- 1Department of Neonatology, Children’s Medical Center, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 2Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Pediatric Surgery, Children’s Medical Center, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
Invasive fungal infections (IFIs) remain an important problem for hospitalized newborn infants receiving intensive care, given their substantial morbidity and mortality. Candida species (Candida spp.) are the major fungal pathogens, which cause the so-called invasive Candida infections (ICIs). Of these, Candida albicans is the most commonly isolated species, followed by Candida parapsilosis. Other identified Candida spp. include Candida glabrata, Candida tropicalis, Candida krusei, etc. However, an increasing shift in the epidemiology of ICIs worldwide has been described, non-albicans Candida (NAC) spp. ICIs pose a growing threat to neonates. Herein, we examine the epidemiology of Candida spp. infections, patterns of antifungal resistance, risk factors, prevention strategies, clinical outcomes, and treatment recommendations for ICIs in hospitalized newborn infants. This review aims to provide a thorough understanding of the current evidence on ICIs to better inform targeted prevention strategies and improved treatments to reduce neonatal morbidity and mortality.
Introduction
Invasive fungal infections (IFIs), primarily invasive Candida infections (ICIs), remain an important problem for hospitalized infants receiving newborn critical care, as they are associated with substantial morbidity and mortality (1). Very low birth weight (VLBW) infants are at high risk for ICIs because of their immature immune systems, frequent exposure to invasive procedures and medical devices, use of broad-spectrum antimicrobial medications, prolonged parenteral nutrition and hospitalization, and postnatal corticosteroid exposure (1, 2). For VLBW infants, the incidence of ICIs across different centers ranges from 2.6%–13.2% and is even higher in extremely low birth weight (ELBW, birth weight <1,000 g) infants, from 6.6%–26.0% (3). The mortality rate remains above 25%, and nearly half of the survivors may develop significant long-term adverse outcomes, particularly neurodevelopmental impairment (NDI) (4, 5). Of note, there is a different pattern of ICIs among susceptible newborn infants in low- and middle-income countries (LMICs) compared to those in high-income countries (HICs). In HICs, ICIs are most commonly reported in ELBW infants, but reports from neonatal units in LMICs indicate ICIs occur in infants beyond this specific group (6–8). Larger infants with congenital malformations requiring surgery are increasingly affected by ICIs as a result of prolonged use of broad-spectrum antibiotics and increased duration of NICU stay in LMICs (6, 9–11).
Despite improvements in neonatal intensive care, advanced life support measures, and aggressive antifungal treatment, ICIs remain a persistent challenge, necessitating a thorough understanding of the current evidence on ICIs to better inform targeted prevention strategies and improved treatments to reduce neonatal morbidity and mortality.
Common pathogens in newborn infants
A positive culture of fungal organisms from blood, urine, cerebrospinal fluid (CSF), or other sterile body fluids collected using techniques to minimize contamination with surface-colonizing organisms remains the standard for the diagnosis of IFIs, and can further identify the specific species (12, 13). IFIs in neonates are predominantly caused by Candida species (Candida spp.), which cause the so-called ICIs. Other fungal pathogens, including the yeast Malassezia and molds such as Aspergillus spp. and Zygomycetes, rarely cause nosocomial and mucocutaneous infections in newborn infants (14–17). In this review, we will focus on ICIs.
Candida is a genus comprising more than 200 fungal species, but only a minority are pathogenic and cause infections in humans (13, 18). Although Candida spp. are usually part of the normal flora and live as commensal organisms on the skin and mucous membranes, such as the oral cavity, and respiratory, gastrointestinal, and genitourinary tracts, they can transform into pathogenic forms under certain conditions (19). In particular, these yeasts have a higher binding affinity for mucosal surfaces than for the skin (18). Candida is present on the mucocutaneous surfaces of 84%–88% of individuals, including both hospitalized patients and healthy adults (20). More importantly, Candida spp. can adhere to and colonize the non-living surfaces of medical devices such as indwelling catheters, endotracheal tubes, and implants, posing important infectious risks (18). Candida albicans shows a greater adherence capability compared to other Candida spp., partially accounting for its higher prevalence and stronger correlation with infections (21). The transition of Candida spp. from a commensal relationship to a pathogenic state is driven by the expression of multiple virulence determinants. Specifically, these mechanisms include the formation of biofilms, the secretion of hydrolytic enzymes (e.g., proteinases, phospholipases, and hemolysins), the ability to adhere to host tissues and medical devices, and the transition to pseudohyphal growth (22, 23). In addition, the antifungal resistance profile has a significant impact on the virulence of Candida spp (19, 24). Mohammadi et al. found, for example, that higher minimum inhibitory concentrations (MICs) of fluconazole for Candida albicans are correlated with increased biofilm formation, elevated phospholipase production, and enhanced hemolysin activity (25). Similarly, Nakamura-Vasconcelos et al. revealed a positive correlation between fluconazole resistance in Candida glabrata and both enhanced adherence efficiency and increased biofilm formation (26).
In the NICU, nearly 75% of infants are colonized with Candida spp. by one month of age, either from maternal vertical transmission or horizontal nosocomial spread (27). Infants vaginally delivered are more likely to be colonized with Candida at birth than those born via cesarean section (C-section) (28), and further evidence supports that vaginal delivery is an important risk factor for neonatal Candida colonization (29, 30). This may be attributed to a significant increase in vaginal Candida colonization during pregnancy, particularly in the third trimester, with reported rates reaching up to 69.2% (31). Candida albicans is the most common Candida strain causing vaginal colonization in pregnant women, and it can be transmitted to their neonates (32, 33). According to Ali et al., Candida albicans accounted for 67.8% of maternal vaginal colonization and 77.7% of preterm infant colonization among all Candida colonization cases (33). Neonatal colonization and onset may predispose infants, particularly preterm infants, to the development of ICIs. Ali et al. reported that ICIs were identified in 22.2% of colonized preterm infants (33). Infants developing ICIs within the first postnatal week are more likely to have vertical transmission of Candida from the mother, with an associated higher mortality rate than those who develop the disease after the first week (34).
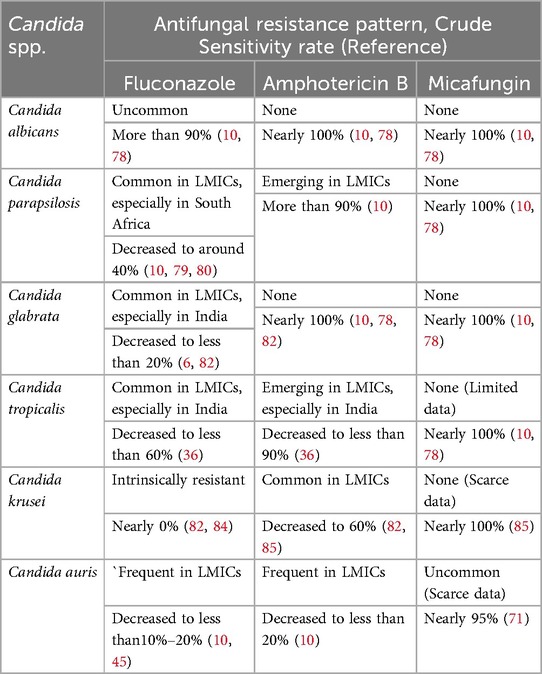
Historically, Candida albicans has been the organism most commonly isolated in ICIs, followed by Candida parapsilosis (35). Other Candida spp. identified include Candida glabrata, Candida tropicalis, Candida krusei, Candida auris, and Candida lusitaniae, et al (1, 11, 13). Consistently, Candida albicans is also the most frequently isolated organism responsible for ICIs in HICs (35). However, an increasing shift in the epidemiology of ICIs worldwide has been described, NAC spp. ICIs are emerging as a growing threat among NICUs in LMICs (10, 11, 36). Among the NAC spp., Candida parapsilosis, Candida glabrata, and Candida tropicalis are the most common isolates. A series of multicenter epidemiological studies present a high prevalence of Candida albicans in Northern Europe (37), the United States (38), England (39), Canada (40), and Saudi Arabiaand (41), accounting for more than 50% of Candida isolates. Candida parapsilosis is usually the most common NAC spp., with a prevalence of more than 35% in Southern Europe (37), Australia (42), and South Africa (43). The highest proportions (22.2%–44.4%) of Candida glabrata were reported in studies that were conducted in the central part of India (44), while Candida tropicalis is widespread in Eastern and Southern India (11, 36). Certainly, we should be aware that the distribution of species is very heterogeneous between regions and even between centers within a region. In recent years, the new pathogen Candida auris has shown an increasing incidence and is responsible for NICU outbreaks in South Africa, India, Colombia, and Venezuela, et al (45). The common pathogenic Candida strains in NICUs and their special profile are presented in Table 1.
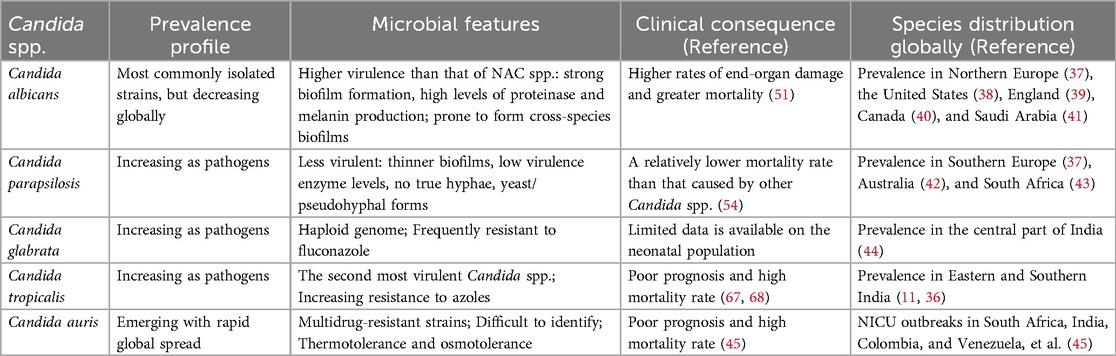
Table 1. Common pathogenic Candida strains of invasive Candida infections (ICIs) in neonatal intensive care units (NICUs).
The incidence of neonatal ICIs usually peaks during the second to third week of life. Oeser et al. reported the median age at diagnosis for different Candida spp. as follows: 11 days for Candida albicans, 18 days for Candida parapsilosis, 9 days for Candida glabrata, 20 days for Candida tropicalis, and 23 days for Candida lusitaniae (46). However, a prospective cohort study from Northern India (6), one of the LMICs where early-onset sepsis is more common (in contrast to HICs) (47, 48), shows that most of Candida spp. were isolated during the first week of life with proven fungal sepsis, much earlier than in HICs. It is also worth noting that Candida albicans is the predominant strain in vertical transmission. Candida parapsilosis ICI more commonly results from nosocomial transmission. It is the most common Candida spp. colonizing the hands of healthcare workers, with a prevalence nearing 60% (49, 50).
In general, Candida albicans is typically more virulent than NAC spp., causes greater end-organ damage, and has higher attributable mortality (51). Makled et al. report that Candida albicans exhibited the highest virulence, featuring strong biofilm formation and high levels of proteinase and melanin production (19). Biofilms can both initiate and prolong infections by serving as a protective niche that resists treatment, enabling cells to invade local tissues and create new foci of infection (21). Proteinases contribute to fungal pathogenesis by degrading host cell membrane proteins, promoting adhesion and tissue invasion, while also disrupting host defense mechanisms to evade antimicrobial responses (52). Melanin production helps Candida evade the immune system, reducing phagocyte effectiveness and altering responses to antifungals (53). In comparison, Candida parapsilosis is generally associated with a relatively lower mortality rate than other Candida spp. A prospective observational study of ICIs in NICUs reported mortality rates of 39.5% for Candida albicans and 11.1% for Candida parapsilosis (54). Candida parapsilosis is less virulent, such as thinner and less structured biofilms, lower levels of virulence-associated enzymes, and the absence of true hyphal formation, existing instead in either a yeast phase or in a pseudohyphal form (55, 56). In addition, this species tends to develop biofilms on central venous catheters (CVCs) and other medical implants. It grows rapidly in parenteral nutrition with high glucose and lipid concentrations. Parenteral nutrition provides a medium that promotes Candida parapsilosis biofilm formation (49, 56, 57), making it more challenging to eradicate (58). Another concern is that Candida spp., particularly Candida albicans, are known for their association with various bacterial spp. in the formation of cross-species biofilms (59). Research has shown that Candida albicans frequently co-occurs in biofilms with a variety of bacteria, including Staphylococcus species, Pseudomonas aeruginosa, Enterococcus faecalis, etc. In the neonatal population, co-infection with Candida albicans and Staphylococcus aureus is frequently observed (21). This multi-species infection form of Candida albicans may be another important factor contributing to significant morbidity and mortality.
Candida glabrata, unlike Candida albicans and many other NAC spp., has a haploid genome, a key distinguishing genetic feature (60). It is frequently resistant to fluconazole, primarily due to the overexpression of efflux pump genes, particularly CDR1, mediated by the transcription factor PDR1, as well as mutations or upregulation of the ERG11 gene, which encodes the target of azoles (61, 62). This resistance mechanism confers a competitive advantage to Candida glabrata in clinical settings where fluconazole is widely used, either for prophylaxis or treatment (57). In a clinical study, isolates of Candida glabrata were found more frequently in preterm infants with a higher gestational age (Candida glabrata: 30 weeks, Candida albicans: 26 weeks, Candida parapsilosis: 27 weeks) and birth weight (Candida glabrata: 1,442 g, Candida albicans: 931 g, Candida parapsilosis: 965 g) compared to those infected with other Candida spp (63).
Candida tropicalis is increasingly becoming an important pathogen in NICUs (64). In a recent systematic review and meta-analysis of neonatal candidiasis, investigators found that among a total of 402 Candida isolates, 9.5% were identified as Candida tropicalis, ranking third after Candida albicans and Candida parapsilosis (41). Genetically, this species is most similar to Candida albicans (65). It is widely regarded as the second most virulent Candida spp., surpassed only by Candida albicans (66). Among the NAC spp., Candida tropicalis generally has a high mortality and a poor prognosis and is classified as a high-priority pathogenic fungus by the WHO (67, 68). Another worrying feature of Candida tropicalis is the increasing rate of resistance to azoles. There are reports of resistance rates of around 15%–20%, compared to the previously observed rate of around 7% (68). However, given the limited availability of high-quality neonatal-specific data, further research is urgently needed to better understand the epidemiology, host-pathogen interactions, and resistance patterns of Candida tropicalis in neonates to better define the clinical impact in this vulnerable population.
