- 1Neonatal Intensive Care Unit, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Clinical Sciences and Community Health, Università degli Studi di Milano, Milan, Italy
- 3San Raffaele Telethon Institute for Gene Therapy (SR-Tiget), IRCCS San Raffaele Scientific Institute, Milan, Italy
- 4Department of Pediatrics, Clínica Universidad de Navarra, Madrid, Spain
- 5Great Ormond Street Institute of Child Health, University College London, London, United Kingdom
- 6Neonatal and Paediatric Surgery Department, Great Ormond Street Hospital for Children NHS Foundation Trust, London, United Kingdom
- 7Pediatric Immunohematology Unit and BMT Program, IRCCS San Raffaele Scientific Institute, Milan, Italy
- 8Maternal and Child Department, Vita-Salute San Raffaele University, Milan, Italy
Necrotizing enterocolitis (NEC) presents a life-threatening intestinal emergency primarily affecting premature infants in neonatal intensive care units. This disease is a significant cause of morbidity and mortality in such newborns. NEC involves inflammation, bacterial overgrowth, and cell death affecting a portion of the bowel wall, commonly the distal ileum. Despite advances in neonatal care, the pathogenesis of NEC remains not fully understood. Although its pathogenesis remains not fully elucidated, the upregulation of Toll-like receptor 4 in the premature intestinal epithelium is recognized as a key factor contributing to epithelial barrier dysfunction. Recent studies have explored the potential of mesenchymal stromal cells (MSCs) in NEC management. MSCs are up-and-coming candidates for preclinical NEC models as they possess anti-inflammatory and immune modulatory properties, which reduce inflammation, help increase intestinal integrity, and help tissue repair. Bone marrow-derived mesenchymal stromal cells (BM-MSCs) have proven impactful in most experimental settings, mitigating injury from NEC and facilitating intestinal development. While MSC therapies hold promise, challenges remain regarding inconsistent isolation and expansion of these cells, variable differentiation, and possible tumorigenicity in vivo. As a result, the focus has been drawn to MSC-derived secretome, especially exosomes, as a novel cell-free therapeutic. These bioactive molecules transported by exosomes can reduce inflammation and facilitate tissue repair, providing a safer and more plausible alternative to treating NEC. Further research is needed to standardize secretome production and evaluate its clinical efficacy and safety. This review aims to provide a comprehensive overview of the mechanisms of action and the available research on human (h)BM-MSCs to support the development of studies that may prevent and/or treat the disease.
Highlight
1. Numerous preclinical studies in murine models have demonstrated the efficacy of bone marrow-derived mesenchymal stromal cells (BM-MSCs) in preventing and treating necrotizing enterocolitis (NEC). However, their use inevitably raises ethical, legal, and biological concerns that require careful consideration.
2. This review provides an overview of exosomes and secretome as viable alternatives that effectively address the aforementioned limitations.
3. This review endeavors to furnish an up-to-date and comprehensive synopsis of the current body of literature, aiming to equip clinicians and researchers with a thorough understanding while also considering the potential for imminent clinical translation.
1 Introduction
Necrotizing enterocolitis (NEC) is a severe inflammatory condition that primarily affects the terminal ileum and is most commonly observed in preterm infants (1). The severity of NEC ranges from mild mucosal injury to transmural necrosis with bowel perforation, and it is associated with high morbidity and mortality rates in neonatal intensive care units (NICUs) (2–4).
The pathogenesis of NEC is multifactorial, involving the underdeveloped intestinal barrier, dysregulated immune responses, and microbial dysbiosis. Notably, upregulation of Toll-like receptor 4 (TLR4) in the premature gut plays a pivotal role in NEC development by impairing epithelial barrier integrity and promoting inflammation (5–9). Aberrant inflammatory signaling and the subsequent release of cytokines further compromise the intestinal lining, making the tissues more susceptible to ischemic injury. Additional contributing factors, such as impaired blood flow and compromised oxygen delivery to the immature intestinal epithelium, exacerbate tissue damage and may culminate in extensive tissue death if left untreated (10, 11).
Despite advancements in neonatal intensive care management, NEC often results in short- and long-term complications, with a profound impact on the affected infant's life (12, 13).
