- 1College of Nursing, Henan University of Chinese Medicine, Zhengzhou, China
- 2Pediatric Hospital of the First Affiliated Hospital of Henan University of Traditional Chinese Medicine, Zhengzhou, China
Objective: To investigate the current status of comorbid anxiety and depression in adolescents with chronic kidney disease and to analyze their associated factors.
Methods: 105 adolescents with chronic kidney disease, hospitalized in the First Affiliated Hospital of Henan University of Traditional Chinese Medicine from May 2022 to May 2023, were selected through convenience sampling. A general information questionnaire, anxiety self-assessment scale, and depression self-assessment scale were used. Factors associated with anxiety and depression were analyzed using univariate analysis, and significant variables underwent multinomial logistic regression.
Results: Among the 105 adolescents, 11 cases (10.5%) had anxiety alone, 6 cases (5.7%) had depression alone, and 26 cases (24.8%) presented both anxiety and depression. Multinomia logistic regression analysis revealed that the illness duration (>12 months) (OR = 34.114), duration of hormone treatment (≥12 months) (OR = 37.585), duration of immunotherapy (≥12 months) (OR = 12.700), and OR values greater than 1 indicate that illness duration (>12 months), duration of hormone treatment (≥12 months), and the duration of immunotherapy (≥12 months) are independent associated factors for nanxiety and depression.
Conclusion: In this cross-sectional cohort study, the illness duration, duration of hormone treatment and duration of immunotherapy are independent associated factors for anxiety and depression. Psychological screening should be prioritized for adolescents with these clinical features, and relevant measures should be taken to reduce the incidence of anxiety and depression in adolescents with CKD. Future longitudinal studies could begin by elucidating whether modifying treatment can reduce mental health risks, taking into account potential bidirectional effects between disease control and mental health.
1 Introduction
Chronic kidney disease (CKD) is a progressive disease with irreversible changes in kidney structure and function caused by various factors (1). It is characterized by lengthy diagnostic and therapeutic cycles, being difficult to cure, and often leading to psychological abnormalities in patients, with depression and anxiety being common psychological problems. Recent years show CKD prevalence in children ranging from 14.9/1,000,000 to 118.8/1,000,000 (2), becoming a serious public health concern as pediatric kidney disease incidence increases annually (3–5). Adolescence is a critical period for children's growth and development. It is not only a peak period for physical development, but also a critical period for the gradual formation of personality, cognition, and worldview. However, adolescents are sensitive to external stimuli and have relatively low tolerance for setbacks and failures (6). Adolescents with CKD face developmental challenges while managing disease-related stressors, potentially leading to psychological and social dysfunction (7). Studies indicate that the global prevalence of anxiety disorders in children and adolescents is 2,235.1 per 100,000 (8), with an anxiety detection rate of 34.6% in adolescents, depressed mood at 28% (9–11), and depression detection rates exceeding 19.7% (9, 12). Current research on anxiety and depression in CKD primarily focuses on adults, with fewer studies examining adolescents (1, 13). There are already many validated anxiety and depression assessment scales, but these tools have mainly been validated in healthy or generally ill adolescents, with a lack of research in adolescents with CKD (14, 15). General scales may not capture stressors unique to adolescents with CKD, such as fear of dialysis/transplantation, body image concerns, academic/social disruption, and concerns about the future, and therefore may not fully reflect their psychological distress. Therefore, this study primarily investigates the current status of anxiety, depression, and comorbidity among adolescents with CKD, and explores their influencing factors. The aim is to provide a basis for the clinical prevention and treatment of anxiety, depression, and comorbidity among adolescents with chronic kidney disease, and to provide a reference for the formulation of psychological intervention treatment plans for adolescents with CKD in the future. The findings are as follows:
2 Information and methods
2.1 Study subjects
1. Inclusion criteria:
a. Children with CKD who have been regularly followed up for ≥6 months at our hospital's Pediatric Nephrology Center;
b. Meet the diagnostic criteria of CKD in Chinese and Western medicine, and the diagnosis is clear;
c. Age ≥12 years old and ≤19 years old;
d. Duration of the disease ≥6 months;
e. Children and their family members signed an informed consent form and voluntarily enrolled in the study.
2. Exclusion criteria:
a. Children who do not meet the diagnostic criteria of CKD in Chinese and Western medicine;
b. Children with CKD whose family members do not cooperate well and cannot maintain clinic attendance;
c. Children with a history of anxiety and depression.
A total of 354 patients who met the criteria were consecutively enrolled, and 123 agreed to participate. A total of 18 participants withdrew for various reasons, resulting in a final study sample of 105 participants.
