- Department of Anaesthesiology, Anhui Provincial Children’s Hospital, Hefei, Anhui, China
Background: Emergence agitation (EA) is characterized by excessive reactivity and sensory impairment that occurs in children after general anesthesia. Alfentanil is a µ-opioid receptor with rapid onset and short duration, widely used in minor surgery. The aim of this meta-analysis is to assess the effect of alfentanil on the incidence of EA in children undergoing general anesthesia.
Methods: PubMed, Cochrane Library, Embase, and Web of Science databases were reviewed to search for related trials published before April 30, 2025. The primary outcome was the incidence of EA. Secondary outcomes included rescue analgesia, postoperative nausea and vomiting (PONV), emergence time, extubation time, and time to discharge from post-anesthesia care unit (PACU).
Results: The study extracted from 5 studies including 532 patients. Compared to saline, alfentanil reduced the incidence of EA in children (RR = 0.54; 95% CI: 0.42–0.70; P < 0.01). In addition, alfentanil decreased the use of rescue analgesic (RR = 0.5; 95% CI: 0.38–0.65; P < 0.01), did not increase the incidence of PONV (RR = 1.39; 95% CI: 0.67–2.88; P = 0.37). According to the GRADE system, the quality of evidence was moderate for the incidence of EA.
Conclusions: Limited available evidence suggests that alfentanil is associated with a lower incidence of EA in children. However, further high-quality studies are needed to verify the effect of alfentanil in preventing the occurrence of EA in children undergoing general anesthesia.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42023448260, PROSPERO CRD42023448260.
Introduction
Emergence agitation (EA) is an acute behavioral disturbance occurring in patients during recovery from general anesthesia (1). EA can occur at any age, but it is more common in pediatric populations, with an incidence rate of about 10%–80% (2, 3). Children with EA typically exhibit irritability, uncooperativeness, and inconsolability, often accompanied by crying, moaning, writhing, and kicking. Although EA is usually self-limiting behavior, it can increase the risks of self-injury, wound dehiscence, bleeding, and dislodgment of catheters. Additionally, children with EA have a higher risk of anxiety, apathy, sleep and eating disorders within 30 days postoperatively (4–6).
The occurrence of EA may be attributed to various factors. Potential risk factors for EA include pre-school children, volatile anesthetics, especially sevoflurane, type of surgery such as otolaryngology and ophthalmic surgeries, body temperature in children, pain, and preoperative anxiety in children and parents (7). The precise mechanism of EA remains unclear, and the search for ways to prevent and treat EA is urgent and necessary. Although there is no universal protocol for managing anesthesia in EA, emerging research has demonstrated the effectiveness of pharmacological methods in preventing EA. A meta-analysis found that dexmedetomidine significantly reduced the incidence of EA in children (8). Multiple studies have shown that drugs such as nalbuphine, midazolam, and melatonin can reduce the occurrence of EA in pediatric patients after general anesthesia (9–11). Besides, magnesium sulfate, a non-competitive NMDA receptor antagonist, emerges as a potential preventive agent against EA (12). Similarly, several studies have found that opioids can effectively reduce the incidence of EA in children (13, 14). Alfentanil is a µ-opioid receptor agonist known for its rapid onset, short duration, and has little effect on cardiovascular and respiratory system, commonly used for minor surgeries (15). Studies have shown that the use of alfentanil in adenotonsillectomy can effectively reduce the incidence of EA (16). However, the addition of alfentanil 10 min before the end of strabismus or epiblepharon repair surgery did not significantly improve the incidence of EA in children (17).
Therefore, the aim of this study was to assess the effect of alfentanil on the incidence of agitation during recovery in children under general anesthesia using systematic review and meta-analysis. It also evaluated the impact of alfentanil on secondary outcome measures such as postoperative nausea and vomiting (PONV). The study provided evidence-based medical evidence for optimizing the management strategy of children's recovery period under general anesthesia.
Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18). The study has been registered in PROSPERO (CRD42023448260).
Search strategy
Two authors independently conducted a literature search in four electronic databases: PubMed, EMBASE, Web of Science, and Cochrane Library, with the search cutoff date set at April 30, 2025. The electronic database search was supplemented by manually searching the reference lists of included articles. The detailed search strategies for each database are available in the Supplementary Material S1.
Study selection
After searching for and removing duplicates, two additional researchers independently reviewed abstracts and full-text articles. Any discrepancies were resolved through discussion with a third researcher to reach a consensus decision.
Criteria for study
The inclusion criteria were as follows: (1) children aged between 0 and 18 years; (2) intervention with alfentanil administered intravenously or intranasally, compared with saline or other drugs (e.g., ketamine); (3) evaluate the incidence of EA; (4) randomized controlled trials; (5) elective non-cardiac surgeries under general anesthesia; (6) publications in English. The exclusion criteria were as follows: (1) cardiac surgery; (2) emergency surgery.
Data extraction
The authors extracted following data from eligible studies: author names, publication year, country of origin, duration of the study, type of surgery, total number of participants, baseline patient characteristics (mean age, gender), details of the intervention and control protocols, outcomes, and details of methodological quality.
