- 1Department of Paediatrics and Child Health, Division of Neonatal Medicine, University of Cape Town, Cape Town, South Africa
- 2Department of Medical Immunology, Institute for Cellular and Molecular Medicine, Faculty of Health Sciences, University of Pretoria, Pretoria, South Africa
- 3Extramural Unit for Stem Cell Research and Therapy, South African Medical Research Council, Pretoria, South Africa
Introduction: Enteral feeding during therapeutic hypothermia (TH) for neonatal hypoxic ischaemic encephalopathy (HIE), is beneficial, but there is insufficient evidence to guide timing and feed advancement strategies. The aim of this study was to describe feed tolerance and outcomes after TH with a standardized progressive early enteral feeding regimen.
Methods: Data were retrospectively reviewed from neonates with HIE who were treated with TH for HIE in the Groote Schuur Hospital (GSH) Neonatal intensive care unit (NICU), between 1 July 2019 and 31 October 2022. Enteral feeds were commenced at age 12–24 h and incremented daily if tolerated, at 12 ml/kg/day for the first 3 days and 24 ml/kg thereafter. Nutritional, morbidity and mortality outcomes were compared between neonates with and without early feed intolerance (EFI) by the fourth day of life.
Results: Thirty three percent (16/48) developed EFI. However, by day six the median (IQR) enteral volumes were, 120 (110–120) and 90 (90–99), in neonates without and with feed intolerance respectively. There were no differences in resuscitation characteristics. Neonates with EFI, had higher HIE grades, more amplitude integrated electro-encephalograph (aEEG) suppression at 48 h (p = 0.002), later attainment of full nutritive sucking or cup feeds (p < 0.001) and longer hospital stays (p = 0.038). There were no differences in other morbidities. Mortality was 6% and necrotising enterocolitis did not occur in either group.
Conclusions: Early feeding was generally well tolerated. Feed intolerance was more frequent in neonates with severe HIE, but most neonates achieved independence from IV fluids by day six.
Introduction
Neonatal encephalopathy (NE) occurring after acute peripartum hypoxia, in the absence of other causes, is referred to as hypoxic ischaemic encephalopathy (HIE) (1). Hypoxic ischaemic encephalopathy is a major cause of neonatal mortality, multiorgan morbidity and subsequent disability, with the highest burden in low and middle-income countries (LMICS) (2). Therapeutic hypothermia (TH) is the only evidence-based therapy known to improve outcomes after moderate to severe NE due to suspected HIE (NESHIE) (3). The vascular fetal adaptive response to hypoxia—redistribution of blood to essential organs—contributes to the multiorgan morbidity which can be associated with HIE and may include necrotizing enterocolitis (NEC) (4–6). The risk for NEC in neonates with HIE is suggested by ultrasound evidence of impaired gastro-intestinal (GIT) blood flow and intestinal pathology in neonates with severe HIE (7, 8). Standard management of HIE, with or without TH, may therefore include restriction of enteral nutrition (9). Despite the potential GIT compromise associated with HIE, the most recent Cochrane systematic review showing the benefit of therapeutic hypothermia (TH) for moderate-severe HIE (10), noted that only three of the 11 included trials reported data on NEC, which occurred in only two trials; at <1% and 3.6% (11, 12). The trials which reported feeding strategies, either withheld enteral nutrition during TH (13, 14) or provided delayed and minimal enteral feeding from 24 h or later (9, 11).
However, the practice of delayed or restricted enteral feeding in term neonates with HIE, has been questioned, since it is associated with adverse outcome in preterm neonates (15). A survey of cooling centres in the United Kingdom (UK) in 2016, showed that only 41% withheld feeds during TH and 29% gave parenteral nutrition (PN) (16). Retrospective cohort studies of neonates treated with TH, comparing early minimal enteral feeding (MEF) to delayed enteral feeding (DEF), have found probable benefit of MEF including; increased breast feeding at discharge (17, 18), no difference in adverse events, NEC or feed intolerance (17–21), reduced length of stay (18, 19), reduced time to full feeds (19, 20), and reduced proinflammatory cytokines (19). The authors of the most recent systematic review comparing the outcomes of delayed vs. early enteral feeding in infants undergoing TH for HIE, concluded that enteral feeding during TH is feasible, safe, and beneficial, but there is insufficient evidence to guide timing of initiation, size and feed advancement strategies (22).
The global knowledge gap of guidance for feeding strategies during TH is emphasized in the South African context by the variable management of early enteral nutrition for neonates receiving TH for moderate to severe HIE. The use of PN and withholding of enteral feeds was standard practice in a South African cohort described by Kali et al. (23), whereas early and progressive enteral feeding is practiced and standardized in a different South African center—the tertiary neonatal intensive care unit (NICU) at Groote Schuur Hospital (GSH) (24). The aim of this study was to provide novel South African data describing feed tolerance, characteristics and outcomes in a cohort of neonates with HIE who were cooled and managed with a standardised progressive early enteral feeding protocol at GSH. The primary objective was to describe the incidence of early feed intolerance (EFI) and the characteristics of affected neonates, during TH for moderate-severe NESHIE. The secondary objectives were: (i) To determine if there are associations between EFI and maternal or neonatal characteristics at birth; (ii) To determine if there are associations between EFI and major morbidities; and (iii) To determine if there are associations between EFI and short-term outcomes.
Methods
Study setting, design and ethical approval
This retrospective, descriptive, study was conducted on data from the GSH Neonatal Unit, a 75-bedded tertiary referral centre for the Metro West Area of Cape Town, with approximately 2000 admissions per annum. The study was approved by the Human Research Ethics Committee (HREC) of the Faculty of Health Sciences (FHS), University of Cape Town (UCT), (HREC 658/2024) and by the GSH hospital management. The study was developed and carried out in accordance with the Declaration of Helsinki, 2013. The study population was a cohort of neonates with HIE who were admitted to GSH, treated with TH, had data collected into the GSH neonatal encephalopathy registry (HREC 087/2010) and were also included, with their consent, in a separate observational national study; Genetic predisposition to death and disability after moderate-severe Neonatal Encephalopathy due to suspected HIE (NESHIE) in cooled infants (HREC 622/2018)—The National NESHIE Study. Data were included from neonates admitted from 1 July 2019 to 31 October 2022, representing the period with the most complete data entry for GSH neonates at the time of study design.
