- 1Department of Medicine, Combined Military Hospital Quetta, Quetta, Pakistan
- 2SMBZAN Institute of Cardiology, Quetta, Pakistan
- 3Department of Pediatrics, CMH Quetta, Quetta, Pakistan
- 4Quetta Institute of Medical Sciences, Quetta, Pakistan
- 5Department of Pediatrics, Combined Military Hospital Quetta, Quetta, Pakistan
- 6Internal Medicine Department, Mirwas Regional Hospital, Kandahar, Afghanistan
Background: Monosomy 45,X is commonly associated with congenital heart defects, particularly coarctation of the aorta (CoA). In this case, the patient developed respiratory distress due to hemodynamic instability from a large bidirectional patent ductus arteriosus (PDA) shunt and systemic hypoperfusion secondary to CoA, which complicated diagnosis and management.
Case presentation: We report a 34-week premature female neonate weighing 1.94 kg, delivered via lower segment cesarean section (LSCS) due to oligohydramnios and intrauterine growth restriction. She exhibited characteristic features of Turner syndrome, including a webbed neck, low-set ears, widely spaced nipples, and lymphedema of the hands and feet. Karyotyping confirmed a 45,X monosomy. Echocardiography revealed a bicuspid aortic valve, juxtaductal coarctation of the aorta, a moderate-sized PDA with a bidirectional shunt, and suspected pulmonary hypertension. A contrast-enhanced CT aortogram confirmed the coarctation. The patient was managed with mechanical ventilation, continuous positive airway pressure (CPAP), surfactant therapy, and phototherapy. Rescue transcatheter balloon angioplasty was performed for the coarctation, followed by PDA ligation and surgical coarctation repair at a tertiary center, resulting in marked clinical improvement.
Introduction
Turner syndrome (TS) is a genetic condition resulting from complete or partial loss of one sex chromosome in females, with an incidence of 1 in 2,500 live-born female infants. It is associated with various multisystem anomalies, among which congenital heart anomalies are most common, particularly coarctation of aorta (CoA), bicuspid aortic valve (BAV), and aortic stenosis (AS) (1).
Studies have shown that in patients with Turner syndrome, CoA is found in one-third of patients while in 25% of cases, BAV is prevalent (2). Turner syndrome presents with multiple sets of clinical features including primary hypogonadism, skeletal anomalies, congenital heart disease, gastrointestinal and hepatic dysfunctions, visual and auditory problems, lymphatic and cutaneous complications, renal malformations, and mental deficits (3). Despite being well-documented, the occurrence of CoA, PDA, and pulmonary hypertension in neonates altogether results in serious hemodynamic instability, presenting a unique range of diagnostic and therapeutic challenges. Turner syndrome can be suspected on routine ultrasound; however, diagnosis can be confirmed by prenatal karyotyping through amniocentesis and chorionic villi sampling (4).
Patient information
A 34-week preterm female neonate was delivered via lower segment cesarean section (LCS) at CMH Quetta on 11 November 2024 due to oligohydramnios and intrauterine growth restriction (IUGR), with a maternal history of gestational diabetes mellitus (GDM) and thalassemia trait. The baby had a low birth weight of 1.94 kg and fronto-occipital circumference (FOC) of 30 cm, with good APGAR scores.
Upon admission
Vitals signs
Pulse 160 bpm, respiratory rate 65/min, and blood pressure 68/45 mmHg. She was admitted to the NICU immediately after birth due to respiratory distress.
On physical examination
The neonate was hypotonic with reduced spontaneous movement. She had dysmorphic features including a webbed neck, low-set ears, widely spaced nipples, and lymphedema of hands and feet.
Cardiovascular examination revealed a prominent precordial impulse, bounding peripheral pulses, reveals large pulmonary component of second heart sound (loud P2), and a continuous machinery murmur best heard at the left upper sternal border.
Respiratory examination showed tachypnea, nasal flaring, and intercostal retractions. Abdominal exam was soft with no organomegaly, and neurologically, the baby was lethargic but responsive to stimuli.
Timeline
The patient was admitted on 11 November 2024 and immediately transferred to the NICU due to respiratory distress. The first echocardiogram was performed on 25 November 2024, followed by a second on 9 December 2024. A contrast-enhanced CT aortogram was conducted on 10 December 2024, confirming juxtaductal coarctation. Rescue transcatheter balloon angioplasty was performed on 12 December 2024, with a follow-up echocardiogram on 13 December 2024 showing improvement. The patient was transferred to a tertiary cardiac institute on 6 January 2025, where she underwent successful PDA ligation and coarctation repair on 29 January 2025.