Primary and secondary outcomes
The primary outcome was the incidence of EA. Secondary outcomes included the number of patients requiring rescue analgesia, PONV, extubation time, emergence time, and time to discharge from PACU. For the measurement of EA, the quadruple scale or the PAED scale were mainly used (19, 20). EA was considered present when the PAED score was more significant than 12 or four-point scale score was ≥3.
Quality and risk of bias assessment
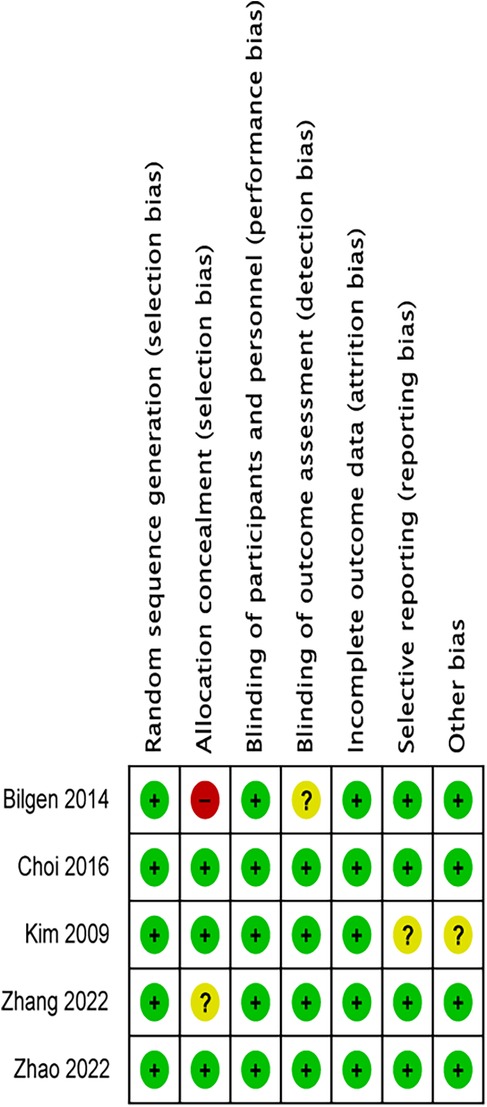
Two independent researchers used the Cochrane Risk of Bias tool to assess the quality based on seven domains (21): random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. According to the Cochrane tool's standards, the risk of bias for each domain was marked as low, unclear, or high.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was employed to assess the level of certainty, yielding four distinct results: high, moderate, low, and very low.
Data analysis
Summary estimates of categorical and continuous variables were presented as risk ratio (RR) and mean difference(MD), respectively, with each effect size accompanied by a 95% confidence interval (CI). Heterogeneity was assessed by I2 between-study and the thresholds of ≥25%, ≥50%, and ≥75% represented low, moderate, and high heterogeneity, respectively. Due to clinical heterogeneity, a random-effects model was used for the meta-analysis (22). This model provides an appropriate estimate of the average treatment effect when studies are statistically heterogeneous, typically resulting in relatively wider CI and thus more conservative effects. This meta-analysis did not perform publication bias because the Cochrane's Handbook onsiders the test to be too low when fewer than 10 studies were included (23). The analysis was conducted using Cochrane Review Manager version 5.4, with a significance level set at P < 0.05.
Results
Study selection and study characteristics
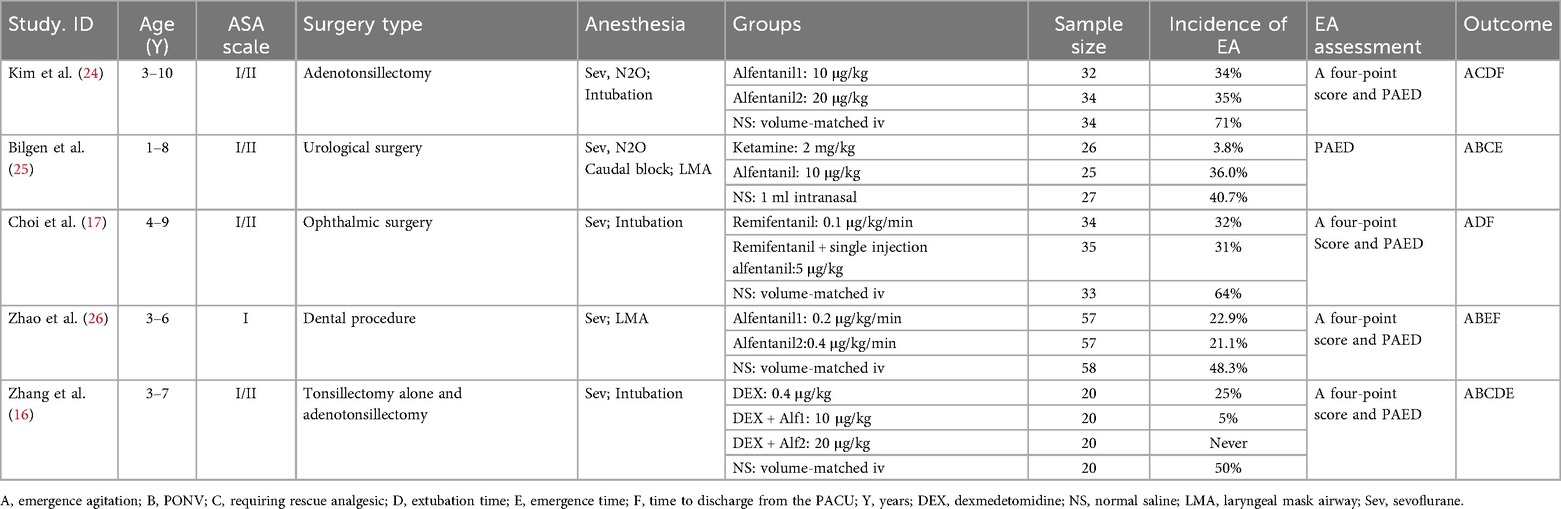
