- 1Pediatric Respiratory Department, Dalian Women and Children’s Medical Group, Dalian, Liaoning, China
- 2Department of Pharmacy, Dalian Women and Children’s Medical Group, Dalian, Liaoning, China
Mycoplasma pneumoniae (MP) is a significant pathogen of community-acquired pneumonia in children, typically following a benign course. However, some cases may progress to severe or refractory MP pneumonia (SMPP or RMPP) and lead to thromboembolic complications. This report describes a rare case of a 9-year-old boy with RMPP complicated by bilateral pulmonary embolism (PE) and pulmonary infarction. The patient initially presented with a fever and cough. Despite 24 days of prior treatment at another hospital, including macrolide, carbapenem, and tetracycline antibiotics and corticosteroids, he remained febrile with persistent wheezing when transferred to our institution. Through some laboratory findings and contrast-enhanced chest computed tomography, he fulfilled the diagnostic criteria for both SMPP and RMPP, accompanied by a PE with pulmonary infarction. A multidisciplinary therapeutic approach combining anti-infective agents (linezolid and moxifloxacin), anti-inflammatory therapy (methylprednisolone), and adjusted anticoagulation (low-molecular-weight heparin followed by rivaroxaban) led to rapid clinical improvement and normalization of inflammatory/coagulation markers. Complete resolution of the PE was further demonstrated by 3-month follow-up imaging. Residual focal necrosis in the right lower lobe was observed. This case highlights the potential for severe thromboembolic events in pediatric RMPP and underscores the importance of early recognition of imaging features (e.g., vascular filling defects and wedge-shaped infarcts) and integrated multidisciplinary management to optimize patient outcomes.
1 Introduction
Mycoplasma pneumoniae (MP) is one of the most common pathogens of community-acquired pneumonia (CAP) in children. An MP infection follows a largely benign course, while pneumonia is the most prominent clinical manifestation. M. pneumoniae pneumonia (MPP) accounts for up to 40% of CAP cases in children, with 18% of children requiring hospitalization (1). Children with MPP have been reported to have a high risk of blood coagulation and thrombosis (2–4). The mechanism of thrombosis caused by MPP is still unknown, but it is probably related to immune modulation (5). A pulmonary embolism (PE), the most common thromboembolism in patients with MPP, is a significant cause of residual atelectasis, organizing pneumonia, and pulmonary necrosis (6). Pediatric patients with a PE caused by MPP have rarely been reported. Herein, we report a case of a 9-year-old patient with refractory MPP (RMPP) who gradually developed a PE, and an etiological examination confirmed a Haemophilus influenzae superinfection. Following the administration of anticoagulation treatment, bronchoscopic interventional therapy, and systemic corticosteroid and antibiotic therapy, the patient demonstrated a favorable prognosis. This study aimed to contribute to the clinical understanding and management of such diseases by sharing practical diagnostic and therapeutic experiences.
2 Patient presentation
