- 1Department of Pediatrics, Hematology Oncology Unit, Saint George Hospital University Medical Center, Beirut, Lebanon
- 2Saint George University, School of Medicine, Beirut, Lebanon
- 3Department of Epidemiology and Population Health, Faculty of Health Sciences, American University of Beirut, Beirut, Lebanon
- 4Department of Pediatrics Hematology Oncology, Sahel Hospital Medical Center (Lebanese University), Beirut, Lebanon
- 5Department of Pediatrics, Rai Hospital, Saida, Lebanon
- 6Department of Pediatrics, Hematology Oncology Lebanese University Hospital Geitewi, Beirut, Lebanon
- 7Department of Pediatrics, Hematology Oncology CHU Notre Dame Des Secours, Byblos, Lebanon
- 8Scientific and Medical Affairs, Emerging Markets, Pfizer Inc., Collegeville, PA, United States
- 9Department of Pediatrics, LAU Gilbert and Rose-Marie Chaghoury School of Medicine, Byblos, Lebanon
- 10Division of Pediatric Hematology Oncology, NINI Hospital, Tripoli, Lebanon
Introduction: The COVID-19 pandemic has significantly impacted the pediatric population worldwide, particularly those with comorbidities who often experience more severe outcomes. However, the impact of COVID-19 on pediatric oncology patients remain poorly understood.
Methods: This retrospective observational study examined data from 85 pediatric oncology patients aged <18 years who tested positive for COVID-19 at five study sites across Lebanon from March 2020 to May 2023. Detailed demographic, clinical, treatment, healthcare resource utilization (HCRU), and disease outcomes were collected. Outcomes was summarized descriptively and two-sided 95% confidence intervals (CI) were calculated using the Clopper-Pearson method.
Results: There was 85 pediatric oncology patients diagnosed with COVID-19, with a mean age of 11.57 years. Of these, 72 patients (84.6%) had hematological malignancies and 13 (15.4%) had solid tumors. Thirteen patients (15.3%) had comorbidities. Treatment delays on oncology care occurred in 61 patients (71.8%). Twelve patients (14.1%) required hospitalization, four of whom needed intensive care unit (ICU) admission, with ICU stays ranging from 1 day to 2 months. COVID-19 related mortality was 4.7%.
Discussion: COVID-19 was associated with changes or delay in oncological treatment. In addition, the burden of COVID related hospitalization, intensive care utilization and death were substantial among pediatric cancer patients. Our findings highlight the importance of vaccination in pediatric oncology patients.
1 Introduction
The outbreak of Coronavirus Disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rapidly evolved into a global health pandemic, as officially declared by the World Health Organization (WHO) (1). The pandemic particularly captured the interest of pediatric oncologists, who closely monitored its impact on children with cancer.
Adult cancer patients with COVID-19 experienced severe illness, with significantly higher mortality rates compared to other age groups (2–5). Many case reports and cohort studies from national and international registries have shown that the majority of pediatric cancer patients experience only clinically mild to moderate symptoms (6–11). In fact, only a small percentage of them progress to severe disease and necessitate critical care (12).
Despite the generally milder impact of COVID-19 on children, the pandemic has introduced numerous challenges to the healthcare sector, particularly affecting the overall management of pediatric cancer patients. These challenges include significant delays in the diagnosis of cancer due to disruptions in routine healthcare services, interruptions in necessary hospitalizations, and major disruptions to ongoing cancer treatments (13–15). The pandemic has also complicated treatment administration and follow-up care, necessitating adjustments in clinical practice to ensure continued care while minimizing exposure to the virus (14, 16). Moreover, families of children with cancer faced a decline in income due to the pandemic, which adversely affected their ability to access care and resources. Finally, the migration crisis, in addition to the economic crisis and sociopolitical instability in Lebanon since 2019 have further exacerbated the challenges for the healthcare sector in general, and the situation for pediatric cancer patients specifically, due to critical shortages of essential resources. This includes a severe lack of medications that are vital for the treatment of the pediatric oncology population (17, 18).
Data on the severity of COVID-19 in pediatric patients with cancer in the Middle East is limited and not well understood. To address this knowledge gap, we hereby describe the impact of COVID-19 on the clinical outcomes and overall care delivery of pediatric oncology patients (≤18 years of age) who tested positive for COVID-19 in Lebanon between March 2020 and May 2023. We also outline the specific challenges that were faced by these patients during the pandemic and the additional support or resources required to manage their care effectively.