Retrospective collection of de-identified patient data into the GSH neonatal encephalopathy registry, of all neonates with HIE who were admitted to GSH and treated with TH, was approved by the HREC FHS UCT (HREC 087/2010). Informed consent was not required for retrospective analysis of this de-identified data, however consent was collected for all patients, for the collection of observational data as part of the procedures for the The National NESHIE TH Study.
Inclusion Criteria for the National NESHIE TH Study, and this study.
All of the following inclusion criteria (a), (b), (c), and (d) were required:
(a) Gestational age ≥36 weeks at birth
(b) Birth weight ≥1,800 g
(c) Intrapartum hypoxia suggested by:
(i) Cord blood or neonatal blood in first hour of life with pH ≤ 7 or base deficit (BD) ≥ 16 mmol/L, or
(ii) A history of sentinel event and first hour pH ≤ 7.15 or BD ≥ 10 mmol/L, or
(iii) Intermittent positive pressure ventilation (IPPV) at 10 min
(d) Moderate or severe encephalopathy by age 6 hours based on:
(i) moderate modified Sarnat grade or a Thompson HIE Score ≥7 (25–28), and
(ii) Abnormal amplitude-integrated electro encephalograph (aEEG) (29, 30).
Exclusion Criteria for the National NESHIE TH Study, and this study.
Neonates with any of the following criteria were excluded:
• Consent for National NESHIE study not available
• Not treated with TH within 6 h from birth
• Moribund or too unstable to cool because of severe or unresponsive persistent pulmonary hypertension (PPHN)/hypoxia/hypotension/bleeding or hypoglycemia.
• Suspected or confirmed congenital infections
• Chromosomal abnormalities or major congenital anomalies affecting neurological outcome
In addition to the above criteria, neonates with missing key data describing characteristics, outcomes and/or feed and fluid management, were excluded from this study.
Standard medical care and feeding definitions
All included neonates were cooled to a core rectal target temperature of 33.5°C for 72 h, using an automated Blanketrol III (Cincinnati Sub-Zero, Cincinnati, Ohio) cooling mat. During rewarming the rectal temperature was increased by 0.2°C per hour until a temperature of 36.5°C was reached. Neonates were sedated with morphine during cooling unless already obtunded, comatose or sedated with anticonvulsants. Heart rate and oxygen saturations were monitored continuously, and blood pressure was documented hourly. Investigation on admission included blood glucose, electrolytes haemoglobin (Hb) and blood culture. First-line antibiotics were commenced on admission but stopped at 36–48 h if infection indicators were negative. A cranial ultrasound scan was performed to exclude other causes of NE.
Intravenous (IV) fluids were initiated at 40 ml/kg/day, with a potassium-free solution containing 10% dextrose, 33 mmol/L sodium chloride and 5 mmol/L calcium gluconate [Sabax Potassium-free Neonatal maintenance Solution, (Adcock Ingram, Midrand, South Africa)]. Total administered fluids (IV and enteral combined) were maintained at 40 ml/kg/day for the first three days—or adjusted according to sodium levels—and increased progressively thereafter by 20–30 ml/kg/day to a final total of 150 ml/kg/day. If hypoglycaemia occurred, the concentration of administered dextrose was increased rather than volume. Enteral feeds were commenced at age 12–24 h, using term formula milk or preferably expressed breast milk (EBM) if available, since EBM was frequently not available, particularly when neonates were outborn. Feeds were incremented daily if tolerated at 12 ml/kg/day for the first 3 days and 24 ml/kg thereafter.
Feed intolerance was defined by the presence of one or more of the following: tense abdominal distention, recurrent vomiting, and/or bloody stools. Early feed intolerance was defined as, a tolerated enteral feed volume on day four, which was less than the volume which would be expected to be attained by day three (<36 ml/kg/day), due to any cause of feed intolerance as defined above.
Encephalopathy assessment methods and aEEG
The clinical grade of HIE was assessed before TH and daily with both the Thompson HIE score (27), and the Modified Sarnat Grade (25, 26). The Thompson HIE score consists of nine clinical signs, scored numerically to a maximum of 22, and a score a of ≥7 was considered to indicate moderate-severe HIE (27). When HIE was assessed by the Modified Sarnat Grade, moderate-severe HIE was defined clinically by the presence of one or more abnormal signs in at least three of six possible clinical categories or the presence of seizures, where the grade severity is determined by the number of signs per grade, and if these are equal, the grade is determined by the level of consciousness (25, 26). An abnormal aEEG was required in addition to clinical signs, to assign a diagnosis of moderate-severe HIE by age 6 h. The aEEG background was classified according to pattern, into continuous normal voltage (CNV), discontinuous normal voltage (DNV), burst suppression (BS), continuous low voltage (CLV), and flat trace (FT)—aEEGs with abnormal backgrounds (DNV, BS, CLV, or FT) and/or those showing seizures were considered abnormal (29, 30). A suppressed background was defined by backgrounds showing BS, CLV or FT which aligns with voltage criteria for suppression described by al Naqeeb et al. (31) and is strongly associated with abnormal neurodevelopmental outcome or death, when present at age 48 h in cooled neonates with moderate-severe HIE (32).
Sample size and data collection
The sample was defined by the number of neonates admitted to GSH with moderate to severe HIE and treated with TH, who were also included in the separate National NESHIE study, at the time of study design, which spanned a period of 39 months. De-identified data were routinely and prospectively collected on all cooled neonates admitted to GSH and entered into an access-controlled Research Electronic Data Capture (REDCap) database, accessible to the departmental research clinicians. Identifying data is kept in a separate password-protected Excel spread sheet, accessible only to senior clinician database managers who ordinarily manage the cooled neonates (limited to the senior authors, ARH and SP). The GSH registry also includes the deidentified study numbers for patients who are included in related trials, including The NESHIE Study. The following de-identified data of patients who were included in the NESHIE Study, were extracted from the ethics-approved REDCap registry (HREC 087/2010) directly into a statistical database using STATA 18.0 BE-Basic edition (StataCorp, Texas, USA):
(i) Baseline maternal, antenatal, labour and neonatal characteristics at birth and before cooling; (ii) Neonatal neurological assessments before cooling, at ages 24 and 48 h and maximum scores during admission; (iii) Fluid and nutrition management during the first four days of life; (iv) Major morbidity data including the occurrence of hypoglycaemia (<2.6 mmol/L), hyperglycaemia (>8.3 mmol/L), hyponatraemia (<130 mmol/L), serum creatinine at 24 h, anaemia (Hb ≤ 12 g), bleeding treated with blood products, mechanical ventilation, FiO2 on admission, hypotension requiring inotropes, early sepsis (positive blood or cerebrospinal fluid culture ≤72 h after birth), late sepsis (positive blood or cerebrospinal fluid culture >72 h after birth), and NEC (modified Bell's stage II or III NEC, shown by abdominal distension, gastric aspirate and/or blood in stools together with abdominal x-ray showing bowel oedema, pneumatosis or pneumoperitoneum) (33); and v) Short-term outcome data including EFI (a tolerated enteral feed volume on day four, which was less than the volume which would be expected to be attained by day three; <36 ml/kg/day), durations of hospital stays, ages at suck/cup feeding, maximum Thompson scores, maximum modified Sarnat grades, suppressed aEEG at 48 h, and mortality.