The search identified 136 relevant studies from 4 databases, 64 studies were excluded due to duplication, and 57 were excluded after title and abstract screening. Additionally, 15 articles were eligible to undergo full-text review, of which 10 were excluded for not meeting the inclusion criteria. A total of 5 randomized studies were ultimately included in the meta-analysis (Figure 1). The five studies included were published from 2009 to 2022 (16, 17, 24–26). The sample size of the included trials ranged from 78 to 172, totaling 532 participants. The age of the subjects ranged from 1 to 10 years. The types of surgery performed were tonsillectomy, adenotonsillectomy, urological surgery, ophthalmic surgery, and dental procedures. All patients were anesthetized with sevoflurane inhalation combined with laryngeal mask or endotracheal intubation. The basic characteristics of the included studies are presented in Table 1.
Risk of bias
The article by Kim 2009 using alfentanil at 10 μg/kg is denoted as Kim 2009–1, and the 20 μg/kg group is Kim 2009–2; in the article by Zhao 2022, alfentanil at 0.2 μg/kg/min is labeled as Zhao 2022–1, and 0.4 μg/kg/min as Zhao 2022–2; in the article by Zhang 2022, the 10 μg/kg alfentanil group is Zhang 2022–1, and the 20 μg/kg dose group is Zhang 2022–2. The risk of bias is summarised in Figure 2.
Meta-analysis of the primary outcome
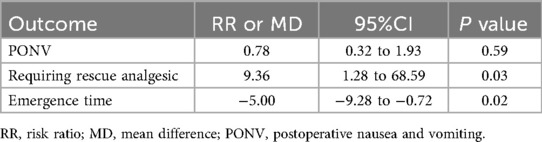
All included studies reported the incidence of postoperative EA (16, 17, 24–26). The comprehensive results of the forest plot showed that the incidence of EA was lower with alfentanil as compared with saline group (RR = 0.54; 95% CI: 0.42–0.70; P < 0.01; I2 = 15%) (Figure 3). Additionally, the study by Sevgi compared the effect of alfentanil and ketamine on EA (25), and the meta-analysis showed that the incidence of EA was lower in ketamine group than in alfentanil group (RR = 9.36; 95% CI: 1.28–68.59; P = 0.03) (Figure 4).
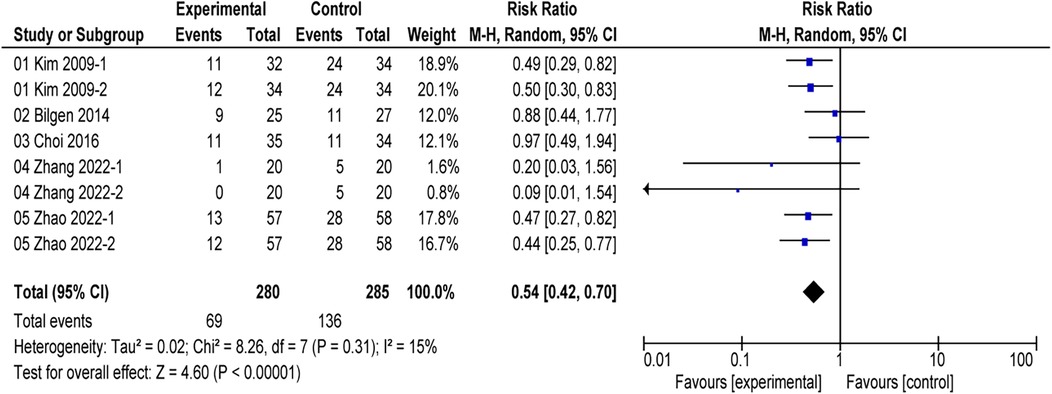
Figure 3. Forest plot of meta-analysis of the incidence of emergence agitation (EA): afentanil vs. Saline.

Figure 4. Forest plot of meta-analysis of the incidence of emergence agitation (EA): afentanil vs. ketamine.