A 9-year-old boy with no significant family history, specifically nothing suggesting thromboembolic disease, presented with a fever and cough but no symptoms of hemoptysis, fatigue, or syncope within the previous 24 days. The chest computed tomography (CT) at approximately 2 weeks of the disease revealed wedge-shaped consolidations with pleural-based, hilar-pointing configurations (Figure 1). Outpatient antibiotic therapy (azithromycin and erythromycin) for 10 days, followed by inpatient dual broad-spectrum antibiotic therapy (minocycline and meropenem) for 13 days and immunosuppressive therapy [methylprednisolone (12 days) and intravenous immunoglobulin (3 days)] were initiated at the other hospital. However, the patient still presented with a persistent fever, persistent paroxysmal coughing, and wheezing. Thus, he was transferred to our hospital on 30 November 2023. At admission, Mycoplasma pneumoniae-polymerase chain reaction (MP-PCR) was positive, accompanied by elevated specific IgM of 48.8 (<20) and IgG >300. Moreover, the laboratory findings were white blood cell (WBC) level of 22.76 × 109 /L, neutrophil ratio (NEUT%) of 74.2% (the neutrophil count was 13.7 × 109 /L), procalcitonin (PCT) level of 0.83 ng/ml, and C-reactive protein (CPR) and serum amyloid A (SAA) levels of 75.05 and 232.3 mg/L, respectively. Moreover, the coagulation analysis revealed an elevated D-dimer level (5.18 mg/L) and elevated fibrin degradation products (FDP) (10.35 mg/L). Therefore, considering a double bacterial infection, he was intravenously administered linezolid (30 mg/kg/day, q8h) combined with oral moxifloxacin (10 mg/kg/day) as an anti-infection therapy, along with methylprednisolone (2 mg/kg/day, q12h) as an anti-inflammatory treatment and low-molecular-weight heparin (LMWH, 3,000 IU/day, qd) as an anticoagulation therapy (Table 1). Within the first 4 days after admission, his fever had gone, but the cough was still persistent. In addition, microbiological testing of sputum, bronchoalveolar lavage fluid, and blood samples for bacteria, fungi, and common respiratory viruses (including respiratory syncytial virus, adenovirus, influenza virus, rhinovirus, and human metapneumovirus) yielded negative results. On day 5, chest contrast-enhanced CT showed multiple filling defects in the bilateral lower lobe pulmonary arteries, involving the initial segments of some basal segmental arteries and their distal branches (Figure 2). Based on the above finding, bilateral PE with bilateral lower lung infarction was suspected. Thus, his dosage of low-molecular-weight heparin was adjusted to 6,000 IU/day, administered every 12 h. However, remarkably, coagulation examinations revealed a decrease in D-dimer level (0.97 mg/L) and fibrinogen (FIB) (4.35 g/L). On day 7, Gram-positive (G+) bacteria were found in sputum culture. After 15 days of hospitalization, the condition of the patient was stable. In addition, the posteroanterior and lateral chest radiographs indicated that the inflammation in the lungs had been partially reduced compared to before. The anticoagulation regimen was transitioned from low-molecular-weight heparin calcium to oral rivaroxaban (15 mg/day). He was discharged after an 18-day stay, with normalized inflammatory markers and clinical improvement. His oral rivaroxaban (15 mg/day) treatment was continued for 3 months. At the 3-month follow-up, he was afebrile with no cough or wheezing. Contrast-enhanced chest CT showed no evidence of thromboembolism in the bilateral pulmonary arteries or veins, but revealed residual sequelae of right lower lobe atelectasis with minimal necrosis (Figure 3).
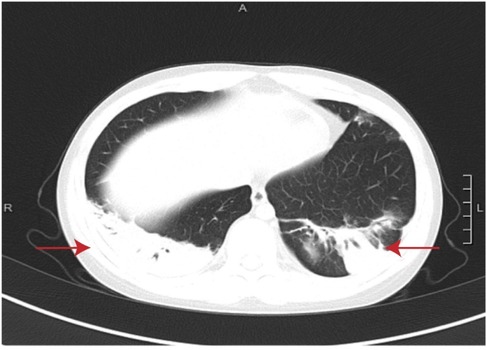
Figure 1. Chest CT in week 2 of the disease. The red arrows indicate wedge-shaped consolidations with pleural-based, hilar-pointing configurations.