Candida auris is an emerging, multidrug-resistant species that poses a significant and growing global public health threat due to its rapid worldwide spread (69, 70). It is frequently resistant to fluconazole, with variable resistance patterns to amphotericin B and echinocandins. Specifically, in general, 60%–90% of Candida auris strains are resistant to fluconazole, 10%–30% have high MICs for amphotericin B, and up to 5% are resistant to echinocandins (71). A more recent systematic review of 24 studies involving 476 neonates revealed a higher prevalence of antifungal resistance: 97% of cases were resistant to fluconazole, and 67% to amphotericin B (45). Candida auris has an unprecedented ability to spread rapidly in healthcare settings, not only through direct patient-to-patient transmission but also via contaminated medical devices such as thermometers. This strain can persist on environmental surfaces and equipment for long periods due to the formation of “dry” biofilms, rendering it thermotolerant and osmotolerant (72). Many commonly used hospital disinfectants are ineffective against it. Although initially identified in 2009 as a rare pathogen (73), Candida auris has emerged as a frequent cause of outbreaks in NICUs in many countries, primarily in LMICs (13, 45). Candida auris ICI has a mortality as high as 42% (45). Another concern is that Candida auris is challenging to identify and may be misidentified as Candida haemulonii, another emerging multidrug-resistant fungus. Such difficulties complicate its management and heighten the health threat (45, 74).
Antifungal resistance patterns of Candida spp
As is known, there is considerable variability among different geographic regions, healthcare centers, and even individual units of Candida spp. that cause ICIs (75), and each Candida spp. poses a unique challenge to antifungal susceptibility profiles. The resistance status of Candida spp. is determined on the basis of clinical breakpoints (CBPs). CBPs are determined taking into account pharmacokinetic/pharmacodynamic parameters, relationships between clinical outcomes and MICs, and distributions of MIC values in wild-type fungal isolates (76). The Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) methods for susceptibility testing of yeasts are standardized and reproducible methods for susceptibility testing of fungi (77). If no MIC breakpoint is established, the epidemiological cutoff value (ECV) can be used (Table 2), which is based on an examination of the distribution of MIC values within a species.
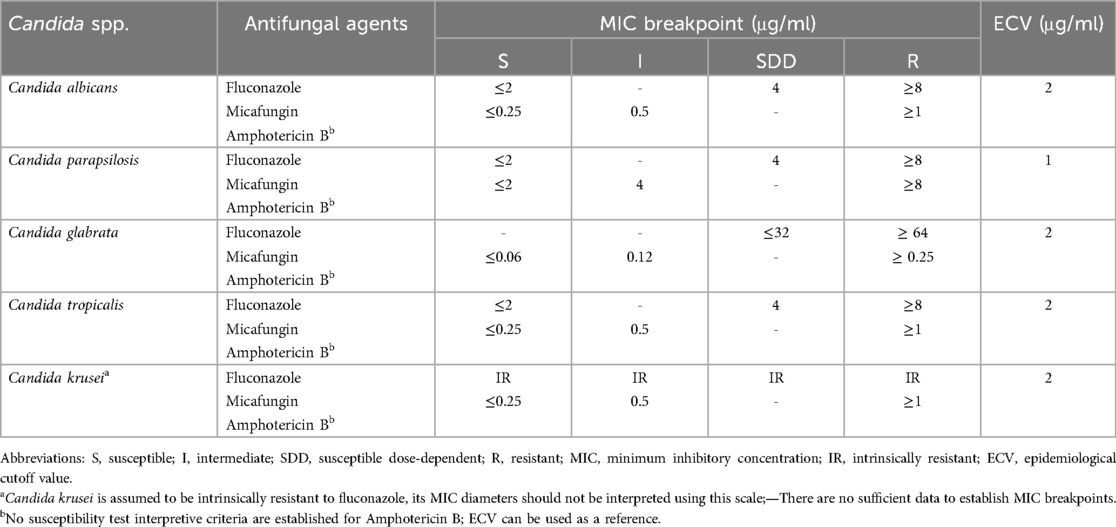
Table 2. Clinical breakpoints for the most commonly used antifungal agents against common Candida spp. in neonates.
In general, antifungal-resistant Candida spp. remain uncommon in HICs. However, over the past decade, an increasing proportion of resistant Candida spp., particularly Candida parapsilosis, Candida krusei, and Candida auris, have emerged in LMICs. NeoOBS data from LMICs reported that among Candida spp., the overall resistance profile showed that 40% were resistant to fluconazole, 18% were resistant to amphotericin B, but there was no resistance to micafungin (10). Even more, a recent study from Eastern India revealed alarming resistance rates of fluconazole and amphotericin B among Candida spp., particularly NAC spp., reaching up to 51% and 35%, respectively (11). Herein, we present the 3 most commonly used drugs against the most common Candida spp. in neonates (Table 3).
At present, resistance to antifungal agents in Candida albicans is still uncommon, although individual isolates may not conform to this general pattern. In a secondary analysis of ELBW infants with ICIs from the NICHD-Neonatal Research Network (NRN) study of HICs, 308 isolates were obtained from 110 infants, of which only two Candida albicans (2/184, susceptibility rate 98.9%) were resistant to fluconazole (78). The NeoOBS substudy of invasive candidiasis in LMICs found that 90.5% (38/42) of Candida albicans isolates were sensitive to fluconazole (10). The antifungal sensitivity to amphotericin B and micafungin was 100% (10, 78).
Resistance of Candida parapsilosis to antifungals is gradually increasing in LMICs. The NICHD-NRN data from HICs showed that none of the 107 Candida parapsilosis isolates were resistant to fluconazole, amphotericin B, or micafungin (78). The NeoOBS data from LMICs reported that the susceptibility rate was only 40.6% (13/32) for fluconazole, 93.1% (27/29) for amphotericin B, but also 100% for micafungin (10). Other South Africa series reported similar data; fluconazole resistance rates were 53%–55% (79, 80).
Candida glabrata is more susceptible to developing fluconazole resistance than other Candida spp (81). It is thought that azole resistance has increased so much in Candida glabrata isolates that it is difficult to rely on these agents for therapy in the absence of susceptibility testing (75). Data from HICs with a low rate indicated that one of the Candida glabrata isolates (1/9) was resistant to fluconazole, but no isolates were resistant to amphotericin B or micafungin (78). In a study from India, the susceptibility rate of Candida glabrata to fluconazole was only 26.3% (5/19), but susceptibility to amphotericin B was 100% (19/19) (82). In another study from India, the fluconazole resistance rate was found to be 87.5% (6). In a previous observational study conducted by our team, we found that the overall susceptibility rate of this isolate to fluconazole decreased significantly (from 85%–40%) after prophylactic fluconazole, although there were no resistant isolates (1). The main reason for this could be related to the frequent occurrence of Candida glabrata. The overall susceptibility rate of Candida spp. to amphotericin B was consistently 100%.
Candida tropicalis, which is prevalent in India, is more often resistant to fluconazole and/or amphotericin B. The resistance rates of this species to fluconazole and amphotericin B were as high as 44% and 14.2%, respectively (36). The susceptibility to micafungin was also 100%, but only limited data were available (10, 78).
Candida krusei, a potentially multidrug-resistant opportunistic species, is emerging in LMICs. It is intrinsically resistant to fluconazole and can also rapidly acquire resistance to other antifungal agents (82, 83). Among the 1,075 Candida krusei isolates tested, the crude percentage of resistance to fluconazole was 96.6%, according to epidemiological data (84). Some data from India indicated that the susceptibility rates to amphotericin B have decreased to 60%–86% (82, 85), and scarce data found no resistance to micafungin (85).
Candida auris, a rapidly emerging multidrug-resistant causative pathogen, usually causes outbreaks in LMICs. NeoOBS data from LMICs showed high resistance to fluconazole (15/17, 88%) and amphotericin B (11/13, 85%) in Candida auris isolates, but no resistance to micafungin; however, many isolates were not tested (10).
Taken together, in neonates, Candida spp. especially NAC spp. are gradually developing resistance to antifungal agents, particularly fluconazole (the most commonly used antifungal agent in neonates), followed by amphotericin B. However, current studies are predominantly retrospective and thus possess inherent limitations; susceptibility testing for Candida spp. remains sparse and restricted, leading to an overall low level of evidence. These data only provide an approximate overview of the antifungal resistance patterns of the different Candida spp. in hospitalized newborn infants. There is an urgent need for large-scale, prospective studies to establish a robust framework for susceptibility testing of Candida spp. in neonatal units. This will allow a better understanding of these patterns and help in formulating strategies for the use of appropriate antifungal agents against ICIs in neonates.
Potential risk factors
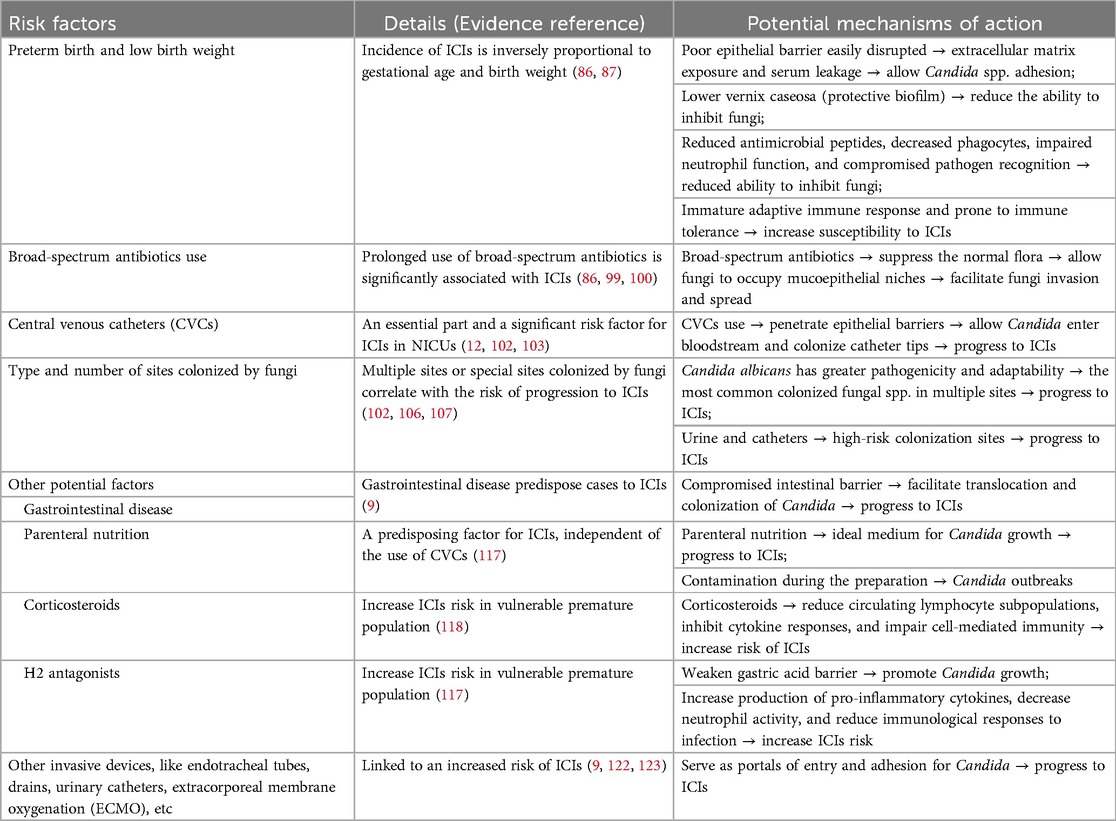
Hospitalized newborn infants are at high risk for ICIs due to host and environmental factors (Figure 1, Table 4).
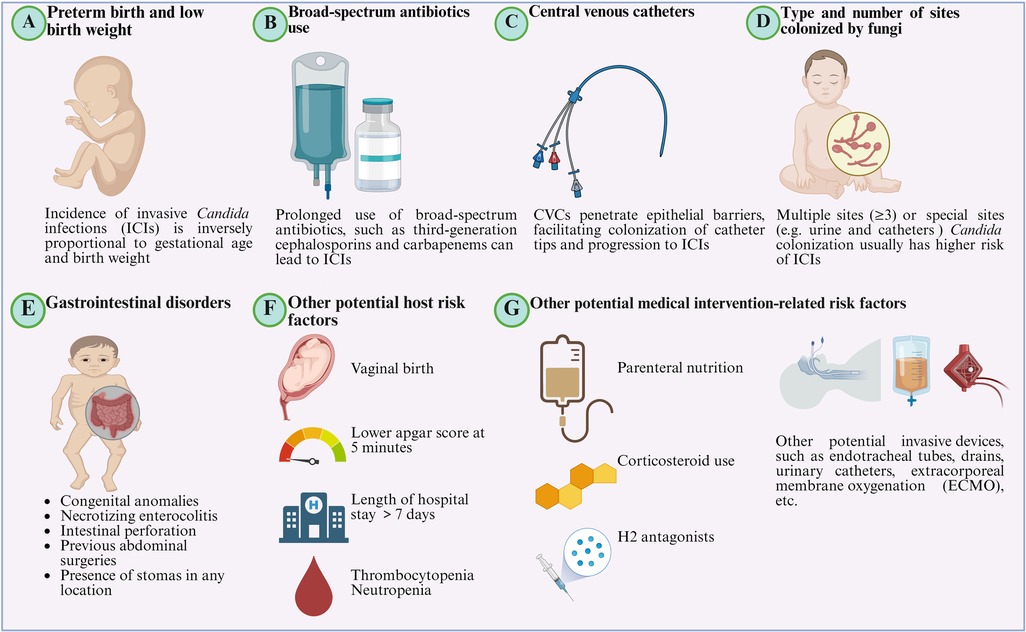
Figure 1. Potential risk factors for the incidence of invasive Candida infections (ICIs). (A) Preterm birth and low birth weight. Gestational age and birth weight are key risk factors for ICIs, with incidence inversely related to both. (B) Broad-spectrum antibiotics use. Prolonged use of broad-spectrum antibiotics, especially third-generation cephalosporins (TGCs) and carbapenems, suppresses normal flora, allowing fungi to colonize mucosal niches and facilitating subsequent invasion and spread. (C) Central Venous Catheters (CVCs). CVCs penetrate epithelial barriers, facilitating colonization of catheter tips and progression to ICIs. (D) Type and number of sites colonized by fungi. Multiple sites (≥3) or special sites (e.g., urine, catheters) colonized by Candida spp. are associated with a higher risk of ICIs. (E) Gastrointestinal disorders. Gastrointestinal disorders, including congenital anomalies, as well as necrotizing enterocolitis (NEC), intestinal perforation, previous abdominal surgeries, and the presence of stomas in any location, predispose cases to ICIs. (F) Other potential risk factors. Other potential host risk factors include vaginal birth, lower Apgar score at 5 min, length of NICU stay >7 days, thrombocytopenia, neutropenia, etc. (G) Other potential medical intervention-related risk factors. Related risk factors also include parenteral nutrition, corticosteroid use, H2 antagonists, and other potential invasive devices, such as endotracheal tubes, drains, urinary catheters, extracorporeal membrane oxygenation (ECMO), etc. (Created in BioRender.com).
