- 1Department of Neonatology, Bharati Vidyapeeth Deemed University Medical College, Pune, India
- 2Department of Community Medicine, Bharati Vidyapeeth Deemed University Medical College, Pune, India
Background: Heel-stick procedures for glucose monitoring are common in neonatal intensive care units (NICUs) and can have adverse physiological and developmental effects on preterm neonates. The National Neonatology Forum of India and the American Academy of Pediatrics recommend the routine use of non-pharmacological measures during such procedures. Our study aimed to evaluate the efficacy of expressed breast milk (EBM), facilitated tucking (FT), and their combination in reducing heel stick pain in preterm neonates, as assessed using the Premature Infant Pain Profile (PIPP) score at 1 and 4 min post-procedure.
Methods: In this randomized controlled trial, preterm neonates born between 27 and 36+6 weeks of gestation, who met the eligibility criterion, were randomly allocated into three categories: EBM (n = 56), FT (n = 56), and EBM + FT (n = 56), following approval from the Institutional Ethics Committee and parenteral consent at a tertiary-level NICU in Pune. Neonates were videotaped by a senior resident/clinical fellow for 2 min before and up to 4 min after the heel-stick procedure. Infants’ pain was determined using the PIPP score at the time of heel-sticking and at 1 and 4 min post-procedure.
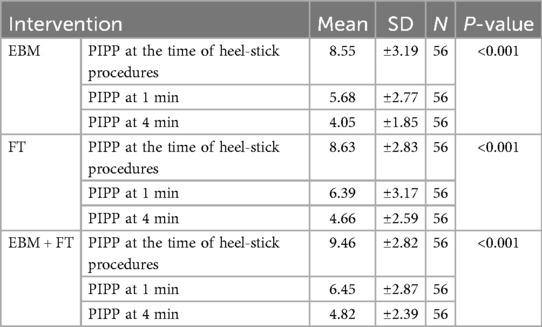
Results: Repeated-measures ANOVA revealed significant reductions in pain scores from baseline (EBM: 8.55 ± 3.19, FT: 8.63 ± 2.83, and EBM + FT: 9.46 ± 2.82; p < 0.001) to both 1 min (EBM: 5.68 ± 2.77, FT: 6.39 ± 3.17, and EBM + FT: 6.45 ± 2.87; p < 0.001) and 4 min (EBM: 4.05 ± 1.85, FT: 4.66 ± 2.59, and EBM + FT: 4.82 ± 2.39; p < 0.001) post-procedure. Bonferroni post-hoc analyses confirmed significant within-group reductions in pain across all time points.
Conclusions: EBM and FT, whether alone or in combination, are effective in reducing pain in preterm neonates during heel-stick procedures.
Clinical Trial Registration: https://ctri.nic.in/Clinicaltrials/login.php, identifier [CTRI/2023/09/057787 (Registered on: 18/09/2023)].
Introduction
During hospital stays, newborns are frequently subjected to invasive and painful procedures, including heel sticks, which might result in adverse physiological, metabolic, or behavioral responses (1). Repeated exposure to pain may also hinder brain development by inducing oxygen desaturation, which leads to the production of free radicals that damage rapidly growing tissues (2, 3). Research has indicated that stress due to neonatal discomfort is related to reduced cognitive and motor functions at corrected ages of 8 and 18 months (4). In addition, such infants may experience increased anxiety, depression, and other behavioral changes at 18 months and even up to 7 years of age (4, 5). These findings necessitate the need for proper interventions to alleviate pain during neonatal intensive care units (NICU) procedures to improve neurodevelopmental outcomes. While numerous pharmacological and non-pharmacological interventions are available for neonatal pain management, the latter are often preferred in preterm infants due to their safety profile and ease of use (6). Expressed breast milk (EBM) represents a promising option, offering advantages such as bioavailability, safety, cost-effectiveness, and the satisfaction a mother feels when she creates an analgesic for her baby (7). There are two possible explanations for the analgesic effect of breast milk: first, its lactose-based sweetness, along with flavor (8) and odor (9), and second, its high tryptophan level (10), which is a melatonin precursor. Beta-endorphin, an endogenous opioid that lessens pain perception, is believed to be released in response to melatonin (11). Similarly, facilitated tucking (FT) involves holding the infant with warm hands to provide tactile and thermal sensory stimulation, thereby helping to lessen pain during invasive procedures (12).