Subgroup analysis of the primary outcome
In the subgroup analysis, two studies (16, 24) indicated that alfentanil compared to saline in tonsillectomy and/or adenoidectomy procedures reduced the incidence of EA (RR = 0.44, 95% CI: 0.32–0.60; P < 0.01; I2 = 0%). Choi's study (17) demonstrated that the combination of remifentanil and alfentanil did not reduce the incidence of EA in ophthalmic surgery compared to remifentanil alone (RR = 0.97; 95% CI: 0.49–1.94; P = 0.93) (Figure 5). The use of alfentanil in combination with midazolam did not reduce the incidence of EA in urological surgery compared to midazolam alone (RR = 0.88; 95% CI: 0.44–1.77; P = 0.73) (25). However, alfentanil reduced the incidence of EA compared with saline in oral surgery (RR = 0.45; 95% CI: 0.31–0.67; P < 0.01; I2 = 0%) (26). Four studies (16, 24–26) found that the use of alfentanil at doses of 10 µg/kg, 20 µg/kg, 0.2 µg/kg/min, and 0.4 µg/kg/min all reduced the incidence of EA (P < 0.01) (Figure 6).
Meta-analysis of the secondary outcomes
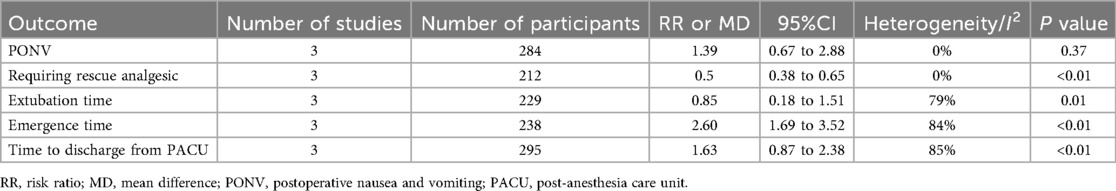
Three studies documented the incidence of PONV (16, 25, 26), postoperative rescue analgesics (16, 24, 25), extubation time (16, 17, 24), emergence time (16, 25, 26) and the time discharge from PACU (17, 24, 26). Compared to the saline group, alfentanil significantly reduce the percentage of children requiring postoperative rescue analgesics (RR = 0.5; 95% CI: 0.38 to 0.65; P < 0.01, I2 = 0%). However, the administration of alfentanil has been linked to prolonged extubation time (MD = 0.85; 95% CI: 0.18 to 1.51; P = 0.01, I2 = 79%), emergence time (MD = 2.60; 95% CI: 1.69 to 3.52; P < 0.01, I2 = 84%), and the time discharge from PACU(MD = 1.63; 95% CI: 0.87 to 2.38; P < 0.01, I2 = 85%). Moreover, alfentanil has no significant effect on PONV (RR = 1.39; 95% CI: 0.67–2.88; P = 0.37, I2 = 0%) (Table 2). In comparison with other drug (25), alfentanil significantly increased the number of children requiring postoperative rescue analgesics compared to ketamine (RR = 9.36; 95% CI: 1.28 to 68.59; P = 0.03), while reducing emergence time compared to ketamine significantly (MD = −5.00; 95% CI: −9.28 to −0.72; P = 0.02). There was also no significant difference in PONV between the two drugs (RR = 0.78; 95% CI: 0.32 to 1.93; P = 0.59) (Table 3).
GRADE assessment
All included studies were randomized trials, and the assessors were blinded. The risk of bias for some outcomes was graded as “serious” due to the high risk for selection bais in one of the included studies. For outcomes with moderate or substantial heterogeneity, we evaluated the level of inconsistency as “serious (30% ≤ I2 < 60%)” or “very serious (I2 > 60%)”. According to the GRADE system, the quality of evidence was moderate for the incidence of EA, PONV and requiring rescue analgesic, while low for Extubation time and Time to discharge from PACU. And the quality of evidence was very low for emergance time (Table 4).

Table 4. Grading of recommendations assessment, development, and evaluation (GRADE) evidence profile (Alfentanil vs. Saline).
Discussion
This meta-analysis indicates that compared with saline, alfentanil can significantly reduce the incidence of EA in children after general anesthesia, decrease postoperative rescue analgesics use, and has no effect on PONV, but it prolongs extubation time, emergence time, and the time of discharge from PACU.
EA is one of the most common complications of pediatric anesthesia, characterized by excitement, restlessness, and other unusual behaviors, such as crying, kicking, inconsolability, and non-cooperation (27, 28). Although the etiology of EA in children remains unclear, certain risk factors for EA generally considered are as follows:preschool age, preoperative anxiety, postoperative pain, and inhalational anesthetic (7, 29). Drug therapy isone of the effective methods. Studies have found that α-receptor agonists such as dexmedetomidine, midazolam, opioid receptor agonists such as nalbuphine, and alfentanil can reduce the incidence of EA in children (30, 31). Consistent with Tan's study (32), this meta-analysis shows that alfentanil significantly reduced the incidence of EA compared to saline. In subgroup analyses of different surgical types on the incidence of EA, the use of alfentanil in ophthalmic and urological surgeries showed no significant impact compared to saline. We speculate that it may be related to the combination of alfentanil with other anesthetic drugs in these two articles. In Bilgen's study, all patients received permedication with oral midazolam 0.5 mg/kg before anesthesia induction. Oral midazolam has been proven to effectively reduce the incidence of EA in children in multiple studies (33, 34). In Choi's study, both the alfentanil group and the control group received continuous infusion of remifentanil during surgery. Remifentanil has also been shown to reduce the incidence of EA in children after general anesthesia (35, 36).