Clinical finding
Initial laboratory investigations revealed a hemoglobin level of 18.3 g/dL, total leukocyte count of 9.9 × 109/L, platelet count of 265 × 109/L, and C-reactive protein of 1 mg/L. Serum sodium was 143 mmol/L, potassium 5.2 mmol/L, calcium 1.82 mmol/L, urea 6.0 mmol/L, creatinine 87 µmol/L, albumin 36 g/L, ALT 10 U/L, and serum total bilirubin 110 µmol/L. Magnesium was 0.8 mmol/L, phosphate 2.66 mmol/L, lactate 4.7 mmol/L, and TSH 2.78 mIU/L. The Coombs test was negative. These findings supported stable general metabolic status, with no evidence of significant infection or metabolic derangement.
Diagnostic assessment
Imaging
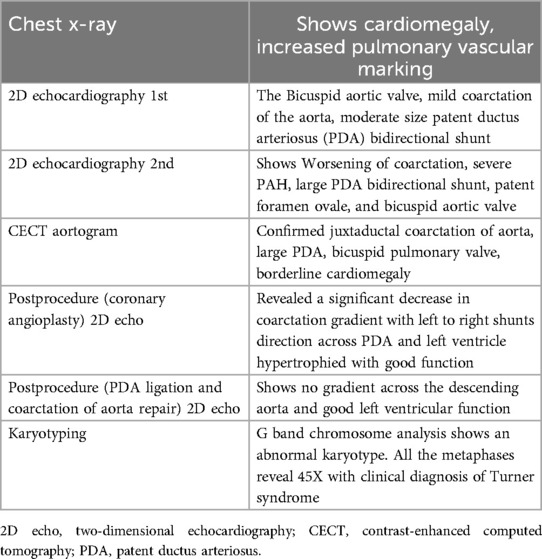
For imaging, see Figures 1 and 2 and Table 1.
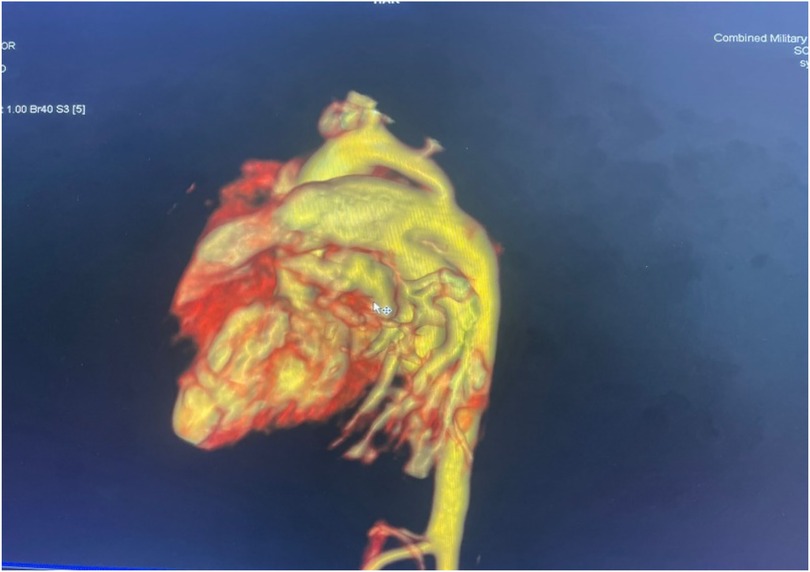
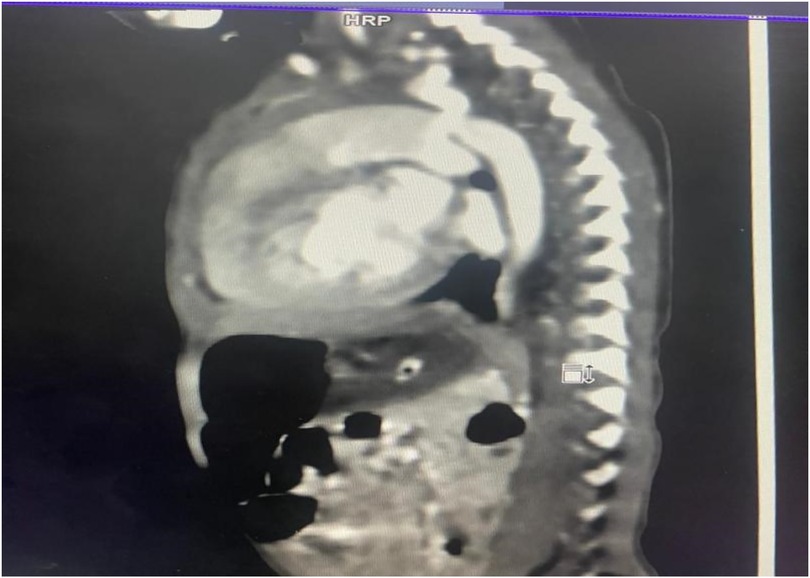
Figure 1. Volume-rendered 3D reconstruction of a CT aortogram showing severe discrete coarctation with a large PDA continuing into the descended aorta.