Preterm birth and low birth weight
Gestational age and birth weight are the most important risk factors for the development of ICIs (86, 87). A large multicenter cohort study of 4,579 ELBW infants revealed significant associations between birth weight/gestational age and the risk of ICIs. Infants with a birth weight of 400–750 g or gestational age of 22–25 weeks had a 3–5 -fold increased risk compared to those weighing 751–1,000 g or 26–28 weeks (11%–10% vs. 3%–2%, 95% CI 2.47–4.19) (86). A large retrospective study of 530,162 infants weighing greater than 1,500 g from 305 NICUs reported a 0.06% ICI incidence (87), indicating that ICIs are rarely observed in neonates born after 32 weeks of gestation and/or with a birth weight greater than 1,500 g, especially in HICs.
The epithelial barrier serves as the first line of defense against exogenous pathogens. In term infants, this barrier is well developed, whereas, in most immature newborns, such as those born preterm or with low birth weight, it is poorly developed and can be easily disrupted (88, 89). Such disruption can lead to exposure of the extracellular matrix and serum leakage into the mucosa. This creates a favorable environment for Candida spp. to establish adhesion as pathogen-derived adhesins form a bridge to the host epithelial surface (90). Furthermore, preterm or low birth weight infants usually have a lower amount of vernix caseosa, a protective biofilm that forms in the hair follicles during the last trimester of pregnancy (89, 91). Vernix caseosa has good antimicrobial properties, is enriched with lysozyme, lactoferrin, and antimicrobial peptides, and has been shown to inhibit common bacterial and fungal pathogens (89, 92). Preterm infants have a range of differences in innate immunity, including reduced levels of antimicrobial peptides, decreased numbers of phagocytes (neutrophils and monocytes), altered neutrophil function, and compromised pathogen recognition due to immature functionality of pattern recognition receptors (PRRs) (91). These immune differences in preterm newborns increase the risk of infection, in particular for ICIs. The adaptive immune response in preterm infants remains immature and is prone to immune tolerance. In neonates, T cells are primarily naive and exhibit limited response to microbial antigens due to a lack of prior exposure during gestation (93). Additionally, neonatal CD4T cells have an intrinsic reduced ability to differentiate into Th17 cells, which play a critical role in controlling Candida proliferation (94). In term infants, antigen-presenting cells (APCs) produce elevated levels of Th17-polarizing cytokines, such as IL-1β and IL-23 (95), which are essential for driving the differentiation of naive CD4T cells into Th17 cells. In contrast, dendritic cells and monocytes from preterm infants, especially those born before 29 weeks of gestation (96, 97), have significantly reduced production of these cytokines and impaired antigen presentation, which further increases their susceptibility to ICIs.
Of particular note, in LMICs, especially in some regions of India, larger infants born after 32 weeks' gestation, with a birth weight ≥1,500 g, or even term infants, account for a larger proportion of ICIs (6, 11). In a tertiary neonatal unit of these regions, a large number of higher birth weight infants are usually admitted after being referred from peripheral units for surgical or cardiac morbidities. Most of these infants have a history of prior exposure to broad-spectrum antibiotics.
Broad-spectrum antibiotics use
In NICUs, broad-spectrum antibiotics such as third-generation cephalosporins (TGCs) and carbapenems are frequently used and sometimes for long durations. Antibiotic exposure poses a risk for ICIs, especially those caused by Candida spp., as they suppress the normal flora, thereby allowing fungi to occupy mucoepithelial niches and facilitating subsequent invasion and spread (98). A multicenter study involving 3,702 ELBW infants found that the average duration of TGC use was significantly associated with the occurrence of ICIs, with a correlation coefficient of 0.67 (P = 0.017) (99). Similarly, another study of 4,579 ELBW infants revealed that the use of TGCs on hospital day 3 significantly increased the risk of subsequent ICIs compared to other antibiotics [15.3% vs. 5.6%, odds ratio (OR) 1.77, 95% CI 1.31–2.38] (86). Hou et al. pointed out that a 10% increase in antibiotic use leads to a 71% increase in the risk of ICIs (OR 1.71, 95% CI 1.41–2.08). Each additional day of antibiotic exposure increases the risk of IFIs by 13% (OR 1.13, 95% CI 1.06–1.20). The use of TGCs and carbapenems was associated with a 17% (95% CI 1.04–1.33) or 18% (95% CI 1.06–1.30) increased risk of ICIs, respectively, for each additional day of exposure (100). Conversely, Aliaga et al. reported that a 10% reduction in the use of broad-spectrum antibiotics was associated with a 3%–7% decrease in ICI episodes for VLBW infants (3).
Central venous catheters (CVCs)
CVCs are an essential part of the care of VLBW infants during their stay in NICUs. The use of CVCs is a well-documented and significant risk factor for ICIs in neonates. CVCs penetrate epithelial barriers, allowing Candida to enter the bloodstream and preferentially colonize the catheter tips, which is often associated with the normal resident skin flora at the insertion site (101).
Colonization with Candida spp. is an essential first step in the pathogenesis of ICIs, and CVC colonization represents a significant risk factor for progression to invasive disease. A previous study of 689 VLBW infants in the NICU found that infants with a Candida-colonized CVC had a nearly tenfold higher risk of progression to ICI than infants without a colonized CVC (OR 10.81, 95% CI 1.45–8.10) (102). Furthermore, CVCs facilitate the formation of Candida biofilms, which play an important role in the development of ICIs in neonates. In addition, a study reported that the risk of ICIs increases with each additional day that CVCs remain in place, with an OR of 1.06 per day of use (95% CI 1.02–1.10) (103). It is particularly crucial to remove CVCs at the earliest opportunity during ICIs when the CVC is suspected to be the source and its removal is feasible (12). The potential for drug-resistant biofilm formation highlights the importance of the timely removal of catheters, especially in ELBW infants with ICIs. Delayed removal has been associated with prolonged ICIs and increased risk of end-organ involvement, NDI, and higher mortality (86, 104). For example, Benjamin et al. observed that mortality and NDI rates were significantly higher in infants in whom catheter removal or replacement was delayed (more than one day after initiation of antifungal treatment), with an OR of 2.69 (95% CI 1.25–5.79), compared with those timely removal (86).
Type and number of sites colonized by fungi
Some studies have shown that the type and number of sites colonized by fungi correlate with the risk of progression to ICIs in neonates. Mahieu et al. found no cases of ICIs in neonates colonized exclusively on the skin. However, in neonates with gastrointestinal colonization, the prevalence of ICIs was 16.6% and increased to 41.7% when both skin and gastrointestinal sites were colonized (105). A cohort of 201 VLBW infants colonized with Candida spp. identified colonization at multiple sites (≥3) as an independent risk factor for the development of ICIs during hospitalization (OR 6.15, 95% CI 2.40–7.69) (102). Subsequently, Manzoni et al. further reported a threefold increase in ICI incidence in VLBW infants colonized at more than three sites (106). One possible explanation for this finding is that Candida albicans is the most common fungal spp. in multiple sites colonized by fungi and has greater pathogenicity and adaptability (107). In addition, Manzoni et al. revealed that high-risk colonization sites such as urine and catheters were associated with a fourfold higher risk of ICIs than low-risk sites like skin, nasopharyngeal secretions, and gastric aspirates (106).
Other potential risk factors
Gastrointestinal disorders, including congenital anomalies such as gastroschisis, omphalocele, and duodenal or ileocolic atresia/stenosis, as well as necrotizing enterocolitis (NEC), intestinal perforation, previous abdominal surgeries, and the presence of stomas in any location, predispose cases to ICIs (9). This predisposition arises from a compromised intestinal barrier that facilitates the translocation of Candida spp. colonizing the gastrointestinal tract (108). Multiple studies have demonstrated a close correlation between gastrointestinal diseases and ICIs in neonates (109–111). Other potential host risk factors include prior Candida colonization (112), vaginal birth (40), lower Apgar score at 5 min (40), length of NICU stay >7 days (113), thrombocytopenia, platelet counts <50,000/mm3 (87), neutropenia, and neutrophil count <1,500/mm3 (114).
Parenteral nutrition is necessary for supporting the nutritional needs of VLBW infants in NICUs. Parenteral nutrition infusions, especially those with high glucose content and rich fat emulsions, provide an ideal medium for Candida growth and promote the formation of drug-resistant biofilms on catheters (49, 115). Moreover, contamination during the preparation of parenteral nutrition solutions continues to be a potential contributing factor to Candida outbreaks in NICUs (116). It is also important to know that parenteral nutrition serves as a predisposing factor for ICIs, independent of the use of CVCs (117). Corticosteroids are commonly used in very premature infants to reduce the need for ventilatory support and its duration, as well as to reduce pulmonary morbidity (118). A recent survey of 397 NICUs across Europe found that the majority of these units administer corticosteroids in the second or third week of life. This practice aims to facilitate extubation and/or prevent bronchopulmonary dysplasia (BPD) in high-risk infants, regardless of the type of ventilatory support (119). It is well documented that corticosteroids can reduce circulating lymphocyte subpopulations, inhibit cytokine responses, and impair cell-mediated immunity in preterm infants, and thus increase the risk of ICIs in this vulnerable population (118). H2 antagonists act by inhibiting gastric acid secretion, resulting in an elevated stomach pH. This weakened gastric acid barrier promotes the growth of Gram-negative bacteria and Candida spp., which can then spread through the gastrointestinal tract (120). Manzoni et al. found that each additional day of exposure to gastric acid inhibitors was associated with a 4.5% (95% CI 1.02–1.07) and 6.3% (95% CI 1.03–1.10) increased risk of fungal colonization and fungal infection, respectively, in preterm infants (120). In addition, H2 antagonists can increase the production of pro-inflammatory cytokines, decrease neutrophil activity, and reduce immunological responses to infection (121). A prospective cohort study including 2,847 infants across multiple NICUs found that the administration of H2 antagonists was associated with a 2.44-fold increased risk (95% CI 1.11–5.29) of developing candidemia in neonates. Other potential invasive devices, like endotracheal tubes, drains, and urinary catheters, similar to CVCs, serve as portals of entry and adhesion for Candida spp. and may contribute to nosocomial transmission (9). In an epidemiologic surveillance study, compared to CVCs and urinary catheters, endotracheal tubes were found to be linked to the highest risk [risk ratio (RR) 22.9, 95% CI 5.6–93.8] of developing nosocomial infection, including those caused by Candida spp (122). This study also found that two catheters increased the relative risk for nosocomial infections by 2.6 times (95% CI 1.3–4.9), while the use of three catheters increased it by 3.6 times (95% CI 1.9–7.1). In a similar pattern, extracorporeal membrane oxygenation (ECMO) procedures may also contribute to the risk of acquired fungal infection, and Candida spp. was the most common pathogen. A greater number of ECMO cannula placement procedures were independently linked to an increased risk of acquired infection, including fungal infection during ECMO treatment [hazard ratio (HR) 2.13, 95% CI 1.22, 3.72] (123).
Prevention strategies
Given the serious consequences of ICIs for survival and NDI in very premature and VLBW infants, proactive prevention emerges as a critical strategy (Table 5).
Antifungal prophylaxis
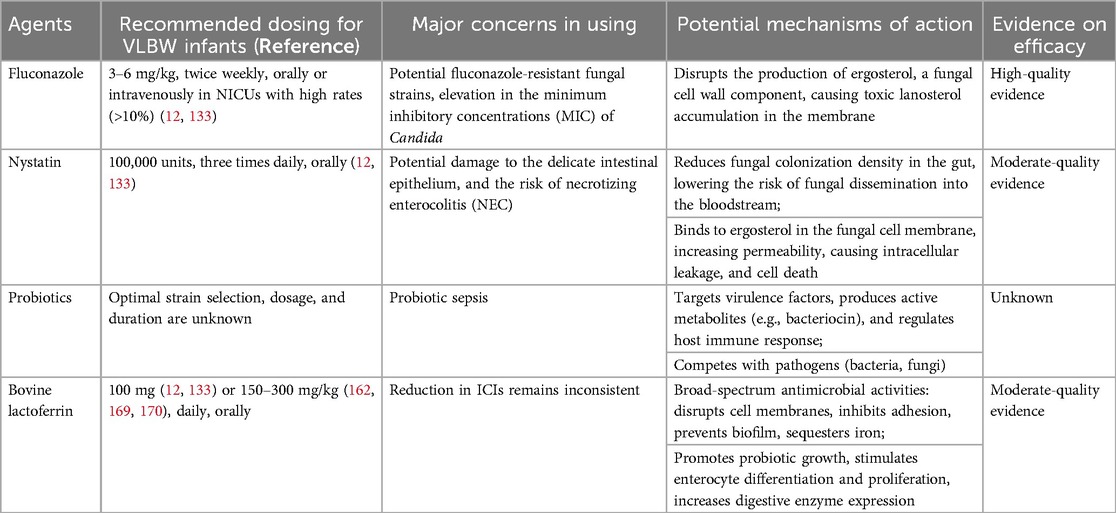
Antifungal prophylaxis has been proven to be effective in reducing fungal colonization and the incidence of ICIs, thereby reducing associated mortality and long-term disability. At birth, most VLBW infants have either no or minimal fungal colonization, creating a critical window for antifungal interventions. These drugs can effectively prevent initial colonization or suppress the growth and spread of yeast in infants already colonized. It is worth highlighting that without antifungal prophylaxis, Candida colonization can affect up to 60% of VLBW infants by their second to third week of life (124).