2.2 Investigative instruments
2.2.1 General information survey
A self-designed general information survey form was used, which included the following items: sex (male, female), age, only child (no, yes), educational Background (Primary school, junior high school, high school, university, college, other), family residence (countryside, cities and towns, municipalities), family status (single-parent family, two-parent family, stepfamily), residence during school (attend a day school, homestay, suspend schooling), parents' educational level (junior high school or below, high school/secondary school, specialized training school, Undergraduate and above), illness duration (6–12 months, more than one year), Type of disease (Nephrotic Syndrome, Purpura Nephritis, Lupus Nephritis, Glomerulonephritis, Renal Failure, Membranous Nephropathy, IgA Nephropathy), Duration of hormone treatment (<3 months, 3–5 months, 6–11 months, ≥12 months), Duration of immunosuppressive therapy (<3 months, 3–5 months, 6–11 months, ≥12 months, not have), Duration of kidney replacement therapy (<3 months, ≥3 months, not have), disease follow-up period (2 weeks, 1 month, 3 months, 6 months). If the child's parents are unaware of certain personal information, the child may seek assistance in completing the form.
2.2.2 Anxiety and depression scales
The Self-Administered Scale of Anxiety (SAS) and Depression Self-Assessment Scale (SDS) were developed by Dr. Zung in 1971 and 1965, respectively, to provide a brief, efficient, self-rated screening tool for anxiety and depressive symptoms in primary care and psychiatric clinical practice. They were designed to rapidly identify individuals who may need further professional evaluation (16, 17). Since their release, SAS and SDS have been widely used worldwide, including in China, especially in large-scale epidemiological investigations, clinical studies, and preliminary screening, favored for their ease of operation, ease of understanding, and implementation. Each scale comprises 20 self-rating scales, addressing the respondent's experience in the preceding week. Examples include “I feel more nervous and anxious than usual” and “I feel depressed and low in mood,” with response options rarely, sometimes, often, and continuously, scored 1–4 (total score 20–80 points). Total scores are converted to index scores using the formula: index = (raw score/80) × 100, and the index score ranges from 25 to 100, Based on clinical experience and early research data, Zung proposed the cut-off criteria of index scores (SAS ≥ 50, SDS ≥ 53) in the original literature to indicate the level of significant anxiety/depression symptoms. Multiple studies have evaluated the reliability of the SAS/SDS. In the general population and in a variety of clinical samples, the Cronbach's α coefficients are typically reported for the SAS between 0.70 and 0.85. The Cronbach's α coefficient for SDS showed a similar level, typically between 0.79 and 0.88 (18, 19).
2.3 Survey method
A questionnaire-based anonymous survey was used. Hospitalized children who met the inclusion criteria received instructions on questionnaire completion from the investigator on the second or third day of hospitalization. Parents were instructed to minimize their participation, and the children completed the survey independently.
2.4 Pre-survey
Using random sampling, a pre-survey was conducted with 15 children of different ages who met the inclusion criteria. The survey revealed that the average completion time was 119–183 s. Children could understand and complete the questionnaire independently, while family members demonstrated high cooperation and minimal participation.
2.5 Data collection and quality control
1. An anonymous QR code was generated using the Questionnaire Star platform, which was set to not collect any personal information. Patients scanned the code themselves and completed the questionnaire independently in a relatively private space, ensuring anonymity from the outset;
2. Researchers present are strictly limited to explaining the process, clarifying the meaning of questions (not answer choices), addressing operational queries, monitoring progress, and reminding parents not to participate. However, if parental personal information is involved, the primary caregiver may provide relevant answers to the patient, and researchers are not medical staff from the same ward;
3. Researchers cannot view real-time answers and cannot link answers to patients before submission;
4. After submission, success is confirmed via platform notifications or backend status (without reviewing answer content). The platform's mandatory response feature and on-site supervision ensure questionnaire completeness;
5. Data is double-checked after export.
6. A total of 105 questionnaires were distributed, with 105 valid responses collected, resulting in a 100% valid response rate.
2.6 Ethics consideration
The study protocols received informed consent from patients and their families and were approved by the hospital's Ethics Committee.
2.7 Statistical methods
SPSS 22.0 software performed the statistical analysis. Variables underwent normality testing, with normally distributed data expressed as x ± s, Non-normal distributions are represented by the median and quartiles. Qualitative data were described by frequency and percentage. Independent sample t-tests compared two groups, while ANOVA or rank-sum tests compared multiple groups to identify variables affecting anxiety and depression in adolescents with CKD. Multinomial logistic regression analyzed significant variables, with P < 0.05 considered statistically significant.