Emerging therapies, specifically mesenchymal stromal cells (MSCs) therapy, have demonstrated potential for prevention and treatment options for NEC. MSCs are multipotent cells capable of differentiating into multiple lineages and secreting a broad range of paracrine factors that support tissue repair (14). In particular, bone marrow-derived MSCs (BM-MSCs) have shown potential in preclinical NEC models, significantly reducing intestinal injury and inflammation. The therapeutic effects of MSCs are primarily mediated through their secretion of anti-inflammatory cytokines and their ability to modulate the endogenous repair process by inducing epithelial regeneration and barrier function (15).
However, several challenges must be addressed before MSC-based therapies can be successfully translated into clinical practice for NEC. These obstacles include variability in MSCs isolation and expansion, limited engraftment efficiency, and potential risks such as tumorigenicity and immune rejection (16–18). Furthermore, the in vivo behavior of MSCs remains incompletely understood, and concerns persist regarding their long-term safety and efficacy (19).
In this context, there is growing interest in cell-free therapeutic approaches, particularly focusing on the MSC-derived secretome, including exosomes and other paracrine factors (20, 21). These strategies offer several advantages, such as a reduced risk of tumorigenicity, simplified manufacturing and storage process, and the potential to harness the therapeutic benefits of MSCs without the complexities associated with cellular therapies.
Despite advances in supportive care, NEC remains a life-threatening condition in preterm infants, associated with significant morbidity and mortality. Innovative therapies, including MSC-based and secretome-based interventions, may provide more effective and targeted strategies for preventing and treating NEC (22–33).
This review aims to summarize the current knowledge regarding BM-MSCs' mechanisms of action, highlight their biological effects in the context of NEC, and discuss the challenges and opportunities for their preclinical and clinical translation, thereby fostering the development of novel therapeutic strategies.
2 NEC overview
2.1 Epidemiology
The incidence of NEC varies from 0.3 to 2.4 per 1,000 live births, with geographic, ethnic, and neonatal care strategy variations (34–36). The incidence of the disease is strongly inversely correlated with the gestational age (GA) at birth, with approximately 90%–95% of cases occurring in infants born before 36 weeks' GA (1). A multicenter cohort analysis conducted in the United States revealed only marginal improvement in the overall incidence of NEC cases among Very Low Birth Weight (VLBW) over the past years, with a decrease from 9% in 2006 to 6% in 2017 (37). However, term infants can also be affected, mainly when associated with other conditions such as congenital heart disease, polycythemia, early-onset bacterial sepsis, hypotension, or maternal drug use (38, 39).
2.2 Risk factors and pathogenesis
Since its description in the 1940s to 1950s, NEC is recognized as a multifactorial disease caused by various risk factors, including prematurity, formula feeding, microbial dysbiosis, immature intestinal barrier, underdeveloped motility, immune regulation, hypoxia, inadequate microcirculation, and intense inflammation (Table 1) (40–57). In this context, the upregulation of TLR4 is pivotal in NEC pathogenesis, directly contributing to the intestinal epithelial barrier impairment (5–7). Because of the central role of TLR-4 in the pathogenesis of the disease, many therapeutic options are moving toward drugs explicitly targeting the signaling pathway associated with this molecule. The inhibitor 2-acetamidopyranoside (C34), identified by Neal et al., mainly exhibits efficacy in inhibiting TLR-4 signaling both in vitro and in vivo. Mice treated with C34 showed preserved intestinal mucosa and a reduced incidence of the disease in an experimental model of NEC (58, 59). The pregnane X receptor (PXR), a xenobiotic sensor that acts as an intermediary for specific host-bacterial metabolites, can inhibit the TLR-4 pathway (60). In PXR knockout mice, symptoms following NEC induction were more severe. Activation of intestinal PXR by lithocholic acid (LCA), a PXR agonist, reduces NEC-induced intestinal inflammation (60, 61). Furthermore, Dai et al. recently demonstrated that high mobility group box 1 (HMGB1), a DNA-binding protein, inhibits enterocyte migration by activating the TLR-4 pathway. Hence, NEC is associated with elevated protein expression (62). In a rat model, glycyrrhizin, an HMGB1 inhibitor, was used to reduce the disease incidence through the TLR-4 and nuclear factor kB (NF-kB) pathways (63).