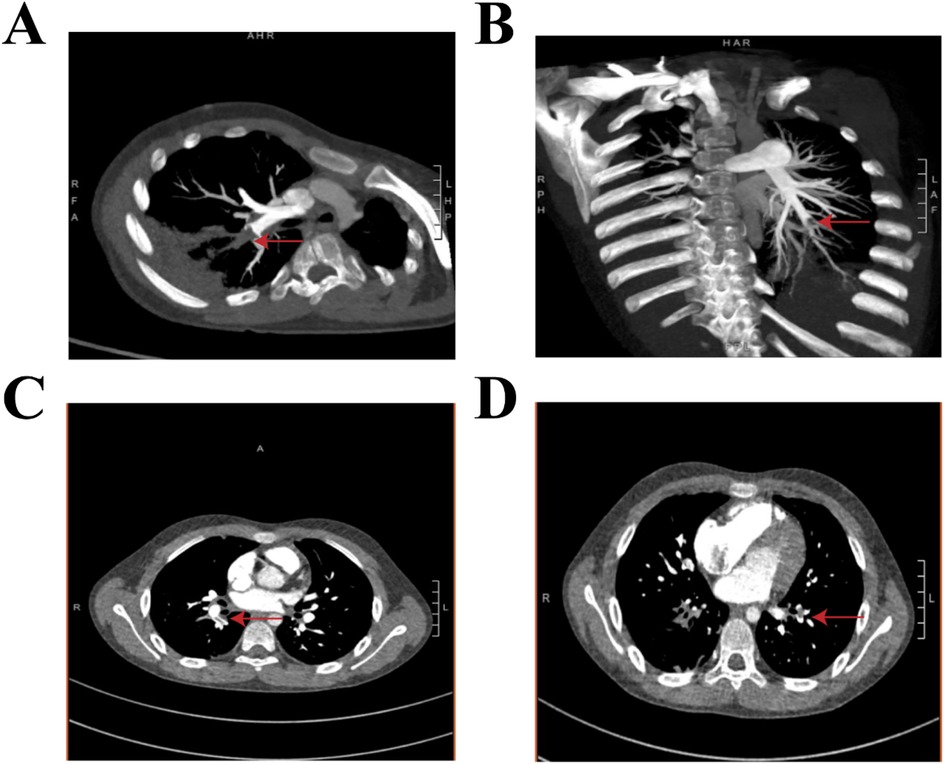
Figure 2. Contrast-enhanced chest CT obtained during the fourth week of illness demonstrates multiple pulmonary emboli in the bilateral lower lobe arteries. (A,C) The right lower lobe pulmonary artery shows filling defects involving the proximal basal segmental arteries (the red arrow) and their terminal branches. (B,D) The left lower lobe pulmonary artery shows occlusions at the origins of the basal segmental arteries (the red arrow), with distal propagation into branch vessels.
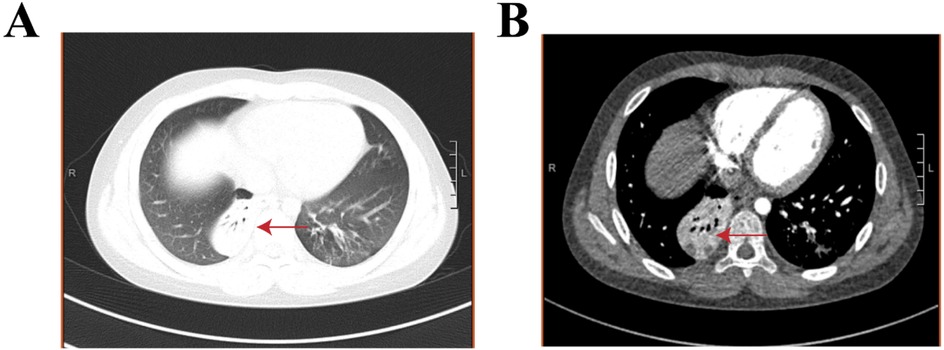
Figure 3. Chest contrast-enhanced CT with a three-dimensional reconstruction in month 4.5 of the disease. (A, B) The red arrows indicate right lower lobe pulmonary atelectasis with small areas of necrosis..
3 Discussion
In 2023, the post-COVID-19 era began, coinciding with a significant surge in MP infections, which reached epidemic levels globally (7). Furthermore, there has been a significant surge in reports of RMPP or severe MPP (SMPP) worldwide, with a particularly notable increase observed in Asia (8, 9). According to the Guidelines for the Management of Community-acquired Pneumonia in Children (2024 revision), RMPP is defined by the following criteria: (1) failure to respond to standard macrolide therapy for at least 1 week, (2) persistent fever, (3) progressive worsening of clinical symptoms, (4) deterioration of pulmonary imaging findings, and (5) presence of extrapulmonary complications involving multiple organ systems (10). RMPP mostly affects school-aged children (11). This child met the diagnostic criteria for both SMPP and RMPP, with the multifactorial pathogenesis attributable to a drug-resistant MP infection, hyperinflammatory response, and concurrent mixed infections. A delayed diagnosis likely contributed to disease progression, further complicating the clinical course. Moreover, the patient had residual sequelae of right lower lobe atelectasis. Early recognition and aggressive management of SMPP and RMPP are critical to minimizing the development of long-term sequelae (12). The optimal therapeutic window lies within 5–10 days post-fever onset (13). If the fever persists beyond 14 days of disease progression with no clinical improvement, patients are at high risk of irreversible complications (10). Given the clinical heterogeneity of MPP, individualized therapeutic regimens should be formulated based on disease subtypes (14, 15). Especially for SMPP cases, targeted multimodal interventions (e.g., combined anti-infective therapy, glucocorticoids, bronchoscopy, and anticoagulation) should be prioritized (16). It is also important to note that management must address both co-infections and precisely identify/control excessive inflammatory responses, including cytokine storms. Failure to promptly control hyperinflammation may significantly increase the risk of secondary infections and long-term sequelae (17).