Several pain scoring systems are available for neonates, one of which is the Premature Infant Pain Profile (PIPP; 28–40 weeks), which can be used to assess both procedural and postoperative pain while adjusting for prematurity. Since the PIPP focuses on scoring facial expressions, it is particularly suitable for preterm infants who may have limited motor function and may not be able to fully express pain through crying or movement.
Apaydin Cirik and Efe assessed pain reduction during orogastric tube insertion using the PIPP score and found that the combination of EBM and swaddling produced the maximum analgesic effect (13).
Both the National Neonatology Forum (NNF) of India and the American Academy of Pediatrics (AAP) strongly recommend the routine use of non-pharmacological interventions, such as oral sucrose/glucose solutions, EBM, FT, non-nutritive sucking (NNS), and swaddling, for procedural pain relief in neonates, including during heel pricks (14, 15).
Despite growing emphasis on such strategies, few studies have directly compared the effectiveness of EBM and FT, either individually or in combination, for heel-stick pain relief in preterm neonates. Most available studies have focused on a single intervention or compared these with sucrose, with limited evidence from Indian settings. Moreover, while the NNF claims high-certainty evidence for pain reduction during and up to 30 s after heel pricks, little is known about the sustained effects of these interventions beyond this window. This represents a significant knowledge gap, particularly in resource-limited settings where sucrose may not be readily accessible.
Our study addresses this gap by evaluating the effectiveness of EBM, FT, and their combination in reducing pain during heel-stick procedures using PIPP scores assessed up to 1 and 4 min post-procedure. To our knowledge, no Indian study has compared these three methods. Although a 24% sucrose solution is recognized as an effective technique for pain reduction in preterm newborns before the heel-stick procedure (16–18), it is not readily accessible across Indian markets. In contrast, our proposed alternatives, EBM and FT, are quick, inexpensive, easy to administer, and readily available in every setting and therefore can be used for pain management before the heel-stick procedure.
Materials and methods
Study design and participants
A three-arm, parallel-group randomized controlled trial was conducted in a tertiary care NICU of Bharati Vidyapeeth (Deemed University) Medical College, Pune, Maharashtra, India, between October 2023 and May 2024. Preterm neonates aged 27–36+6 week gestational age (GA) admitted to the NICU within 48 h of birth were eligible for inclusion. The study was approved by the Institutional Ethics Committee (IEC) and registered with the CTRI (CTRI/2023/09/057787). Neonates were excluded if they were on invasive ventilation, hemodynamically unstable, had any major congenital malformations or genetic abnormalities, had encephalopathy, or had received sedatives 24 h prior to the interventions. To assess the intra-class correlation (ICC) of the PIPP score, a pilot study was conducted prior to commencing the main study. The ICC values demonstrated acceptable margins across the EBM, FT, and EBM + FT groups: 0.954 for EBM, 0.985 for FT, and 0.942 for EBM + FT.
Randomization and sample size
Our sample size was determined using data from a study by Apaydin Cirik and Efe (13), which reported mean PIPP scores of 7.9 and 6.6 in the EBM and EBM + FT groups, respectively, with 31 participants per group. Based on these values, the sample size was calculated to be 56 participants per group, assuming a 95% confidence interval (CI), 80% power, an acceptable error margin of 1.30, a pooled standard deviation (SD) of 2.455, and group-specific SDs of 2.6 for EBM and 2.3 for EBM + FT. Participants were randomly assigned to the EBM, FT, or EBM + FT group using computer-generated numbers. Allocation concealment was ensured using sequentially numbered, opaque, sealed envelopes (SNOSEs). Written informed consent was obtained.
Interventions
Regardless of the interventions, all infants were positioned supine with rolled towels for support at least 30 min prior to the heel-stick procedure.
Expressed breast milk group: A sterile cotton gauze soaked in EBM was placed in the oral cavity of the infant 2 min prior to the procedure and maintained up to 4 min after the heel-stick procedure. The breast milk volume was adjusted as per GA: 0.5 mL for 27–27+6 weeks; 1.0 mL for 28–29+6 weeks; 1.5 mL for 30–31+6 weeks; 2 mL for 32–36+6 weeks (19).
Facilitated tucking group: Infants were held gently by a qualified nurse or physician with warm hands for 2 min before, during, and up to 4 min after the heel-stick procedure. The infant was positioned in a flexed, midline posture with the limbs adjacent to their body while constraining the head and body.