Fluconazole prophylaxis
Among antifungal agents, fluconazole (oral or intravenous), a member of the azole class, has emerged as the most commonly used prophylactic option for high-risk neonates. Fluconazole acts by selectively inhibiting cytochrome P450 enzymes, particularly 14α-demethylase, a critical enzyme in the synthesis of ergosterol, which is essential for the integrity of the fungal cell membrane (125). When ergosterol production is disrupted, toxic precursors such as lanosterol accumulate in the membrane and impair its structure and function. This leads to damage to the fungal cells and effectively suppresses the growth and reproduction of fungi.
Numerous RCTs and retrospective studies have shown fluconazole to be safe and effective in reducing the incidence of ICIs in VLBW or ELBW infants, although the effect on mortality reduction in these studies is inconsistent (1, 126–130). An earlier meta-analysis of 578 VLBW infants in the United States comparing fluconazole prophylaxis with placebo showed that fluconazole significantly reduced the likelihood of Candida colonization (OR 0.28, 95% CI 0.18–0.41) and ICIs (OR 0.20, 95% CI 0.08–0.51). However, there was no significant reduction in mortality (OR 0.68, 95% CI 0.40–1.13) in those receiving fluconazole (131). A recent meta-analysis of 1,635 VLBW infants found that fluconazole prophylaxis significantly reduced the rate of fungal colonization (RR 0.32, 95% CI 0.24–0.41), the incidence of ICIs (RR 0.37, 95% CI 0.21–0.65) and ICI-related mortality (RR 0.17, 95% CI 0.05–0.64) compared with the control group (132). The Infectious Diseases Society of America (IDSA) and ESCMID guidelines recommend 6 weeks of prophylactic fluconazole use (3–6 mg/kg, twice weekly, either orally or intravenously) for VLBW infants in NICUs where the incidence of ICIs exceeds 10% (12, 133). The decision between 3 mg/kg and 6 mg/kg fluconazole prophylaxis administered twice weekly should be guided by local MIC data and resistance patterns for ICIs (134).
A major concern is that fluconazole prophylaxis may promote the development of fluconazole-resistant fungal strains. Although the emergence of resistance is rare and remains controversial, sporadic reports indicate an increase in fluconazole-resistant strains and a slight elevation in the MICs of Candida among exposed preterm infants (14, 135–137). This finding was also corroborated in our previous study (1). There is evidence that Candida spp. may cooperate after exposure to a low dose of fluconazole, leading to a gradual increase in MICs and an expansion of the azole resistance spectrum (107, 138).
Oral nystatin
Nystatin is the most frequently used oral, non-absorbable agent for the prophylaxis of Candida infections (139). Non-absorbable antifungals, which are not absorbed systemically, aim to reduce the density of fungal colonization in the gastrointestinal tract and thus reduce the risk of fungal dissemination from the intestine into the bloodstream (133). In addition, nystatin binds to ergosterol in the fungal cell membrane, increasing membrane permeability and leading to leakage of intracellular components and eventual cell death (13). Although it has not been studied as extensively as fluconazole prophylaxis, several studies have shown that nystatin is also effective in reducing Candida colonization and ICIs (138, 140, 141). However, data on mortality associated with prophylactic nystatin remain limited.
In an RCT of 278 VLBW infants, prophylactic administration of nystatin significantly reduced the incidence of Candida colonization (11.7% vs. 42.9%) and ICIs (4.3% vs. 16.5%) compared to the control group, but no significant difference was found between the two groups in terms of deaths due to ICIs (1.1% vs. 3.3%) (138). A Cochrane meta-analysis of 1,800 VLBW infants evaluated the efficacy of oral non-absorbable antifungal prophylaxis, primarily nystatin, compared to placebo. The analysis showed a significant reduction in the incidence of ICIs (RR 0.20, 95% CI 0.14–0.27), but also no significant effect on mortality (RR 0.87, 95% CI 0.72–1.05) (142). Similarly, a recent systematic review and meta-analysis involving 1,750 VLBW infants showed that oral nystatin was associated with a significant reduction in Candida colonization (RR 0.34, 95% CI 0.24–0.48) and ICIs (RR 0.15, 95% CI 0.12–0.19) compared to the control group, with no significant difference in mortality observed between the two groups either (RR 0.87, 95% CI 0.64–1.18) (143).
A potential concern with the use of nystatin is the risk of inadvertent damage to the delicate intestinal epithelium of preterm infants, which may contribute to the development of NEC (133). An RCT of 80 VLBW infants compared the efficacy of oral nystatin with fluconazole prophylaxis (144). The study was terminated prematurely due to the unfavorable prognosis associated with nystatin. Despite being underpowered, the findings indicated a significantly higher mortality rate in the nystatin group compared to the fluconazole group (7.5% vs. 0.0%, six deaths in the nystatin group, four of which were related to NEC). Furthermore, oral administration of nystatin has its limitations due to the frequent dosing regimen and lack of suitability for infants with gastrointestinal problems, a condition commonly observed in very premature and VLBW infants. The IDSA recommends six weeks of treatment with oral nystatin at a dosage of 100,000 units administered three times daily as an alternative when fluconazole is not available or contraindicated due to resistance (12), which is consistent with the moderate recommendation of ESCMID (133).
Probiotics
Probiotics are live bacterial organisms that, when administered in sufficient quantities, provide health benefits to the host through the modulation of the gut microbiota (145). The potential benefits of probiotic supplementation for VLBW infants have been an ongoing focus of numerous studies in the past two decades. Probiotics have been shown to have an antifungal effect by targeting the virulence factor of fungi, producing active metabolites, particularly bacteriocin, regulating the host immune response, etc (146). The gastrointestinal commensal microbiota could also compete with pathogenic organisms such as bacteria or fungi (146, 147). Meanwhile, the gastrointestinal tract is an important site for Candida colonization and a mucosal surface for translocation. Broad-spectrum antibiotics may lead to dysbiosis and increase the risk of ICIs; it follows that the reintroduction of commensal microbiota may reduce this risk; however, this has not been confirmed for hospitalized newborns in rigorous clinical trials.
In an RCT of 62 extremely preterm infants (born at <290/7 weeks of gestation and weighing ≤1,000 g), Alshaikh et al. found that multi-strain probiotics significantly increased fecal levels of Bifidobacterium and Lactobacillus, while markedly reducing the abundance of Candida in the stool (148). Two prospective, randomized comparative studies were conducted in VLBW infants. In both cases, prophylactic supplementation of Lactobacillus reuteri and Saccharomyces boulardii showed similar efficacy to nystatin prophylaxis in reducing fungal colonization and ICIs (149, 150). However, a meta-analysis of eight RCTs found no significant benefit of probiotics in preventing ICIs (RR 0.89, 95% CI 0.44–1.78). Another meta-analysis of seven RCTs, which included 1,371 preterm infants administered with strains of Bifidobacterium, Lactobacillus, or a combination of both, demonstrated a reduced incidence of Candida colonization compared to infants who did not receive probiotics (RR 0.43, 95% CI 0.27–0.68). However, there was no statistically significant impact observed on the incidence of ICIs (RR 0.88, 95% CI 0.44–1.78) (151). Even more, in a multicenter-matched cohort study of 2,178 preterm infants from 392 NICUs, infants receiving probiotics had an elevated risk of ICIs relative to those not receiving probiotics (OR 2.23, 95% CI 1.29–3.85). However, the absolute difference in the incidence of Candida infection was relatively minor (1.0% in the probiotic group vs. 0.4% in the non-probiotic group) (152). To sum up, conflicting data presents its efficacy in preterm infants, which may be based on the massive heterogeneity of study protocols, such as the strain selection, dosage, and duration of administration, needing further and ongoing investigation.
Another concern regarding the administration of probiotics in preterm infants is the potential risk of triggering sepsis, also termed “probiotic sepsis”. Probiotic sepsis is severe and sometimes life-threatening. This complication is defined as positive cultures from blood or CSF that isolate the strain of the administered probiotics, along with clinical signs of infection (153). Although rare, several reports have documented individual cases or case series of sepsis associated with probiotic supplementation in preterm infants (154–156). The efficacy and safety of probiotic strains administered are therefore all potential barriers to the use of prophylactic probiotics in preterm infants. Of particular importance is the American Academy of Pediatrics (AAP) statement opposing the routine use of probiotics, as well as the regulatory restrictions imposed by the US Food and Drug Administration (FDA) on their use in preterm infants, especially ELBW infants, the highest risk population for ICIs.
Bovine lactoferrin (BLfcin)
Lactoferrin (Lfcin), a mammalian glycoprotein found in milk and belonging to the transferrin family, is an important bioactive component of whey protein and accounts for about 10%–20% of the total protein content of milk (157). It plays a key role in the innate immune response of mammals to infections. Multiple studies have demonstrated its broad spectrum of antimicrobial properties, including mechanisms such as disruption of microbial cell membranes, inhibition of microbial adhesion to host cells, prevention of biofilm formation, and sequestration of iron (158, 159). In addition, Lfcin facilitates the growth of probiotic bacteria, stimulates differentiation and proliferation of enterocytes, increases the expression of digestive enzymes, and shows direct immunomodulatory and anti-inflammatory effects in the gut (160). Very premature infants often receive little or no milk in the early postnatal period, resulting in a low intake of Lfcin. This can be exacerbated by delays in the introduction of enteral feeding. To address this immunodeficiency, enteral supplementation of bovine Lfcin (bLfcin) has been proposed as a simple strategy (161). BLfcin shares approximately 70% homology to human Lfcin (hLfcin) but has a higher antimicrobial activity due to the different three-dimensional structures (162, 163). Specifically, bLfcin in solution forms a β-sheet conformation containing a group of aligned hydrophobic residues that are well suited for interactions with biological membranes, whereas hLfcin in solution forms a coiled structure that lacks these aligned residues and therefore has weaker interactions with target cells (164–166).
A secondary analysis of a multicenter RCT involving 472 preterm infants in Italy found a significantly lower incidence of ICIs in VLBW infants receiving bLfcin alone (0.7%) or in combination with Lactobacillus rhamnosus GG (2.0%) compared to the placebo group (7.7%). However, the fungal colonization rates were comparable across the three groups (17.6%, 16.6%, and 18.5%, respectively) (167). Nonetheless, a UK-based RCT involving 2,203 infants born before 32 weeks of gestation found no significant difference in the incidence of late-onset sepsis (LOS), including ICIs, between the infants receiving bLfcin supplementation and the placebo group (29% vs. 31%, RR 0.95, 95% CI 0.86–1.04). Of particular note, the study found a low prevalence of invasive candidiasis with a total of only five episodes (0.3% vs. 0.2%), which aligns with UK population surveillance data. In a systematic review of ten RCTs enrolling 3,679 preterm infants (<37 weeks gestation), supplementation with Lfcin, either alone or in combination with probiotics, significantly reduced the incidence of all types of LOS (RR 0.56, 95% CI 0.36–0.86). However, for fungal sepsis in particular, the reduction did not reach statistical significance (RR 0.27, 95% CI 0.08–1.00), especially in very premature infants (RR 0.30, 95% CI 0.04–2.23) (168). In contrast, in another systematic review of 12 RCTs including 5,452 preterm infants, Pammi et al. found that Lfcin supplementation significantly reduced the incidence of LOS (RR 0.80, 95% CI 0.72–0.89) compared to placebo. The same effect was observed for fungal sepsis, both with Lfcin alone (RR 0.23, 95% CI 0.10–0.54) and in combination with probiotics (RR 0.24, 95% CI 0.08–0.71) (169).
According to the IDSA guidelines, oral bLfcin at a dose of 100 mg per day may be effective in VLBW infants, but the recommendation is graded as weak (12). The ESCMID guideline gives a moderate recommendation for daily treatment with 100 mg Lfcin, either as monotherapy or in combination with 106 CFU Lactobacillus, starting on the third day of life and continuing until the sixth week of life or until discharge from NICU, to reduce the risk of fungal infections (133). Alternatively, many studies have shown that birth weight-dependent doses of 150–300 mg/kg/day are considered optimal (162, 169, 170).
Clinical and neurodevelopmental outcomes
ICIs can affect multiple organs and tissues throughout the body, such as the brain, heart, kidneys, eyes, liver, spleen, lungs, bones, etc (171). The central nervous system (CNS) is often damaged, with meningitis being the most common form (172). Of particular note, this population usually has high mortality and subsequent severe NDI regardless of adequate antifungal treatment (109, 127), and this proportion is higher than in those infected with bacterial pathogens. There is an inverse relationship between birth weight or gestational age and the mortality rate of infants with ICIs, and this rate can be as high as 50% in ELBW infants (173). One study reported that infants with Candida infection had the highest risk of death and/or NDI among preterm infants with late-onset infection (174). Similarly, according to the study by Benjamin DK et al., among ELBW infants, those who developed candidiasis had the highest rate of NDI at 57%, while the rate among infants with bacterial infections or no infections was relatively low at 36% (86). Even more, nearly 70% of ELBW (birth weight <750 g) infants are reported to either die from or experience severe NDI following ICIs, despite treatment (127).
Treatment and recommendations
Prevention of infection should be the most important goal in newborn infants, as it is a severe threat to this vulnerable population, who are at high risk of infection. However, if an infection is suspected or confirmed, prompt and appropriate treatment has been shown to reduce morbidity and mortality (109). Infections in infants tend to spread to multiple critical organs. In general, blood and urine cultures should be taken. If neonates have positive Candida spp. cultures from blood and/or urine, a lumbar puncture, and a dilated retinal examination are recommended to further assess for potential dissemination to the CNS and retina. In instances where Candida blood cultures remain persistently positive, disseminated ICIs should be suspected, and an ultrasound evaluation of the urogenital tract, liver, and spleen should also be performed (12). When selecting antifungal agents, it is important to determine whether the infection involves the CNS or the urinary tract. Removal of all foreign bodies, such as tubes, shunts, implants, or catheters, should be considered if possible, as Candida spp. tend to form biofilms on most materials, which are difficult to penetrate and hinder the effective eradication of the infection (18).