In subgroup analyses of different doses of alfentanil, we found that alfentanil at doses of 10 µg/kg, 20 µg/kg, 0.2 µg/kg/min, and 0.4 µg/kg/min all reduced the incidence of EA compared to saline, while alfentanil at 5 µg/kg showed no effect on the incidence of EA compared to saline. However, only 3 and 2 of the 5 included articles recorded the effects of 10 µg/kg and 20 µg/kg of alfentanil on EA, while only 1 article recorded 5 ug/kg, 0.2 µg/kg/min and 0.4 µg/kg/min. Due to the limited research available, it still cannot determine the optimal and minimum dose of alfentanil for preventing EA in children after general anesthesia. Meanwhile, the effect of using alfentanil at different times on EA remains unclear. Thus, The optimal level of alfentanil to control EA should be determined in future studies.
Postoperative acute pain is recognized as an important risk factor for EA, and inadequate analgesia can lead to it, as described in the PAED scale (37). Three studies reported the need for postoperative rescue analgesics. The results of our meta-analysis showed that the use of alfentanil significantly reduced the use of postoperative rescue analgesics compared with the use of control group. Therefore, the effect of pain on postoperative agitation could not be completely excluded in this study. Alfentanil is a synthetic, short-acting µ-opioid agonist that can effectively alleviate pain and may prevent the occurrence of EA (32). It is worth noting that pediatric patients, due to their limited language expression abilities, often exhibit pain-related defensive movements (such as kicking and resisting treatment). These behaviors can be difficult to distinguish from the irrational agitation caused by disorientation during the anesthesia emergence period. This phenotypic overlap may lead to an overestimation of the proportion of non-painful agitation during emergence from anesthesia (6, 38). Face, Legs, Activity, Cry, Consolability (FLACC) scores is a common tool to assess the degree of pain in infants and young children (39). It scores patients based on five behavioral indicators, helping healthcare professionals quantify the pain level and develop appropriate interventions. Cai's team proposed a method to differentiate postoperative pain from emergence agitation using both the FLACC and PAED (Pediatric Assessment of Emergence Delirium) scales (40). For children with a FLACC score of ≥4, acetaminophen or fentanyl is administered to mitigate the potential impact of pain on the assessment of postoperative emergence agitation (40).
Moreover, this meta-analysis shows that alfentanil prolong extubation time, emergence time, and the time discharge from PACU. However, it must be emphasized that the results of these three time indicators all exhibit a high heterogeneity (I2: 79%; 84%; 85%). We used sensitivity analysis to eliminate the included literature one by one, but the heterogeneity was still high. This suggests that heterogeneity may be caused by a combination of methodological or clinical differences between studies. In Zhang's study, the use of dexmedetomidine may have affected the extubation time and emergence time (16). In addition, the definition of emergence time varies in different studies, including spontaneous eye opening time, time from discontinuation of sevoflurane until the children acted on command, and first eye opening or crying (16, 25, 26). In terms of the time to discharge from PACU, although the Aldrete score was used in all studies, there were differences in the threshold (9 or 10 points) and implementation details (whether parents accompanied them) that affected the consistency of the decision to leave the room (16, 17, 24).
This meta-analysis still has limitations. First, the sample size is relatively small, which consequently compromises the overall precision of the findings. Future research necessitates the conduct of additional high-quality multicenter randomized controlled trials. Second, the literature included in this study was only published in English, which might lead to certain publication bias. Third, the literature included in this meta-analysis used the PAED scale and the 4-point scale, and we did not separate these two scales for statistics. This is because we only analyzed the incidence of EA, but not the severity of EA. Future studies are needed to further analyze the severity of EA to provide a more precise rationale for clinical treatment. Fourth, whether different opioids affect the incidence of EA requires further study. Although study showed that an additional single dose of alfentanil before the end of surgery did not significantly reduce the incidence of EA when remifentanil is continuously infused (17). However, it remains to be studied whether the incidence of EA is different with continuous infusion of alfentanil and other opioids. Fifth, the heterogeneity of some of the secondary outcomes was high. In addition, the GRADE approach assessed the quality of evidence for these outcomes as low and very low quality. This indicates the uncertainty in the results.
