- 1Respiratory Department II, Children's Hospital of Hebei Province (Hebei Provincial Clinical Research Center for Child Health and Disease; Hebei Provincial Medical Key Discipline), Shijiazhuang, China
- 2Lung Function Laboratory, Children's Hospital of Hebei Province (Hebei Provincial Clinical Research Center for Child Health and Disease; Hebei Provincial Medical Key Discipline), Shijiazhuang, China
Objective: To evaluate the effectiveness of integrated nursing and medical management in children with lobar pneumonia, focusing on symptom relief, pulmonary function recovery, inflammation control, length of hospital stay, and caregiver satisfaction.
Methods: Fifty pediatric patients with lobar pneumonia were randomly assigned to receive either routine nursing care or an integrated medical and nursing intervention. Key clinical outcomes—including the duration of symptoms, pulmonary function indices, inflammatory markers, length of hospital stay, treatment efficacy, caregiver satisfaction, and adverse events—were compared between groups.
Results: Compared with the control group, the observation group demonstrated significantly shorter durations of fever, cough, and pulmonary rales, reduced antibiotic usage, and shorter hospital stays (all P < 0.001). Pulmonary function indices improved markedly (P = 0.001), and inflammatory markers showed more substantial reductions (P < 0.001). The overall treatment effectiveness in the observation group was 100%, with a caregiver satisfaction rate of 96.00% and a complication rate of 8.00%, all significantly better than those in the control group (P < 0.05).
Conclusion: Integrated nursing and medical management significantly improves clinical outcomes for pediatric lobar pneumonia, accelerating recovery, enhancing pulmonary function, reducing complications, and increasing caregiver satisfaction. These findings support its broader application in clinical practice.
ClinicalTrials.gov Identifier: https://clinicaltrials.gov/study/NCT06945991, NCT06945991 (16th/April/2025).
1 Introduction
Lobar pneumonia remains a leading cause of morbidity and hospitalization among children worldwide, with pediatric patients exhibiting distinct clinical characteristics such as persistent high fever, cough, and pulmonary rales, often resulting in significant impairment of lung function and prolonged recovery periods (1). The condition is predominantly caused by bacterial infections, with common pathogens of community-acquired lobar pneumonia including Streptococcus pneumoniae (2), Klebsiella pneumoniae (3), and Mycoplasma pneumoniae (4). Despite advances in antibiotic therapy, the burden of pediatric lobar pneumonia remains substantial (5). Recent epidemiological studies indicate a rising incidence among children, especially in China, where it constitutes up to 30% of community-acquired pneumonia cases (6–8). Notably, children are more susceptible to severe disease and complications due to their unique anatomical and immunological profiles, underscoring the need for tailored management strategies.
Traditional management typically relies on routine nursing and empirical antibiotic therapy, yet challenges persist. Fragmented care, limited multidisciplinary collaboration, and inadequate family engagement are common issues, often resulting in suboptimal outcomes such as delayed symptom resolution and impaired pulmonary recovery (9, 10). Furthermore, current care models seldom incorporate pulmonary rehabilitation or dynamic inflammatory monitoring—elements increasingly recognized as crucial for improving pediatric outcomes (11, 12).
In recent years, integrated medical and nursing care has emerged as an innovative management model, demonstrating notable advantages in the management of chronic diseases and critical illnesses (13, 14). This approach emphasizes multidisciplinary team collaboration, standardized clinical pathways, and continuous engagement of patients and their families. For example, in adult patients with chronic obstructive pulmonary disease (COPD), community-based integrated care—coordinating pulmonologists, nurses, rehabilitation therapists, and nutritionists—has significantly reduced hospital stays and improved pulmonary function (15). However, research on integrated care in the context of pediatric lobar pneumonia remains limited, particularly with respect to high-quality evidence derived from randomized controlled trials (RCTs). Moreover, existing studies primarily focus on short-term clinical outcomes, with insufficient systematic evaluation of long-term effects on pulmonary function recovery, inflammation control, and family-centered interventions.
This single-blind randomized controlled trial assessed whether integrated nursing and medical care, compared to conventional treatment, could enhance pulmonary function, reduce hospital stay, and lower inflammatory markers in children with lobar pneumonia, while also evaluating clinical feasibility and caregiver satisfaction. We hypothesized that multidisciplinary collaboration and family engagement would accelerate symptom resolution, improve respiratory recovery, and decrease complication rates—offering evidence to optimize pediatric pneumonia management and inform efficient healthcare resource allocation.
2 Methods
2.1 Study design
This study was a single-blind, parallel-group, repeated-measures randomized controlled trial conducted from January 2023 to January 2025 in the Department of Respiratory Medicine at a hospital in Hebei, China. Participants randomly assigned to the intervention group received both integrated medical and nursing care in addition to routine nursing, while those assigned to the waitlist control group received only routine nursing care. Outcome measures were assessed at baseline (T0) and at discharge (T1).