MP infections can give rise to both pulmonary and extrapulmonary complications, such as necrotizing pneumonia (NP), plastic bronchitis (PB), thromboembolism, myocarditis, hemolytic anemia, Stevens–Johnson syndrome, and erythema multiforme (18–22). Significantly, there has been a rising number of reported cases of MPP-associated thromboembolism (23, 24). The potential mechanisms underlying MP-associated thromboembolism include the following (25). First, MP can directly invade and damage pulmonary vascular endothelial cells, leading to endothelial activation and exposure of tissue factor, thus initiating the extrinsic coagulation cascade and promoting localized thrombus formation. Second, the MP infection triggers a robust inflammatory response characterized by elevated cytokines, thus inducing vascular wall inflammation and thrombotic vascular occlusion through endothelial dysfunction. Finally, through molecular mimicry, MP triggers autoimmune responses characterized by autoantibody production (antiphospholipid antibodies and antiprothrombin antibodies) and complement system activation, thereby elevating coagulation factor levels and establishing a systemic hypercoagulable state that predisposes the patient to thrombus formation. Thromboembolic events associated with MP infection can occur in any anatomical site, including PE, deep vein thrombosis (DVT) of the lower extremities, intracardiac thrombosis, aortic thrombosis, cerebral infarction, cerebral venous sinus thrombosis (CVST), pulmonary venous thrombosis, renal vein thrombosis, and splenic infarction (26–29). PE is the most common manifestation and serves as a major contributor to pulmonary necrosis, residual atelectasis, and organizing pneumonia (6). In this pediatric case, the patient developed a pulmonary embolism, pulmonary infarction, and residual right lower lobe atelectasis with focal necrosis during the disease course. The trigger for these complications may be a prolonged and excessive inflammatory response. The pathogenesis of this dysregulated hyperinflammation likely stems from multiple factors: delayed initiation and inadequate dosing of corticosteroid therapy, macrolide antibiotic resistance, and concurrent bacterial co-infection. Therefore, the early recognition of and prophylactic intervention for a PE hold significant clinical relevance, particularly in mitigating life-threatening complications and improving long-term outcomes.
Chest pain represents the most frequently reported symptom of a PE in children with an MP infection (6, 30). While dyspnea and hemoptysis may also occur, these manifestations are often overshadowed by the overlapping symptoms of MPP, complicating their identification. Notably, one-seventh to one-third of pediatric PE cases present asymptomatically (20, 31). Beyond clinical evaluation, D-dimer levels provide a valuable diagnostic clue for a PE in MP infections (32). Despite its high sensitivity for detecting a PE, the D-dimer level lacks specificity, as it can also be markedly elevated in patients with SMPP without thrombotic events (3, 33, 34). In addition, the most common diagnostic method for MPP combined with a PE is chest contrast-enhanced CT (35). The direct imaging signs of a PE on CT include filling defects or complete obstruction within the vascular lumen (36). Other associated findings may involve a localized reduction in pulmonary vascular markings, patchy lung shadows, and dilated bronchial arteries (37). A PE can often lead to secondary pulmonary infarction, with its classic imaging manifestation being a wedge-shaped consolidation beneath the pleura, with the apex pointing toward the hilum (38). However, some patients may also exhibit a reversed halo sign on imaging (39). These signs are critical for guiding the early diagnosis of a pulmonary