Data analysis
Differences in characteristics, morbidity and short-term outcomes, between neonates with and without early feed tolerance were analysed using STATA 18.0 BE-Basic edition (StataCorp, Texas, USA). Categorical variables were analysed with Chi square or Fischer's exact tests and continuous variables were analysed with student t-test or Wilcoxon rank sum, with significance assigned as p < 0.05.
Results
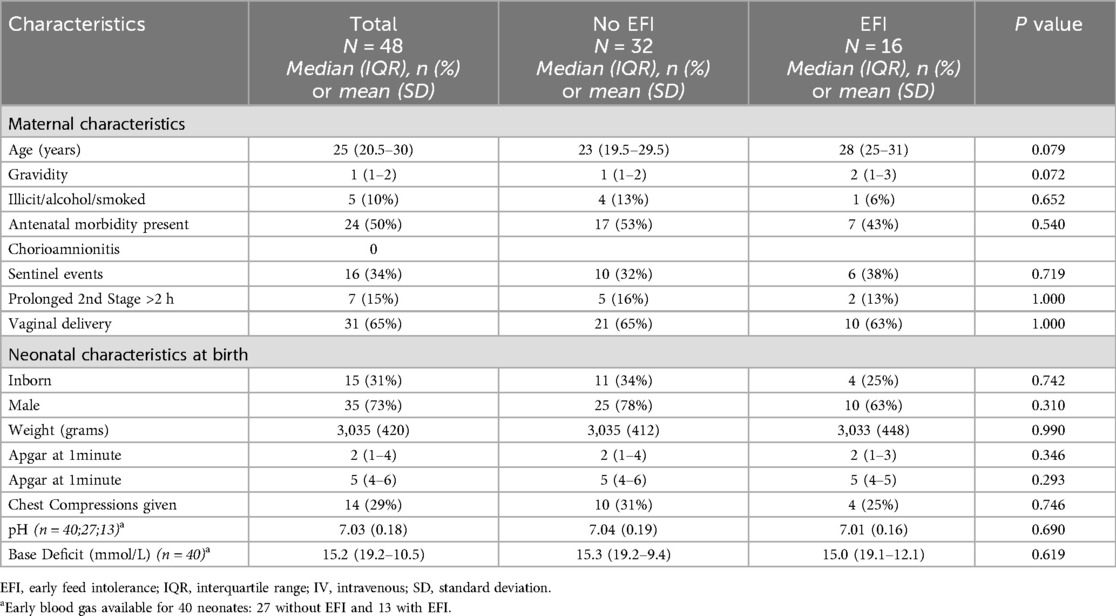
The application of inclusion and exclusion criteria is shown a flow chart in Figure 1. Forty-eight neonates with complete data were included in our cohort, after 46 neonates were excluded. The perinatal characteristics, grouped according to EFI, are shown in Table 1. Sixteen (33%) neonates in the study had EFI. There were no differences in perinatal characteristics. Notably, sentinel events were only reported in 34%, a minority of neonates were inborn (15/48; 31%), the mean ± SD weight was 3,035 ± 420 g, the median (IQR) 5 min Apgar was 5 (4–6) and the median (IQR) base deficit (BD) was 15.2 (19.2–10.5).
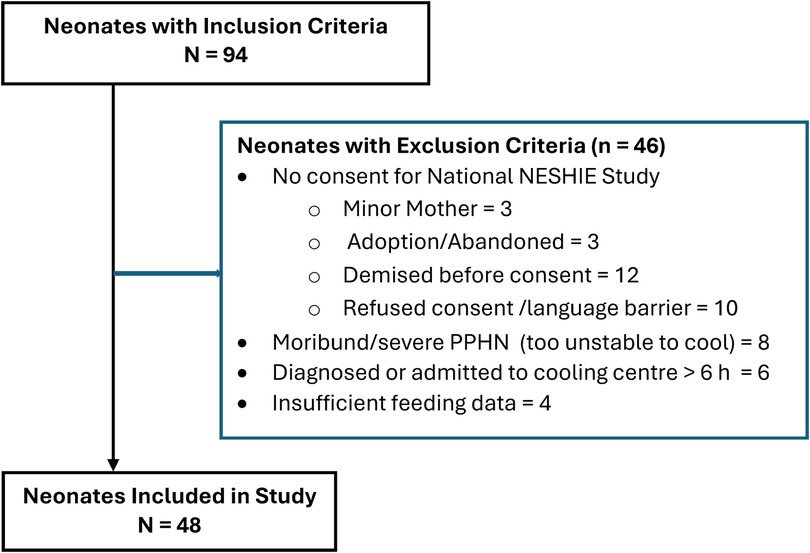
Figure 1. Application of inclusion and exclusion criteria. H, hours; PPHN, persistent pulmonary hypertension.