2.2 Participants
Pediatric patients diagnosed with lobar pneumonia were recruited from the Department of Respiratory Medicine at a hospital using convenience sampling. Informed consent was obtained from the child's legal guardians.
2.2.1 Inclusion criteria
(1) Met the diagnostic criteria for lobar pneumonia, presenting with symptoms such as fever, cough, and pulmonary rales, and confirmed by laboratory tests and chest x-ray; (2) Aged between 1 and 12 years.
2.2.2 Exclusion criteria
(1) Presence of other concurrent respiratory diseases; (2) Congenital or acquired airway malformations, or airway foreign bodies; (3) Impaired hepatic or renal function; (4) Presence of other systemic diseases; (5) Cognitive impairment.
2.3 Sample size
Sample size calculation for the randomized controlled design was performed using G*Power software (version 3.1.9.4). Sample size estimation was based on Li et al. (16), who reported significant improvements in FEV and FVC with integrated care in severe pneumonia. Using FEV as the primary outcome, a Cohen's d of 0.83 was applied to reflect the large observed effect size. Based on a significance level of 0.05, a power of 0.80, and two groups, the calculated sample size was 48 participants. To account for an anticipated 15% attrition rate, 56 participants were enrolled.
2.4 Randomization and blinding
Participants were randomly assigned to the intervention or control group using block randomization (block size of eight) generated online (http://www.randomizer.org). Allocation was concealed in advance by an independent assistant using sealed envelopes, which were opened sequentially after baseline data collection. Outcome assessors were blinded to group assignment.
2.5 Implementation of the intervention
In the control group, children received standard inpatient care following current pediatric community-acquired pneumonia (CAP) guidelines. This included: Medication management: Weight-based antibiotics (typically amoxicillin or ceftriaxone as per IDSA/PIDS guidelines), along with antipyretics and intravenous fluids guided by physician orders. Therapy was reviewed on Days 1, 3, and 7. Respiratory support: Nebulized inhalation (e.g., β2-agonists or saline) administered twice daily for approximately 15 min, with vital signs monitored throughout. Caregivers were taught chest physiotherapy (back patting) upon admission, with nursing staff ensuring daily execution and documentation. Environmental and ward measures: Daily ward air disinfection using ultraviolet lamps (30 min each morning) and regular ventilation per hospital protocol. Monitoring and assessment: Nurses performed twice-daily checks of SpO2, respiratory rate, heart rate, temperature, and conducted routine lab tests (CBC, CRP, PCT) and chest imaging as ordered. Any SpO2 < 94% or respiratory distress triggered immediate physician notification. Nutrition support: Within 24 h of admission, a dietitian assessed patients and prescribed age-appropriate nutrition. Nurses recorded daily intake/output and monitored hydration status. Nurse-physician communication: Routine ward rounds occurred twice daily, with verbal handovers at each shift. Nurses conveyed abnormal findings promptly to physicians, though no formal multidisciplinary protocol was in place.
The observation group received integrated medical and nursing care in addition to routine care during hospitalization, representing a coordinated intervention that integrates multiple medical resources. This integrated care included two components: (1) A multidisciplinary team collaboration for a two-week inpatient health management period (17, 18); (2) Family intervention (19). Experts, including pulmonologists, clinical nurses, rehabilitation therapists, and nutritionists, reviewed the draft of the integrated medical and nursing intervention plan. Feedback from the experts indicated that the plan was simple, easy to understand, and applicable to children with lobar pneumonia at this hospital. Consequently, the intervention plan was adopted without any changes. The components of the plan are outlined as follows.
1. Multidisciplinary Team Collaboration for Health Management (Supplementary Table S1)
2. Family intervention (Supplementary Table S2)
2.6 Data collection
Researchers collected data from the hospital medical record database, including information such as gender (male, female), age (years), disease duration (days), lung lobe infection sites, and clinical symptoms. The clinical symptoms recorded included: Duration of fever (days), Duration of cough (days), Duration of lung crackles (days), Duration of antibiotic use (days), Duration of hospitalization (days).
Lung function tests [FEV1 (L), FVC (L), and FEV1/FVC (%)] and laboratory inflammatory markers [CRP (mg/L), WBC (×109/L), PCT (ng/ml), and LDH (U/L)] were measured on the day of admission and the day before discharge. Data were collected accordingly.
According to the PIDS/IDSA CAP guidelines for children (20), efficacy was classified as cure (complete symptom resolution and radiological recovery), improvement (partial symptom relief and lesion absorption), or ineffective (no significant change). The total effective rate included both cure and improvement. Family satisfaction was rated on a four-point scale, and adverse events and complications were recorded throughout the intervention.