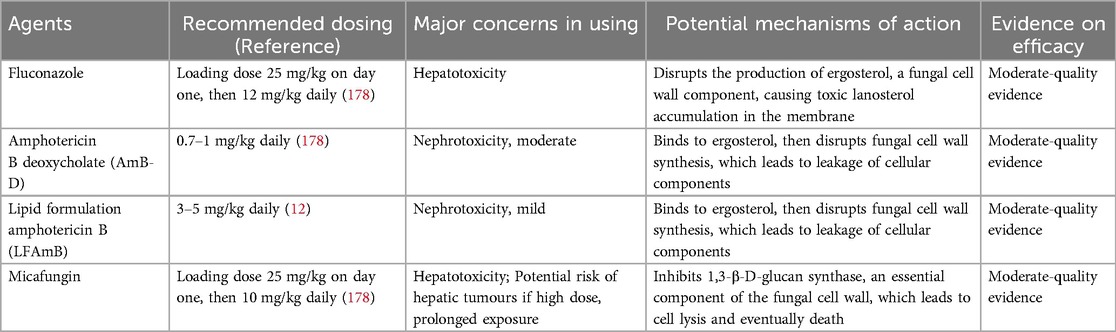
Timely initiation of empiric antifungal therapy is critical to treatment success, especially considering that it takes an average of 36 h for cultures to become positive, or up to 42 h if the infant is receiving antifungal prophylaxis (175). Empiric antifungal therapy has been shown to increase the survival rate without NDI in infants with ICIs (176). In addition to timely pharmacologic intervention, optimal antifungal agent selection and appropriate dosing are also important. When selecting agents for ICIs, clinicians should consider the predominant isolates of Candida spp. in their NICUs, Candida antifungal resistance patterns, and whether the infant has received prior antifungal prophylaxis, as well as the specific prophylactic agents. The current major antifungal agents for the treatment of neonates with ICIs are azoles, polyenes, and echinocandins (Table 6).
Fluconazole, an azole antifungal agent, is the most studied agent for the prevention and treatment of fungal infections in neonates due to its favorable nephrotoxicity profile. It has high antifungal activity against Candida spp. and penetrates well into the CSF, making it ideal for CNS infections. It is mainly excreted via the kidneys and reaches high concentrations in the urine, which is beneficial in the treatment of Candida infections of the urinary tract. According to IDSA guidelines, fluconazole treatment at 12 mg/kg daily, either intravenously or orally, is a reasonable alternative for infants without previous fluconazole prophylaxis (12). Concurrently, fluconazole treatment should be avoided in infants if Candida glabrata or Candida krusei infection is suspected or confirmed. A loading dose of fluconazole at 25 mg/kg has demonstrated a more rapid achievement of target concentrations and does not increase the risk of hepatotoxicity, a rare adverse effect linked to fluconazole (177, 178).
Amphotericin B (AmB), a polyene antifungal agent, includes AmB deoxycholate (AmB-D) and lipid formulation AmB (LFAmB). It targets fungal cell wall synthesis by binding to ergosterol, triggering pore formation and subsequent leakage of intracellular components, and eventually presents fungicidal activity against susceptible organisms (179). Of the available AmB formulations, AmB-D is currently the major option for ICIs in neonates, with a recommended dose of 1 mg/kg daily (12, 178). Infusion-related reactions to this agent are virtually absent in neonates, and the toxicity risk in neonates is considered low, even though increased serum creatinine and hypokalemia have been observed (180). LFAmB is considered to have less renal toxicity due to its renal protective properties, as lower concentrations of the lipid formulation are present in the urinary tract. Therefore, LFAmB can be considered at a dose of 3–5 mg/kg daily when there is no urinary tract involvement in neonates (12).
Echinocandins are attractive agents given their efficacy against Candida biofilms and their extended spectrum against Candida spp., including often-resistant species such as Candida parapsilosis, Candida glabrata, Candida krusei, and others (181). The IDSA guidelines recommend that echinocandins should be used as a salvage therapy or in cases where resistance or toxicity precludes the use of AmB-D or fluconazole (12). Of particular note, echinocandins fail to attain therapeutic concentrations in the urine (182), rendering them unsuitable for the treatment of urinary tract infections. Furthermore, echinocandins do not penetrate the CSF, but they have demonstrated the ability to reach the brain parenchyma (183) and have proven effective in the treatment of Candida meningoencephalitis in neonatal animal models (184). Of the echinocandins, micafungin is the most preferred agent because of its efficacy and safety in the neonatal population, despite the observation of micafungin treatment discontinuation due to abnormal liver tests in multicenter trials (185). The mechanism of action involves the inhibition of β- (1, 3)-D-glucan synthesis, a critical component of the fungal cell wall, resulting in cell lysis and subsequent cell death (186). The recommended dosing of micafungin is a loading dose of 15 mg/kg on day one, then 10 mg/kg daily (178). We should be aware that micafungin carries a black box warning due to its association with hepatic tumors observed in murine models under conditions of high doses and prolonged exposure; thus, long-term safety remains in need of careful consideration in this vulnerable group of neonates. However, to date, such conditions and effects have not been observed in human subjects.
Current first-line agent recommendations include AmB-D or fluconazole (if the isolate is susceptible), with second-line and salvage therapies, including LFAmB and micafungin (12). The duration of antifungal therapy should continue for at least 2 weeks after documented clearance, which must be confirmed by microbiologic clearance, and in the absence of signs or symptoms indicating persistent infection (12). In the first week of therapy, the aim is to attain microbiologic clearance of the infection, which includes securing at least two blood cultures negative ≥24 h apart, as well as a negative urine culture, and a CSF culture (13). If microbiologic clearance does not occur during the first week, an alternative antifungal agent should be considered, such as micafungin, and source control must also be reassessed. In addition, special attention should be paid to the fact that a Candida infection of the urinary tract in premature infants, also known as “Candiduria”, generally means a disseminated Candida infection and is usually associated with a substantial risk of death or NDI (182). Positive urine cultures obtained from either sterile urethral catheterization or suprapubic aspiration should be considered equivalent to positive blood cultures. Meanwhile, this population should undergo a systemic workup (abdominal ultrasound, blood, and CSF cultures) for disseminated Candida infection that warrants treatment (12).
Conclusions and future perspectives
ICIs continue to be a major clinical challenge for hospitalized newborn infants receiving intensive care, primarily affecting very premature and VLBW infants in HICs, whereas larger infants are more commonly affected in LMICs. The implementation of a series of proactive prevention and control strategies, such as the expanded use of prophylactic fluconazole, the prudent reduction of broad-spectrum antibiotics, and the increased adoption of empiric antifungal therapies, has contributed to a gradual decline in the incidence of ICIs in HICs. However, this problem is also a serious threat in LMICs, where limited access to these interventions and non-judicious use of broad-spectrum antibiotics remain key barriers. When ICIs are suspected, timely initiation of empirical antifungal therapy, along with prompt removal of compromised catheters, has been shown to improve clinical outcomes of neonates. Certainly, evidence-based prophylactic measures are critical for reducing the burden of ICIs in hospitalized newborn infants. Future efforts should prioritize standardized protocols for evidence-based prevention across intensive care units and track the trends of ICIs, particularly in LMICs with limited data and a large number of “outborn” newborns.
Author contributions
DZ: Funding acquisition, Writing – original draft. DX: Writing – original draft. HY: Funding acquisition, Writing – review & editing. NH: Conceptualization, Writing – review & editing. WD: Supervision, Writing – review & editing. XL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Research Foundation of Southwest Medical University (2017-ZRQN-164, ZXX2023QN090).
Acknowledgments
We would like to thank BioRender (https://app.biorender.com) for providing the illustration support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang D, Xie D, He N, Wang X, Dong W, Lei X. Prophylactic use of fluconazole in very premature infants. Front Pediatr. (2021) 9:726769. doi: 10.3389/fped.2021.726769
2. Baptista MI, Nona J, Ferreira M, Sampaio I, Abrantes M, Tomé MT, et al. Invasive fungal infection in neonatal intensive care units: a multicenter survey. J Chemother. (2016) 28:37–43. doi: 10.1179/1973947814Y.0000000222
3. Aliaga S, Clark RH, Laughon M, Walsh TJ, Hope WW, Benjamin DK, et al. Changes in the incidence of candidiasis in neonatal intensive care units. Pediatrics. (2014) 133:236–42. doi: 10.1542/peds.2013-0671
4. Fu J, Ding Y, Jiang Y, Mo S, Xu S, Qin P. Persistent candidemia in very low birth weight neonates: risk factors and clinical significance. BMC Infect Dis. (2018) 18:558. doi: 10.1186/s12879-018-3487-9
5. Zhou Q, Kelly E, Luu TM, Ye XY, Ting J, Shah PS, et al. Fungal infection and neurodevelopmental outcomes at 18–30 months in preterm infants. Front Pediatr. (2023) 11:1145252. doi: 10.3389/fped.2023.1145252
6. Jajoo M, Manchanda V, Chaurasia S, Sankar MJ, Gautam H, Agarwal R, et al. Alarming rates of antimicrobial resistance and fungal sepsis in outborn neonates in North India. PLoS One. (2018) 13:e0180705. doi: 10.1371/journal.pone.0180705
7. Ballot DE, Bosman N, Nana T, Ramdin T, Cooper PA. Background changing patterns of neonatal fungal sepsis in a developing country. J Trop Pediatr. (2013) 59:460–4. doi: 10.1093/tropej/fmt053
8. Morkel G, Bekker A, Marais BJ, Kirsten G, van Wyk J, Dramowski A. Bloodstream infections and antimicrobial resistance patterns in a South African neonatal intensive care unit. Paediatr Int Child Health. (2014) 34:108–14. doi: 10.1179/2046905513Y.0000000082
9. De Rose DU, Santisi A, Ronchetti MP, Martini L, Serafini L, Betta P, et al. Invasive Candida infections in neonates after major surgery: current evidence and new directions. Pathogens. (2021) 10:319. doi: 10.3390/pathogens10030319
10. Cook A, Ferreras-Antolin L, Adhisivam B, Ballot D, Berkley JA, Bernaschi P, et al. Neonatal invasive candidiasis in low- and middle-income countries: data from the NeoOBS study. Med Mycol. (2023) 61:myad010. doi: 10.1093/mmy/myad010
11. Saha AK, Saha B. Profile of neonatal candidiasis in tertiary neonatal intensive care unit: a report from a developing country. J Neonatal Perinatal Med. (2023) 16:501–6. doi: 10.3233/NPM-231204
12. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin Infect Dis. (2016) 62:e1–50. doi: 10.1093/cid/civ933
13. Kilpatrick R, Scarrow E, Hornik C, Greenberg RG. Neonatal invasive candidiasis: updates on clinical management and prevention. Lancet Child Adolesc Health. (2022) 6:60–70. doi: 10.1016/S2352-4642(21)00272-8
14. Chen I-T, Chen C-C, Huang H-C, Kuo K-C. Malassezia furfur emergence and candidemia trends in a neonatal intensive care unit during 10 years: the experience of fluconazole prophylaxis in a single hospital. Adv Neonatal Care. (2020) 20:E3–8. doi: 10.1097/ANC.0000000000000640
15. Mehler K, Cornely O, Seifert H, Zweigner J, Janssen S, Oberthuer A. Molds and more: rare fungal infections in preterm infants <24 weeks of gestation. Pediatr Infect Dis J. (2022) 41:352–7. doi: 10.1097/INF.0000000000003407
16. Kucinskiene V, Sutkute A, Valiukeviciene S. Cutaneous fungal infection in a neonatal intensive care unit patient: a case report and literature review. Pediatr Dermatol. (2014) 31:267–70. doi: 10.1111/pde.12323
17. Weimer KED, Smith PB, Puia-Dumitrescu M, Aleem S. Invasive fungal infections in neonates: a review. Pediatr Res. (2022) 91:404–12. doi: 10.1038/s41390-021-01842-7
18. Malinovská Z, Čonková E, Váczi P. Biofilm formation in medically important Candida species. J Fungi. (2023) 9:955. doi: 10.3390/jof9100955
19. Makled AF, Ali SAM, Labeeb AZ, Salman SS, Shebl DZM, Hegazy SG, et al. Characterization of Candida species isolated from clinical specimens: insights into virulence traits, antifungal resistance and molecular profiles. BMC Microbiol. (2024) 24:388. doi: 10.1186/s12866-024-03515-x
20. Staniszewska M. Virulence factors in Candida species. Curr Protein Pept Sci. (2020) 21:313–23. doi: 10.2174/1389203720666190722152415
21. Lohse MB, Gulati M, Johnson AD, Nobile CJ. Development and regulation of single- and multi-species Candida albicans biofilms. Nat Rev Microbiol. (2018) 16:19–31. doi: 10.1038/nrmicro.2017.107