2 Materials and methods
This retrospective, observational database study included all pediatric patients aged 0–18 years diagnosed with any type of cancer, including both hematologic malignancies and solid tumors, and who tested positive for SARS-CoV-2 between March 2020 and May 2023 during any visit at one of the five participating hospitals and referral centers in Lebanon. These included: Saint George Hospital University Medical Center in Achrafieh, Beirut (Mid Lebanon); NINI Hospital in Tripoli (North Lebanon); Notre Dame de Secours Hospital in Jbeil (Mount Lebanon); Lebanese Hospital Geitaoui in Achrafieh, Beirut (Mid Lebanon) and Al Raii Hospital in Sidon (South Lebanon). Collectively, these five centers account for approximately 40%–45% of all pediatric oncology patients in Lebanon. The study data were extracted from patient medical records and hospital databases for further analyses (N = 210). Patients were categorized based on their COVID-19 status, with two distinct groups: 85 patients who tested positive for COVID-19 and 125 patients who tested negative by PCR. Exclusion criteria included any patient who received a COVID-19 vaccine prior to their SARS-CoV-2 infection. Institutional Review Board (IRB) approval for this study was obtained from all participating centers. As this was an observational, non-interventional study with anonymized data, patients' caregiver consent was not needed.
The primary endpoint of the study was to determine the proportion of pediatric oncology patients, categorized by specific demographics, clinical, and hematologic characteristics, who experienced changes in their outcomes and therapy due to COVID-19 infection. The secondary endpoints of the study included several key measures. First, the proportion of pediatric oncology patients who experienced delays or interruptions in their treatment course, based on pre-established criteria, both with and without COVID-19 infection. A delay was defined as a postponement of seven or more days from the originally scheduled date of treatment. “Treatment delay” encompasses all types of oncologic therapies, including chemotherapy, radiotherapy, surgery, and scheduled imaging. Second, the proportion of these patients who were hospitalized specifically due to COVID-19 were assessed. Third, the study evaluated the rate of COVID-19 re-infections among pediatric oncology patients. Fourth, it examined the proportion of COVID-19 positive pediatric oncology patients who demonstrated pre-defined measures of disease progression and oncologic severity related to their COVID-19 status. Fifth, the incidence of thrombosis among COVID-19 positive pediatric oncology patients was also analyzed. Finally, the study assessed the healthcare resource utilization (HCRU) related to the sequelae of COVID-19 among this patient population.
All statistical analyses were conducted using Stata 18. The primary analysis used descriptive statistics to summarize the clinical and demographic characteristics of pediatric oncology patients, categorized by their COVID-19 status (positive or negative). Continuous variables, such as age and hematological measurements (e.g., hemoglobin levels), were reported as means with standard deviations (mean ± SD). Categorical variables, including gender, cancer type, and comorbidities, were presented as counts and percentages. In order to estimate the precision of these percentages, two-sided 95% confidence intervals (CIs) for the point estimates were calculated using the Clopper-Pearson exact method. Missing data were addressed by including missing values in the analysis without imputation. The presence of missing values was also noted and reported in the relevant tables.
Comparative analyses were conducted to assess differences in demographic and clinical characteristics between COVID-19 positive and negative pediatric cancer patients. Categorical variables were compared using the Chi-square test when expected cell counts were ≥5, and the Fisher's exact test was applied for 2 × 2 tables with small cell sizes. For continuous variables (white blood cell count, hemoglobin level, platelet count,…), independent samples t-tests with unequal variances (Welch's t-test) were employed. A two-sided p-value <0.05 was considered indicative of statistical significance.
3 Results
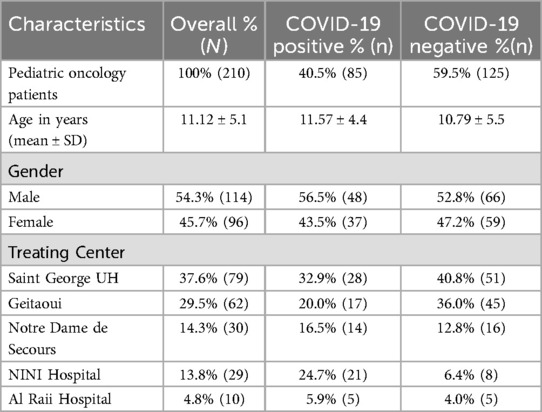
During the study period, we monitored a total of 210 pediatric oncology patients undergoing active chemotherapy. Among these, we identified 85 (40.5%) patients who tested positive for COVID-19 through RT-PCR. This cohort included 48 (56.5%) males and 37 (43.5%) females, with a mean age of 11.6 + 4.4 years. More details on the demographic characteristics of the pediatric oncology children across all five centers are presented in Table 1.