2.7 Statistical analysis
SPSS 26.0 (IBM Corp., Armonk, NY, USA) statistical softwares were used for data analysis. The α level was 0.05 by bilateral statistical test. If the P-value is less than 0.05, it is considered significant. The Shapiro–Wilk test and Paired t-test were used to evaluate the normality of continuous data. The normal distribution of measurement data was expressed as. Non-normally distributed continuous data were expressed as median (minimum, maximum), and the Mann–Whitney U-test was used for comparisons between groups. Independent sample t-test was used for comparison between groups. The statistical data were expressed by [case number (%)] and χ2 test was used between groups. P < 0.05 was considered statistically significant.
3 Results
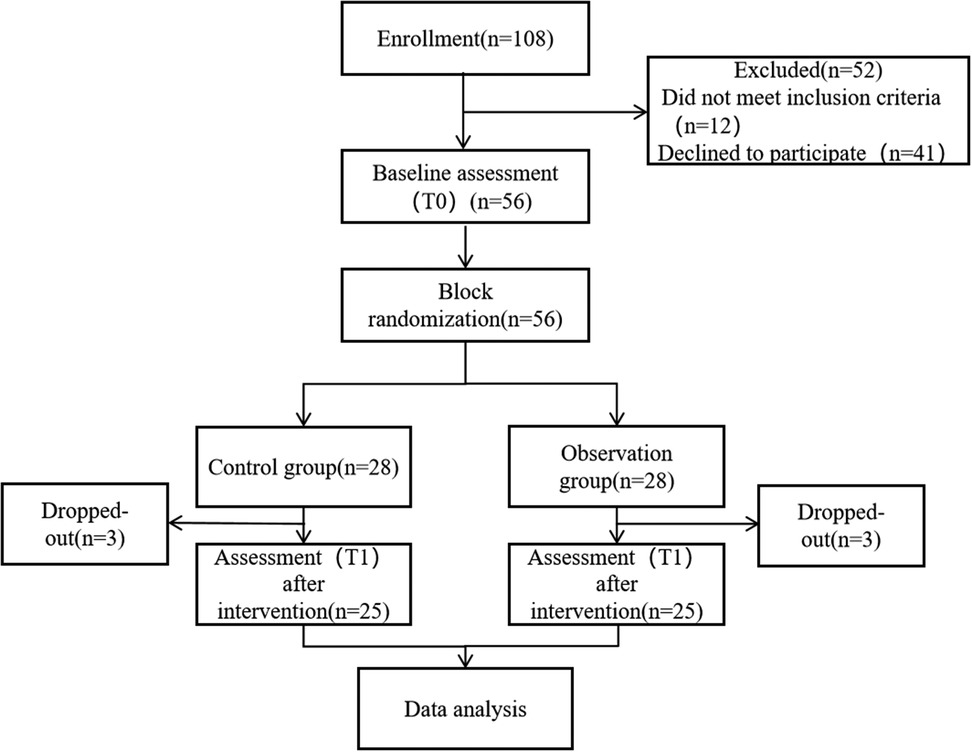
3.1 CONSORT flowchart of the study
A total of 108 pediatric patients diagnosed with lobar pneumonia were assessed for eligibility. Among them, 52 were excluded, including 12 who did not meet the inclusion criteria and 41 who declined to participate. The remaining 56 participants underwent baseline assessment (T0) and were subsequently randomized into either the control group (n = 28) or the observation group (n = 28) using a permuted block randomization method. During the intervention period, three participants dropped out from each group due to various reasons, resulting in 25 participants in each group completing the post-intervention assessment (T1). All 50 participants who completed the study were included in the final data analysis. As shown in Figure 1.
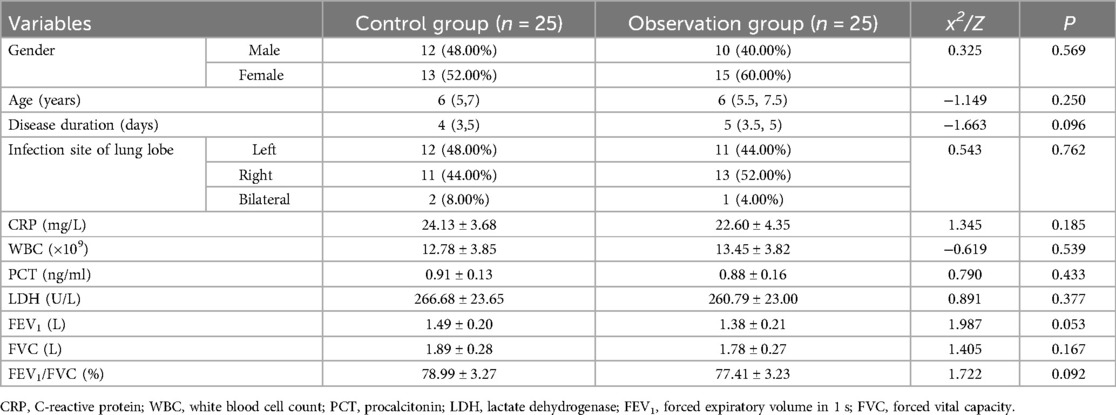
3.2 Comparison of baseline data
There were no significant differences between the control and observation groups in terms of gender, age, disease duration, ethnicity, or infection site (P > 0.05). Laboratory parameters (CRP, WBC, PCT, LDH) and lung function indicators (FEV1, FVC, FEV1/FVC) also showed no statistically significant differences between the two groups (P > 0.05). As shown in Table 1.