EBM + FT group: Both interventions, as mentioned above, were applied concurrently.
Heel-stick procedure and pain assessment
Heel-stick procedure: Heel sticks were performed by a neonatal nurse using proper aseptic precautions. An Accusure™ safety lancet (Microgene Diagnostic Systems Private Limited, India) of 28 gauge was used for the procedure. To collect the blood sample, gentle pressure was applied without squeezing the heel after the heel stick.
Pain assessment: PIPP was utilized to evaluate procedural pain during heel sticks. Scoring was performed by the principal investigator using video recordings of the infants' facial expressions captured during the heel-stick procedure and at 1 and 4 min post-procedure. Videos were recorded by NICU on-call personnel (senior resident/clinical fellow) who were proficiently trained in capturing the facial expressions of newborns. Concurrently, heart rate and oxygen saturation visible displayed on the bedside pulse oximeter were documented. Due to the nature of the interventions, blinding of the personnel administering the interventions and assessing the PIPP scores was not feasible. Pain indicators were assessed in the following sequence: GA, heart rate, sleep/wake state, oxygen saturation, eye squeeze, brow bulge, and nasolabial furrow. Gestational age was determined using first-trimester dating scans or the last menstrual period; when these were unavailable, the New Ballard Scoring System was employed.
Physiological parameters: Heart rate and oxygen saturation were continuously monitored using bedside pulse oximeters. An oxygen saturation probe was affixed to the right upper limb of each infant. Baseline readings were documented before the heel-stick procedure.
Statistical analysis