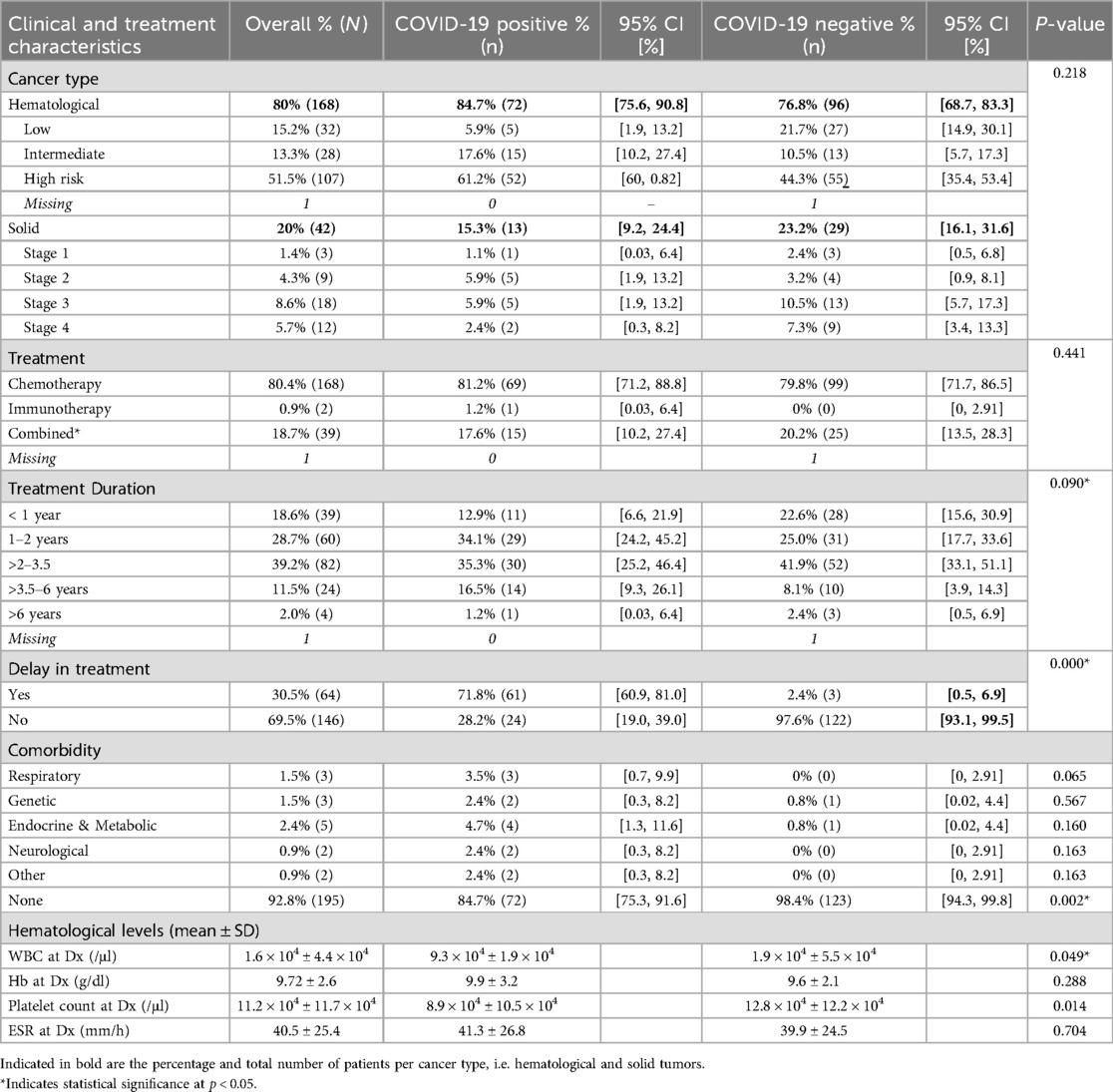
A total of 72 COVID patients (84.7%) had a hematological malignancy, while 13 (15.3%) had solid tumors (Table 2). At the time of COVID-19 diagnosis, 69 patients (81.2%) were receiving chemotherapy, 1 patient (1.2%) was receiving immunotherapy, and the remaining 15 patients (17.6%) were on a combined regimen. Of the COVID-19 positive patients, 13 patients (15.3%) had comorbidities, which included respiratory, genetic, endocrine, metabolic, and neurological among other comorbidities (Table 2). While individual comorbidity types were not significantly different between groups, the overall absence of comorbidities was significantly more common among COVID-negative patients (p = 0.002). When compared to their COVID-19 negative counterparts, we observed that COVID-19 positive patients had lower white blood count (WBC) 9.3 × 104 ± 1.9 × 104 and platelet count (8.9 × 104 ± 10.5 × 104) and higher erythrocyte sedimentation rate (ESR) levels (41.3 ± 26.8). WBC count (p = 0.049) and platelet count (p = 0.014) were significantly different between groups, whereas hemoglobin (p = 0.288) and ESR (p = 0.704) were not, implicating that COVID-19 may have a notable impact on hematological parameters in pediatric oncology patients. Among the 85 patients who were in the midst of anticancer therapy, 61 patients (71.8%) experienced modifications or delay in their treatment as a result of COVID-19 infection (Table 2). This delay was significantly more frequent among COVID-positive patients compared to COVID-negative ones (p < 0.001).