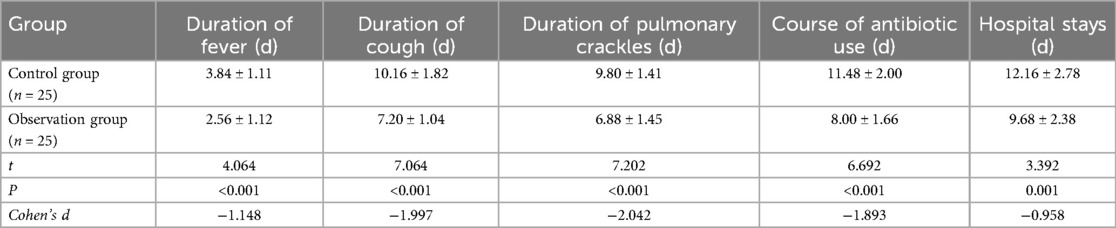
3.3 Comparison of duration of clinical symptoms between two groups
Compared with the control group, the observation group showed significantly shorter durations of fever (2.56 ± 1.12 vs. 3.84 ± 1.11 days, P < 0.001), cough (7.20 ± 1.04 vs. 10.16 ± 1.82 days, P < 0.001), lung rales (6.88 ± 1.45 vs. 9.80 ± 1.41 days, P < 0.001), antibiotic therapy (8.00 ± 1.66 vs. 11.48 ± 2.00 days, P < 0.001), and hospital stays (9.68 ± 2.38 vs. 12.16 ± 2.78 days, P = 0.001). These differences were statistically significant. As shown in Table 2.
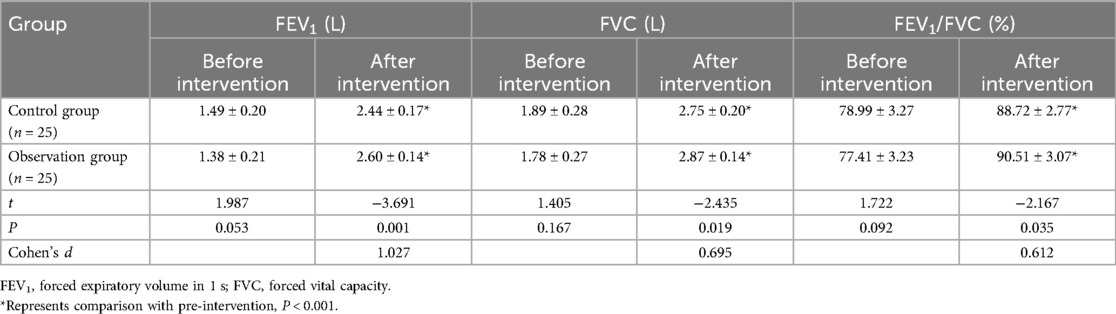
3.4 Comparison of pulmonary function indexes between two groups
Before intervention, no significant differences were observed between the two groups in FEV1, FVC, and FEV1/FVC (P > 0.05). After intervention, the observation group demonstrated significantly greater improvements in FEV1 (2.60 ± 0.14 vs. 2.44 ± 0.17 L, P = 0.001), FVC (2.87 ± 0.14 vs. 2.75 ± 0.20 L, P = 0.019), and FEV1/FVC ratio (90.51 ± 3.07% vs. 88.72 ± 2.77%, P = 0.035) compared to the control group. As shown in Table 3.