3 Results
3.1 General information of survey respondents
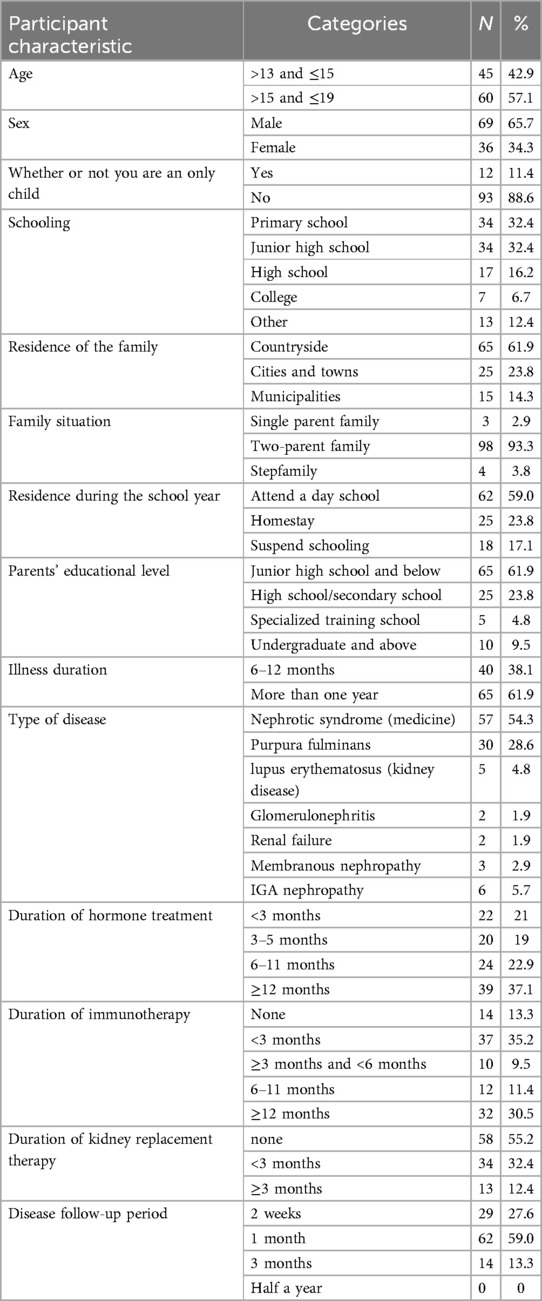
Table 1 describes the demographic and chronic kidney disease-related characteristics of the sample. A total of 105 valid questionnaires were obtained, with a response rate of 100%. Among the study participants, 62 children (59.0%) did not meet the threshold for anxiety or depression, 11 children (10.5%) had anxiety alone, 6 children (5.7%) had only depression, and 26 children (24.8%) had both anxiety and depression. Among them, 37 children had anxiety symptoms, with a detection rate of 35.2%; 32 children had depression symptoms, with a depression detection rate of 30.5%. See Table 1.
3.2 Univariate analysis of anxiety and depression scores among adolescents with chronic kidney disease with different demographic characteristics
Schooling, parents' educational level, illness duration, duration of hormone treatment, duration of immunotherapy, and duration of kidney replacement were significant factors associated with anxiety symptoms (P < 0.05); schooling, illness duration, duration of hormone treatment, duration of immunotherapy were significant factors associated with depression symptoms (P < 0.05) see Table 2.
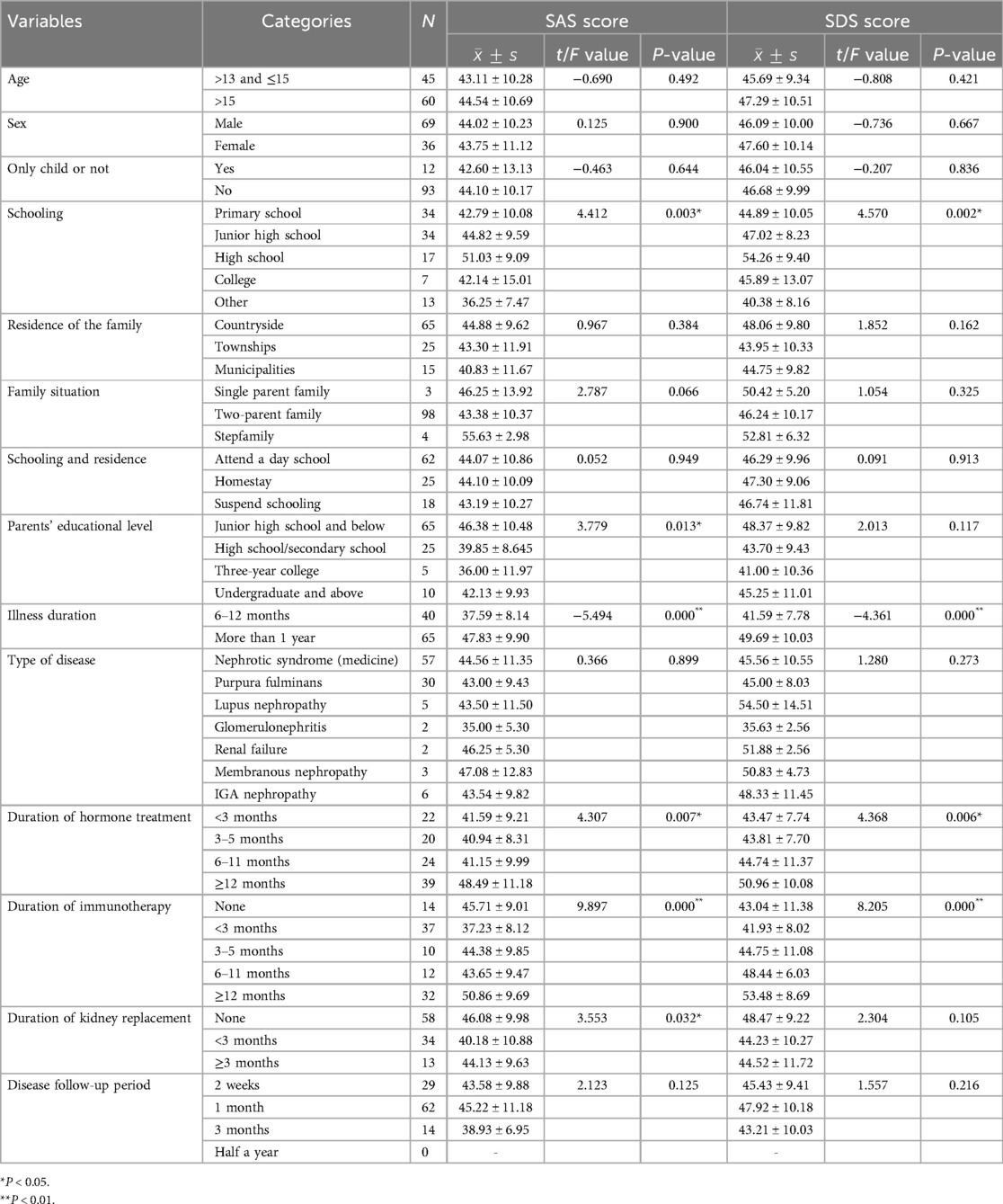
Table 2. Univariate analysis of anxiety and depression scores in adolescents with chronic kidney disease (n = 105, `x ± s, points).
3.3 Multinomial logistic analysis of factors influencing anxiety and depression levels
Taking anxiety, depression, anxiety and depression as dependent variables, the statistically significant variables in the univariate analysis, namely disease-related variables (illness duration, duration of hormone treatment, duration of immunotherapy, and use of renal replacement therapy), and demographic variables (schooling, parents' educational level) as independent variables, were used for multinomial logistic regression analysis. The results showed that:
In isolated anxiety, patients with a disease duration of >12 months had an odds ratio for anxiety that was nearly 13 times higher than that of patients with a disease duration of 6–12 months (reference group). In isolated depression, the odds of having depression among patients with a disease duration of >12 months were nearly 8 times higher than those with a disease duration of 6–12 months (reference group), but this difference was not statistically significant; in anxiety and depression, the odds of having anxiety and depression among patients with a disease duration of >12 months were nearly 34.5 times higher than those with a disease duration of 6–12 months (reference group).
In isolated anxiety, patients who used hormones for 3–5 months, 6–11 months, and ≥12 months had odds of anxiety that were nearly 2-fold, 2-fold, and 3.5-fold higher than those who used hormones for less than 3 months (reference group), but these differences were not statistically significant. In isolated depression, patients who used hormones for 3–5 months, 6–11 months, and ≥12 months had odds of depression that were approximately 0.7 times, 0.5 times, and 1.5 times those of patients who used hormones for less than 3 months (reference group), respectively, but these differences were not statistically significant; In anxiety and depression, the odds of having anxiety and depression among patients with hormone use duration ≥12 months were approximately 38 times higher than those with hormone use duration <3 months (reference group); Patients who used hormones for 3–5 months and 6–11 months had odds of anxiety and depression that were approximately 0.7 times and 3.5 times those of the reference group (less than 3 months), respectively, but these differences were not statistically significant.