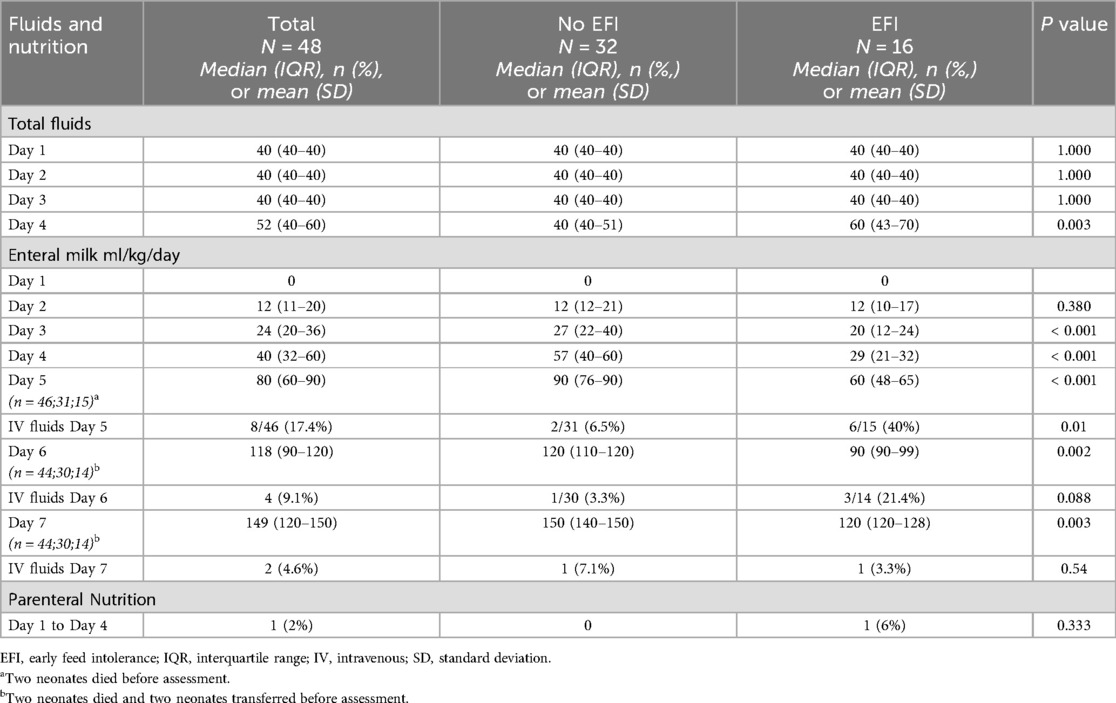
The fluid and nutritional management data, grouped according to EFI, from day 1–4 and enteral feeds on day 7, are shown in Table 2. Total fluid intake did not differ between groups on day 1–3, however on day 4 those with EFI received 20 ml/kg more fluid (p = 0.003). There were no differences in enteral feeds on day 1 and 2 between both groups, however significantly less feeds were given (tolerated) on days 3 and 4 in the EFI group (p < 0.001)—this difference persisted to day 7, where those with EFI were only receiving 120 ml/kg/d compared to 150 ml/kg/d in those without EFI (p = 0.003). Only one baby required PN.
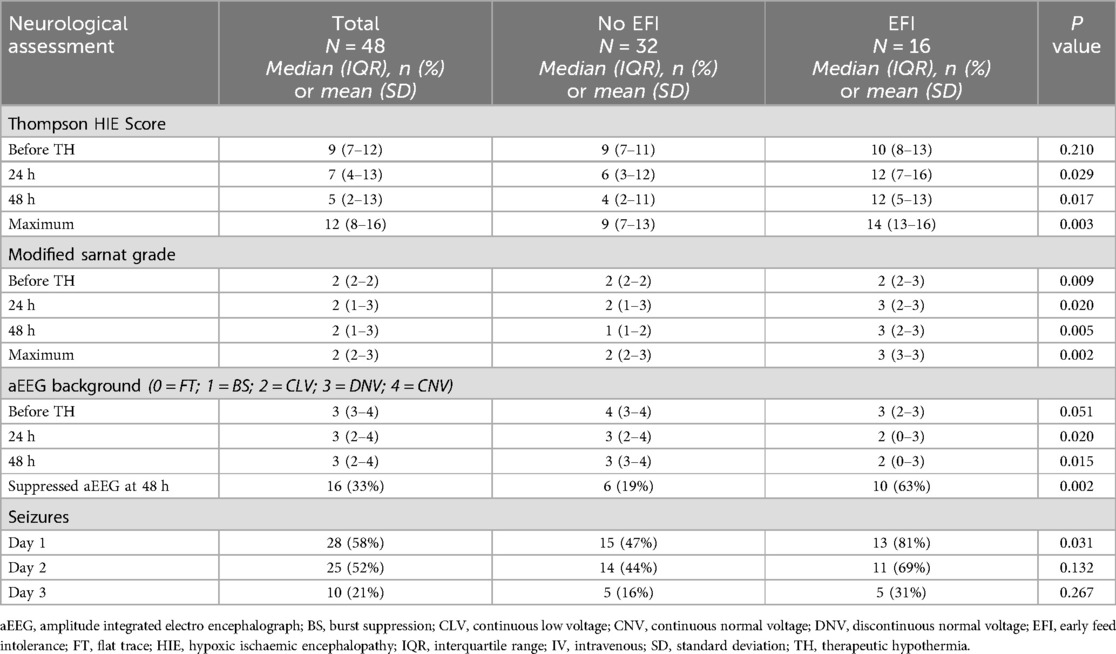
The differences in neurological assessments, grouped according to EFI, are shown in Table 3. There were no differences in pre-cooling Thompson scores between groups, however considerably higher Thompson scores were found in the EFI group at 24 and 48 h (p = 0.029 and 0.017 respectively) and these neonates also had significantly higher maximum Thompson scores (p = 0.003)—these data are depicted graphically in Figure 2. Assessment with the Modified Sarnat grade, showed higher HIE grades in the neonates with EFI at all stages of assessment, and the maximum HIE grade was most often grade 3 [median (IQR): 3 (3–3), p = 0.002].
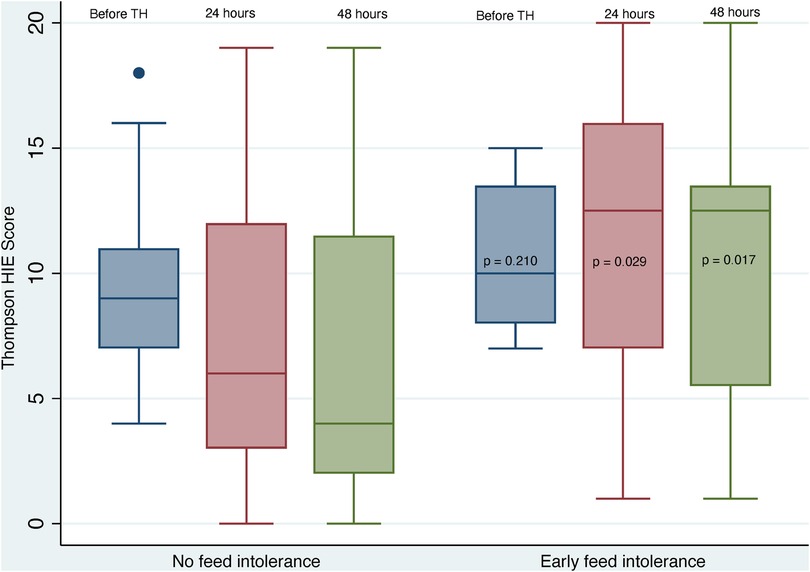
Figure 2. Early thompson scores grouped according to feed tolerance. HIE, hypoxic ischaemic encephalopathy; TH, therapeutic hypothermia.