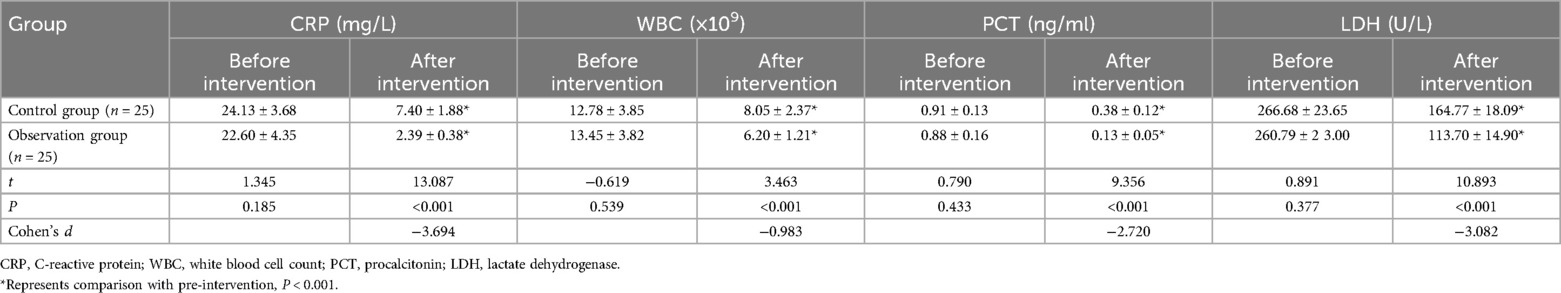
3.5 Comparison of inflammatory markers between the two groups
Prior to intervention, there were no significant differences in CRP, WBC, PCT, and LDH levels between the control and observation groups (P > 0.05). After intervention, the observation group exhibited significantly greater reductions in inflammatory markers, including CRP (2.39 ± 0.38 vs. 7.40 ± 1.88 mg/L, P < 0.001), WBC (6.20 ± 1.21 vs. 8.05 ± 2.37 × 109/L, P < 0.001), and PCT (0.13 ± 0.05 vs. 0.38 ± 0.12 ng/ml, P < 0.001), as well as in LDH (113.70 ± 14.90 vs. 164.77 ± 18.09 U/L, P < 0.001), compared to the control group. As shown in Table 4.
3.6 Comparison of treatment efficiency, parental satisfaction, and complication rate between the two groups
The observation group achieved significantly better outcomes than the control group. The total effective rate was higher in the observation group (100% vs. 84.00%, P = 0.037), with more cases of complete recovery (76.00% vs. 64.00%) and no ineffective cases, while the control group had a 16% non-response rate. Parental satisfaction was also greater in the observation group (96.00% vs. 76.00%, P = 0.042), with no reports of dissatisfaction compared to 12.00% in the control group. Furthermore, the incidence of adverse events was lower in the observation group (8.00% vs. 20.00%, P = 0.034), with only one case of bronchospasm (4.00%) and no other complications, whereas the control group had higher rates of laryngeal edema, pneumothorax, bronchospasm, and airway mucosal injury. As shown in Table 5.
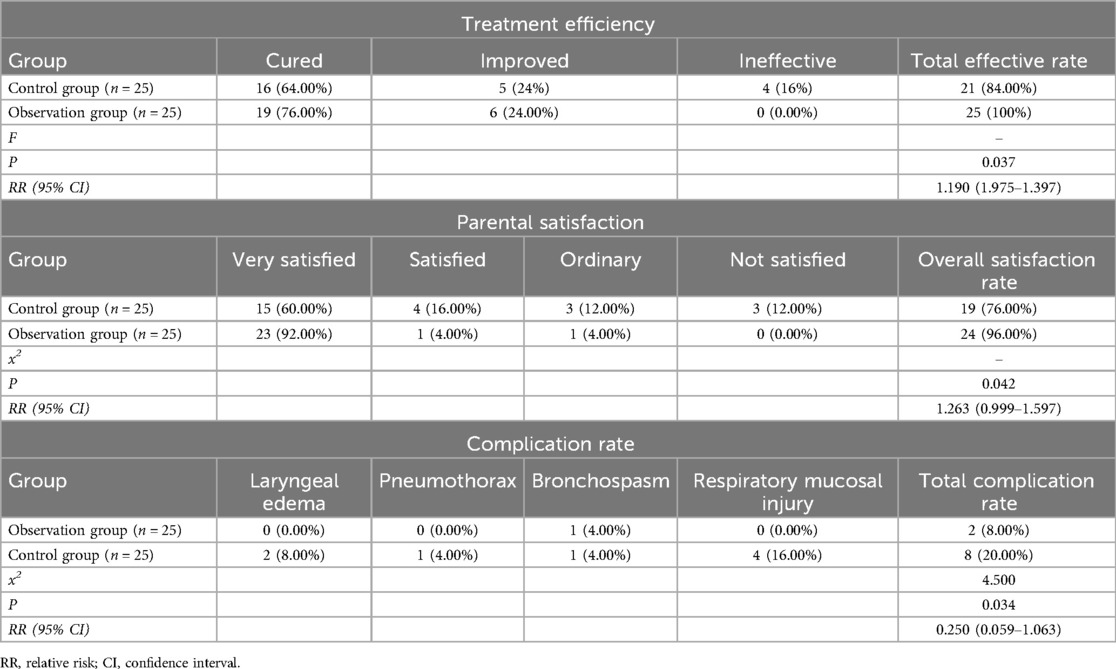
Table 5. Comparison of treatment efficiency, parental satisfaction, and complication rate between the two groups.