Statistical analyses were conducted using SPSS version 29 software. Descriptive statistics such as mean and standard deviation summarized quantitative variables (GA, birth weight, heart rate, SpO2, age/time at heel stick, and PIPP score), while frequency and percentages described categorical variables (gender and mode of delivery). Using repeated-measures ANOVA, we analyzed the significant mean change in PIPP scores from baseline to 1 min and up to 4 min post-procedure within each intervention group. Pairwise comparisons were performed using Bonferroni post-hoc analysis. ANOVA was used to assess the mean difference in PIPP scores from baseline to 1 min and from baseline to 4 min between the intervention groups. A P-value <0.05 was considered statistically significant.
Results
Infant characteristics
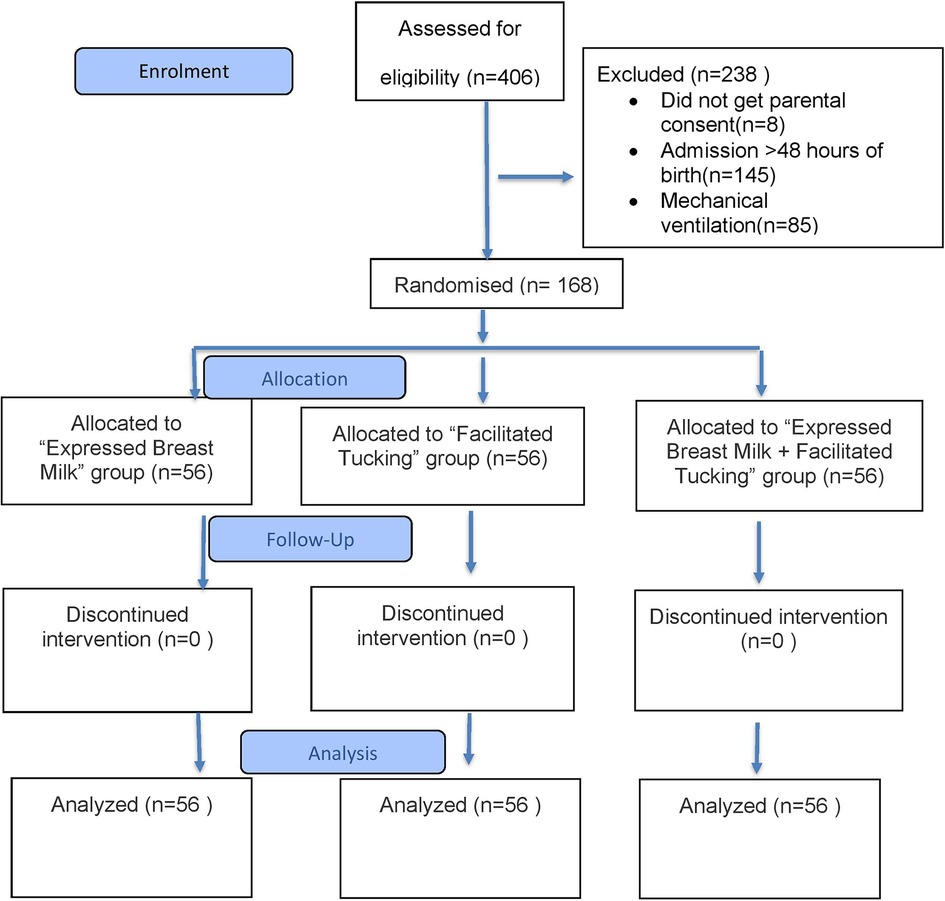
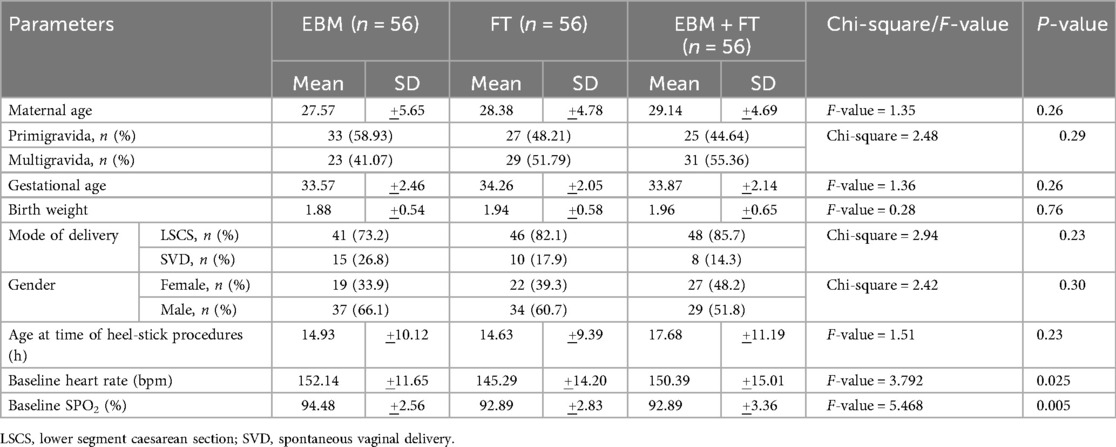
A total of 406 preterm neonates were assessed for eligibility, of whom 238 were excluded and 168 were enrolled in the study (Figure 1). No participants were lost to follow-up or discontinued from the allocated intervention. The mean gestational ages were 33.57 ± 2.46, 34.26 ± 2.05, and 33.97 ± 2.14 weeks in the EBM, FT, and EBM + FT groups, respectively. The mean birth weights were 1.88 ± 0.54, 1.94 ± 0.58, and 1.96 ± 0.65 kg in the EBM, FT, and EBM + FT groups, respectively. Baseline characteristics of infants were comparable across the three groups, with no statistically significant differences except for baseline heart rate and baseline oxygen saturation (Table 1), which, however, were not clinically significant.
Differences in pain measurement parameters at the time of heel stick and at 1 and 4 min post-procedure
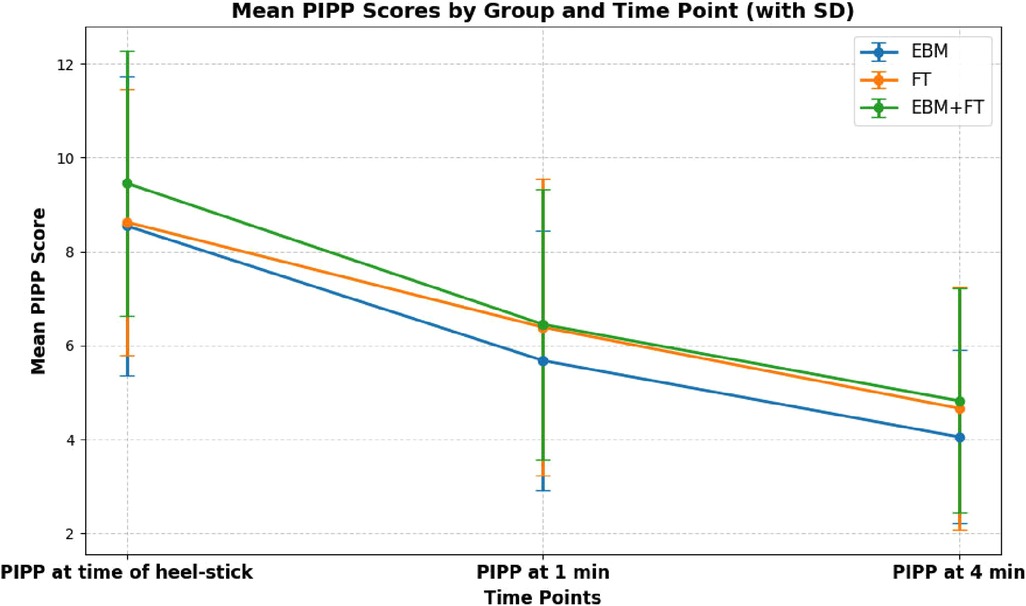
The efficacy of EBM, FT, and EBM + FT in minimizing pain during heel-stick procedures was examined. Table 2 shows that the mean PIPP score decreased significantly in all three intervention groups from baseline to 1 and 4 min after the heel-stick procedure. This indicates that all three methods are equally effective in reducing pain, as depicted graphically in Figure 2.