22. Caetano CF, Gaspar C, Oliveira AS, Palmeira-de-Oliveira R, Rodrigues L, Gonçalves T, et al. Study of ecological relationship of yeast species with Candida albicans in the context of vulvovaginal infections. Microorganisms. (2023) 11:2398. doi: 10.3390/microorganisms11102398
23. Jalal M, Azam Ansari M, Alshamrani M, Ghazanfar Ali S, Jamous YF, Alyahya SA, et al. Crinum latifolium mediated biosynthesis of gold nanoparticles and their anticandidal, antibiofilm and antivirulence activity. J Saudi Chemi Soc. (2023) 27:101644. doi: 10.1016/j.jscs.2023.101644
24. Bohner F, Papp C, Gácser A. The effect of antifungal resistance development on the virulence of Candida species. FEMS Yeast Res. (2022) 22:foac019. doi: 10.1093/femsyr/foac019
25. Mohammadi F, Ghasemi Z, Familsatarian B, Salehi E, Sharifynia S, Barikani A, et al. Relationship between antifungal susceptibility profile and virulence factors in Candida albicans isolated from nail specimens. Rev Soc Bras Med Trop. (2020) 53:e20190214. doi: 10.1590/0037-8682-0214-2019
26. Nakamura-Vasconcelos SS, Fiorini A, Zanni PD, Bonfim-Mendonça PdS, Godoy JR, Almeida-Apolonio AA, et al. Emergence of Candida glabrata in vulvovaginal candidiasis should be attributed to selective pressure or virulence ability? Arch Gynecol Obstet. (2017) 296:519–26. doi: 10.1007/s00404-017-4465-y
27. Bendel CM. Nosocomial neonatal candidiasis. Pediatr Infect Dis J. (2005) 24:831–2. doi: 10.1097/01.inf.0000178291.40568.ef
28. Filippidi A, Galanakis E, Maraki S, Galani I, Drogari-Apiranthitou M, Kalmanti M, et al. The effect of maternal flora on Candida colonisation in the neonate. Mycoses. (2014) 57:43–8. doi: 10.1111/myc.12100
29. Henderickx JGE, de Weerd H, Groot Jebbink LJ, van Zoeren-Grobben D, Hemels MAC, van Lingen RA, et al. The first fungi: mode of delivery determines early life fungal colonization in the intestine of preterm infants. Microbiome Res Rep. (2022) 1:7. doi: 10.20517/mrr.2021.03
30. Zisova LG, Chokoeva AA, Amaliev GI, Petleshkova PV, Miteva-Katrandzhieva TМ, Krasteva MB, et al. Vulvovaginal candidiasis in pregnant women and its importance for Candida colonization of newborns. Folia Med. (2016) 58:108–14. doi: 10.1515/folmed-2016-0018
31. Caramalac DA, da Silva Ruiz L, de Batista GCM, Birman EG, Duarte M, Hahn R, et al. Candida isolated from vaginal mucosa of mothers and oral mucosa of neonates: occurrence and biotypes concordance. Pediatr Infect Dis J. (2007) 26:553–7. doi: 10.1097/INF.0b013e31806166d7
32. Gedefie A, Shimeles G, Motbainor H, Kassanew B, Genet C. Vaginal colonization and vertical transmission of Candida species: prevalence and associated factors among pregnant women and their neonates at public health facilities of Northeast Ethiopia. BMC Pregnancy Childbirth. (2025) 25:22. doi: 10.1186/s12884-024-07103-9
33. Ali GY, Algohary EHSS, Rashed KA, Almoghanum M, Khalifa AA. Prevalence of Candida colonization in preterm newborns and VLBW in neonatal intensive care unit: role of maternal colonization as a risk factor in transmission of disease. J Matern Fetal Neonatal Med. (2012) 25:789–95. doi: 10.3109/14767058.2011.622005
34. Barton M, Shen A, O’Brien K, Robinson JL, Davies HD, Simpson K, et al. Early-onset invasive candidiasis in extremely low birth weight infants: perinatal acquisition predicts poor outcome. Clin Infect Dis. (2017) 64:921–7. doi: 10.1093/cid/cix001
35. He B, Yang Q. Updates in laboratory identification of invasive fungal infection in neonates. Microorganisms. (2023) 11:1001. doi: 10.3390/microorganisms11041001
36. Ferreras-Antolin L, Chowdhary A, Warris A. Neonatal invasive candidiasis: current concepts. Indian J Pediatr. (2025) 92:765–73. doi: 10.1007/s12098-025-05593-9
37. Warris A, Pana Z-D, Oletto A, Lundin R, Castagnola E, Lehrnbecher T, et al. Etiology and outcome of candidemia in neonates and children in Europe: an 11-year multinational retrospective study. Pediatr Infect Dis J. (2020) 39:114–20. doi: 10.1097/INF.0000000000002530
38. Benedict K, Roy M, Kabbani S, Anderson EJ, Farley MM, Harb S, et al. Neonatal and pediatric candidemia: results from population-based active laboratory surveillance in four US locations, 2009–2015. J Pediatric Infect Dis Soc. (2018) 7:e78–85. doi: 10.1093/jpids/piy009
39. Oeser C, Vergnano S, Naidoo R, Anthony M, Chang J, Chow P, et al. Neonatal invasive fungal infection in England 2004–2010. Clin Microbiol Infect. (2014) 20:936–41. doi: 10.1111/1469-0691.12578
40. Ting JY, Roberts A, Synnes A, Canning R, Bodani J, Monterossa L, et al. Invasive fungal infections in neonates in Canada: epidemiology and outcomes. Pediatr Infect Dis J. (2018) 37:1154–9. doi: 10.1097/INF.0000000000001968
41. Molla A, Albadrani M. Prevalence and Species distribution of neonatal candidiasis: a systematic review and meta-analysis. Diseases. (2024) 12:154. doi: 10.3390/diseases12070154
42. Blyth CC, Chen SCA, Slavin MA, Serena C, Nguyen Q, Marriott D, et al. Not just little adults: candidemia epidemiology, molecular characterization, and antifungal susceptibility in neonatal and pediatric patients. Pediatrics. (2009) 123:1360–8. doi: 10.1542/peds.2008-2055
43. Pillay D, Naidoo L, Swe Swe-Han K, Mahabeer Y. Neonatal sepsis in a tertiary unit in South Africa. BMC Infect Dis. (2021) 21:225. doi: 10.1186/s12879-021-05869-3
44. Caggiano G, Lovero G, De Giglio O, Barbuti G, Montagna O, Laforgia N, et al. Candidemia in the neonatal intensive care unit: a retrospective, observational survey and analysis of literature data. Biomed Res Int. (2017) 2017:7901763. doi: 10.1155/2017/7901763
45. Sokou R, Palioura AE, Kopanou Taliaka P, Konstantinidi A, Tsantes AG, Piovani D, et al. Candida auris infection, a rapidly emerging threat in the neonatal intensive care units: a systematic review. J Clin Med. (2024) 13:1586. doi: 10.3390/jcm13061586
46. Oeser C, Lamagni T, Heath PT, Sharland M, Ladhani S. The epidemiology of neonatal and pediatric candidemia in England and Wales, 2000–2009. Pediatr Infect Dis J. (2013) 32:23–6. doi: 10.1097/INF.0b013e318275612e
47. Kumar J, Soni PK, Angrup A, Saini SS, Sundaram V, Mukhopadhyay K, et al. Antimicrobial resistance patterns among neonates referred to pediatric emergency in North India: a prospective cohort study. Pediatr Infect Dis J. (2023) 42:1007–11. doi: 10.1097/INF.0000000000004056
48. Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. (2016) 4:e752–760. doi: 10.1016/S2214-109X(16)30148-6
49. Tóth R, Nosek J, Mora-Montes HM, Gabaldon T, Bliss JM, Nosanchuk JD, et al. Candida parapsilosis: from genes to the bedside. Clin Microbiol Rev. (2019) 32:e00111–18. doi: 10.1128/CMR.00111-18
50. Bonassoli LA, Bertoli M, Svidzinski TIE. High frequency of Candida parapsilosis on the hands of healthy hosts. J Hosp Infect. (2005) 59:159–62. doi: 10.1016/j.jhin.2004.06.033
51. Benjamin DK, Poole C, Steinbach WJ, Rowen JL, Walsh TJ. Neonatal candidemia and end-organ damage: a critical appraisal of the literature using meta-analytic techniques. Pediatrics. (2003) 112:634–40. doi: 10.1542/peds.112.3.634
52. Naglik J, Albrecht A, Bader O, Hube B. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. (2004) 6:915–26. doi: 10.1111/j.1462-5822.2004.00439.x
53. Liu S, Youngchim S, Zamith-Miranda D, Nosanchuk JD. Fungal melanin and the mammalian immune system. J Fungi. (2021) 7:264. doi: 10.3390/jof7040264
54. Roilides E, Farmaki E, Evdoridou J, Dotis J, Hatziioannidis E, Tsivitanidou M, et al. Neonatal candidiasis: analysis of epidemiology, drug susceptibility, and molecular typing of causative isolates. Eur J Clin Microbiol Infect Dis. (2004) 23:745–50. doi: 10.1007/s10096-004-1210-9
55. Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. (2008) 21:606–25. doi: 10.1128/CMR.00013-08
56. Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. (2012) 36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x
57. Tortorano AM, Prigitano A, Morroni G, Brescini L, Barchiesi F. Candidemia: evolution of drug resistance and novel therapeutic approaches. Infect Drug Resist. (2021) 14:5543–53. doi: 10.2147/IDR.S274872
58. Hartung de Capriles C, Mata-Essayag S, Azpiróz A, Ponente A, Magaldi S, Pérez C, et al. Neonatal candidiasis in Venezuela: clinical and epidemiological aspects. Rev Latinoam Microbiol. (2005) 47:11–20. Available online at: https://pubmed.ncbi.nlm.nih.gov/17061542/17061542
59. Khan F, Bamunuarachchi NI, Pham DTN, Tabassum N, Khan MSA, Kim Y-M. Mixed biofilms of pathogenic Candida-bacteria: regulation mechanisms and treatment strategies. Crit Rev Microbiol. (2021) 47:699–727. doi: 10.1080/1040841X.2021.1921696
60. Fidel PL, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. (1999) 12:80–96. doi: 10.1128/CMR.12.1.80
61. Xia C, Liu R, Zhang S, Shen J, Wang Z. Fluconazole-induced changes in azole resistance and biofilm production in Candida glabrata in vitro. Diagn Microbiol Infect Dis. (2025) 111:116683. doi: 10.1016/j.diagmicrobio.2025.116683
62. Yenişehirli G, Alıcı A, Yenişehirli A. Antifungal drug susceptibility profiles and molecular mechanisms of azole resistance in Candida blood stream isolates. Indian J Med Microbiol. (2023) 45:100389. doi: 10.1016/j.ijmmb.2023.100389
63. Fairchild KD, Tomkoria S, Sharp EC, Mena FV. Neonatal Candida glabrata sepsis: clinical and laboratory features compared with other Candida species. Pediatr Infect Dis J. (2002) 21:39–43. doi: 10.1097/00006454-200201000-00009
64. Goel S, Mittal S, Chaudhary U. Role of non albicans Candida spp. and biofilm in neonatal ICU. Infect Disord Drug Targets. (2016) 16:192–8. doi: 10.2174/1871526516666160818150148
65. Butler G, Rasmussen MD, Lin MF, Santos MAS, Sakthikumar S, Munro CA, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. (2009) 459:657–62. doi: 10.1038/nature08064
66. Zuza-Alves DL, Silva-Rocha WP, Chaves GM. An update on Candida tropicalis based on basic and clinical approaches. Front Microbiol. (2017) 8:1927. doi: 10.3389/fmicb.2017.01927
67. Ko J-H, Jung DS, Lee JY, Kim HA, Ryu SY, Jung S-I, et al. Poor prognosis of Candida tropicalis among non-albicans candidemia: a retrospective multicenter cohort study, Korea. Diagn Microbiol Infect Dis. (2019) 95:195–200. doi: 10.1016/j.diagmicrobio.2019.05.017
68. Keighley C, Kim HY, Kidd S, Chen SC-A, Alastruey A, Dao A, et al. Candida tropicalis—a systematic review to inform the world health organization of a fungal priority pathogens list. Med Mycol. (2024) 62:myae040. doi: 10.1093/mmy/myae040
69. Meis JF, Chowdhary A. Candida auris: a global fungal public health threat. Lancet Infect Dis. (2018) 18:1298–9. doi: 10.1016/S1473-3099(18)30609-1
70. Ettadili H, Vural C. Current global status of Candida auris an emerging multidrug-resistant fungal pathogen: bibliometric analysis and network visualization. Braz J Microbiol. (2024) 55:391–402. doi: 10.1007/s42770-023-01239-0
71. Alp Ş, Arıkan Akdağlı S. Candida auris and mechanisms of antifungal drug resistance. Mikrobiyol Bul. (2021) 55:99–112. doi: 10.5578/mb.20217
72. Ahmad S, Alfouzan W. Candida auris: epidemiology, diagnosis, pathogenesis, antifungal susceptibility, and infection control measures to combat the spread of infections in healthcare facilities. Microorganisms. (2021) 9:807. doi: 10.3390/microorganisms9040807
73. Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. (2009) 53:41–4. doi: 10.1111/j.1348-0421.2008.00083.x
74. Reséndiz-Sánchez J, Ortiz-Álvarez J, Casimiro-Ramos A, Hernández-Rodríguez C, Villa-Tanaca L. First report of a catheter-related bloodstream infection by Candida haemulonii in a children’s hospital in Mexico city. Int J Infect Dis. (2020) 92:123–6. doi: 10.1016/j.ijid.2019.12.037
75. Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY antimicrobial surveillance program (2008 to 2009). J Clin Microbiol. (2011) 49:396–9. doi: 10.1128/JCM.01398-10
76. Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D. CLSI subcommittee for antifungal susceptibility testing. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat. (2010) 13:180–95. doi: 10.1016/j.drup.2010.09.002
77. Clinical Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi. 3rd ed. M38M51S. Wayne, PA: Clinical and Laboratory Standards Institute (2022).