Conclusion
Compared to saline, alfentanil reduces the incidence of EA and the requirement for rescue analgesics in children undergoing general anesthesia, without increasing the incidence of PONV. However, it prolongs extubation time, emergence time, and time to discharge from PACU. Practical decisions should weigh core benefits against potential costs. For children at high risk of EA, a controlled recovery delay might be a reasonable choice to exchange for stable awakening. For low-risk or cases requiring rapid turnover, the need for medication should be individually evaluated and the dosing strategy optimized. Given the limitations of small sample size and low-quality evidence, future high-quality research is necessary to provide further effective estimates of the effect of alfentanil in preventing EA in pediatric surgical patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YX: Writing – original draft, Formal analysis, Investigation, Methodology, Conceptualization. KD: Methodology, Formal analysis, Writing – review & editing, Investigation. XZ: Writing – review & editing, Investigation, Formal analysis. YS: Investigation, Methodology, Conceptualization, Writing – review & editing, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1607279/full#supplementary-material
References
1. Wong DD, Bailey CR. Emergence delirium in children. Anaesthesia. (2015) 70(4):383–7. doi: 10.1111/anae.13043
2. Welborn LG, Hannallah RS, Norden JM, Ruttimann UE, Callan CM. Comparison of emergence and recovery characteristics of sevoflurane, desflurane, and halothane in pediatric ambulatory patients. Anesth Analg. (1996) 83(5):917–20. doi: 10.1097/00000539-199611000-00005
3. Cravero J, Surgenor S, Whalen K. Emergence agitation in paediatric patients after sevoflurane anaesthesia and No surgery: a comparison with halothane. Paediatr Anaesth. (2000) 10(4):419–24. doi: 10.1046/j.1460-9592.2000.00560.x
4. Kain ZN, Caldwell-Andrews AA, Weinberg ME, Mayes LC, Wang SM, Gaal D, et al. Sevoflurane versus halothane: postoperative maladaptive behavioral changes: a randomized, controlled trial. Anesthesiology. (2005) 102(4):720–6. doi: 10.1097/00000542-200504000-00005
5. Stargatt R, Davidson AJ, Huang GH, Czarnecki C, Gibson MA, Stewart SA, et al. A cohort study of the incidence and risk factors for negative behavior changes in children after general anesthesia. Paediatr Anaesth. (2006) 16(8):846–59. doi: 10.1111/j.1460-9592.2006.01869.x
6. Somaini M, Engelhardt T, Fumagalli R, Ingelmo PM. Emergence delirium or pain after anaesthesia–how to distinguish between the two in young children: a retrospective analysis of observational studies. Br J Anaesth. (2016) 116(3):377–83. doi: 10.1093/bja/aev552
7. Lee SJ, Sung TY. Emergence agitation: current knowledge and unresolved questions. Korean J Anesthesiol. (2020) 73(6):471–85. doi: 10.4097/kja.20097
8. Yang X, Hu Z, Peng F, Chen G, Zhou Y, Yang Q, et al. Effects of dexmedetomidine on emergence agitation and recovery quality among children undergoing surgery under general anesthesia: a meta-analysis of randomized controlled trials. Front Pediatr. (2020) 8:580226. doi: 10.3389/fped.2020.580226
9. He J, Zhang L, Tao T, Wen X, Chen D, Zheng X, et al. Nalbuphine reduces the incidence of emergence agitation in children undergoing adenotonsillectomy: a prospective, randomized, double-blind, multicenter study. J Clin Anesth. (2023) 85:111044. doi: 10.1016/j.jclinane.2022.111044
10. Mihara T, Nakamura N, Ka K, Oba MS, Goto T. Effects of melatonin premedication to prevent emergence agitation after general anaesthesia in children: a systematic review and meta-analysis with trial sequential analysis. Eur J Anaesthesiol. (2015) 32(12):862–71. doi: 10.1097/eja.0000000000000323