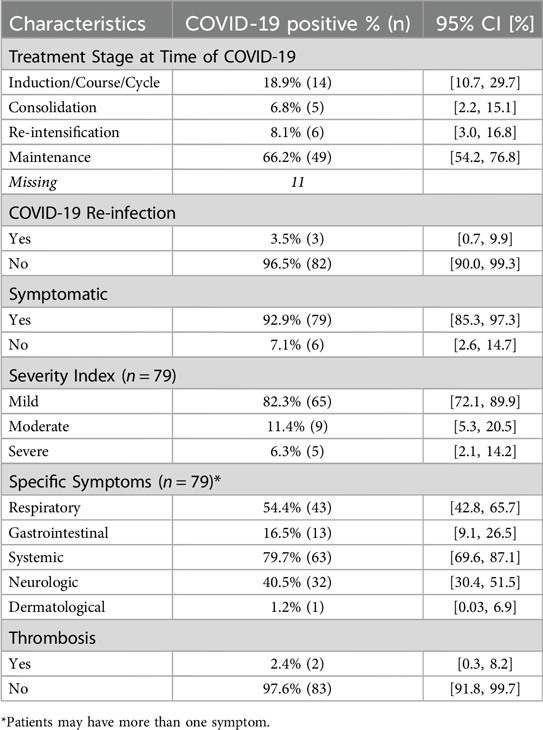
In terms of COVID-19 signs and symptoms, the majority of the patients (92.9%) were symptomatic but had mild disease symptoms (82.3%). Of these patients, the most common symptoms were systemic (fever, chills, tiredness/weakness) (79.7%), respiratory symptoms (54.4%), and neurologic symptoms (headaches) (40.5%). Notably, two patients developed thrombosis in the form of pulmonary embolism and received low-molecular-weight heparin (LMWH) (Table 3). All patients received appropriate treatment for the management of their COVID-19-related symptoms, including antibiotic and steroid therapies. None of the children received hydroxychloroquine, remdesivir, favipiravir, or tocilizumab.
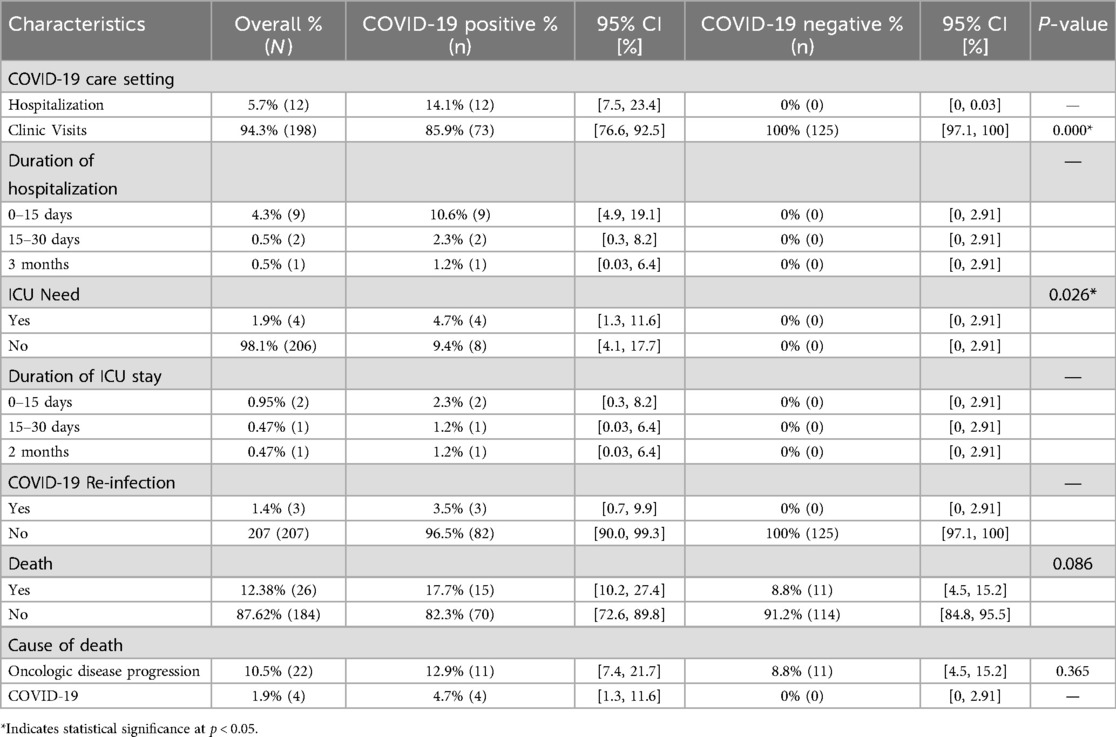
Out of the COVID-positive cohort, a total of 12 (14.1%) patients required hospital admission due to COVID-19 for a duration ranging from short stays of 0–15 days (10.6%, n = 9), 15–30 days (2.3%, n = 2), and up to three months (1.2%, n = 1). Among these cases, four (1.9%) patients necessitated admission to the intensive care unit (ICU), with lengths of stay ranging anywhere from one day to two months (Table 4). The overall mortality rate among patients who tested positive for COVID-19 was 17.7%. Of these, 11 deaths were attributed to progression of oncologic disease, while four were due to COVID-19. However, the difference in mortality between COVID-positive and negative groups was not statistically significant (p = 0.086), nor was the difference in cause of death due to cancer progression or COVID-19 (p = 0.365). Notably, all four COVID-19-related deaths occurred in patients with refractory/progressive disease or relapse. Moreover, three patients (3.5%) tested positive again for COVID-19, defined as a new positive PCR test following documented resolution of their initial infection at the beginning of the study (Table 4).