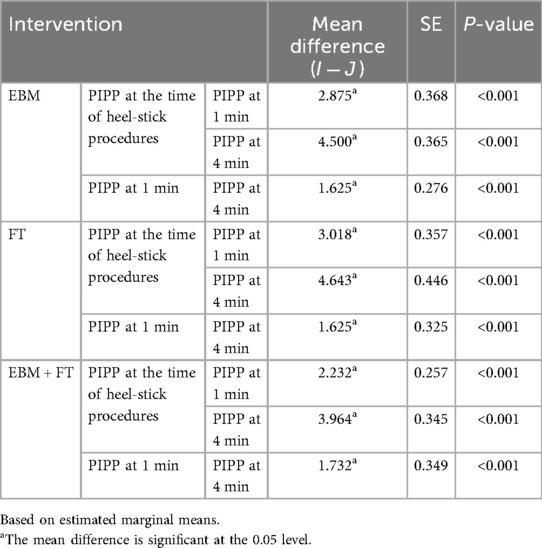
Further Bonferroni post-hoc pairwise analysis (Table 3) demonstrated that the mean difference in PIPP scores decreased statistically significantly within each intervention group across all time points: from baseline (i.e., from time of heel stick) to 1 min, from 1 to 4 min, and from baseline to 4 min. Subgroup analyses were not performed because stratified randomization by gestational age was not conducted.
No adverse events or complications were reported in any of the groups.
Discussion
Pain management during procedures such as heel sticks is essential because this may have detrimental physical and developmental effects on preterm newborns in the short and long term (4, 5). The present research examined the effectiveness of EBM and FT, used either alone or in combination, in reducing pain in preterm infants' (as determined by PIPP scores) at various time points after the heel-stick procedure. According to our research, EBM and FT, either used alone or in combination, effectively reduced pain in preterms.
Our results align with those of Peng et al., who reported that combinations of NNS, EBM, and FT significantly reduced pain during heel-stick procedures, as assessed by PIPP scores, compared to routine care (19). While their study evaluated multi-modal interventions, ours was specifically focused on the independent and combined effects of EBM and FT. To the best of our knowledge, this is the first study from India that solely investigated the synergistic analgesic effect of EBM combined with FT for preterm neonates undergoing heel-stick procedures.
Multiple studies have supported the analgesic efficacy of breast milk. Ribeiro et al. reported that breast milk was nearly as effective as sucrose in reducing pain during ophthalmoscopy for retinopathy of prematurity (20). Simomse et al., in their study, found no discernible difference in mean PIPP scores between newborns receiving sucrose (5.5) and breast milk (6.1), with a mean difference of 0.6 (95% CI −1.6 to 2.8; P = 0.58) (21). The analgesic effect of EBM was further reinforced in the study by Upadhyay et al., who found that feeding 5 mL of EBM before venepuncture effectively reduced pain in term neonates, as assessed by the Neonatal Facial Coding System (NFCS) scores (22).
However, other studies have suggested that sweet solutions like sucrose or dextrose might be more effective than EBM. Bueno et al. reported poorer effects of EBM compared to 24% sucrose during the heel-stick procedure, as assessed via PIPP scores and crying time (23). Similarly, Sahoo et al. found that 25% dextrose was more effective than EBM in lowering the mean PIPP scores following venipuncture (24).