11. Cho EJ, Yoon SZ, Cho JE, Lee HW. Comparison of the effects of 0.03 and 0.05 Mg/Kg midazolam with placebo on prevention of emergence agitation in children having strabismus surgery. Anesthesiology. (2014) 120(6):1354–61. doi: 10.1097/aln.0000000000000181
12. Abdulatif M, Ahmed A, Mukhtar A, Badawy S. The effect of magnesium sulphate infusion on the incidence and severity of emergence agitation in children undergoing adenotonsillectomy using sevoflurane anaesthesia. Anaesthesia. (2013) 68(10):1045–52. doi: 10.1111/anae.12380
13. Xu N, Chen Q, Huang ST, Sun KP, Cao H. Sufentanil reduces emergence delirium in children undergoing transthoracic device closure of Vsd after sevoflurane-based cardiac anesthesia. Braz J Cardiovasc Surg. (2020) 35(5):660–5. doi: 10.21470/1678-9741-2019-0334
14. Wu J, Gui Q, Wang J, Ye J, Xia Z, Wang S, et al. Oxycodone preemptive analgesia after endoscopic plasma total adenotonsillectomy in children: a randomized controlled trial. Medicine. (2020) 99(6):e19004. doi: 10.1097/md.0000000000019004
15. Larijani GE, Goldberg ME. Alfentanil hydrochloride: a new short-acting narcotic analgesic for surgical procedures. Clin Pharm. (1987) 6(4):275–82.2889559
16. Zhang YZ, Wei XL, Tang B, Qin YY, Ou M, Jiang XH, et al. The effects of different doses of alfentanil and dexmedetomidine on prevention of emergence agitation in pediatric tonsillectomy and adenoidectomy surgery. Front Pharmacol. (2022) 13:648802. doi: 10.3389/fphar.2022.648802
17. Choi YH, Kim KM, Lee SK, Kim YS, Kim SJ, Hwang WS, et al. Effects of remifentanil and remifentanil-alfentanil administration on emergence agitation after brief ophthalmic surgery in children. BMC Anesthesiol. (2016) 16(1):50. doi: 10.1186/s12871-016-0213-2
18. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
19. Kim MS, Moon BE, Kim H, Lee JR. Comparison of propofol and fentanyl administered at the End of anaesthesia for prevention of emergence agitation after sevoflurane anaesthesia in children. Br J Anaesth. (2013) 110(2):274–80. doi: 10.1093/bja/aes382
20. Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. (2004) 100(5):1138–45. doi: 10.1097/00000542-200405000-00015
21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc. (2015) 13(3):196–207. doi: 10.1097/xeb.0000000000000065
24. Kim JY, Chang YJ, Lee JY, Park HY, Kwak HJ. Post-induction alfentanil reduces sevoflurane-associated emergence agitation in children undergoing an adenotonsillectomy. Acta Anaesthesiol Scand. (2009) 53(5):678–81. doi: 10.1111/j.1399-6576.2009.01943.x
25. Bilgen S, Köner Ö, Karacay S, Sancar NK, Kaspar EC, Sözübir S. Effect of ketamine versus alfentanil following midazolam in preventing emergence agitation in children after sevoflurane anaesthesia: a prospective randomized clinical trial. J Int Med Res. (2014) 42(6):1262–71. doi: 10.1177/0300060514543039
26. Zhao N, Zeng J, Fan L, Zhang C, Wu Y, Wang X, et al. The effect of alfentanil on emergence delirium following general anesthesia in children: a randomized clinical trial. Paediatr Drugs. (2022) 24(4):413–21. doi: 10.1007/s40272-022-00510-5
27. Wang Z, Wang X, Yang Y, He X, Jia W, Yao X, et al. The effect of repeated maternal voice orientation on postoperative emergence agitation in children following tonsillectomy and adenoidectomy: a randomized controlled trial. J Clin Anesth. (2025) 104:111851. doi: 10.1016/j.jclinane.2025.111851
28. Zhou D, Yang XD, Wu HY, Xiong GL, Wang LK. Determination of the Ed90 of dexmedetomidine infusion to prevent emergence agitation in children undergoing dental rehabilitation with sevoflurane anesthesia: a biased-coin up-and-down sequential allocation trial. Anesth Analg. (2024) 139(4):761–9. doi: 10.1213/ane.0000000000006626
29. Voepel-Lewis T, Malviya S, Tait AR. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. (2003) 96(6):1625–30. doi: 10.1213/01.Ane.0000062522.21048.61