4 Discussion
The present study demonstrates considerable burden of morbidity and mortality rates, treatment delays, and need for hospitalization and intensive care among pediatric cancer patients with COVID-19 in Lebanon. Our findings also highlight that COVID-19 infection may have had a notable impact on hematological parameters in pediatric oncology patients and contributed to higher hospitalization and ICU admission with longer stay in children with oncologic conditions. These data underscore the importance of effective preventive measure in this at-risk population.
In our study, 61 (71.8%) COVID patients had their treatment delayed suggesting a substantial impact of COVID-19 pandemic on healthcare system and practice related to the management of pediatric oncology patients. Balancing the interruption of cancer treatment against the risk of malignancy progression can be challenging, especially in newly diagnosed patients (19, 20). However, a small case series by Kakunje et al. reported no delays in chemotherapy treatment among children with cancer diagnosed with COVID-19 (21). Additional large studies are needed to quantify the true impact of COVID-19 on treatment delay and disruption of healthcare services not only during this past 4 years but also in the years to come.
Comorbidities in pediatric cancer patients with COVID-19 may further complicate their clinical management and overall prognosis. Toluney et al. reported that four patients had comorbidities unrelated to COVID-19 in addition to their cancer diagnosis; however, no mortality was observed in these patients. Similarly, in our study, 13 (15%) pediatric oncology patients who tested positive for COVID-19 had various comorbidities, including respiratory, genetic, endocrine, metabolic, neurological among others. Notably, 11 patients passed away due to comorbidities unrelated to COVID-19, primarily as a result of disease progression.
Data from other studies have shown that the most common COVID-19 symptoms observed in pediatric patients were cough and fever, a finding consistent with studies involving cancer patients (21–26). In our study, fever and cough were also among the predominant symptoms. When evaluating the severity of COVID-19 among pediatric oncology patients, we found that most children were symptomatic but experienced mild forms of the disease. This is consistent with other reports from the literature (24, 25).
The mortality rate of 4.7% (four deaths) reported in the present study within the COVID-19 patients is comparable to other studies of COVID-19 infection in pediatric oncology patients (27–30). All four dead patients had refractory/progressive disease or were in relapse at the time of COVID-19 infection. One study by Parker et al. and de Rojas et al. reported no COVID-19 deaths (19). However, the mortality rate from COVID-19 was found to be 28% in a study by Arous et al. and 4.4% in a study by Tolunay et al. (26, 31). Hospitalization rates among pediatric oncology patients with COVID-19 infection have been a growing concern in recent studies. Parker et al. found a significant rate of COVID-19-related hospitalizations, with more than one in four patients and over one in three symptomatic patients requiring admission (19). Similarly, in our study we also had 12 (5.7%) cases of hospitalization. The need for ICU admission was also reported by several studies. Antúnez-Montes et al. noted that among children with immunodeficiency, the need for ICU rose to 11.5%, despite the overall rate of immunodeficient children being only 4.4% (32). Likewise, among patients with a history of chemotherapy, the ICU requirement was 7.7%, compared to an overall rate of 3.4% (32). Moreover, in a study by Toluney et al., the rate of ICU admission was reported at 8.9% among pediatric cancer patients (26). In our cohort of pediatric oncology patients who tested positive for COVID-19, the rate of ICU admission was found to be 4.7%.
Reinfection of pediatric oncology patients with COVID-19 raises important concerns for both clinical management and patient outcomes. In our study, three (3.5%) patients tested positive for COVID-19 for a second time. Similarly, Hashmi et al. reported a higher rate of COVID reinfection in 11/110 (10%) patients with hematological malignancy (20).
While initial infections can lead to a range of symptoms, reinfections may present differently, potentially resulting in varying degrees of severity (sometimes more severe in nature) and new challenges. Given the immunocompromised status of many pediatric oncology patients due to their underlying conditions and treatments, they may be at higher risk for both initial infections and subsequent reinfections, particularly with the rise of new COVID-19 strains and variants (20, 33). While this study provides valuable insights, several limitations should be acknowledged. First, the retrospective nature of the study may introduce biases that could impact the findings. Additionally, the heterogeneity in cancer treatment phases and types—ranging from early aggressive treatments to mild maintenance therapies, as well as the variations in treatment of their basic disease—from chemotherapy alone to multimodal treatment approaches—may affect the generalizability of our results. In addition, our study included a limited number of oncology patients in a small country, therefore, caution should be exercised when interpreting the findings from this study.
5 Conclusion
Our study indicates that in Lebanon, COVID-19 was related to treatment changes or delay in oncological children. In addition, a higher proportion of hospitalization, need for intensive care and death were more observed among pediatric cancer patients that tested positive for SARS-CoV-2. Maintaining timely cancer treatment is essential for pediatric oncology patients and should not be interrupted, even in the presence of COVID-19 symptoms, as delays in oncological care can significantly impact patient outcomes. It is crucial that healthcare providers adopt strategies to manage both cancer and COVID-19 concurrently. Vaccination remains essential to provide optimal protection against COVID-19 for this vulnerable population, particularly given the emergence of new SARS-CoV-2 variants (34–38).