A numeric value was aligned with the aEEG background, where “0” is most severe and “4” is normal. The neonates with EFI had more severe aEEG background patterns at 24 and 48 h and at 48 hours they had the highest proportion of suppressed aEEG background (63% vs. 19% in those without early feed intolerance; p = 0.002). The neonates with EFI had more seizures prior to cooling (81% vs. 47%; p = 0.031), but the difference did not persist.
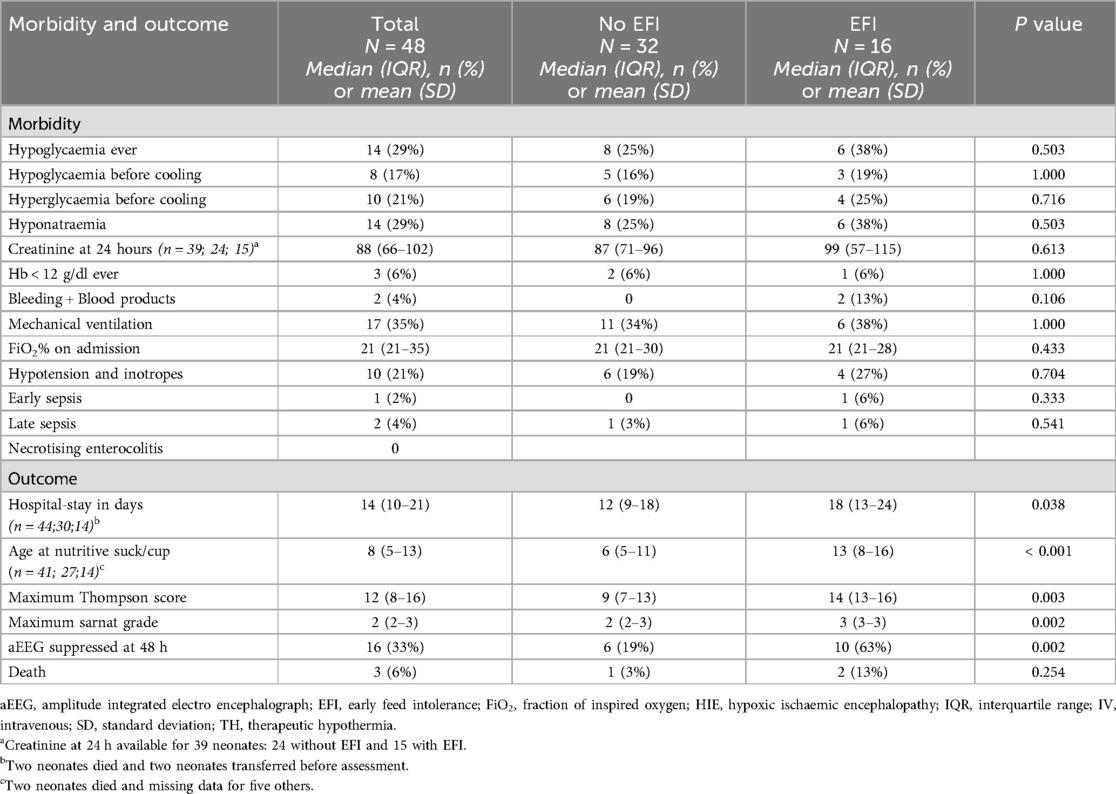
The morbidity and short-term outcomes, grouped according to EFI, are shown in Table 4. There were no differences in the proportions of morbidities between groups—notably, 35% required mechanical ventilation and 21% required inotropes, but early and late sepsis occurred in only 2% and 4% respectively and NEC did not occur. However, the EFI group had longer hospital stays (p = 0.038), higher Thompson scores (p = 0.003), higher Sarnat grades (p = 0.002) and 3 times more frequent suppressed aEEG at 48 h (p = 0.002). Only 6% of neonates died, and there were no differences in mortality between the groups.
Discussion
This observational study of 48 neonates who were treated with TH for moderate-severe HIE, and were progressively enterally fed during TH, showed that EFI by day 4 of life, occurred in a third of neonates, and was strongly associated with the severity of encephalopathy at admission and 48 h, but was not associated with perinatal characteristics at birth nor with major morbidities. Most neonates did not require IV fluids after day 5, irrespective of early feed intolerance and NEC did not occur.
Necrotising enterocolitis
The low rate of NEC with early enteral feeding during TH is well described, and previous studies described NEC rates varying from 0%–4.3% when defined as Bell stage II or higher—the only study reporting NEC rate above 0%, included neonates at 35 weeks’ gestation, however two other studies including neonates at ≥35 weeks’ gestation, reported 0% NEC (19–21, 34, 35). The inclusion of neonates at 35 weeks’ gestation in some studies, may limit comparison, since feeding intolerance is more frequent in preterm neonates (15). However the mean gestational age in studies which included neonates at 35 weeks’ gestation, was ≥38 weeks’ gestation (19, 20, 35). A large population-level retrospective cohort study of 6,030 neonates with HIE and TH, from the UK National Neonatal Research Database found that severe NEC, confirmed at surgery or causing death occurred in 0.1% (18). The low rate of NEC in this context is in keeping with data from very low birthweight infants managed with early MEF, which was associated with increased GI motility, increased GI hormone secretion, decreased GI inflammation, more beneficial microflora, improved GI tract maturation and decreased feeding intolerance without increasing the risk of developing NEC (15). Moreover, neonates with moderate-to-severe HIE who are treated with TH have less feeding intolerance compared to non-cooled neonates (17, 19, 36).