The disruption of the TLR4 pathway and the other risk factors are closely linked to alterations in the normal proliferation/apoptosis cycle within the intestinal tissue. Specifically, the Wingless and Int-1 (Wnt) signaling pathway, originating from Paneth cells, drives crypt division and plays a pivotal role (64–66). After apoptosis, which leads to the average turnover of epithelial cells, there is an autophagic clearance of apoptotic remnants and shedding of cell remnants into the lumen (66). Under normal conditions, there is a balance between cell proliferation and loss through processes such as apoptosis, autophagy, and shedding. In contrast, in experimental models and humans, NEC is defined by intestinal necrosis, epithelial cell loss, and increased apoptosis and autophagy (67–70). The loss of this delicate intestinal stem cell niche disrupts the balance between cell propagation and loss, while also impairing cell migration (71). Intestinal epithelial apoptosis triggers severe bowel necrosis in an experimental neonatal rodent model that resembles necrotizing enterocolitis in human newborns (72).
2.3 Diagnosis and treatment
Clinical features, radiographic findings, and laboratory values based on Bell's staging criteria support the diagnosis of NEC. They were initially described in 1978 and subsequently refined by Walsh and Kliegman in 1986, extending the original 3-stage classification to the current 6-stage classification (73–75). Despite their well-recognized pitfalls, Modified Bell's criteria are widely employed to stage the severity of NEC, with higher stages associated with a greater risk of adverse outcomes (74).
The condition's severity is commonly classified into “medical NEC” vs. “surgical NEC”, a distinction that has critical implications, as surgically managed disease carries higher mortality and worse long-term outcomes (76).
In clinical practice, the early manifestation of NEC typically shows wide variation in infants, and its non-specific nature makes it challenging to make an early diagnosis. The disease exhibits various clinical symptoms and signs, including apnea, bradycardia, lethargy, irritability, and temperature instability. This holds for other conditions in preterm infants, such as sepsis (59, 77). Concurrent gastrointestinal symptoms, such as abdominal distension, blood in the stools (either gross or occult), gastric residual volumes, and bilious vomiting, may also support the diagnosis of NEC (78, 79). Radiographic findings and laboratory values help evaluate diseases and exclude other conditions from the differential diagnosis of NEC (80). Abdominal x-rays can indicate intestinal dilation, ileus, pneumatosis intestinalis, portal venous gas, or pneumoperitoneum, which is almost pathognomonic for NEC (81, 82). Additionally, abdominal ultrasound is increasingly recognized as a promising method for enhancing NEC diagnosis and guiding clinical decision-making (83, 84).
Acute complications include fulminant sepsis, peritonitis, abscess formation, thrombocytopenia, granulocytopenia, disseminated intravascular coagulation (DIC), and hypotensive shock (85, 86).
NEC medical management involves implementing measures such as bowel rest, nasogastric or orogastric drainage, fluid balance, parenteral nutrition, pain medication, and the empiric administration of broad-spectrum antibiotics. Critically affected patients often require ventilatory support, vasopressors for blood pressure stabilization, and blood transfusions (87, 88).
While numerous NEC cases are treated medically, around 20%–70% of affected infants require surgical procedures (89).
The only definitive indication for surgical intervention is the presence of gastrointestinal tract (GIT) perforation, as determined by radiographic evidence of pneumoperitoneum or paracentesis showing enteric content (90). However, surgery may be required when infants do not display improvement despite medical management or in patients with particular symptoms (91, 92).
Surgical management includes placing a peritoneal drain or performing a laparotomy, resecting non-viable segments of the gastrointestinal tract, and potentially creating ostomies or primary anastomoses (93–97).
2.4 Short- and long-term outcomes
Despite significant advances in neonatal intensive care, NEC-related mortality rates remain alarmingly high, peaking at up to 42% in infants weighing less than 750 g at birth (98, 99). Patients with “medical NEC” have a 21% mortality rate, while those with “surgical NEC” face a mortality rate of up to 35%–50% (100, 101). Additionally, infants with extensive gut resection often face complications such as wound dehiscence, nutrient imbalance, a high rate of abscesses, a high incidence of strictures, and short bowel syndrome (12, 76, 100, 102). Moreover, extended use of total parenteral nutrition (TPN) raises the risk of infections, cholestasis, and liver failure (98, 103). Additionally, NEC patients have been shown to experience worsened neurological outcomes in both the short and long term (13, 104).
2.5 Preventive strategies
NEC research should emphasize prevention over treatment, which can alter outcomes and reduce morbidity. Preventive strategies have centered on standardized protocols, breastfeeding and nutrition with human milk, antenatal maternal steroid administration, prophylactic probiotics, careful antibiotic use, and avoiding histamine type II receptor antagonists (22–29, 31–33, 105).
