A, Syed W. Young patient with pneumonia complicated by bronchopleural fistula. Am J Emerg Med. (2021) 46:797.e3–97.e5. doi: 10.1016/j.ajem.2021.01.057
11. Grant AA, Lineen EB, Klima A, Vianna R, Loebe M, Ghodsizad A. Refractory traumatic bronchopleural fistula: is extracorporeal membrane oxygenation the new gold standard? J Card Surg. (2019) 35(1):242–45. doi: 10.1111/jocs.14298
12. Khan NU, Al-Aloul M, Khasati N, Machaal A, Leonard CT, Yonan N. Extracorporeal membrane oxygenator as a bridge to successful surgical repair of bronchopleural fistula following bilateral sequential lung transplantation: a case report and review of literature. J Cardiothorac Surg. (2007) 2(1):28. doi: 10.1186/1749-8090-2-28
13. Grant AA, Hart VJ, Lineen EB, Badiye A, Byers PM, Patel A, et al. A weaning protocol for venovenous extracorporeal membrane oxygenation with a review of the literature. Artif Organs. (2018) 42(6):605–10. doi: 10.1111/aor.13087
14. Garlick J, Maxson T, Imamura M, Green J, Prodhan P. Differential lung ventilation and venovenous extracorporeal membrane oxygenation for traumatic bronchopleural fistula. Ann Thorac Surg. (2013) 96(5):1859–60. doi: 10.1016/j.athoracsur.2013.01.094
Keywords: lung abscess, bronchopleural fistula, endobronchial blocker, extracorporeal membrane oxygenation, case report
Citation: Chen S, Lu S, Sun Y, Wang W, Chen Y, Li J and Liu C (2025) Endobronchial blocker and extracorporeal membrane oxygenation use for lung abscess complicated by a bronchopleural fistula in a pediatric patient: a case report and literature review. Front. Pediatr. 13:1604298. doi: 10.3389/fped.2025.1604298
Received: 1 April 2025; Accepted: 4 June 2025;
Published: 19 June 2025.
Edited by:
Suresh Keshavamurthy, University of Texas Southwestern Medical Center, United StatesReviewed by:
Daniel Dirnberger, Alfred I. duPont Hospital for Children, United StatesPriya Prabhakaran, University of Alabama at Birmingham, United States
Copyright: © 2025 Chen, Lu, Sun, Wang, Chen, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengjun Liu, bGl1Y3dkQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Song Chen
Song Chen Siwei Lu
Siwei Lu Yuelin Sun
Yuelin Sun Wenlei Wang
Wenlei Wang Yingfu Chen
Yingfu Chen Jing Li
Jing Li Chengjun Liu
Chengjun Liu