78. Autmizguine J, Tan S, Cohen-Wolkowiez M, Cotten CM, Wiederhold N, Goldberg RN, et al. Antifungal susceptibility and clinical outcome in neonatal candidiasis. Pediatr Infect Dis J. (2018) 37:923–9. doi: 10.1097/INF.0000000000001913
79. Shuping L, Mpembe R, Mhlanga M, Naicker SD, Maphanga TG, Tsotetsi E, et al. Epidemiology of culture-confirmed candidemia among hospitalized children in South Africa, 2012–2017. Pediatr Infect Dis J. (2021) 40:730–7. doi: 10.1097/INF.0000000000003151
80. Govender NP, Patel J, Magobo RE, Naicker S, Wadula J, Whitelaw A, et al. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: results from laboratory-based sentinel surveillance in South Africa. J Antimicrob Chemother. (2016) 71:1994–2004. doi: 10.1093/jac/dkw091
81. Luparia M, Landi F, Mesini A, Militello MA, Galletto P, Farina D, et al. Fungal ecology in a tertiary neonatal intensive care unit after 16 years of routine fluconazole prophylaxis: no emergence of native fluconazole-resistant strains. Am J Perinatol. (2019) 36:S126–33. doi: 10.1055/s-0039-1691808
82. Juyal D, Sharma M, Pal S, Rathaur VK, Sharma N. Emergence of non-albicans Candida species in neonatal candidemia. N Am J Med Sci. (2013) 5:541–5. doi: 10.4103/1947-2714.118919
83. Jamiu AT, Albertyn J, Sebolai OM, Pohl CH. Update on Candida krusei, a potential multidrug-resistant pathogen. Med Mycol. (2021) 59:14–30. doi: 10.1093/mmy/myaa031
84. Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida Species. Front Microbiol. (2016) 7:2173. doi: 10.3389/fmicb.2016.02173
85. Biswas B, Sharma AK, Seema K, Kumar A, Boipai M, Kumar M. Emerging threat of candida resistance among neonates at a teaching institute of Jharkhand. J Family Med Prim Care. (2023) 12:946–52. doi: 10.4103/jfmpc.jfmpc_2104_22
86. Benjamin DK, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics. (2006) 117:84–92. doi: 10.1542/peds.2004-2292
87. Lee JH, Hornik CP, Benjamin DK, Herring AH, Clark RH, Cohen-Wolkowiez M, et al. Risk factors for invasive candidiasis in infants >1,500 g birth weight. Pediatr Infect Dis J. (2013) 32:222–6. doi: 10.1097/INF.0b013e3182769603
88. Rutter N. Clinical consequences of an immature barrier. Semin Neonatol. (2000) 5:281–7. doi: 10.1053/siny.2000.0014
89. Taïeb A. Skin barrier in the neonate. Pediatr Dermatol. (2018) 35(Suppl 1):s5–9. doi: 10.1111/pde.13482
90. Wächtler B, Citiulo F, Jablonowski N, Förster S, Dalle F, Schaller M, et al. Candida albicans-epithelial interactions: dissecting the roles of active penetration, induced endocytosis and host factors on the infection process. PLoS One. (2012) 7:e36952. doi: 10.1371/journal.pone.0036952
91. Michalski C, Kan B, Lavoie PM. Antifungal immunological defenses in newborns. Front Immunol. (2017) 8:281. doi: 10.3389/fimmu.2017.00281
92. Marchini G, Lindow S, Brismar H, Ståbi B, Berggren V, Ulfgren A-K, et al. The newborn infant is protected by an innate antimicrobial barrier: peptide antibiotics are present in the skin and vernix caseosa. Br J Dermatol. (2002) 147:1127–34. doi: 10.1046/j.1365-2133.2002.05014.x
93. Dowling DJ, Levy O. Ontogeny of early life immunity. Trends Immunol. (2014) 35:299–310. doi: 10.1016/j.it.2014.04.007
94. de Roock S, Stoppelenburg AJ, Scholman R, Hoeks SBEA, Meerding J, Prakken BJ, et al. Defective TH17 development in human neonatal T cells involves reduced RORC2 mRNA content. J Allergy Clin Immunol. (2013) 132:754–756.e3. doi: 10.1016/j.jaci.2013.04.014
95. Vanden Eijnden S, Goriely S, De Wit D, Goldman M, Willems F. Preferential production of the IL-12(p40)/IL-23(p19) heterodimer by dendritic cells from human newborns. Eur J Immunol. (2006) 36:21–6. doi: 10.1002/eji.200535467
96. Sharma AA, Jen R, Kan B, Sharma A, Marchant E, Tang A, et al. Impaired NLRP3 inflammasome activity during fetal development regulates IL-1β production in human monocytes. Eur J Immunol. (2015) 45:238–49. doi: 10.1002/eji.201444707
97. Lavoie PM, Huang Q, Jolette E, Whalen M, Nuyt AM, Audibert F, et al. Profound lack of interleukin (IL)-12/IL-23p40 in neonates born early in gestation is associated with an increased risk of sepsis. J Infect Dis. (2010) 202:1754–63. doi: 10.1086/657143
98. de Nies L, Kobras CM, Stracy M. Antibiotic-induced collateral damage to the microbiota and associated infections. Nat Rev Microbiol. (2023) 21:789–804. doi: 10.1038/s41579-023-00936-9
99. Cotten CM, McDonald S, Stoll B, Goldberg RN, Poole K, Benjamin DK, et al. The association of third-generation cephalosporin use and invasive candidiasis in extremely low birth-weight infants. Pediatrics. (2006) 118:717–22. doi: 10.1542/peds.2005-2677
100. Hou S, Wang X, Yu Y, Ji H, Dong X, Li J, et al. Invasive fungal infection is associated with antibiotic exposure in preterm infants: a multi-centre prospective case-control study. J Hosp Infect. (2023) 134:43–9. doi: 10.1016/j.jhin.2023.01.002
101. Pitiriga V, Kanellopoulos P, Bakalis I, Kampos E, Sagris I, Saroglou G, et al. Central venous catheter-related bloodstream infection and colonization: the impact of insertion site and distribution of multidrug-resistant pathogens. Antimicrob Resist Infect Control. (2020) 9:189. doi: 10.1186/s13756-020-00851-1
102. Manzoni P, Farina D, Leonessa M, d’Oulx EA, Galletto P, Mostert M, et al. Risk factors for progression to invasive fungal infection in preterm neonates with fungal colonization. Pediatrics. (2006) 118:2359–64. doi: 10.1542/peds.2006-1311
103. Feja KN, Wu F, Roberts K, Loughrey M, Nesin M, Larson E, et al. Risk factors for candidemia in critically ill infants: a matched case-control study. J Pediatr. (2005) 147:156–61. doi: 10.1016/j.jpeds.2005.02.021
104. Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. (2004) 17:638–80. doi: 10.1128/CMR.17.3.638-680.2004
105. Mahieu LM, Van Gasse N, Wildemeersch D, Jansens H, Ieven M. Number of sites of perinatal Candida colonization and neutropenia are associated with nosocomial candidemia in the neonatal intensive care unit patient. Pediatr Crit Care Med. (2010) 11:240–5. doi: 10.1097/PCC.0b013e3181b808fb
106. Manzoni P, Farina D, Galletto P, Leonessa M, Priolo C, Arisio R, et al. Type and number of sites colonized by fungi and risk of progression to invasive fungal infection in preterm neonates in neonatal intensive care unit. J Perinat Med. (2007) 35:220–6. doi: 10.1515/JPM.2007.055
107. Kaufman DA, Gurka MJ, Hazen KC, Boyle R, Robinson M, Grossman LB. Patterns of fungal colonization in preterm infants weighing less than 1000 grams at birth. Pediatr Infect Dis J. (2006) 25:733–7. doi: 10.1097/01.inf.0000226978.96218.e6
108. Yan L, Yang C, Tang J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol Res. (2013) 168:389–95. doi: 10.1016/j.micres.2013.02.008
109. Barton M, O’Brien K, Robinson JL, Davies DH, Simpson K, Asztalos E, et al. Invasive candidiasis in low birth weight preterm infants: risk factors, clinical course and outcome in a prospective multicenter study of cases and their matched controls. BMC Infect Dis. (2014) 14:327. doi: 10.1186/1471-2334-14-327
110. Shetty SS, Harrison LH, Hajjeh RA, Taylor T, Mirza SA, Schmidt AB, et al. Determining risk factors for candidemia among newborn infants from population-based surveillance: Baltimore, Maryland, 1998–2000. Pediatr Infect Dis J. (2005) 24:601–4. doi: 10.1097/01.inf.0000168751.11375.d6
111. Yu Y, Du L, Yuan T, Zheng J, Chen A, Chen L, et al. Risk factors and clinical analysis for invasive fungal infection in neonatal intensive care unit patients. Am J Perinatol. (2013) 30:589–94. doi: 10.1055/s-0032-1329688
112. Dermitzaki N, Baltogianni M, Tsekoura E, Giapros V. Invasive Candida infections in neonatal intensive care units: risk factors and new insights in prevention. Pathogens. (2024) 13:660. doi: 10.3390/pathogens13080660
113. Montagna MT, Lovero G, De Giglio O, Iatta R, Caggiano G, Montagna O, et al. Invasive fungal infections in neonatal intensive care units of Southern Italy: a multicentre regional active surveillance (AURORA project). J Prev Med Hyg. (2010) 51:125–30. Available online at: https://pubmed.ncbi.nlm.nih.gov/21361118/21361118
114. Ramy N, Hashim M, Abou Hussein H, Sawires H, Gaafar M, El Maghraby A. Role of early onset neutropenia in development of candidemia in premature infants. J Trop Pediatr. (2018) 64:51–9. doi: 10.1093/tropej/fmx029
115. Swindell K, Lattif AA, Chandra J, Mukherjee PK, Ghannoum MA. Parenteral lipid emulsion induces germination of Candida albicans and increases biofilm formation on medical catheter surfaces. J Infect Dis. (2009) 200:473–80. doi: 10.1086/600106
116. Guducuoglu H, Gultepe B, Otlu B, Bektas A, Yildirim O, Tuncer O, et al. Candida albicans outbreak associated with total parenteral nutrition in the neonatal unit. Indian J Med Microbiol. (2016) 34:202–7. doi: 10.4103/0255-0857.180303
117. Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in neonatal intensive care unit patients. The national epidemiology of mycosis survey study group. Pediatr Infect Dis J. (2000) 19:319–24. doi: 10.1097/00006454-200004000-00011
118. Pera A, Byun A, Gribar S, Schwartz R, Kumar D, Parimi P. Dexamethasone therapy and Candida sepsis in neonates less than 1250 grams. J Perinatol. (2002) 22:204–8. doi: 10.1038/sj.jp.7210699
119. Moretti C, Gizzi C, Gagliardi L, Petrillo F, Ventura ML, Trevisanuto D, et al. A survey of the union of European neonatal and perinatal societies on neonatal respiratory care in neonatal intensive care units. Children. (2024) 11:158. doi: 10.3390/children11020158
120. Manzoni P, García Sánchez R, Meyer M, Stolfi I, Pugni L, Messner H, et al. Exposure to gastric acid inhibitors increases the risk of infection in preterm very low birth weight infants but concomitant administration of lactoferrin counteracts this effect. J Pediatr. (2018) 193:62–67.e1. doi: 10.1016/j.jpeds.2017.09.080
121. Santos VS, Freire MS, Santana RNS, Martins-Filho PRS, Cuevas LE, Gurgel RQ. Association between histamine-2 receptor antagonists and adverse outcomes in neonates: a systematic review and meta-analysis. PLoS One. (2019) 14:e0214135. doi: 10.1371/journal.pone.0214135
122. Becerra MR, Tantaleán JA, Suárez VJ, Alvarado MC, Candela JL, Urcia FC. Epidemiologic surveillance of nosocomial infections in a pediatric intensive care unit of a developing country. BMC Pediatr. (2010) 10:66. doi: 10.1186/1471-2431-10-66
123. Cashen K, Reeder R, Dalton HJ, Berg RA, Shanley TP, Newth CJL, et al. Acquired infection during neonatal and pediatric extracorporeal membrane oxygenation. Perfusion. (2018) 33:472–82. doi: 10.1177/0267659118766436
124. Kaufman DA. “Getting to zero”: preventing invasive Candida infections and eliminating infection-related mortality and morbidity in extremely preterm infants. Early Hum Dev. (2012) 88(Suppl 2):S45–49. doi: 10.1016/S0378-3782(12)70014-2
125. Yoshida Y. Cytochrome P450 of fungi: primary target for azole antifungal agents. Curr Top Med Mycol. (1988) 2:388–418. doi: 10.1007/978-1-4612-3730-3_11
126. Manzoni P, Stolfi I, Pugni L, Decembrino L, Magnani C, Vetrano G, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. (2007) 356:2483–95. doi: 10.1056/NEJMoa065733
127. Benjamin DK, Hudak ML, Duara S, Randolph DA, Bidegain M, Mundakel GT, et al. Effect of fluconazole prophylaxis on candidiasis and mortality in premature infants: a randomized clinical trial. JAMA. (2014) 311:1742–9. doi: 10.1001/jama.2014.2624
128. Healy CM, Campbell JR, Zaccaria E, Baker CJ. Fluconazole prophylaxis in extremely low birth weight neonates reduces invasive candidiasis mortality rates without emergence of fluconazole-resistant Candida species. Pediatrics. (2008) 121:703–10. doi: 10.1542/peds.2007-1130
129. Kaufman DA, Morris A, Gurka MJ, Kapik B, Hetherington S. Fluconazole prophylaxis in preterm infants: a multicenter case-controlled analysis of efficacy and safety. Early Hum Dev. (2014) 90(Suppl 1):S87–90. doi: 10.1016/S0378-3782(14)70026-X
130. Silva-Rios J, Camargos P, Correa L, Romanelli R. Prophylactic regimens with fluconazole for candidiasis in neonates under 1.500g: a retrospective chart review of two cohorts. J Neonatal Perinatal Med. (2019) 12:29–36. doi: 10.3233/NPM-17121
131. Ericson JE, Kaufman DA, Kicklighter SD, Bhatia J, Testoni D, Gao J, et al. Fluconazole prophylaxis for the prevention of candidiasis in premature infants: a meta-analysis using patient-level data. Clin Infect Dis. (2016) 63:604–10. doi: 10.1093/cid/ciw363
132. Xie J, Zeng J, Zheng S. The efficacy and safety of fluconazole in preventing invasive fungal infection in very low birth weight infants: a systematic review and meta-analysis. Ital J Pediatr. (2023) 49:51. doi: 10.1186/s13052-023-01460-5
133. Hope WW, Castagnola E, Groll AH, Roilides E, Akova M, Arendrup MC, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. (2012) 18(Suppl 7):38–52. doi: 10.1111/1469-0691.12040