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The raw data supporting the conclusions of this article will be made available by the authors on request. Requests to access these datasets should be directed to Adlette Inati,YWRsZXR0ZS5pbmF0aUBsYXUuZWR1Lmxi; Peter Noun,cGV0ZXJub3VuQGdtYWlsLmNvbQ==.
Ethics statement
The studies involving humans were approved by The Lebanese Hospital Geitaoui-University Medical Center (study approval code 2022-IRB021 on 5th December 2022), the University of Balmand/Saint George Hospital Medical Center (study approval code IRB-REC/O/044-22/1522 on 8th November 2022), the CHU-Notre Dame des Secours (study approval code 4/2022 on 13th December 2022), the Raee Hospital (on 3rd July 2023) and the NiNi Hospital (on 20th January 2023). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
PN: Conceptualization, Supervision, Writing – review & editing, Writing – original draft, Methodology. MF: Software, Data curation, Validation, Formal analysis, Writing – review & editing, Visualization, Writing – original draft. HK: Writing – review & editing, Investigation, Resources. JY: Investigation, Writing – review & editing, Resources. MF: Investigation, Resources, Writing – review & editing. HK: Writing – review & editing, Investigation, Resources. HH: Funding acquisition, Writing – review & editing. JM: Writing – review & editing. FL: Project administration, Writing – review & editing. SR: Methodology, Supervision, Writing – review & editing, Writing – original draft. JS: Conceptualization, Writing – review & editing. NA: Conceptualization, Project administration, Writing – review & editing, Funding acquisition, Methodology. MK: Project administration, Writing – original draft, Supervision, Methodology, Conceptualization, Writing – review & editing. AI: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Pfizer Inc. as collaborative research (grant no: 73421001).
Acknowledgments
The authors thank Kristen Allen from Pfizer for her support and assistance in this study.
Conflict of interest
NK, JS, HH, JM, FH, SV, MK are employees of Pfizer and may hold stock or stock options of Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. (2020) 91(1):157–60. doi: 10.23750/abm.v91i1.9397
2. Majeed A, Wright T, Guo B, Arora RS, Lam CG, Martiniuk AL. The global impact of COVID-19 on childhood cancer outcomes and care delivery—a systematic review. Front Oncol. (2022) 12:869752. doi: 10.3389/fonc.2022.869752
3. Sengar M, Chinnaswamy G, Ranganathan P, Ashok A, Bhosale S, Biswas S, et al. Outcomes of COVID-19 and risk factors in patients with cancer. Nat Cancer. (2022) 3(5):547–51. doi: 10.1038/s43018-022-00363-4
4. Turtle L, Elliot S, Drake TM, Thorpe M, Khoury EG, Greenhalf W, et al. Changes in hospital mortality in patients with cancer during the COVID-19 pandemic (ISARIC-CCP-UK): a prospective, multicentre cohort study. Lancet Oncol. (2024) 25(5):636–48. doi: 10.1016/S1470-2045(24)00107-4
5. Nolan MB, Piasecki TM, Smith SS, Baker TB, Fiore MC, Adsit RT, et al. Relations of current and past cancer with severe outcomes among 104,590 hospitalized COVID-19 patients: the COVID EHR cohort at the University of Wisconsin. Cancer Epidemiol Biomarkers Prev. (2023) 32(1):12–21. doi: 10.1158/1055-9965.EPI-22-0500
6. Perez-Martinez A, Guerra-Garcia P, Melgosa M, Frauca E, Fernandez-Camblor C, Remesal A, et al. Clinical outcome of SARS-CoV-2 infection in immunosuppressed children in Spain. Eur J Pediatr. (2021) 180(3):967–71. doi: 10.1007/s00431-020-03793-3
7. Radhakrishnan V, Gangopadhyay D. Repeat-positive SARS-CoV-2 in a child with cancer. Pediatr Blood Cancer. (2021) 68(3):e28744. doi: 10.1002/pbc.28744
8. Schied A, Trovillion E, Moodley A. SARS-CoV-2 infection in a neutropenic pediatric patient with leukemia: addressing the need for universal guidelines for treatment of SARS-CoV-2-positive, immunocompromised patients. Pediatr Blood Cancer. (2020) 67(9):e28546. doi: 10.1002/pbc.28546
9. Bernar B, Kropshofer G, Crazzolara R, Kapelari K, Griesmacher A, Muller T, et al. SARS-CoV-2 infection in a 7-year-old girl with pancytopenia during acute lymphocytic leukemia maintenance therapy. Pediatr Blood Cancer. (2020) 67(11):e28391. doi: 10.1002/pbc.28391