Facilitated tucking has also been validated as an effective intervention for pain reduction in neonates. Studies by Reyhani et al., Lopez et al., and Ranjbar et al. demonstrated that FT significantly reduces pain in preterm neonates undergoing painful procedures (25–27). The findings of our study are consistent with this evidence and suggest that FT, being non-invasive and easy to apply, represents a robust intervention for pain management in preterm neonates.
Differences across studies may arise from variability in pain assessment tools, choice of comparison groups, type of procedural pain evaluated, and the kind of intervention combinations tested. Also, some studies assessed pain only during or immediately after the procedure, while our study evaluated for an extended time of 4 min.
Our findings are consistent with the broader literature supporting non-pharmacological approaches. A Cochrane review by Stevens et al. reported that 24% sucrose, administered alone or in combination with other interventions such as NNS, significantly reduced procedural pain during heel lancing in preterm neonates, as measured by PIPP scores at both 30 and 60 s post-procedure (17). However, this benefit was not evaluated beyond 1 min, and long-term effects remain unclear. Unlike many of those trials, our study extended the assessment window to 4 min post-intervention, demonstrating sustained analgesic effects of EBM and FT, thus presenting a novel perspective on prolonged benefit, going beyond the current evidence base and the 30 s window cited in the NNF guidelines.
From a practical perspective, EBM and FT are viable and scalable interventions for Indian NICUs, where 24% sucrose is often either unavailable or inconsistently used. Because these methods do n't require specialized materials, extensive training, or pharmacological medications, they are particularly well-suited for routine pain management in resource-constrained settings.
Our results indicate that FT or EBM, whether used alone or in combination, may be able to provide pain relief to preterm newborns who are unable to be breastfed because of severe sickness or who are unable to obtain oral sucrose. Most Indian NICUs do not have easy access to sucrose products, even though oral sucrose, either by itself or in combination with NNS, has been demonstrated to alleviate procedural pain in preterm infants (28, 29) along with their crying/fussy state (30). The results of our study offer a substitute for sucrose in treating pain in preterm neonates.
Our study adds to the growing body of evidence and supports integrating EBM and FT into national neonatal care protocols while also advocating for further multi-center studies and implementation research to scale these interventions widely.
Strengths and limitations
Our investigation offers several advantages. This randomized controlled study was conducted using a sizable enough sample, employed standardized pain scoring through video recordings, and adhered to strict inclusion/exclusion criteria. To reduce mistakes, a senior resident or clinical fellow with extensive training performed the video recording. To the best of our knowledge, this is the first study from India to evaluate the combined effects of EBM and FT during the heel-stick procedure. These non-pharmacological interventions are quick, easy to learn, and require only a short training session for neonatal nurses. Thus, these interventions can be applied even at peripheral health centers catering to newborn care. Despite its strength, the study was not without limitations. It was conducted at a single center. Gestational age-wise stratification was not performed, hindering subgroup analyses. Blinding the principal investigator to the allotted interventions while assessing the PIPP score was not feasible. Also, PIPP scores were not documented before the start of the procedure, making it rather difficult to discern whether the changes were because of pain management or because of decreased stress levels due to intervention. Furthermore, we simply measured pain scores as an outcome. Future research should include other outcome factors, such as oxygen needs and sleep and salivary cortisol levels.