In isolated anxiety, patients who used immunosuppressants for less than 3 months, 3–5 months, 6–11 months, and ≥12 months had odds ratios of approximately 1-fold, 7-fold, 9.5-fold, and 2-fold, respectively, compared to those who used immunosuppressants for other durations (reference group), but these differences were not statistically significant; In isolated depression, patients who used immunosuppressants for less than 3 months, 3–5 months, 6–11 months, and ≥12 months had odds ratios of approximately 0.4, 3, 7.5, and 0.7, respectively, compared to those who used immunosuppressants for none (reference group), but these differences were not statistically significant. In anxiety and depression, the odds ratio for patients with immunosuppressant use duration ≥12 months was approximately 13 times that of the reference group (other durations). For patients with anxiety and depression who used immunosuppressants for less than 3 months, 3–5 months, and 6–11 months, the odds were approximately 0.2 times, 1 times, and 1 times, respectively, compared to those who used immunosuppressants for other durations (reference group), but these differences were not statistically significant.
In isolated anxiety, the odds of anxiety in patients with renal replacement therapy duration of less than 3 months and 3–5 months were nearly 6 times and 1.5 times higher, respectively, than in the reference group (other durations), but these differences were not statistically significant; In isolated depression, the odds of having depression during the first 3 months and 3–5 months of renal replacement therapy were nearly 2.5 times and 1.5 times higher, respectively, compared to other durations of renal replacement therapy (reference group), but these differences were not statistically significant; In anxiety and depression, the odds of anxiety and depression in patients with a renal replacement therapy duration of less than 3 months and 3–5 months were approximately 2 times and 1.5 times higher, respectively, compared to the reference group (other durations), but these differences were not statistically significant (see Table 3).
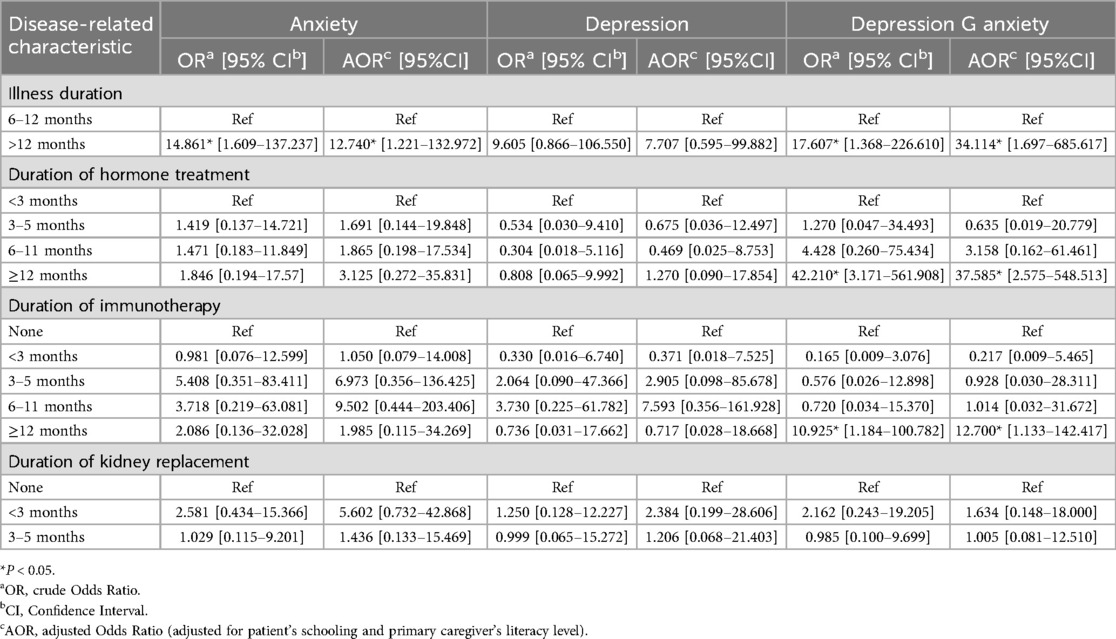
Table 3. Multinomial regression of associations between disease-related characteristics and anxiety and depression Among adolescents with chronic kidney disease.
4 Discussion
Due to the complexity of CKD's etiology, the extended treatment period, and the challenges in diagnosis and treatment, patients frequently experience anxiety, depression, and other negative emotions that adversely affect therapeutic outcomes (20). This study's results revealed that Among 105 adolescents with CKD, 37 (35.2%) had anxiety symptoms and 32 (30.5%) had depression symptoms,the illness duration, duration of hormone and duration of immunotherapy have factors associated with anxiety and depression. These findings align with both international and domestic studies (21, 22).
Studies have shown (23) that care levels help reduce anxiety and depression in adolescents, and parents' education and cultural background may influence their understanding and support of their child's illness. Parents with higher education levels better analyze information, show greater initiative, and pay more attention to their children's illnesses. They also demonstrate higher acceptance of healthcare professionals' health promotion guidance (24). Conversely, parents with lower education levels may lack disease knowledge and coping strategies, potentially increasing their children's adverse emotions. In this study, 61.9% of caregivers had junior high school or lower education levels. Caregivers with lower educational attainment may have a less complete understanding of disease progression and prognosis (25). This limited understanding has been associated with caregiver distress and adjustment difficulties in other studies (26), which could, in turn, potentially contribute to higher levels of anxietyand depression observed in their children in this study.