10. Yadav SP, Thakkar D, Bhoyar RC, Jain A, Wadhwa T, Imran M, et al. Asymptomatic reactivation of SARS-CoV-2 in a child with neuroblastoma characterised by whole genome sequencing. IDCases. (2021) 23:e01018. doi: 10.1016/j.idcr.2020.e01018
11. Zhou X, Wang G, Chen L, Meng F, Huang L, Huang L, et al. Clinical characteristics of hematological patients concomitant with COVID-19. Cancer Sci. (2020) 111(9):3379–85. doi: 10.1111/cas.14544
12. Nikolopoulou GB, Maltezou HC. COVID-19 in children: where do we stand? Arch Med Res. (2022) 53(1):1–8. doi: 10.1016/j.arcmed.2021.07.002
13. Vasquez L, Sampor C, Villanueva G, Maradiegue E, Garcia-Lombardi M, Gomez-Garcia W, et al. Early impact of the COVID-19 pandemic on paediatric cancer care in Latin America. Lancet Oncol. (2020) 21(6):753–5. doi: 10.1016/S1470-2045(20)30280-1
14. Ding YY, Ramakrishna S, Long AH, Phillips CA, Montiel-Esparza R, Diorio CJ, et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr Blood Cancer. (2020) 67(9):e28427. doi: 10.1002/pbc.28427
15. Nerli RB, Sanikop AC, Sharma M, Ghagane SC. COVID-19 pandemic: a challenge to a child with cancer. Pediatr Blood Cancer. (2020) 67(9):e28512. doi: 10.1002/pbc.28512
16. Jazieh AR, Akbulut H, Curigliano G, Rogado A, Alsharm AA, Razis ED, et al. Impact of the COVID-19 pandemic on cancer care: a global collaborative study. JCO Glob Oncol. (2020) 6:1428–38. doi: 10.1200/GO.20.00351
17. Eid D, Jabbour J, Moujaes E, Kourie HR, Safieddine M, Kattan J. Impact of the economic crisis and drug shortage on Lebanese cancer patients’ care. World J Clin Oncol. (2024) 15(5):644–52. doi: 10.5306/wjco.v15.i5.644
18. Saab R, Jeha S, Khalifeh H, Zahreddine L, Bayram L, Merabi Z, et al. Displaced children with cancer in Lebanon: a sustained response to an unprecedented crisis. Cancer. (2018) 124(7):1464–72. doi: 10.1002/cncr.31273
19. Parker RS, Le J, Doan A, Aguayo-Hiraldo P, Pannaraj PS, Rushing T, et al. COVID-19 outcomes in children, adolescents and young adults with cancer. Int J Cancer. (2022) 151(11):1913–24. doi: 10.1002/ijc.34202
20. Hashmi SK, Bodea J, Patni T, Angel S, Bhakta NH, Jeha S, et al. COVID-19 in pediatric patients with acute lymphoblastic leukemia or lymphoma. JAMA Netw Open. (2024) 7(2):e2355727. doi: 10.1001/jamanetworkopen.2023.55727 Karius in the form of testing for investigator-initiated research, nonfinancial support from Scynexis in the form of medication and therapeutic drug monitoring for a single patient treatment plan, financial support (to the institution) from Merck for participating in industry-initiated sponsored research, and grants from Pfizer, all outside the submitted work. Dr Pui received personal fees from Novartis for serving on the data monitoring board outside the submitted work. Dr Inaba received grants from Servier and nonfinancial support from Amgen and Incyte in the form of medication for investigator-initiated research, all outside the submitted work. No other disclosures were reported.38363571
21. Kakunje M, Bharadwaj N, Alex KS, Bharadwaj V, Prakash A. COVID-19 pandemic and childhood cancer: lessons learnt from a pediatric oncology unit in a developing country. Asian Pac J Cancer Care. (2023) 8(1):213–8. doi: 10.31557/apjcc.2023.8.1.213-218
22. Nakamura S, Kanemasa Y, Atsuta Y, Fujiwara S, Tanaka M, Fukushima K, et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: a single-center retrospective observational study in Tokyo, Japan. Int J Clin Oncol. (2021) 26(3):485–93. doi: 10.1007/s10147-020-01837-0
23. Gampel B, Lucas AGT, Broglie L, Gartrell-Corrado RD, Lee MT, Levine J, et al. COVID-19 disease in New York city pediatric hematology and oncology patients. Pediatr Blood Cancer. (2020) 67(9):e28420. doi: 10.1002/pbc.28420
24. Węcławek-Tompol J, Zakrzewska Z, Gryniewicz-Kwiatkowska O, Pierlejewski F, Bień E, Zaucha-Prażmo A, et al. COVID-19 in pediatric cancer patients is associated with treatment interruptions but not with short-term mortality: a Polish national study. J Hematol Oncol. (2021) 14:1–10. doi: 10.1186/s13045-021-01181-4