Conclusion
For preterm newborns experiencing heel-sticking pain, EBM and FT, whether used separately or in combination, demonstrate a favorable analgesic impact. As a result, they can be suggested as a means of managing pain during the heel-stick procedures. In addition, these interventions are low-cost and do not require policy development implications, which increases the likelihood of their use in clinical practice.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Ethics Committee of Bharati Vidyapeeth (deemed to be university) Medical College, Pune, India (DHR Reg. No.: EC/NEW/INST/2022/MH/0150). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
RDa: Methodology, Writing – review & editing, Investigation, Writing – original draft, Data curation. VD: Data curation, Writing – review & editing, Investigation. AS: Writing – review & editing, Investigation, Data curation. PS: Supervision, Conceptualization, Validation, Writing – review & editing, Methodology, Visualization. RDe: Writing – review & editing, Software, Formal analysis. NM: Writing – original draft, Visualization, Validation, Writing – review & editing, Methodology, Supervision, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anand KJS, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. (1989) 36(4):795–822. doi: 10.1016/S0031-3955(16)36722-0
2. Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Ann Neurol. (2012) 71(3):385–96. doi: 10.1002/ana.22267
3. Bellieni CV, Iantorno L, Perrone S, Rodriguez A, Longini M, Capitani S, et al. Even routine painful procedures can be harmful for the newborn. Pain. (2009) 147(1–3):128–31. doi: 10.1016/j.pain.2009.08.025
4. Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. (2009) 143(1–2):138–46. doi: 10.1016/j.pain.2009.02.014
5. Ranger M, Synnes AR, Vinall J, Grunau RE. Internalizing behaviours in school-age children born very preterm are predicted by neonatal pain and morphine exposure. Eur J Pain. (2014) 18(6):844–52. doi: 10.1002/j.1532-2149.2013.00431.x
6. Gao H, Li M, Gao H, Xu G, Li F, Zhou J, et al. Effect of non-nutritive sucking and sucrose alone and in combination for repeated procedural pain in preterm infants: a randomized controlled trial. Int J Nurs Stud. (2018) 83:25–33. doi: 10.1016/j.ijnurstu.2018.04.006
7. Palomaa AK, Korhonen A, Pölkki T. Factors influencing parental participation in neonatal pain alleviation. J Pediatr Nurs. (2016) 31(5):519–27. doi: 10.1016/j.pedn.2016.05.004
8. Blass EM. Milk-induced hypoalgesia in human newborns. Pediatrics. (1997) 99:825–9. doi: 10.1542/peds.99.6.825
9. Badiee Z, Asghari M, Mohammadizadeh M. The calming effect of maternal breast milk odor on premature infants. Pediatr Neonatol. (2013) 54(5):322–5. doi: 10.1016/j.pedneo.2013.04.004
10. Heine WE. The significance of tryptophan in infant nutrition. Adv Exp Med Biol. (1999) 467:705–10. doi: 10.1007/978-1-4615-4709-9_91
11. Barrett T, Kent S, Voudouris N. Does melatonin modulate beta-endorphin, corticosterone, and pain threshold? Life Sci. (2000) 66(6):467–76. doi: 10.1016/s0024-3205(99)00616-5
12. Axelin A, Salanterä S, Lehtonen L. Facilitated tucking by parents’ in pain management of preterm infants-a randomized crossover trial. Early Hum Dev. (2006) 82(4):241–7. doi: 10.1016/j.earlhumdev.2005.09.012
13. Apaydin Cirik V, Efe E. The effect of expressed breast milk, swaddling and facilitated tucking methods in reducing the pain caused by orogastric tube insertion in preterm infants: a randomized controlled trial. Int J Nurs Stud. (2020) 104:103532. doi: 10.1016/j.ijnurstu.2020.103532
14. National Neonatology Forum of India. Assessment and Management of Pain in the Newborn [Internet]. New Delhi: National Neonatology Forum of India (2023). Available online at: https://www.nnfi.org/nnf-cpg-guidelines.php
15. AAP Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine. Prevention and management of procedural pain in the neonate: an update. Pediatrics. (2016) 137(2):e20154271. doi: 10.1542/peds.2015-4271
16. Bueno M, Yamada J, Harrison D, Khan S, Ohlsson A, Adams-Webber MLS, et al. A systematic review and meta-analyses of nonsucrose sweet solutions for pain relief in neonates. Pain Res Manag. (2013) 18(3):153–61. doi: 10.1155/2013/956549
17. Stevens B, Yamada J, Ohlsson A, Haliburton S, Shorkey A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev. (2016) 7(7):CD001069. doi: 10.1002/14651858.CD001069.pub5