embolism. Throughout the clinical course, this child did not exhibit typical symptoms of a PE. However, his D-dimer levels exceeded 5 mg/L by week 4 of illness, and contrast-enhanced chest CT at week 5 confirmed a PE with pulmonary infarction. Notably, wedge-shaped consolidations with pleural-based, hilar-pointing configurations were already evident in the bilateral lower lobes on CT imaging in approximately week 2 of the disease. For such cases, a PE should be suspected early despite atypical presentations to mitigate thromboembolic complications and long-term sequelae. For SMPP complicated by a PE, anticoagulation is the cornerstone of therapy. Systemic thrombolysis should be considered if hemodynamic instability develops. Unfractionated heparin or LMWH is mostly used as an initial anticoagulation therapy. Moreover, for sequential anticoagulation, LMWH, warfarin, or rivaroxaban are generally selected (39). Anticoagulation therapy is typically continued for 3–6 months (40). In this case, the child received LMWH for 15 days during hospitalization, followed by oral rivaroxaban for 3 months, resulting in the resolution of the PE and favorable clinical outcomes. A phase 3 clinical trial evaluating rivaroxaban for pediatric acute venous thromboembolism reported that, compared to standard anticoagulants (LMWH or vitamin K antagonists), rivaroxaban reduced the risk of thrombotic recurrence without increasing bleeding risk (41). To prevent an MP-associated PE, the following strategies are recommended (10). First, early recognition and timely treatment of the MP infection to reduce progression to SMPP is recommended, thereby minimizing thrombotic complications. In addition, measures should be taken to minimize the risk of thrombosis, including ensuring adequate fluid intake to prevent dehydration caused by persistent high fever and avoiding prolonged immobility, among other precautions. Finally, it is necessary to monitor D-dimer levels in children with SMPP who have prolonged fever, extensive pulmonary consolidation, or elevated inflammatory markers. Clinicians should initiate prophylactic anticoagulation if the patient’s D-dimer level is elevated and there is no bleeding risk. Continuous assessment of bleeding risk during anticoagulation therapy is also important.
CT technology can only detect differences in tissue density, whereas magnetic resonance imaging (MRI) technology offers superior tissue contrast, multiplanar imaging capabilities, sensitivity to blood flow, and absence of ionizing radiation. These features make MRI particularly suitable for detecting and diagnosing soft tissue lesions in the chest, especially for radiation-sensitive populations (e.g., children). Chest soft tissues include the heart, mediastinum, pleura, and chest wall. However, MRI has limited utility in evaluating pulmonary diseases due to motion artifacts caused by physiological respiratory movements, low signal intensity from air-filled lungs, and magnetic field inhomogeneities at air/soft tissue interfaces. Currently, some noble gas MRI contrast agents, such as hyperpolarized (HP) butane gas (42), HP diethyl ether (DE) gas (43), parahydrogen-hyperpolarized propane-d6 gas (44), are revolutionizing functional pulmonary MRI. These agents enable high-resolution lung imaging and are compatible with virtually any MRI system, including emerging portable bedside low-field MRI systems.
4 Conclusion