Diagnostic assessments
The diagnostic workup included a chest radiograph showing cardiomegaly with increased pulmonary vascular markings. Echocardiography confirmed a bicuspid aortic valve, juxtaductal coarctation of the aorta, and a moderate-sized PDA with a bidirectional shunt. A follow-up echocardiogram demonstrated worsening coarctation and suspected pulmonary hypertension. A CT angiogram provided detailed anatomical confirmation of the coarctation and PDA. Although pulmonary hypertension was suspected based on echocardiographic findings and clinical signs such as respiratory distress and a loud P2, we acknowledge that in neonates with critical left-sided obstructive lesions like CoA, reduced systemic pressures can mimic elevated pulmonary pressures. Direct catheter-based pressure measurements were not feasible in our setting, and echocardiographic estimates were limited by technical challenges. The hemodynamic compromise was thus interpreted as multifactorial, primarily due to ductal steal physiology and systemic hypoperfusion from severe coarctation rather than isolated pulmonary arterial hypertension.
Differential diagnosis
The diagnostic workup included Noonan Syndrome, 46XX Gonadal Dysgenesis, Takayasu Arteritis, Hypoplastic Left Heart Syndrome.
Therapeutic intervention
Respiratory distress was initially managed with CPAP ventilation. An intravenous line was secured, first-line antibiotics were initiated, and orogastric feeding was started. Management followed the transient tachypnea of the newborn protocol, with surfactant administration as needed. Cardiac issues were initially treated with low-dose diuretics and fluid restriction. Jaundice developed on day 1 of life and was successfully managed with standard phototherapy.
Despite 15 days of non-invasive ventilation, the infant’s respiratory distress worsened, necessitating mechanical ventilation. As congenital cardiac facilities were not available at our center, rescue transcatheter coarctation ballooning was performed on day 32 of life by a pediatric cardiologist via the femoral artery approach. A 5 × 15 mm coronary angioplasty balloon was used for the procedure. Postprocedure echocardiography showed a significant reduction in the coarctation gradient, with a left-to-right shunt across the PDA and a hypertrophied but well-functioning left ventricle. After the procedure, the infant showed marked clinical improvement, with reduced oxygen requirements and respiratory distress.
On day 57 of life, the patient was successfully weaned off oxygen support and referred to the tertiary care cardiac institute for coarctation repair. PDA ligation surgery was successfully performed on day 80 of life.
Follow-up and clinical outcome
On follow-up, the patient is doing well. Echocardiography showed no gradient across the descending aorta and preserved left ventricular function. The parents were advised to perform hemoglobin electrophoresis at 6 months of age and to ensure regular endocrinology follow-ups for thyroid function and growth monitoring.
Discussion
Congenital heart diseases occur in 25%–50% of individuals with Turner syndrome. Among these, BAV is nearly 60 times more common than in the general female population (4), while CoA affects approximately 7%–18% of affected individuals (5).
In this case, a preterm neonate presented with features suggestive of Turner syndrome and was found to have BAV, CoA, and a moderate PDA. The initial clinical suspicion of pulmonary arterial hypertension (PAH) was based on echocardiographic findings of a bidirectional PDA and clinical signs such as a loud P2. However, in neonates with critical left-sided obstruction, such as CoA, a bidirectional or right-to-left shunt across a PDA may result from low systemic postductal pressure rather than elevated pulmonary arterial pressure (5). In our resource-limited setting, invasive hemodynamic assessment was not possible, and echocardiographic estimates (e.g., tricuspid regurgitation gradient and pulmonary acceleration time) were not reliably obtainable due to technical limitations. Therefore, PAH in this case is best interpreted as suspected rather than confirmed.
One of the key management decisions in this case was the use of transcatheter balloon angioplasty as a bridge to definitive surgical repair (6). Although surgery is typically preferred for CoA in neonates, balloon angioplasty has been employed in selected cases where immediate surgical intervention is not feasible or when clinical instability requires urgent decompression of the aortic gradient. In our context, where pediatric cardiac surgery was unavailable and timely referral was challenging, this approach provided temporary stabilization and allowed successful weaning from mechanical ventilation.
This case highlights the importance of early diagnosis and a multidisciplinary approach, especially in low-resource settings. Despite the complexity of the case and limitations in diagnostic precision, clinical stabilization was achieved through prompt supportive care and staged intervention. Long-term follow-up will be essential to monitor for complications such as restenosis, aneurysm formation, and hormonal issues related to TS, including the initiation of estrogen replacement therapy (ERT) and growth monitoring (2, 7).