CKD is a complex condition characterized by prolonged treatment cycles and a high risk of recurrence. According to Kogon et al. (27), the timing of CKD diagnosis may correlate with anxiety and depression, suggesting that patients' burden and stress increase over time, leading to these conditions. CKD's long-term progressive nature, regular follow-ups, disease progression, and continuous medication use affect children's adaptability and interpersonal skills. According to Nair's study, school-age adolescents with CKD often cannot attend school normally due to recurrent episodes of the disease (28). Frequent follow-up visits and outpatient appointments lead to missed classes, school absences, or missed group activities, depriving adolescents of opportunities to develop essential social and academic skills the prolonged course of the disease imposes a heavy psychological burden on adolescents (29, 30). Roshan (31) found that adolescents' cognitive and personality development is not yet mature, making them more susceptible to external stimuli. Teenagers with CKD are in a crucial period of developing interpersonal communication skills and social adaptability. Due to the recurrence of the disease, they are unable to attend school regularly, and the time spent with classmates and teachers is greatly reduced. These interpersonal relationship deficiencies and limited relationships will aggravate negative emotions. Meanwhile, the age group of this study mainly consists of junior and senior high school students, who have heavy learning tasks and great learning pressure. Frequent medical visits lead to repeated absences from classes, which affects studies. As grades decline, pressure increases and learning difficulties arise, anxiety and depression symptoms may be triggered or exacerbated. Therefore, the longer the course of the illness, the more likely it is to lead to the emergence of negative emotions.
CKD is mainly treated with hormone drugs, but long-term use of hormones can cause changes in the body, thereby triggering anxiety related to appearance. According to Trautmann et al. (32), children receiving long-term glucocorticoid maintenance therapy usually have full-moon faces, buffalo backs, and hirsute skin, while adolescent children are more self-conscious and care more about their external image. Children with chronic diseases often experience side effects related to their appearance due to long-term steroid medication, leading to labels such as “sick,” “unpopular,” or “boring,” which can result in rejection, mockery, isolation, and bullying by peers (33). Additionally,in the face of changes in appearance and body shape brought about by medication, they develop more negative emotions. This is consistent with the results of this study. The children in this study are in adolescence, when psychological changes are in a more sensitive stage, and they pay more attention to their self-image. Due to the long-term use of hormonal drugs for disease treatment, appearance anxiety arising from the side effects of the drugs increases the occurrence of anxiety and depression symptoms.
CKD not only requires the use of hormones but also the periodic use of immunosuppressants. The use of immunosuppressants may lead to disorders and suppression of the immune system, increasing the risk of infections and other complications. Restrictions on social activities, sleep, and diet can easily aggravate the anxiety of children with CKD. Additionally, the long-term special treatment of these children and the protective measures required for treatment give them a sense of “disease shame.” Especially on campus, they may be ridiculed because of their disease, become alienated from their peers, and prone to depression (34). For patients with advanced CKD, renal replacement therapy, such as dialysis or kidney transplantation, may be required. This treatment process can significantly impact the child's lifestyle, self-image, and social activities, increasing the risk of anxiety and depressive symptoms. Therefore, healthcare professionals should promptly recognize these factors and take appropriate measures to help reduce anxiety and depression in adolescents with CKD and improve their quality of life and survival.
5 Limitations of this study
Due to the limitations of the conditions, only CKD adolescent patients attending one hospital were selected as research subjects. There may be bias in subject selection; the sample is not representative enough. The sample size included in this study was small and statistically underpowered. These limitations affect the study's conclusions. In future research, the selection scope should be expanded, and the sample size increased to improve the conclusions' accuracy. This study is investigative; future pilot studies could examine the effects of targeted nursing interventions on anxiety and depression in adolescents with CKD and develop standardized treatment plans to reduce their anxiety and depression.
6 Conclusions
In summary, the prevalence of comorbid anxiety and depression among CKD adolescents is high. The illness duration, duration of hormone treatment and duration of immunotherapy are independent associated factors for anxiety and depression. Clinical measures should address these factors, monitor CKD adolescents' mood changes dynamically, actively support family and school participation, assist in stress relief, and provide psychological support through active listening to reduce anxiety and depression incidence in adolescents with CKD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Henan University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants or the participants' legal guardians/next of kin.