This case highlights the critical risk of a PE in pediatric RMPP, even without classic thrombotic symptoms. Early recognition of subtle clinical clues, such as persistent fever unresponsive to conventional therapy and elevated inflammatory and coagulation markers (e.g., D-dimer), combined with contrasted chest CT, is essential for timely diagnosis. The successful outcome in this patient was achieved through a multidisciplinary approach integrating targeted anti-infective therapy, adjusted anticoagulation (escalated low-molecular-weight heparin followed by rivaroxaban), and immunomodulation. The complete resolution of the PE on follow-up imaging supports the efficacy and safety of a 3-month oral anticoagulation regimen in children. However, the residual pulmonary necrosis observed in this case emphasizes the importance of long-term monitoring for chronic sequelae. Proactive coagulation screening and early imaging in refractory cases are warranted. Further studies are essential to optimize pediatric-specific anticoagulation protocols and risk stratification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Dalian Women and Children Medicine Group Medical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JZ: Data curation, Investigation, Resources, Writing – original draft. ZhZ: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. ZiZ: Data curation, Formal Analysis, Writing – original draft. LC: Methodology, Writing – original draft. YS: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the patient and staff associated with the clinical study discussed here.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin Microbiol Rev. (2017) 30(3):747–809. doi: 10.1128/CMR.00114-16
2. Li TYH, Hou WLZ, Han CWL. Children with MPP have a higher risk of blood coagulation and thrombosis. J Int Med Res. (2017) 46(5):2110–8. doi: 10.1177/0300060517709613
3. Jin X, Zhu Y, Zhang Y, Chen J, Rong L, Zhao X. Assessment of levels of D-dimer and interferon-γ in pediatric patients with Mycoplasma pneumoniae pneumonia and its clinical implication. Exp Ther Med. (2018) 16(6):5025–30. doi: 10.3892/etm.2018.6873
4. Brown SM, Padley S, Bush A, Cummins D, Davidson S, Buchdahl R. Mycoplasma pneumonia and pulmonary embolism in a child due to acquired prothrombotic factors. Pediatr Pulmonol. (2008) 43(2):200–2. doi: 10.1002/ppul.20739
5. Chen Y, Huang P, Chen Q, Lin Z, Tian W. Two separated thrombi in deep veins associated with pulmonary embolism after Mycoplasma pneumoniae infection: a case in adolescent female. Transl Pediatr. (2013) 2(4):198–201. doi: 10.3978/j.issn.2224-4336.2013.10.01
6. China, N. H. C o t P s R o. Guidelines for diagnosis and treatment of Mycoplasma pneumoniae pneumonia in children. China Licensed Pharm. (2023) 20(3):16–24. doi: 10.3760/cma.j.cn331340-20230217-00023
7. Sauteur PM M, Beeton ML, European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Mycoplasma and Chlamydia Infections (ESGMAC), and the ESGMAC Mycoplasma pneumoniae Surveillance (MAPS) study group. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. (2024) 5(2):e100–1. doi: 10.1016/S2666-5247(23)00344-0
8. Sztrymf B, Jacobs F, Fichet J, Hamzaoui O, Prat D, Avenel A, et al. Pneumopathie à mycoplasmeune cause rare de syndrome de détresse respiratoire aiguë (SDRA) et de résistance potentielle aux antibiotiques [Mycoplasma-related pneumonia: a rare cause of acute respiratory distress syndrome (ARDS) and of potential antibiotic resistance]. Rev Mal Respir. (2013) 30(1):77–80. doi: 10.1016/j.rmr.2012.06.012
9. Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T. Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect. (2008) 57(3):223–8. doi: 10.1016/j.jinf.2008.06.012
10. Subspecialty Group of Respiratory, the Society of Pediatrics, Chinese Medical Association; Editorial Board, Chinese Journal of Pediatrics; China Medicine Education Association Committee on Pediatrics. Guidelines for the management of community-acquired pneumonia in children (2024 revision). Zhonghua Er Ke Za Zhi. (2024) 62(10):920–30. doi: 10.3760/cma.j.cn112140-20240728-00523
11. Colin AA, Yousef S, Forno E, Korppi M. Treatment of Mycoplasma pneumoniae in pediatric lower respiratory infection. Pediatrics. (2014) 133(6):1124–5. doi: 10.1542/peds.2014-0871