4 Discussion
This randomized controlled trial evaluated the effectiveness of integrated medical and nursing management for pediatric lobar pneumonia. The findings suggest that, compared with conventional nursing care, the integrated intervention may offer benefits in reducing the duration of symptoms (fever, cough, pulmonary crackles), shortening antibiotic use and hospitalization, and improving pulmonary function and inflammatory markers. Higher rates of effective treatment and family satisfaction, along with a lower incidence of complications, were observed in the intervention group. However, these results should be interpreted with caution given the study's sample size and single-center design.
First, based on the results of this study, the integrated medical and nursing intervention was able to promote the relief of clinical symptoms in children within a short period, particularly in controlling high fever, severe cough, and lung inflammation absorption during the infection process. Notably, the effect sizes for these differences were large. This success can be attributed, to some extent, to the establishment of the daily joint rounds by the intervention team. Respiratory physicians and specialized nurses collaboratively developed individualized treatment plans and made timely adjustments to anti-infection strategies through dynamic assessments on the 1st, 3rd, and 7th days of hospitalization, which made disease control more targeted and timely. Additionally, lung function training programs, such as vibration positive expiratory pressure training and postural drainage, led by rehabilitation therapists, effectively improved alveolar ventilation, facilitated sputum expulsion, and accelerated the absorption of pulmonary lesions. The precise guidance on antibiotic usage paths provided by pharmacists also helped reduce the risk of antibiotic resistance. Through an interdisciplinary collaboration model, resources from specialized physicians, nurses, and rehabilitation therapists were integrated, reducing redundant work between disciplines. Nurses transitioned from passive executors to active participants, which enhanced their sense of responsibility and involvement. Furthermore, by participating in the management of complex cases, nurses gained professional growth opportunities and, compared to conventional nursing, experienced higher job pride.
Improvements in pulmonary function (FEV1, FVC, FEV1/FVC) were also more pronounced in the intervention group after treatment, with Cohen's d values indicating moderate to large effect sizes (0.612–1.027). This highlights the intervention's practical significance in promoting respiratory recovery beyond statistical significance. This finding aligns with the results of Khan et al. (21), who found that multidisciplinary collaboration improves lung ventilation function in the management of diffuse parenchymal lung diseases. Furthermore, the observation group demonstrated greater reductions in inflammation-related markers (CRP, WBC, PCT, LDH), which may indicate a more rapid control of inflammatory responses. This finding is broadly consistent with the report by Hart et al. (22), who suggested that family-centered care during the COVID-19 pandemic could facilitate faster recovery in patients with pneumonia. This perspective is also supported by Bellizzi et al. (23). Notably, the family nursing interventions in this study were somewhat innovative, such as the introduction of standardized video teaching, nebulization therapy simulation assessments, “parent classrooms”, and feedback mechanisms through the “Nursing Log”. These innovations not only improved the nursing capabilities of family members but also strengthened communication and trust between healthcare providers and families, further enhancing overall treatment adherence and satisfaction.
With respect to service quality, the satisfaction rate in the observation group was 96%, notably higher than the 76% observed in the control group. This suggests that integrated medical and nursing management may enhance the overall care experience, potentially by addressing both technical and emotional needs of families. The establishment of effective communication mechanisms between staff and families likely contributed to this result. Similar improvements in patient and family satisfaction with multidisciplinary collaborative care have been reported in other pediatric populations [Cowpe et al. (24)]. Furthermore, as demonstrated by Wei et al. (25), the integration of continuous multidisciplinary care and nutritional support can further improve patient outcomes. However, it is important to note that satisfaction is a subjective outcome and may be influenced by expectations, the novelty of the intervention, or other unmeasured factors.
Furthermore, the incidence of adverse events in the observation group was 8%, compared to 20% (RR = 0.250, 95% CI: 0.059–1.063) in the control group. Severe complications—including laryngeal edema, bronchospasm, pneumothorax, and respiratory mucosal injury—were observed only in the control group, while the observation group reported only one mild case of bronchospasm and no other serious events. This difference may be partly attributable to the use of a smart monitoring system in the intervention group, which provided real-time transmission of SpO2 and respiratory rate via bedside ECG monitors and incorporated a three-tiered alert threshold system to facilitate prompt clinical responses. Previous studies have shown that early warning systems can reduce delayed clinical responses and unexpected deterioration in pediatric patients [Bates et al. (26)], and the benefits of intelligent monitoring devices in critical care settings have been further supported by Malycha et al. (27) and Li et al. (28). However, the impact of such technology should be interpreted with caution, as other factors and the study's sample size may also have influenced the observed outcomes.