Author contributions
CB: Investigation, Writing – original draft, Software, Methodology, Formal analysis. MW: Conceptualization, Resources, Writing – review & editing, Funding acquisition, Supervision. SH: Data curation, Writing – original draft. JF: Writing – review & editing, Investigation, Visualization. GN: Investigation, Writing – review & editing, Supervision. WZ: Validation, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Henan Province Chinese medicine scientific research special projects (2022ZY1025).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bikbov B, Purcell C, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2020) 395(10225):709–33. doi: 10.1016/s0140-6736(20)30045-3
2. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. (2012) 27(3):363–73. doi: 10.1007/s00467-011-1939-1
3. Tu J, Chen CY, Yang HX, Jia Y, Geng HY, Li HR. Clinical presentation and prognosis in children over 10-year-old with primary nephrotic syndrome. Zhonghua Er Ke Za Zhi. (2023) 61(8):708–13. doi: 10.3760/cma.j.cn112140-20230104-00007
4. Alenazi SA. Incidence and pathological patterns of nephrotic syndrome among infants and children: a systematic review. Cureus. (2024) 16(4):e58331. doi: 10.7759/cureus.58331
5. Shlipak MG, Tummalapalli SL, Boulware LE, Grams ME, Ix JH, Jha V, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. (2021) 99(1):34–47. doi: 10.1016/j.kint.2020.10.012
6. Brent DA, Brunwasser SM, Hollon SD, Weersing VR, Clarke GN, Dickerson JF, et al. Effect of a cognitive-behavioral prevention program on depression 6 years after implementation among at-risk adolescents: a randomized clinical trial. JAMA Psychiatry. (2015) 72(11):1110–8. doi: 10.1001/jamapsychiatry.2015.1559
7. Stapersma L, van den Brink G, Szigethy EM, Escher JC, Utens E. Systematic review with meta-analysis: anxiety and depression in children and adolescents with inflammatory bowel disease. Aliment Pharmacol Ther. (2018) 48(5):496–506. doi: 10.1111/apt.14865
8. Piao J, Huang Y, Han C, Li Y, Xu Y, Liu Y, et al. Alarming changes in the global burden of mental disorders in children and adolescents from 1990 to 2019: a systematic analysis for the Global Burden of Disease study. Eur Child Adolesc Psychiatry. (2022) 31(11):1827–45. doi: 10.1007/s00787-022-02040-4
9. Rao WW, Xu DD, Cao XL, Wen SY, Che WI, Ng CH, et al. Prevalence of depressive symptoms in children and adolescents in China: a meta-analysis of observational studies. Psychiatry Res. (2019) 272:790–6. doi: 10.1016/j.psychres.2018.12.133
10. Wang S, Sun Q, Zhai L, Bai Y, Wei W, Jia L. The prevalence of depression and anxiety symptoms among overweight/obese and non-overweight/non-obese children/adolescents in China: a systematic review and meta-analysis. Int J Environ Res Public Health. (2019) 16(3):340. doi: 10.3390/ijerph16030340
11. Thapar A, Eyre O, Patel V, Brent D. Depression in young people. Lancet. (2022) 400(10352):617–31. doi: 10.1016/s0140-6736(22)01012-1
12. Ren Z, Zhou G, Wang Q, Xiong W, Ma J, He M, et al. Associations of family relationships and negative life events with depressive symptoms among Chinese adolescents: a cross-sectional study. PLoS One. (2019) 14(7):e0219939. doi: 10.1371/journal.pone.0219939
13. Zhang Z, He P, Liu M, Zhou C, Liu C, Li H, et al. Association of depressive symptoms with rapid kidney function decline in adults with normal kidney function. Clin J Am Soc Nephrol. (2021) 16(6):889–97. doi: 10.2215/cjn.18441120
14. O'Connor S, Ferguson E, Carney T, House E, O'Connor RC. The development and evaluation of the paediatric index of emotional distress (PI-ED). Soc Psychiatry Psychiatr Epidemiol. (2016) 51(1):15–26. doi: 10.1007/s00127-015-1134-y
15. Christensen KS, Haugen W, Sirpal MK, Haavet OR. Diagnosis of depressed young people–criterion validity of WHO-5 and HSCL-6 in Denmark and Norway. Fam Pract. (2015) 32(3):359–63. doi: 10.1093/fampra/cmv011
16. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. (1971) 12(6):371–9. doi: 10.1016/s0033-3182(71)71479-0
17. Zung WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
18. Setyowati A, Chung M-H, Yusuf A. Development of self-report assessment tool for anxiety among adolescents: Indonesian version of the Zung self-rating anxiety scale. J Public Health Africa. (2019) 10(s1):1172. doi: 10.4081/jphia.2019.1172