25. Hsiao Y-W, Chiu C-C, Lin Y-T, Wen Y-C, Yang S-H, Wang Y-L, et al. Clinical outcomes of COVID-19 in pediatric hematology-oncology patients: a single-institution experience. Pediatr Neonatol. (2023) 64(6):679–80. doi: 10.1016/j.pedneo.2023.05.004
26. Tolunay O, Celik U, Arslan I, Tutun B, Ozkaya M. Evaluation of clinical findings and treatment results of coronavirus disease 2019 (COVID-19) in pediatric cancer patients: a single center experience. Front Pediatr. (2022) 10:848379. doi: 10.3389/fped.2022.848379
27. Parker RS, Le J, Doan A, Aguayo-Hiraldo P, Pannaraj PS, Rushing T, et al. COVID-19 outcomes in children, adolescents and young adults with cancer. Int J Cancer. (2022) 151(11):1913–24. doi: 10.1002/ijc.34202
28. Tolunay O, Çelik Ü, Arslan I, Tutun B, Özkaya M. Evaluation of clinical findings and treatment results of coronavirus disease 2019 (COVID-19) in pediatric cancer patients: a single center experience. Front Pediatr. (2022) 10:848379. doi: 10.3389/fped.2022.848379
29. Arous R, Djillali IS, Rouis NO, Boudiaf H, Amhis W, Ziane H, et al. High mortality of COVID-19 in children with cancer in a single center in Algiers, Algeria. Pediatr Blood Cancer. (2021) 68(6):e28898. doi: 10.1002/pbc.28898
30. Antúnez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Saráchaga M, Salcedo-Lozada P, et al. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. (2021) 40(1):e1–6. doi: 10.1097/INF.0000000000002949
31. Arous R, Djillali IS, Rouis NO, Boudiaf H, Amhis W, Ziane H, et al. High mortality of COVID-19 in children with cancer in a single center in Algiers, Algeria. Pediatr Blood Cancer. (2021) 68(6):e28898. doi: 10.1002/pbc.28898
32. Antunez-Montes OY, Escamilla MI, Figueroa-Uribe AF, Arteaga-Menchaca E, Lavariega-Sarachaga M, Salcedo-Lozada P, et al. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. (2021) 40(1):e1–6. doi: 10.1097/INF.0000000000002949
33. Eichholz T, Arendt A-M, Holzer U, Seitz C, Rabsteyn A, Ganzenmueller T, et al. Recurrent SARS-CoV-2 infection and impaired immunologic response in a pediatric oncologic patient while treated with radiochemotherapy. Pediatr Infect Dis J. (2022) 41(6):e259–62. doi: 10.1097/INF.0000000000003515
34. Lazar Neto F, Mercadé-Besora N, Raventós B, Pérez-Crespo L, Castro Junior G, Ranzani OT, et al. Effectiveness of COVID-19 vaccines against severe COVID-19 among patients with cancer in Catalonia, Spain. Nat Commun. (2024) 15(1):5088. doi: 10.1038/s41467-024-49285-y
35. Kurucu N, Kutluk T, Kartal İ, Yeşil Ş, Vural Ö, Dinçer OS, et al. Safety and efficacy of COVID-19 vaccines in children and adolescents with cancer. Turk J Pediatr. (2024) 66(4):412–20. doi: 10.24953/turkjpediatr.2024.4512
36. Kam B, Wang Y, Qin F, Long AH, Klein OR, Aftandilian C. COVID-19 Vaccine response in pediatric oncology patients. Pediatr Blood Cancer. (2025) 72(4):e31572. doi: 10.1002/pbc.31572
37. Miao J, Zhang JY, Liang J, Zhao FY, Song H, Xu WQ, et al. Efficacy of inactivated SARS-CoV-2 vaccination in pediatric hematology/oncology patients: a real-world study. World J Pediatr. (2023) 19(10):1017–21. doi: 10.1007/s12519-023-00737-3
Keywords: COVID-19, oncology, pediatric, children, epidemiology, management, diagnosis, care
Citation: Noun P, Farhat M, Khalife H, Younis JB, Farhat M, Kabbout H, Haridy H, Moussa J, Lefebvre d’Hellencourt F, Rao Valluri S, Spinardi J, Al Akoury N, Kyaw MH and Inati A (2025) Impact of COVID-19 on clinical outcomes and care delivery in pediatric oncology patients in Lebanon in 2020–2023: a retrospective study. Front. Pediatr. 13:1608740. doi: 10.3389/fped.2025.1608740
Received: 11 April 2025; Accepted: 23 June 2025;
Published: 9 July 2025.
Edited by:
Luca Giacomelli, Polistudium srl, ItalyReviewed by:
Lidia Fraquelli, Garrahan Hospital, ArgentinaSelma Cakmakci, Yuksek Ihtisas Training and Research Hospital, Türkiye
Copyright: © 2025 Noun, Farhat, Khalife, Younis, Farhat, Kabbout, Haridy, Moussa, Lefebvre d’Hellencourt, Rao Valluri, Spinardi, Al Akoury, Kyaw and Inati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adlette Inati, YWRsZXR0ZS5pbmF0aUBsYXUuZWR1Lmxi
 Peter Noun1,2
Peter Noun1,2 May Farhat
May Farhat Hassan Khalife
Hassan Khalife Jihane Moussa
Jihane Moussa Srinivas Rao Valluri
Srinivas Rao Valluri Moe H. Kyaw
Moe H. Kyaw