Feeding intolerance and time to full enteral feeding
Few studies of feeding and TH have reported the rates of feeding intolerance. Similar to the 33% EFI in our study, Hu et al. (35), reported 26% feeding intolerance, in the early enteral feeding group in their RCT, where enteral feeds were commenced and advanced daily with “<20 ml/kg/day” breast milk/formula, at a mean ± SD age of 2 ± 0.91 days; compared to 32% feeding intolerance in the delayed group, where enteral feeds were commenced 4.84 ± 0.97 days. Severe HIE occurred in 33% of neonates in both studies, and although Hu et al. included neonates at 35 weeks’ gestation, the mean gestation in the early feeding group was 39.9 weeks. Feeding intolerance was reported in only 9% of neonates in a retrospective multicentre five-year review of minimal enteral nutrition (MEN) during TH (21). However, only 12% had severe HIE, and the feeding strategy during TH was not described.
Time to full enteral feeding (TTFEF) can be considered as an indirect indication of feed tolerance. Several studies have defined and reported TTFEF with early enteral feeding during TH, but with substantial variation in the definition of TTFEF; 150 ml/kg/day for at least three consecutive days (mean ± SD TTFEF; 7.6 ± 3.5 days) (20), “no requirement for intravenous fluid” [median (IQR) TTFEF; 7 (5–11) days] (34), 120 ml/kg/day (mean ± SD TTFEF; 9.91 ± 1.88 days) (35), 120 ml/kg/day for 24 h [median (IQR) TTFEF; 6 (5–9) days] (21), and “time between birth and the time when parenteral nutrition was terminated” [median (IQR) TTFEF 6 (3–19) days] (17). The early enteral feeding volumes and strategies in these studies were variable; feed with 12–24 ml/kg/day if not hypotensive, coaguloapthic, hypoxic or acidotic—usually on or after the third day of life (20), initiate and advance feeds at 10–20 ml/kg/day after 24 h as tolerated when considered “stable” (34), initiate and advance feeds as tolerated at <20 ml/kg/day, if “medically feasible” during TH/rewarming (35), start “MEN after 12–24 hours of life” (21), and feeds initiated at median 23.6 h with 1–2 ml/kg/bolus every 3 h (8–16 ml/kg/d) and increased if tolerated (17).
In our study, a regimen of 3-hourly bolus feeds at 12 ml/kg/day, initiated after 12–24 h, resulted in median enteral feed volumes of 120 ml/kg/day by day 6 in neonates without EFI, and by day 7 in neonates with EFI. By day 6 of life, only 3% and 21%, of neonates without and with EFI respectively, were receiving IV fluids. These data suggest that our cohort, including the neonates with feed intolerance, had comparable or faster TTFEF, when compared to other studies. The tolerance of feeds and low proportion of neonates receiving IV fluids by day 6 in our cohort, may be related to our protocol of restricted total fluid volume of 40 ml/kg/day for the first three days, which is different to some centres which administer 60 ml/kg/day (37). While more evidence is needed to guide the use of fluid restriction, our approach avoids a positive fluid balance which has been associated with death and increased severity of brain injury in neonates with HIE (38, 39).
Feeding intolerance and disease severity
Previous studies have reported an increased frequency of delayed feeding initiation and/or delayed attainment of full enteral feeding in neonates with severe HIE and/or cardio-respiratory instability, including mechanical ventilation and inotrope use (20, 34, 37, 40). We found a similar association with severe HIE. The neonates with EFI in our study had significantly higher Thompson HIE scores and Modified Sarnat HIE Grades than neonates without EFI, and they had a three times higher frequency of suppressed aEEG at 48 h. The predominance of severe HIE in neonates with EFI in our study, also aligned with their prolonged hospital stay and later age of attaining nutritive suck/cup feeding. The progressively increasing difference between Thompson HIE scores of neonates with and without EFI, before and during cooling (Figure 2), may represent disease progression and less response to TH in the neonates with severe HIE and EFI.
In addition to the anti-inflammatory effects of TH, it may reduce GI injury after hypoxia-ischaemia, by modulating or reducing the excessive GI blood flow which can occur during the reperfusion phase of injury in neonates with moderate HIE—however, severe HIE is associated with decreased GI blood flow which may not be improved by TH (7, 8). This mechanism may be related to the increased EFI in neonates with severe HIE. There is ongoing research into the potential value of doppler studies of GIT blood flow velocities, to assess intestinal health and guide enteral feeding approaches (41, 42).
We did not find an association between mechanical ventilation, inotrope use, or other morbidities and EFI—this may be related to our standardised approach of initiating enteral feeds in all neonates with a normal abdominal examination, irrespective of ventilation or inotrope requirement. The variable morbidity and EFI in other TH and feeding studies may be affected by the severity of intrapartum hypoxia reflected in the variable requirement of early acidosis as an inclusion criteria for cooling, where BD can vary from ≥10 to ≥16; comparisons will be more meaningful if global and/or regional criteria for TH are standardised (28). We found no association between maternal characteristics and neonatal characteristics at birth, including Apgar scores and blood gas analysis—suggesting that the extent of individual foetal/neonatal response or (mal)adaptation to the hypoxic-ischaemic exposure is more predictive than the exposure alone.
Limitations and strengths
The primary limitation of this study is that it is a retrospective analysis of data where the sample size was determined by time and completeness relative to a separate study—it is therefore subject to selection bias and may lack power for variables with high or low frequencies. A second major limitation is the lack of data on the type of milk per feed—breast milk vs. formula—which may influence feed tolerance. However, the data represent the real-world scenario of limited availability of EBM, the data were collected prospectively, and the study provides novel descriptive data from a setting with a standardized and well-established protocol for nutritional and fluid management of HIE.
Conclusion
Our study addresses a knowledge gap identified by studies showing benefit of early enteral nutrition in neonates with HIE who are treated with TH including a systematic review (22) and several subsequent studies (20, 21, 37)—to provide data and outcomes for specific feeding strategies. It is the first study from South Africa to provide nutritional outcome data in the context of standardised early and progressive feeding of neonates with HIE who are treated with TH.
The progressive, standardised, early feeding regimen of neonates at GSH with HIE who were treated with TH, was generally well tolerated. Independence from IV fluids was achieved by neonates in this study, at comparable or earlier times than were observed in other studies. Early feed intolerance was more frequent in neonates with severe HIE, but most neonates did not require IV fluids after day 5 of life, NEC did not occur and there was a low mortality rate. Further studies should focus on accurately quantifying and standardizing the approach to enteral feeds for neonates, including the use of EBM and donor EBM, according to severity of HIE.