18. Witt N, Coynor S, Edwards C, Bradshaw H. A guide to pain assessment and management in the neonate. Curr Emerg Hosp Med Rep. (2016) 4(1):1–10. doi: 10.1007/s40138-016-0089-y
19. Peng HF, Yin T, Yang L, Wang C, Chang YC, Jeng MJ, et al. Non-nutritive sucking, oral breast milk, and facilitated tucking relieve preterm infant pain during heel-stick procedures: a prospective, randomized controlled trial. Int J Nurs Stud. (2018) 77:162–70. doi: 10.1016/j.ijnurstu.2017.10.001
20. Ribeiro LM, Castral TC, Montanholi LL, Daré MF, De Araújo Silva AC, Antonini SRR, et al. Human milk for neonatal pain relief during ophthalmoscopy. Rev Esc Enferm. (2013) 47(5):1039–45. doi: 10.1590/S0080-623420130000500005
21. Simonse E, Mulder PGH, Van Beek RHT. Analgesic effect of breast milk versus sucrose for analgesia during heel lance in late preterm infants. Pediatrics. (2012) 129(4):657–63. doi: 10.1542/peds.2011-2173
22. Upadhyay A, Aggarwal R, Narayan S, Joshi M, Paul VK, Deorari AK. Analgesic effect of expressed breast milk in procedural pain in term neonates: a randomized, placebo-controlled, double-blind trial. Acta Paediatr Int J Paediatr. (2004) 93(4):518–22. doi: 10.1080/08035250410022792
23. Bueno M, Stevens B, De Camargo PP, Toma E, Krebs VLJ, Kimura AF. Breast milk and glucose for pain relief in preterm infants: a noninferiority randomized controlled trial. Pediatrics. (2012) 129(4):664–70. doi: 10.1542/peds.2011-2024
24. Sahoo JP, Rao S, Nesargi S, Ranjit T, Ashok C, Bhat S. Expressed breast milk vs 25% dextrose in procedural pain in neonates: a double blind randomized controlled trial. Indian Pediatr. (2013) 50(2):203–7. doi: 10.1007/s13312-013-0067-3
25. Reyhani T, Mohebi T, Boskabadi H, Gholami H, Ghavami ghanbarabadi V. The effect of facilitated tucking during venipuncture on pain and physiological parameters in preterm infants. Evid Based Care. (2012) 2:47–56. doi: 10.22038/ebcj.2012.399
26. Lopez O, Subramanian P, Rahmat N, Theam LC, Chinna K, Rosli R. The effect of facilitated tucking on procedural pain control among premature babies. J Clin Nurs. (2015) 24(1–2):183–91. doi: 10.1111/jocn.12657
27. Ranjbar A, Bernstein C, Shariat M, Ranjbar H. Comparison of facilitated tucking and oral dextrose in reducing the pain of heel stick in preterm infants: a randomized clinical trial. BMC Pediatr. (2020) 20(1):162. doi: 10.1186/s12887-020-2020-7
28. Cignacco EL, Sellam G, Stoffel L, Gerull R, Nelle M, Anand KJS, et al. Oral sucrose and “facilitated tucking” for repeated pain relief in preterms: a randomized controlled trial. Pediatrics. (2012) 129(2):299–308. doi: 10.1542/peds.2011-1879
29. Thakkar P, Arora K, Goyal K, Das RR, Javadekar B, Aiyer S, et al. To evaluate and compare the efficacy of combined sucrose and non-nutritive sucking for analgesia in newborns undergoing minor painful procedure: a randomized controlled trial. J Perinatol. (2016) 36(1):67–70. doi: 10.1038/jp.2015.122
30. Liaw JJ, Yang L, Lee CM, Fan HC, Chang YC, Cheng LP. Effects of combined use of non-nutritive sucking, oral sucrose, and facilitated tucking on infant behavioural states across heel-stick procedures: a prospective, randomised controlled trial. Int J Nurs Stud. (2013) 50(7):883–94. doi: 10.1016/j.ijnurstu.2012.08.021
Keywords: preterm, heel-stick, PIPP score, expressed breast milk, facilitated tucking
Citation: Das R, Deshmukh V, Sohane A, Suryawanshi P, Deshmukh R and Malshe N (2025) Efficacy of expressed breast milk, facilitated tucking, and their combination for pain management during heel stick in preterm neonates: a randomized controlled trial. Front. Pediatr. 13:1622357. doi: 10.3389/fped.2025.1622357
Received: 3 May 2025; Accepted: 11 August 2025;
Published: 5 September 2025.
Edited by:
Victoria J. Kain, Griffith University, AustraliaReviewed by:
Enrique Gomez-Pomar, St Bernards Regional Medical Center, United StatesMohamed Farouk Omar, Minia University, Egypt
Copyright: © 2025 Das, Deshmukh, Sohane, Suryawanshi, Deshmukh and Malshe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nandini Malshe, bWFsc2hlbmFuZGluaUBnbWFpbC5jb20=
 Rupam Das
Rupam Das Vikrant Deshmukh
Vikrant Deshmukh Arpit Sohane
Arpit Sohane Pradeep Suryawanshi
Pradeep Suryawanshi Rupeshkumar Deshmukh
Rupeshkumar Deshmukh Nandini Malshe
Nandini Malshe