This study has several limitations. First, the observed effect sizes are substantial, the study's single-center design and limited sample size may have influenced the robustness of these findings. Second, although our study demonstrated significant short-term improvements in clinical symptoms, pulmonary function, and inflammatory markers, the long-term effects of the intervention remain unclear. In particular, data on pulmonary function at 3 or 6 months after discharge were not collected. Future studies should incorporate extended follow-up periods to better evaluate the durability of these clinical benefits. Third, the intervention was relatively complex and required considerable human and technical resources, which may restrict its feasibility in resource-limited settings. These aspects warrant further investigation and refinement in future studies. Furthermore, factors such as the quality of communication among team members, consistency in protocol implementation, and family engagement may have influenced the effectiveness of the intervention. These aspects warrant further investigation and refinement in future studies.
5 Conclusion
In conclusion, this study confirms the effectiveness and safety of integrated healthcare interventions in managing pediatric lobar pneumonia, demonstrating significant advantages over conventional care. The approach, which is child-centered, supported by multidisciplinary collaboration, and enhanced by intelligent tools, improves disease control, pulmonary function, reduces inflammation, increases satisfaction, and lowers complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Hebei Children's Hospital (No. 202222-17). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
YH: Writing – original draft, Methodology, Conceptualization, Resources. QG: Writing – original draft, Formal analysis, Data curation. XL: Writing – original draft, Visualization, Project administration. WL: Validation, Writing – review & editing, Software. LL: Investigation, Supervision, Writing – review & editing, Formal analysis.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Hebei Province Medical Science Research Project (No. 20231172).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1612618/full#supplementary-material
References
1. Zinserling VA, Swistunov VV, Botvinkin AD, Stepanenko LA, Makarova AE. Lobar (croupous) pneumonia: old and new data. Infection. (2022) 50:235–42. doi: 10.1007/s15010-021-01689-4
2. Basse P, Gregory J, Lavenne R, Foucrier A. Right pan-lobar pneumonia due to Streptococcus pneumoniae. Intensive Care Med. (2022) 48:1647. doi: 10.1007/s00134-022-06844-5
3. Mohamed ER, Aly SA, Halby HM, Ahmed SH, Zakaria AM, El-Asheer OM. Epidemiological typing of multidrug-resistant Klebsiella pneumoniae, which causes paediatric ventilator-associated pneumonia in Egypt. J Med Microbiol. (2017) 66:628–34. doi: 10.1099/jmm.0.000473
4. Zheng Y, Mao G, Dai H, Li G, Liu L, Chen X, et al. Early predictors of delayed radiographic resolution of lobar pneumonia caused by Mycoplasma pneumoniae in children: a retrospective study in China. BMC Infect Dis. (2024) 24:414. doi: 10.1186/s12879-024-09289-x
5. Lassi ZS, Das JK, Haider SW, Salam RA, Qazi SA, Bhutta ZA. Systematic review on antibiotic therapy for pneumonia in children between 2 and 59 months of age. Arch Dis Child. (2014) 99:687–93. doi: 10.1136/archdischild-2013-304023
6. Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. (2013) 381:1405–16. doi: 10.1016/S0140-6736(13)60222-6
7. Liu YN, Zhang YF, Xu Q, Qiu Y, Lu QB, Wang T, et al. Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. (2023) 4:e330–9. doi: 10.1016/S2666-5247(23)00031-9
8. Shen L, Wu J, Lu M, Jiang Y, Zhang X, Xu Q, et al. Advancing risk factor identification for pediatric lobar pneumonia: the promise of machine learning technologies. Front Pediatr. (2025) 13:1490500. doi: 10.3389/fped.2025.1490500
9. Grant CJ, Doig LF, Everson J, Foster N, Doig CJ. Impact of patient and family involvement in long-term outcomes. Crit Care Nurs Clin North Am. (2020) 32:227–42. doi: 10.1016/j.cnc.2020.02.005
10. Holland AE, Cox NS, Houchen-Wolloff L, Rochester CL, Garvey C, ZuWallack R, et al. Defining modern pulmonary rehabilitation. An official American thoracic society workshop report. Ann Am Thorac Soc. (2021) 18:e12–29. doi: 10.1513/AnnalsATS.202102-146ST