This case also highlights the successful use of balloon angioplasty as a bridge to definitive surgical correction. However, a key limitation was the lack of invasive hemodynamic assessment to confirm pulmonary arterial hypertension, relying instead on echocardiographic estimates, which may be confounded by severe aortic obstruction and PDA shunting.
Conclusion
This case underscores the importance of integrated care, proactive management, and comprehensive follow-up in TS patients with multiple congenital heart diseases, essential in improving survival and quality of life.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Combined Military Hospital Institutional Ethical Review Board (CMH QTA-IERB/106/2025). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MK: Data curation, Visualization, Investigation, Writing – original draft, Conceptualization, Supervision, Writing – review & editing. SI: Supervision, Writing – review & editing, Resources, Writing – original draft, Validation. MS: Resources, Investigation, Supervision, Writing – review & editing, Writing – original draft, Validation. MT: Resources, Writing – review & editing, Writing – original draft, Investigation. SZ: Writing – original draft, Validation, Writing – review & editing. AS: Methodology, Writing – original draft, Writing – review & editing. AK: Writing – review & editing, Investigation, Writing – original draft, Supervision, Resources, Validation, Methodology. KK: Writing – review & editing, Investigation, Methodology, Writing – original draft, Validation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1607621/full#supplementary-material
Abbreviations
LCS, lower segment cesarian section; PDA, patent ductus arteriosus; CECT, contrast-enhanced computed tomography; CPAP, continuous positive airway pressure; TS, Turner syndrome; CoA, coarctation of aorta; BAV, bicuspid aortic valve; as, aortic stenosis; GDM, gestational diabetes mellitus; FOC, fronto-occipital circumference; NICU, neonatal intensive care unit; 2D Echo, 2 dimensional echocardiography.
References
1. Chou YY, Wang CJ, Lin CH, Chung HT, Lo FS. Association between cardiovascular anomalies and karyotypes in Turner syndrome patients in Taiwan: a local cohort study. Pediatr Neonatol. (2020) 61(2):188–94. doi: 10.1016/j.pedneo.2019.10.001
2. Khan N, Farooqui A, Ishrat R. Turner syndrome where are we? Orphanet J Rare Dis. (2024) 19(1):314. doi: 10.1186/s13023-024-03337-0
3. Tallón-Walton V, Sánchez-Molins M, Hu W, Martínez-Abadías N, Casado A, Manzanares-Céspedes MC. Comprehensive oral diagnosis and management for women with Turner syndrome. Diagnostics (Basel). (2024) 14(7):769. doi: 10.3390/diagnostics14070769
4. Huang AC, Olson SB, Maslen CL. A review of recent developments in Turner syndrome research. J Cardiovasc Dev Dis. (2021) 8(11):138. doi: 10.3390/jcdd8110138
5. Donadille B, Christin-Maitre S. Heart and Turner syndrome. Ann Endocrinol (Paris). (2021) 82(3–4):135–40. doi: 10.1016/j.ando.2020.12.004
6. Shahri HMM, Mortezaeian H, Firouzi A, Khajali Z, Birjandi H, Nezafati MH, et al. Safety of aortic coarctation treatment in patients with Turner syndrome: a single-country case series and literature review. Ann Vasc Surg. (2022) 85:292–8. doi: 10.1016/j.avsg.2022.02.020
Keywords: Turner syndrome, coarctation of aorta, karyotyping, echocardiography, congenital heart disease, balloon angioplasty, case report
Citation: Khan M, Imtiaz S, Shoaib M, Tariq M, Zahra S, Sardar A, Khan AU and Kamil KA (2025) Association of coarctation of aorta with Turner syndrome: a case report. Front. Pediatr. 13:1607621. doi: 10.3389/fped.2025.1607621
Received: 7 April 2025; Accepted: 8 July 2025;
Published: 14 August 2025;
Corrected: 20 August 2025.
Edited by:
Ornella Milanesi, University of Padua, ItalyReviewed by:
Claudio Cavalli, Maggiore Hospital, ItalySimone Bonetti, IRCCS Azienda Ospedaliero-Universitaria Policlinico di S.Orsola, Italy
Copyright: © 2025 Khan, Imtiaz, Shoaib, Tariq, Zahra, Sardar, Khan and Kamil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamil Ahmad Kamil, ZHJrYW1pbGFobWFkMUBnbWFpbC5jb20=
†ORCID:
Musawer Khan
orcid.org/0009-0002-2082-638X
Malaika Tariq
orcid.org/0009-0009-0235-1158
Sadia Zahra
orcid.org/0009-0006-0714-0307
Kamil Ahmad Kamil
orcid.org/0009-0000-8208-0689
 Musawer Khan
Musawer Khan Sana Imtiaz
Sana Imtiaz Muhammad Shoaib3
Muhammad Shoaib3 Kamil Ahmad Kamil
Kamil Ahmad Kamil