12. Yang TI, Chang TH, Lu CY, Chen JM, Lee PI, Huang LM, et al. Mycoplasma pneumoniae in pediatric patients: do macrolide-resistance and/or delayed treatment matter? J Microbiol Immunol Infect. (2019) 52(2):329–35. doi: 10.1016/j.jmii.2018.09.009
13. Yan C, Xue GH, Zhao HQ, Feng YL, Cui JH, Yuan J. Current status of Mycoplasma pneumoniae infection in China. World J Pediatr. (2024) 20(1):1–4. doi: 10.1007/s12519-023-00783-x
14. Corlateanu A, Mendez Y, Wang Y, Garnica RJA, Botnaru V, Siafakas N. Chronic obstructive pulmonary disease and phenotypes: a state-of-the-art. Pulmonology. (2020) 26(2):95–100. doi: 10.1016/j.pulmoe.2019.10.006
15. Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. (2004) 350(26):2645–53. doi: 10.1056/NEJMoa032158
16. Kant R, Kumar N, Malik YS, Everett D, Saluja D, Launey T, et al. Critical insights from recent outbreaks of Mycoplasma pneumoniae: decoding the challenges and effective interventions strategies. Int J Infect Dis. (2024) 147:107200. doi: 10.1016/j.ijid.2024.107200
17. Hardy RD, Jafri HS, Olsen K, Hatfield J, Iglehart J, Rogers BB, et al. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect Immun. (2002) 70(2):649–54. doi: 10.1128/IAI.70.2.649-654.2002
18. Poddighe D. Extra-pulmonary diseases related to Mycoplasma pneumoniae in children: recent insights into the pathogenesis. Curr Opin Rheumatol. (2018) 30(4):380–7. doi: 10.1097/BOR.0000000000000494
19. Cherry JD. Anemia and mucocutaneous lesions due to Mycoplasma pneumoniae infections. Clin Infect Dis. (1993) 17(Suppl 1):S47–51. doi: 10.1093/clinids/17.Supplement_1.S47
20. Liu J, He R, Wu R, Wang B, Xu H, Zhang Y, et al. Mycoplasma pneumoniae pneumonia associated thrombosis at Beijing children’s hospital. BMC Infect Dis. (2020) 20(1):51. doi: 10.1186/s12879-020-4774-9
21. Hahn DW, Atkinson CE, Le M. Multiple anatomic sites of infarction in a pediatric patient with M. pneumoniae infection, a case report. BMC Pediatr. (2021) 21(1):372. doi: 10.1186/s12887-021-02845-3
22. Jin P, Han C, Guo W, Xu Y. Mycoplasma pneumoniae pneumonia-associated thromboembolism with plastic bronchitis: a series of five case reports and literature review. Ital J Pediatr. (2024) 50(1):117. doi: 10.1186/s13052-024-01690-1
23. Chen L, Yin J, Liu X, Liu J, Xu B, Shen K. Thromboembolic complications of Mycoplasma pneumoniae pneumonia in children. Clin Respir J. (2023) 17(3):187–96. doi: 10.1111/crj.13584
24. Han C, Zhang T, Zheng J, Jin P, Zhang Q, Guo W, et al. Analysis of the risk factors and clinical features of Mycoplasma pneumoniae pneumonia with embolism in children: a retrospective study. Ital J Pediatr. (2022) 48(1):153. doi: 10.1186/s13052-022-01344-0
25. Liu J, Li Y. Thrombosis associated with Mycoplasma pneumoniae infection (review). Exp Ther Med. (2021) 22(3):967. doi: 10.3892/etm.2021.10399
26. Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. (2004) 17(4):697–728. doi: 10.1128/CMR.17.4.697-728.2004
27. Kalicki B, Sadecka M, Wawrzyniak A, Kozinski P, Dziekiewicz M, Jung A. Absence of inferior vena cava in 14-year-old boy associated with deep venous thrombosis and positive Mycoplasma pneumoniae serum antibodies–a case report. BMC Pediatr. (2015) 15:40. doi: 10.1186/s12887-015-0357-0
28. Li X, Zhai B, Tang Y, Zhang L, Wang J, Xu C, et al. Clinical features of intracardiac thrombotic complication in patients with severe Mycoplasma pneumoniae pneumonia. Ital J Pediatr. (2025) 51(1):42. doi: 10.1186/s13052-025-01890-3
29. Jin X, Zou Y, Zhai J, Liu J, Huang B. Refractory Mycoplasma pneumoniae pneumonia with concomitant acute cerebral infarction in a child: a case report and literature review. Medicine (Baltimore). (2018) 97(13):e0103. doi: 10.1097/MD.0000000000010103