Data availability statement
The datasets presented in this article are not readily available because informed consent was not taken for that type of data storage and access. However, access to the de-identified raw data set, for purposes of collaborative sub-studies with the authors, pending relevant ethics committee approval, can be obtained from the corresponding author. Requests to access the datasets should be directed toYWxhbi5ob3JuQHVjdC5hYy56YQ==.
Ethics statement
The studies involving humans were approved by Human Research Ethics Committee (HREC) of the Faculty of Health Sciences (FHS), University of Cape Town (UCT), (HREC 658/2024). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the study involved retrospective analysis of de-identified observational data only, from an ethics-approved neonatal encephalopathy registry. There were no interventions.
Author contributions
IS: Conceptualization, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. MSP: Conceptualization, Writing – review & editing. SP: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. ARH: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. IS was funded in part by Department of Paediatrics and Child Health, University of Cape Town. The NESHIE project is funded by the Gates Foundation [grant numbers: INV-022216, INV-041062]; the South African Medical Research Council (SAMRC); and the University of Pretoria through the Institute for Cellular and Molecular Medicine. No additional external funding was received for this study.
Acknowledgments
We acknowledge the support from the academic administrative staff in the Department of Paediatrics and Child Health and the Division of Neonatology, University of Cape Town.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chalak L, Ferriero DM, Gressens P, Molloy E, Bearer C. A 20 years conundrum of neonatal encephalopathy and hypoxic ischemic encephalopathy: are we closer to a consensus guideline? Pediatr Res. (2019) 86:548–9. doi: 10.1038/s41390-019-0547-9
2. Kukka AJ, Waheddoost S, Brown N, Litorp H, Wrammert J, Ashish K. Incidence and outcomes of intrapartum-related neonatal encephalopathy in low-income and middle-income countries: a systematic review and meta-analysis. BMJ Glob Health. (2022) 7:e010294. doi: 10.1136/bmjgh-2022-010294
3. Abate BB, Bimerew M, Gebremichael B, Mengesha Kassie A, Kassaw M, Gebremeskel T, et al. Effects of therapeutic hypothermia on death among asphyxiated neonates with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis of randomized control trials. PLoS One. (2021) 16:e0247229. doi: 10.1371/journal.pone.0247229
4. Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol. (1999) 123:221–34. doi: 10.1016/s1095-6433(99)00057-4
5. Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F346–F58. doi: 10.1136/archdischild-2015-309639
6. Lu Q, Cheng S, Zhou M, Yu J. Risk factors for necrotizing enterocolitis in neonates: a retrospective case-control study. Pediatr Neonatol. (2017) 58:165–70. doi: 10.1016/j.pedneo.2016.04.002
7. Ilves P, Lintrop M, Talvik I, Muug K, Maipuu L. Changes in cerebral and visceral blood flow velocities in asphyxiated term neonates with hypoxic-ischemic encephalopathy. J Ultrasound Med. (2009) 28:1471–80. doi: 10.7863/jum.2009.28.11.1471
8. Faingold R, Cassia G, Prempunpong C, Morneault L, Sant'Anna GM. Intestinal ultrasonography in infants with moderate or severe hypoxic-ischemic encephalopathy receiving hypothermia. Pediatr Radiol. (2016) 46:87–95. doi: 10.1007/s00247-015-3447-0
9. Azzopardi D. Clinical management of the baby with hypoxic ischaemic encephalopathy. Early Hum Dev. (2010) 86:345–50. doi: 10.1016/j.earlhumdev.2010.05.008
10. Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. (2013) (1):CD003311. doi: 10.1002/14651858.CD003311.pub3
11. Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. (2009) 361:1349–58. doi: 10.1056/NEJMoa0900854
12. Jacobs SE, Morley CJ, Inder TE, Stewart MJ, Smith KR, McNamara PJ, et al. Whole-body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med. (2011) 165:692–700. doi: 10.1001/archpediatrics.2011.43
13. Gluckman P, Wyatt J, Azzopardi D, Ballard R, Edwards A, Ferriero D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. (2005) 365:663–70. doi: 10.1016/S0140-6736(05)17946-X
14. Simbruner G, Mittal RA, Rohlmann F, Muche R, neo.nEURO.networkTrialParticipants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RCT. Pediatrics. (2010) 126:e771–8. doi: 10.1542/peds.2009-2441
15. Konnikova Y, Zaman MM, Makda M, D'Onofrio D, Freedman SD, Martin CR. Late enteral feedings are associated with intestinal inflammation and adverse neonatal outcomes. PLoS One. (2015) 10:e0132924. doi: 10.1371/journal.pone.0132924
16. Hazeldine B, Thyagarajan B, Grant M, Chakkarapani E. Survey of nutritional practices during therapeutic hypothermia for hypoxic-ischaemic encephalopathy. BMJ Paediatr Open. (2017) 1:e000022. doi: 10.1136/bmjpo-2017-000022
17. Thyagarajan B, Tillqvist E, Baral V, Hallberg B, Vollmer B, Blennow M. Minimal enteral nutrition during neonatal hypothermia treatment for perinatal hypoxic-ischaemic encephalopathy is safe and feasible. Acta Paediatr. (2015) 104:146–51. doi: 10.1111/apa.12838
18. Gale C, Longford NT, Jeyakumaran D, Ougham K, Battersby C, Ojha S, et al. Feeding during neonatal therapeutic hypothermia, assessed using routinely collected national neonatal research database data: a retrospective, UK population-based cohort study. Lancet Child Adolesc Health. (2021) 5:408–16. doi: 10.1016/S2352-4642(21)00026-2
19. Chang LL, Wynn JL, Pacella MJ, Rossignol CC, Banadera F, Alviedo N, et al. Enteral feeding as an adjunct to hypothermia in neonates with hypoxic-ischemic encephalopathy. Neonatology. (2018) 113:347–52. doi: 10.1159/000487848
20. Costa S, Rizzo ID, Fattore S, Serrao F, Priolo F, Corsello M, et al. Enteral nutritional strategy during therapeutic hypothermia: who? When? What? Front Pediatr. (2024) 12:1357831. doi: 10.3389/fped.2024.1357831