19. Knight RG, Waal-Manning HJ, Spears GF. Some norms and reliability data for the State–Trait Anxiety Inventory and the Zung Self-Rating Depression scale. Br J Clin Psychol. (1983) 22(Pt 4):245–9. doi: 10.1111/j.2044-8260.1983.tb00610.x
20. Pollock C, Carrero JJ, Kanda E, Ofori-Asenso R, Palmer E, Niklasson A, et al. The lived experience of patients with chronic kidney disease: insights from DISCOVER CKD. Am J Nephrol. (2024) 55(6):618–28. doi: 10.1159/000541064
21. Liu M, Zhang Y, Yang S, Wu Q, Ye Z, Zhou C, et al. Bidirectional relations between depression symptoms and chronic kidney disease. J Affect Disord. (2022) 311:224–30. doi: 10.1016/j.jad.2022.05.104
22. Zhang H. Anxiety and depression in patients with different stages of chronic kidney disease. Chin J Ophthalmol. (2024) 51(01):219-21+48. doi: 10.13479/j.cnki.jip.2024.01.063
23. Geyan G, Jiali M, Wenying G, Ying Z. The influence of family management adjustment and the trajectory of transition readiness in adolescents with chronic diseases and caregivers on their quality of life. Chin J Nurs. (2022) 57(16):1969–75. doi: 10.3761/j.issn.0254-1769.2022.16.008
24. Li N, Hao J, Fu T, Du Y. Evaluating the quality of life of 231 children with primary nephrotic syndrome and assessing parental awareness of the disease. Front Pediatr. (2021) 9:745444. doi: 10.3389/fped.2021.745444
25. Glick AF, Yin HS, Dreyer BP. Health literacy and pediatric health. Stud Health Technol Inform. (2020) 269:72–94. doi: 10.3233/shti200024
26. Fisher HR. The needs of parents with chronically sick children: a literature review. J Adv Nurs. (2001) 36(4):600–7. doi: 10.1046/j.1365-2648.2001.02013.x
27. Kogon AJ, Vander Stoep A, Weiss NS, Smith J, Flynn JT, McCauley E. Depression and its associated factors in pediatric chronic kidney disease. Pediatr Nephrol. (2013) 28(9):1855–61. doi: 10.1007/s00467-013-2497-5
28. Nair N, Prashant Kadatane S, Yaacoub RM, Sandhu JM, Bhavaraju S, Raina R. Challenges and unique considerations of adolescents with chronic kidney disease. Acta Paediatr. (2023) 112(6):1165–76. doi: 10.1111/apa.16752
29. Craven S, Brumbach BH, Richardson KL. Patient- and caregiver-reported factors associated with school absenteeism in children with chronic kidney disease. Pediatr Nephrol. (2023) 38(5):1591–8. doi: 10.1007/s00467-022-05780-2
30. Hudson AC, van Zwieten A, Mallitt KA, Durkan A, Hahn D, Guha C, et al. School attendance and sport participation amongst children with chronic kidney disease: a cross-sectional analysis from the kids with CKD (KCAD) study. Pediatr Nephrol. (2024) 39(4):1229–37. doi: 10.1007/s00467-023-06198-0
31. Roshan FS, Rahmani N, Nikrouz L. Investigating various interventions to improve the quality of life of children and adolescents suffering from chronic diseases—a systematic review. Int J Adolesc Med Health. (2024) 36(6):525–40. doi: 10.1515/ijamh-2024-0166
32. Trautmann A, Vivarelli M, Samuel S, Gipson D, Sinha A, Schaefer F, et al. IPNA Clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. (2020) 35(8):1529–61. doi: 10.1007/s00467-020-04519-1
33. Meurillon R, Stheneur C, Le Roux E. Discrimination against adolescents with chronic diseases: a systematic review. Eur J Pediatr. (2024) 184(1):74. doi: 10.1007/s00431-024-05829-4
Keywords: adolescents, anxiety, depression, risk factors, chronic kidney disease
Citation: Bai C, Wei M, Huang S, Fu J, Ning G and Zhai W (2025) Associations between demographic and disease related factors and anxiety and depression among adolescents with chronic kidney disease. Front. Pediatr. 13:1616765. doi: 10.3389/fped.2025.1616765
Received: 23 April 2025; Accepted: 5 August 2025;
Published: 22 August 2025.
Edited by:
Ana Cristina Simões E. Silva, Federal University of Minas Gerais, BrazilReviewed by:
Ali Asghar Anwar Lanewala, Sindh Institute of Urology and Transplantation, PakistanRobert J. Wellman, UMass Chan Medical School, United States
Copyright: © 2025 Bai, Wei, Huang, Fu, Ning and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingjie Wei, d2VpbWluZ2ppZTkwOUBzaW5hLmNvbQ==
 Changchang Bai
Changchang Bai Mingjie Wei2*
Mingjie Wei2*