30. Sheng CQ, Yang CF, Ao Y, Zhao ZY, Li YM. Mycoplasma pneumoniae pneumonia with pulmonary embolism: a study on pediatric cases in Jilin province of China. Exp Ther Med. (2021) 21(3):201. doi: 10.3892/etm.2021.9634
31. Fu Y, Zhang TQ, Dong CJ, Xu YS, Dong HQ, Ning J. Clinical characteristics of 14 pediatric Mycoplasma pneumoniae pneumonia associated thrombosis: a retrospective study. BMC Cardiovasc Disord. (2023) 23(1):1. doi: 10.1186/s12872-022-03030-9
32. Huang X, Li D, Liu F, Zhao D, Zhu Y, Tang H. Clinical significance of D-dimer levels in refractory Mycoplasma pneumoniae pneumonia. BMC Infect Dis. (2021) 21(1):14. doi: 10.1186/s12879-020-05700-5
33. Biss TT, Brandão LR, Kahr WH, Chan AK, Williams S. Clinical probability score and D-dimer estimation lack utility in the diagnosis of childhood pulmonary embolism. J Thromb Haemost. (2009) 7(10):1633–8. doi: 10.1111/j.1538-7836.2009.03572.x
34. Hennelly KE, Baskin MN, Monuteuax MC, Hudgins J, Kua E, Commeree A, et al. Detection of pulmonary embolism in high-risk children. J Pediatr. (2016) 178:214–218.e3. doi: 10.1016/j.jpeds.2016.07.046
35. Moore AJE, Wachsmann J, Chamarthy MR, Panjikaran L, Tanabe Y, Rajiah P. Imaging of acute pulmonary embolism: an update. Cardiovasc Diagn Ther. (2018) 8(3):225–43. doi: 10.21037/cdt.2017.12.01
36. Song S, Xu Y. A retrospective study of the clinical characteristics of 9 children with pulmonary embolism associated with Mycoplasma pneumoniae pneumonia. BMC Pediatr. (2023) 23(1):370. doi: 10.1186/s12887-023-04188-7
37. Simmons BP, Aber RC. Mycoplasma pneumoniae pneumonia. Symptoms mimicking pulmonary embolism with infarction. JAMA. (1979) 241(12):1268–9. doi: 10.1001/jama.1979.03290380044027
38. Zhang X, Sun R, Hou J, Jia W, Li P, Song C, et al. Clinical characteristics and risk factors of pulmonary embolism with Mycoplasma pneumoniae pneumonia in children. Sci Rep. (2024) 14(1):24043. doi: 10.1038/s41598-024-74302-x
39. Ross C, Kumar R, Pelland-Marcotte MC, Mehta S, Kleinman ME, Thiagarajan RR, et al. Acute management of high-risk and intermediate-risk pulmonary embolism in children: a review. Chest. (2022) 161(3):791–802. doi: 10.1016/j.chest.2021.09.019
40. Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. (2018) 2(22):3292–316. doi: 10.1182/bloodadvances.2018024786
41. Male C, Lensing AWA, Palumbo JS, Kumar R, Nurmeev I, Hege K, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. (2020) 7(1):e18–27. doi: 10.1016/S2352-3026(19)30219-4
42. Ariyasingha NM, Samoilenko A, Chowdhury MRH, Nantogma S, Oladun C, Birchall JR, et al. Developing hyperpolarized butane gas for ventilation lung imaging. Chem Biomed Imaging. (2024) 2(10):698–710. doi: 10.1021/cbmi.4c00041
43. Ariyasingha NM, Chowdhury MRH, Samoilenko A, Salnikov OG, Chukanov NV, Kovtunova LM, et al. Toward lung ventilation imaging using hyperpolarized diethyl ether gas contrast agent. Chemistry. (2024) 30(25):e202304071. doi: 10.1002/chem.202304071
Keywords: refractory Mycoplasma pneumoniae pneumonia, severe Mycoplasma pneumoniae pneumonia, pulmonary embolism, children, infection
Citation: Zhang J, Zhang Z, Zhu Z, Cheng L and Shan Y (2025) Case Report: Refractory Mycoplasma pneumoniae pneumonia complicated by pulmonary embolism and infarction in a child. Front. Pediatr. 13:1608233. doi: 10.3389/fped.2025.1608233
Received: 8 April 2025; Accepted: 21 May 2025;
Published: 23 June 2025.
Edited by:
Zhongjie Shi, Wayne State University, United StatesReviewed by:
Huang Chengjiao, Hubei Maternal and Child Health Hospital, ChinaSócrates Vargas Naranjo, Unidade Local de Saúde do Norte Alentejano, Portugal
Boris Garber, Case Western Reserve University, United States
Copyright: © 2025 Zhang, Zhang, Zhu, Cheng and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxia Shan, c3l4MTE1MTA1QDEyNi5jb20=
†These authors have contributed equally to this work
 Jianqin Zhang1,†
Jianqin Zhang1,† Yuxia Shan
Yuxia Shan