11. Sun M, Yan Z, Sun R, Tian W, Yi W, Zhang J. Dynamic monitoring and a clinical correlation analysis of the serum vitamin A, D, and E levels in children with recurrent respiratory tract infections. Am J Transl Res. (2022) 14:3533–8.35702083
12. Yadav KK, Awasthi S. Childhood pneumonia: what’s unchanged, and what’s new? Indian J Pediatr. (2023) 90:693–9. doi: 10.1007/s12098-023-04628-3
13. Hove E, Hazelton MJ, Santangelo P, Wilson RL. Integrated nursing care for people with combined mental health and substance use disorders. Int J Ment Health Nurs. (2023) 32:378–401. doi: 10.1111/inm.13094
14. Kuzmarov IW, Ferrante A. The development of anti-cancer programs in Canada for the geriatric population: an integrated nursing and medical approach. Aging Male. (2011) 14:4–9. doi: 10.3109/13685538.2010.524954
15. Hernandez C, Alonso A, Garcia-Aymerich J, Serra I, Marti D, Rodriguez-Roisin R, et al. Effectiveness of community-based integrated care in frail COPD patients: a randomised controlled trial. NPJ Prim Care Respir Med. (2015) 25:15022. doi: 10.1038/npjpcrm.2015.22
16. Li H. Evaluation of the effect of integrated medical care on pulmonary function of patients with severe pneumonia complicated with respiratory failure. Chin Community Doctors. (2021) 37:141–2. doi: 10.3969/j.issn.1007-614x.2021.17.067
17. Goodhew E, Mayr R, Earing K, Seckam A. The role of an inpatient aphasia-friendly choir for people with post-stroke communication impairment from the perspective of the multidisciplinary team: an exploratory study. Int J Lang Commun Disord. (2025) 60:e13143. doi: 10.1111/1460-6984.13143
18. Chaudhuri N, Spencer L, Greaves M, Bishop P, Chaturvedi A, Leonard C. A review of the multidisciplinary diagnosis of interstitial lung diseases: a retrospective analysis in a single UK specialist centre. J Clin Med. (2016) 5:66. doi: 10.3390/jcm5080066
19. Price D, Ostrem A, Thomas M, Welte T. Dual bronchodilation in COPD: lung function and patient-reported outcomes—a review. Int J Chron Obstruct Pulmon Dis. (2017) 12:141–68. doi: 10.2147/COPD.S116719
20. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the Infectious Diseases Society of America. Clin Infect Dis. (2011) 53:e25–76. doi: 10.1093/cid/cir531
21. Khan MA, Sherbini N, Alyami S, Al-Harbi A, Alrajhi S, Abdullah R, et al. Role of multidisciplinary team meetings in the diagnosis and management of diffuse parenchymal lung diseases in a tertiary care hospital. Avicenna J Med. (2023) 13:230–6. doi: 10.1055/s-0043-1776063
22. Hart JL, Turnbull AE, Oppenheim IM, Courtright KR. Family-centered care during the COVID-19 era. J Pain Symptom Manage. (2020) 60:e93–7. doi: 10.1016/j.jpainsymman.2020.04.017
23. Bellizzi S, Pichierri G, Panu Napodano CM. Family-centered care for children during pandemics. Public Health. (2021) 198:e9–10. doi: 10.1016/j.puhe.2021.04.016
24. Cowpe Jebson E, Hanson B, Smith CH. What do parents of children with dysphagia think about their MDT? A qualitative study. BMJ Open. (2014) 4:e005934. doi: 10.1136/bmjopen-2014-005934
25. Wei W, Chen F, Wang Y. Effect of multidisciplinary team-style continuity of care and nutritional nursing on lung cancer: randomized study. Future Oncol. (2024) 20:3009–18. doi: 10.1080/14796694.2024.2407757
26. Bates DW, Levine D, Syrowatka A, Kuznetsova M, Craig KJT, Rui A, et al. The potential of artificial intelligence to improve patient safety: a scoping review. NPJ Digit Med. (2021) 4:54. doi: 10.1038/s41746-021-00423-6
27. Malycha J, Bacchi S, Redfern O. Artificial intelligence and clinical deterioration. Curr Opin Crit Care. (2022) 28:315–21. doi: 10.1097/MCC.0000000000000945
Keywords: lobar pneumonia, pediatrics, integrated nursing and medical management, pulmonary function, inflammatory markers, satisfaction
Citation: Hu Y, Guo Q, Liu X, Lv W and Liu L (2025) Integrated nursing and medical management improves outcomes in pediatric lobar pneumonia: a randomized controlled study. Front. Pediatr. 13:1612618. doi: 10.3389/fped.2025.1612618
Received: 16 April 2025; Accepted: 14 July 2025;
Published: 28 July 2025.
Edited by:
Orkun Tolunay, Univesity of Health Sciences Ankara Bilkent City Hospital, TürkiyeReviewed by:
Dilber Ademhan Tural, Hacettepe University, TürkiyeÜmmühan Çay, Cukurova University, Türkiye
Merve Kılıç Çil, Ministry of Health, Türkiye
Saliha Kanık-Yüksek, Ankara Yildirim Beyazit University, Türkiye
Copyright: © 2025 Hu, Guo, Liu, Lv and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linlin Liu, bGl1bGlubGluYWE4QDE2My5jb20=
 Yuxiao Hu1
Yuxiao Hu1 Linlin Liu
Linlin Liu