30. Alassaf HM, Sobahi AM, Alshahrani NS. The efficacy and safety of dexmedetomidine in preventing emergence delirium in paediatric patients following ophthalmic surgery: a systematic review and meta-analysis of randomised controlled trials. J Anesth Analg Crit Care. (2022) 2(1):48. doi: 10.1186/s44158-022-00079-y
31. Leister N, Trieschmann U, Yücetepe S, Ulrichs C, Muenke N, Wendt S, et al. Nalbuphine as analgesic in preschool children undergoing ophthalmic surgery and the occurrence of emergence delirium. Br J Ophthalmol. (2023) 107(10):1522–5. doi: 10.1136/bjo-2022-321575
32. Tan Y, Shi Y, Ding H, Kong X, Zhou H, Tian J. Μ-Opioid agonists for preventing emergence agitation under sevoflurane anesthesia in children: a meta-analysis of randomized controlled trials. Paediatr Anaesth. (2016) 26(2):139–50. doi: 10.1111/pan.12815
33. El Batawi HY. Effect of preoperative oral midazolam sedation on separation anxiety and emergence delirium among children undergoing dental treatment under general anesthesia. J Int Soc Prev Community Dent. (2015) 5(2):88–94. doi: 10.4103/2231-0762.155728
34. Keles S, Kocaturk O. Comparison of oral dexmedetomidine and midazolam for premedication and emergence delirium in children after dental procedures under general anesthesia: a retrospective study. Drug Des Devel Ther. (2018) 12:647–53. doi: 10.2147/dddt.S163828
35. Dong YX, Meng LX, Wang Y, Zhang JJ, Zhao GY, Ma CH. The effect of remifentanil on the incidence of agitation on emergence from sevoflurane anaesthesia in children undergoing adenotonsillectomy. Anaesth Intensive Care. (2010) 38(4):718–22. doi: 10.1177/0310057(1003800416
36. Na HS, Song IA, Hwang JW, Do SH, Oh AY. Emergence agitation in children undergoing adenotonsillectomy: a comparison of sevoflurane vs. Sevoflurane-remifentanil administration. Acta Anaesthesiol Scand. (2013) 57(1):100–5. doi: 10.1111/aas.12006
37. Kanaya A. Emergence agitation in children: risk factors, prevention, and treatment. J Anesth. (2016) 30(2):261–7. doi: 10.1007/s00540-015-2098-5
38. Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth. (2000) 10(3):303–18. doi: 10.1046/j.1460-9592.2000.00530.x
39. Merkel SI, Voepel-Lewis T, Shayevitz JR, Malviya S. The flacc: a behavioral scale for scoring postoperative pain in young children. Pediatr Nurs. (1997) 23(3):293–7.9220806
Keywords: emergence agitation, alfentanil, pediatric, general anesthesia, review
Citation: Xu Y, Ding K, Zhao X and Sun Y (2025) The effects of alfentanil on emergence agitation in children under general anesthesia: a meta-analysis of randomized controlled trials. Front. Pediatr. 13:1607279. doi: 10.3389/fped.2025.1607279
Received: 7 April 2025; Accepted: 29 September 2025;
Published: 9 October 2025.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Yitian Yang, Henan Provincial People’s Hospital, ChinaHabtamu Aniley, St Paul Millenium Medical College, Ethiopia
Copyright: © 2025 Xu, Ding, Zhao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingying Sun, YWhzZXR5eXN1bnlpbmd5aW5nQDE2My5jb20=
 Yuanling Xu
Yuanling Xu Kun Ding
Kun Ding Yingying Sun
Yingying Sun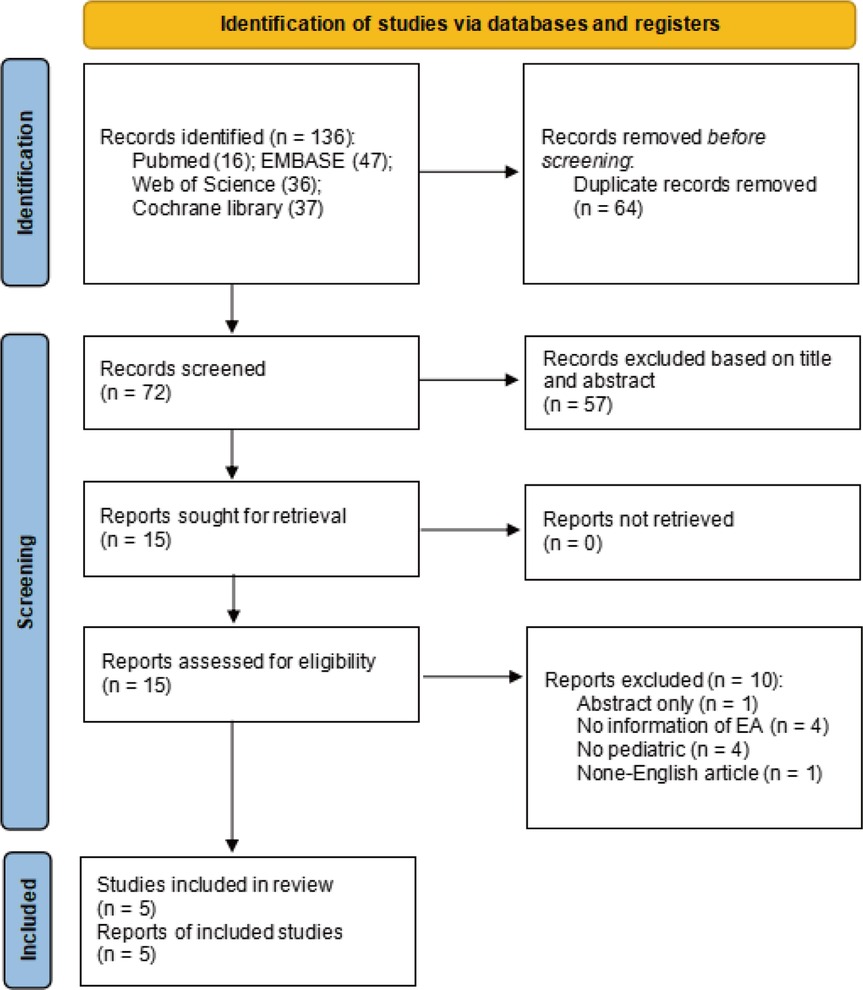

![Forest plot showing the risk ratios and confidence intervals from various studies comparing experimental and control groups at different dosages. The studies are grouped by dosage levels: 1.4.1 (5 micrograms per kilogram), 1.4.2 (1.0 micrograms per kilogram), 1.4.3 (2.0 micrograms per kilogram), 1.4.4 (0.2 micrograms per kilogram per minute), and 1.4.5 (0.4 micrograms per kilogram per minute). Overall, the plot indicates a risk ratio of 0.52 [0.41, 0.66], favoring the experimental group with statistically significant results.](https://www.frontiersin.org/files/Articles/1607279/fped-13-1607279-HTML/image_m/fped-13-1607279-g006.jpg)