134. Hornik CD, Bondi DS, Greene NM, Cober MP, John B. Review of fluconazole treatment and prophylaxis for invasive candidiasis in neonates. J Pediatr Pharmacol Ther. (2021) 26:115–22. doi: 10.5863/1551-6776-26.2.115
135. Autmizguine J, Smith PB, Prather K, Bendel C, Natarajan G, Bidegain M, et al. Effect of fluconazole prophylaxis on Candida fluconazole susceptibility in premature infants. J Antimicrob Chemother. (2018) 73:3482–7. doi: 10.1093/jac/dky353
136. Chen I-L, Chiu N-C, Chi H, Hsu C-H, Chang J-H, Huang DT-N, et al. Changing of bloodstream infections in a medical center neonatal intensive care unit. J Microbiol Immunol Infect. (2017) 50:514–20. doi: 10.1016/j.jmii.2015.08.023
137. Kirpal H, Gathwala G, Chaudhary U, Sharma D. Prophylactic fluconazole in very low birth weight infants admitted to neonatal intensive care unit: randomized controlled trial. J Matern Fetal Neonatal Med. (2016) 29:624–8. doi: 10.3109/14767058.2015.1013933
138. Aydemir C, Oguz SS, Dizdar EA, Akar M, Sarikabadayi YU, Saygan S, et al. Randomised controlled trial of prophylactic fluconazole versus nystatin for the prevention of fungal colonisation and invasive fungal infection in very low birth weight infants. Arch Dis Child Fetal Neonatal Ed. (2011) 96:F164–168. doi: 10.1136/adc.2009.178996
139. Ozturk MA, Gunes T, Koklu E, Cetin N, Koc N. Oral nystatin prophylaxis to prevent invasive candidiasis in neonatal intensive care unit. Mycoses. (2006) 49:484–92. doi: 10.1111/j.1439-0507.2006.01274.x
140. Sims ME, Yoo Y, You H, Salminen C, Walther FJ. Prophylactic oral nystatin and fungal infections in very-low-birthweight infants. Am J Perinatol. (1988) 5:33–6. doi: 10.1055/s-2007-999649
141. Rundjan L, Wahyuningsih R, Oeswadi CA, Marsogi M, Purnamasari A. Oral nystatin prophylaxis to prevent systemic fungal infection in very low birth weight preterm infants: a randomized controlled trial. BMC Pediatr. (2020) 20:170. doi: 10.1186/s12887-020-02074-0
142. Austin N, Cleminson J, Darlow BA, McGuire W. Prophylactic oral/topical non-absorbed antifungal agents to prevent invasive fungal infection in very low birth weight infants. Cochrane Database Syst Rev. (2015) 2015:CD003478. doi: 10.1002/14651858.CD003478.pub5
143. Al-Matary A, Almahmoud L, Masmoum R, Alenezi S, Aldhafiri S, Almutairi A, et al. Oral nystatin prophylaxis for the prevention of fungal colonization in very low birth weight infants: a systematic review and meta-analysis of randomized controlled trials. Cureus. (2022) 14:e28345. doi: 10.7759/cureus.28345
144. Violaris K, Carbone T, Bateman D, Olawepo O, Doraiswamy B, LaCorte M. Comparison of fluconazole and nystatin oral suspensions for prophylaxis of systemic fungal infection in very low birthweight infants. Am J Perinatol. (2010) 27:73–8. doi: 10.1055/s-0029-1224871
145. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat rev Gastroenterol Hepatol. (2014) 11:506. doi: 10.1038/nrgastro.2014.66
146. Cong L, Chen C, Mao S, Han Z, Zhu Z, Li Y. Intestinal bacteria-a powerful weapon for fungal infections treatment. Front Cell Infect Microbiol. (2023) 13:1187831. doi: 10.3389/fcimb.2023.1187831
147. Hou K, Wu Z-X, Chen X-Y, Wang J-Q, Zhang D, Xiao C, et al. Microbiota in health and diseases. Signal Transduct Target Ther. (2022) 7:135. doi: 10.1038/s41392-022-00974-4
148. Alshaikh B, Samara J, Moossavi S, Ferdous T, Soraisham A, Dersch-Mills D, et al. Multi-strain probiotics for extremely preterm infants: a randomized controlled trial. Pediatr Res. (2022) 92:1663–70. doi: 10.1038/s41390-022-02004-z
149. Oncel MY, Arayici S, Sari FN, Simsek GK, Yurttutan S, Erdeve O, et al. Comparison of lactobacillus reuteri and nystatin prophylaxis on Candida colonization and infection in very low birth weight infants. J Matern Fetal Neonatal Med. (2015) 28:1790–4. doi: 10.3109/14767058.2014.968842
150. Demirel G, Celik IH, Erdeve O, Saygan S, Dilmen U, Canpolat FE. Prophylactic Saccharomyces boulardii versus nystatin for the prevention of fungal colonization and invasive fungal infection in premature infants. Eur J Pediatr. (2013) 172:1321–6. doi: 10.1007/s00431-013-2041-4
151. Hu H-J, Zhang G-Q, Zhang Q, Shakya S, Li Z-Y. Probiotics prevent Candida colonization and invasive fungal sepsis in preterm neonates: a systematic review and meta-analysis of randomized controlled trials. Pediatr Neonatol. (2017) 58:103–10. doi: 10.1016/j.pedneo.2016.06.001
152. Gray KD, Messina JA, Cortina C, Owens T, Fowler M, Foster M, et al. Probiotic use and safety in the neonatal intensive care unit: a matched cohort study. J Pediatr. (2020) 222:59–64.e1. doi: 10.1016/j.jpeds.2020.03.051
153. Kulkarni T, Majarikar S, Deshmukh M, Ananthan A, Balasubramanian H, Keil A, et al. Probiotic sepsis in preterm neonates-a systematic review. Eur J Pediatr. (2022) 181:2249–62. doi: 10.1007/s00431-022-04452-5
154. Dani C, Coviello C C, Corsini I I, Arena F, Antonelli A, Rossolini GM. Lactobacillus sepsis and probiotic therapy in newborns: two new cases and literature review. AJP Rep. (2016) 6:e25–29. doi: 10.1055/s-0035-1566312
155. Esaiassen E, Cavanagh P, Hjerde E, Simonsen GS, Støen R, Klingenberg C. Bifidobacterium longum subspecies infantis bacteremia in 3 extremely preterm infants receiving probiotics. Emerg Infect Dis. (2016) 22:1664–6. doi: 10.3201/eid2209.160033
156. Bertelli C, Pillonel T, Torregrossa A, Prod’hom G, Fischer CJ, Greub G, et al. Bifidobacterium longum bacteremia in preterm infants receiving probiotics. Clin Infect Dis. (2015) 60:924–7. doi: 10.1093/cid/ciu946
157. Conesa C, Sánchez L, Rota C, Pérez M-D, Calvo M, Farnaud S, et al. Isolation of lactoferrin from milk of different species: calorimetric and antimicrobial studies. Comp Biochem Physiol B Biochem Mol Biol. (2008) 150:131–9. doi: 10.1016/j.cbpb.2008.02.005
158. Embleton ND, Berrington JE, McGuire W, Stewart CJ, Cummings SP. Lactoferrin: antimicrobial activity and therapeutic potential. Semin Fetal Neonatal Med. (2013) 18:143–9. doi: 10.1016/j.siny.2013.02.001
159. Lu J, Francis J, Doster RS, Haley KP, Craft KM, Moore RE, et al. Lactoferrin: a critical mediator of both host immune response and antimicrobial activity in response to streptococcal infections. ACS Infect Dis. (2020) 6:1615–23. doi: 10.1021/acsinfecdis.0c00050
160. Legrand D. Overview of lactoferrin as a natural immune modulator. J Pediatr. (2016) 173:S10–15. doi: 10.1016/j.jpeds.2016.02.071
161. Lingappan K, Arunachalam A, Pammi M. Lactoferrin and the newborn: current perspectives. Expert Rev Anti Infect Ther. (2013) 11:695–707. doi: 10.1586/14787210.2013.811927
162. ELFIN trial investigators group. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet. (2019) 393:423–33. doi: 10.1016/S0140-6736(18)32221-9
163. Fernandes KE, Carter DA. The antifungal activity of lactoferrin and its derived peptides: mechanisms of action and synergy with drugs against fungal pathogens. Front Microbiol. (2017) 8:2. doi: 10.3389/fmicb.2017.00002
164. Farnaud S, Spiller C, Moriarty LC, Patel A, Gant V, Odell EW, et al. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol Lett. (2004) 233:193–9. doi: 10.1016/j.femsle.2004.01.039
165. Hunter HN, Demcoe AR, Jenssen H, Gutteberg TJ, Vogel HJ. Human lactoferricin is partially folded in aqueous solution and is better stabilized in a membrane mimetic solvent. Antimicrob Agents Chemother. (2005) 49:3387–95. doi: 10.1128/AAC.49.8.3387-3395.2005
166. Alexander DB, Iigo M, Yamauchi K, Suzui M, Tsuda H. Lactoferrin: an alternative view of its role in human biological fluids. Biochem Cell Biol. (2012) 90:279–306. doi: 10.1139/o2012-013
167. Manzoni P, Stolfi I, Messner H, Cattani S, Laforgia N, Romeo MG, et al. Bovine lactoferrin prevents invasive fungal infections in very low birth weight infants: a randomized controlled trial. Pediatrics. (2012) 129:116–23. doi: 10.1542/peds.2011-0279
168. Razak A, Hussain A. Lactoferrin supplementation to prevent late-onset sepsis in preterm infants: a meta-analysis. Am J Perinatol. (2021) 38:283–90. doi: 10.1055/s-0039-1696676
169. Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. (2020) 3:CD 007137. doi: 10.1002/14651858.CD007137.pub6
170. Tarnow-Mordi WO, Abdel-Latif ME, Martin A, Pammi M, Robledo K, Manzoni P, et al. The effect of lactoferrin supplementation on death or major morbidity in very low birthweight infants (LIFT): a multicentre, double-blind, randomised controlled trial. Lancet Child Adolesc Health. (2020) 4:444–54. doi: 10.1016/S2352-4642(20)30093-6
171. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. (2015) 373:1445–56. doi: 10.1056/NEJMra1315399
172. Han T, Qiu M, Niu X, Wang S, Wang F, Cao J, et al. End-organ damage from neonatal invasive fungal infection: a 14-year retrospective study from a tertiary center in China. BMC Infect Dis. (2024) 24:521. doi: 10.1186/s12879-024-09360-7
173. Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc. (2017) 6:S3–S11. doi: 10.1093/jpids/pix046
174. Adams-Chapman I, Bann CM, Das A, Goldberg RN, Stoll BJ, Walsh MC, et al. Neurodevelopmental outcome of extremely low birth weight infants with Candida infection. J Pediatr. (2013) 163:961–967.e3. doi: 10.1016/j.jpeds.2013.04.034
175. Schelonka RL, Moser SA. Time to positive culture results in neonatal Candida septicemia. J Pediatr. (2003) 142:564–5. doi: 10.1067/mpd.2003.188
176. Greenberg RG, Benjamin DK, Gantz MG, Cotten CM, Stoll BJ, Walsh MC, et al. Empiric antifungal therapy and outcomes in extremely low birth weight infants with invasive candidiasis. J Pediatr. (2012) 161:264–269.e2. doi: 10.1016/j.jpeds.2012.01.053
177. Piper L, Smith PB, Hornik CP, Cheifetz IM, Barrett JS, Moorthy G, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. (2011) 30:375–8. doi: 10.1097/INF.0b013e318202cbb3
178. Cornely OA, Sprute R, Bassetti M, Chen SC-A, Groll AH, Kurzai O, et al. Global guideline for the diagnosis and management of candidiasis: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect Dis. (2025) 25:e280–93. doi: 10.1016/S1473-3099(24)00749-7
179. Carolus H, Pierson S, Lagrou K, Van Dijck P. Amphotericin B and other polyenes-discovery, clinical use, mode of action and drug resistance. J Fungi. (2020) 6:321. doi: 10.3390/jof6040321
180. Le J, Adler-Shohet FC, Nguyen C, Lieberman JM. Nephrotoxicity associated with amphotericin B deoxycholate in neonates. Pediatr Infect Dis J. (2009) 28:1061–3. doi: 10.1097/INF.0b013e3181af6201
181. Botero-Calderon L, Benjamin DK, Cohen-Wolkowiez M. Advances in the treatment of invasive neonatal candidiasis. Expert Opin Pharmacother. (2015) 16:1035–48. doi: 10.1517/14656566.2015.1031108
182. Wynn JL, Tan S, Gantz MG, Das A, Goldberg RN, Adams-Chapman I, et al. Outcomes following candiduria in extremely low birth weight infants. Clin Infect Dis. (2012) 54:331–9. doi: 10.1093/cid/cir800
183. Lestner JM, Smith PB, Cohen-Wolkowiez M, Benjamin DK, Hope WW. Antifungal agents and therapy for infants and children with invasive fungal infections: a pharmacological perspective. Br J Clin Pharmacol. (2013) 75:1381–95. doi: 10.1111/bcp.12025
184. Hope WW, Mickiene D, Petraitis V, Petraitiene R, Kelaher AM, Hughes JE, et al. The pharmacokinetics and pharmacodynamics of micafungin in experimental hematogenous Candida meningoencephalitis: implications for echinocandin therapy in neonates. J Infect Dis. (2008) 197:163–71. doi: 10.1086/524063
185. Manzoni P, Wu C, Tweddle L, Roilides E. Micafungin in premature and non-premature infants: a systematic review of 9 clinical trials. Pediatr Infect Dis J. (2014) 33:e291–298. doi: 10.1097/INF.0000000000000434
Keywords: antifungal resistance, invasive Candida infections (ICIs), newborn infants, pathogens, prevention strategies, risk factors, treatments
Citation: Zhang D, Xie D, Yuan H, He N, Dong W and Lei X (2025) Addressing the silent threat: managing invasive Candida infections in hospitalized newborns. Front. Pediatr. 13:1613832. doi: 10.3389/fped.2025.1613832
Received: 17 April 2025; Accepted: 8 July 2025;
Published: 17 July 2025.
Edited by:
Jogender Kumar, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Tushar B. Parikh, KEM Hospital Research Centre, IndiaRobert Lewis Schelonka, Oregon Health and Science University, United States
Anindya Kumar Saha, Institute of Postgraduate Medical Education & Research, India
Copyright: © 2025 Zhang, Xie, Yuan, He, Dong and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Dong, ZG9uZ3dlbmJpbjIwMjBAc3dtdS5lZHUuY24=; Xiaoping Lei, bGVpeGlhb3BpbmcyMDIwQHN3bXUuZWR1LmNu
†These authors have contributed equally to this work
 Deshuang Zhang
Deshuang Zhang Dongke Xie3,†
Dongke Xie3,† Haokun Yuan
Haokun Yuan Na He
Na He Wenbin Dong
Wenbin Dong Xiaoping Lei
Xiaoping Lei