21. Sharma S, Kallesh A, Aradhya AS, Diggikar S, Veeraiah PS, Subbareddy NN, et al. Feasibility of minimal enteral nutrition during therapeutic hypothermia for perinatal asphyxia: a five-year multicenter experience from south India. Indian J Pediatr. (2023) 90:513–5. doi: 10.1007/s12098-022-04456-x
22. Kumar J, Anne RP, Meena J, Sundaram V, Dutta S, Kumar P. To feed or not to feed during therapeutic hypothermia in asphyxiated neonates: a systematic review and meta-analysis. Eur J Pediatr. (2023) 182:2759–73. doi: 10.1007/s00431-023-04950-0
23. Kali GT, Martinez-Biarge M, Van Zyl J, Smith J, Rutherford M. Therapeutic hypothermia for neonatal hypoxic-ischaemic encephalopathy had favourable outcomes at a referral hospital in a middle-income country. Acta Paediatr. (2016) 105:806–15. doi: 10.1111/apa.13392
24. Sichula M, Pillay S, Nakibuuka V, Harrison MC, Horn AR. Hyperglycaemia and outcome in neonates with hypoxic-ischaemic encephalopathy. S Afr J Child Health. (2024) 18:83–9. doi: 10.7196/SAJCH.2024.v18i2.1028
25. Shalak LF, Laptook AR, Velaphi SC, Perlman JM. Amplitude-integrated electroencephalography coupled with an early neurologic examination enhances prediction of term infants at risk for persistent encephalopathy. Pediatrics. (2003) 111:351–7. doi: 10.1542/peds.111.2.351
26. Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. (2005) 353:1574–84. doi: 10.1056/NEJMcps050929
27. Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. (1997) 86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x
28. Horn AR, Swingler GH, Myer L, Linley LL, Raban MS, Joolay Y, et al. Early clinical signs in neonates with hypoxic ischemic encephalopathy predict an abnormal amplitude-integrated electroencephalogram at age 6 hours. BMC Pediatr. (2013) 13:52. doi: 10.1186/1471-2431-13-52
29. Hellström-Westas L, Rosén I. Continuous brain-function monitoring: state of the art in clinical practice. Seminars in Fetal and Neonatal Medicine. (2006) 11:503–11. doi: 10.1016/j.siny.2006.07.011
30. Toet MC, Hellstrom-Westas L, Groenendaal F, Eken P, de Vries LS. Amplitude integrated EEG 3 and 6 hours after birth in full term neonates with hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. (1999) 81:F19–23. doi: 10.1136/fn.81.1.f19
31. al Naqeeb N, Edwards AD, Cowan FM, Azzopardi D. Assessment of neonatal encephalopathy by amplitude-integrated electroencephalography. Pediatrics. (1999) 103:1263–71. doi: 10.1542/peds.103.6.1263
32. Thoresen M, Hellstrom-Westas L, Liu X, de Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics. (2010) 126:e131–9. doi: 10.1542/peds.2009-2938
33. Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. (1986) 33:179–201. doi: 10.1016/s0031-3955(16)34975-6
34. Gunes AO, Karadag N, Topcuoglu S, Ozalkaya E, Toptan HH, Dincer E, et al. Factors associated with the transition time to full enteral feeding in newborns with hypoxic ischemic encephalopathy. Arch Iran Med. (2022) 25:547–51. doi: 10.34172/aim.2022.87
35. Hu Y, Chen F, Xiang X, Wang F, Hua Z, Wei H. Early versus delayed enteral nutrition for neonatal hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia: a randomized controlled trial. Ital J Pediatr. (2022) 48:146. doi: 10.1186/s13052-022-01342-2
36. Thornton KM, Dai H, Septer S, Petrikin JE. Effects of whole body therapeutic hypothermia on gastrointestinal morbidity and feeding tolerance in infants with hypoxic ischemic encephalopathy. Int J Pediatr. (2014) 2014:643689. doi: 10.1155/2014/643689
37. Markus M, Giannakis S, Ruhfus M, Stein A, Heep A, Plagemann T, et al. Fluid supply and feeding practices in cooled asphyxiated newborns. Children (Basel). (2021) 8:899. doi: 10.3390/children8100899
38. Kumar J. Fluid restriction in moderate and severe hypoxemic-ischemic encephalopathy: more evidence is needed. J Perinatol. (2019) 39:342–3. doi: 10.1038/s41372-018-0284-7
39. Ottolini KM, Basu SK, Herrera N, Govindan V, Mashat S, Vezina G, et al. Positive fluid balance is associated with death and severity of brain injury in neonates with hypoxic-ischemic encephalopathy. J Perinatol. (2021) 41:1331–8. doi: 10.1038/s41372-021-00988-w
40. Malviya M, Murthi S, Jayaraj D, Ramdas V, Nazir Malik F, Nair V, et al. Effects of therapeutic hypothermia and minimal enteral nutrition on short-term outcomes in neonates with hypoxic-ischemic encephalopathy: a 10-year experience from Oman. Children (Basel). (2024) 12:23. doi: 10.3390/children12010023
41. Sakhuja P, More K, Ting JY, Sheth J, Lapointe A, Jain A, et al. Gastrointestinal hemodynamic changes during therapeutic hypothermia and after rewarming in neonatal hypoxic-ischemic encephalopathy. Pediatr Neonatol. (2019) 60:669–75. doi: 10.1016/j.pedneo.2019.04.003
Keywords: neonate, hypoxia ischaemia -brain, hypothermia -induced, Africa south of the Sahara, neonatal encephalopathy, nutrition-enteral
Citation: Samaai I, Pepper MS, Pillay S and Horn AR (2025) Minimal impact of feed intolerance during therapeutic hypothermia for hypoxic ischaemic encephalopathy in a South African cohort with a standardised feeding regimen. Front. Pediatr. 13:1611619. doi: 10.3389/fped.2025.1611619
Received: 14 April 2025; Accepted: 14 July 2025;
Published: 30 July 2025.
Edited by:
Fiammetta Piersigilli, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Iliana Bersani, Bambino Gesù Children’s Hospital (IRCCS), ItalyPeter Cooper, University of the Witwatersrand, South Africa
Katherine Carkeek, Cliniques Universitaires Saint-Luc, Belgium
Copyright: © 2025 Samaai, Pepper, Pillay and Horn. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alan R. Horn, YWxhbi5ob3JuQHVjdC5hYy56YQ==
†These authors have contributed equally to this work and share senior authorship
‡These authors have contributed equally to this work and share last authorship
 Ilhaam Samaai
Ilhaam Samaai Michael S. Pepper
Michael S. Pepper Shakti Pillay
Shakti Pillay Alan R. Horn
Alan R. Horn