3 Stem and stromal cells in NEC prevention and therapy
Stem and stromal cells are unspecialized cells that can self-renew and differentiate into various cell types (106). They can be categorized as totipotent, pluripotent, and unipotent based on their differentiation capabilities (107). Totipotent cells can differentiate in various directions; pluripotent cells can develop into different tissues, while unipotent cells can only change into one cell type (108). The intestinal epithelium renews rapidly, typically every 4–5 days. Stem cells located in the crypts divide swiftly and differentiate into various cell types, including enterocytes, enteroendocrine cells, goblet cells, and Paneth cells, before migrating to the tips of the villi (109).
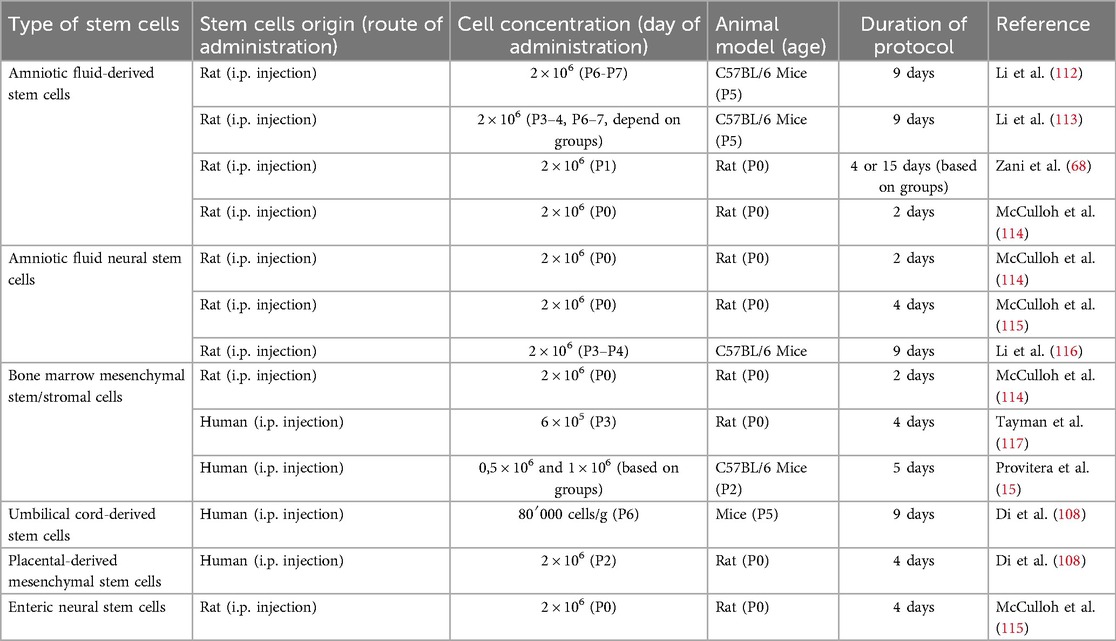
Stem cells possess anti-inflammatory properties and can improve tissue healing in various disease models (110). Similarly, preclinical studies have observed encouraging outcomes regarding their application in the therapy and prevention of NEC (111). Table 2 summarizes the type of stem cells, their origin (including the route of administration), cell concentration, timing of administration, animal model (including age), and protocol duration. Substantial heterogeneity in methodologies and experimental designs is evident across studies.
3.1 Mesenchymal stromal cells
MSCs are a versatile type of adult stem cell (ASCs) localized in specialized niches within various tissues throughout the body, including the bone marrow, adipose tissue, and umbilical cord. MSCs play a crucial role in tissue homeostasis, repair, and regeneration. They are multipotent cells capable of differentiating into several cell types, including bone, cartilage, fat, and connective tissue, making them a valuable resource for regenerative medicine (14).
MSCs can be easily isolated in vitro by plastic adherence and expanded in culture as fibroblast-like cells expressing CD90, CD73, and CD105 to reach an appropriate number for clinical use (14, 118). Also, they need to lack the expression of hematopoietic cell surface markers CD34, CD45, CD11a, CD19, and HLA-DR and to adhere to plastic surfaces under culture conditions (119, 120). Clinical and preclinical data reported the therapeutic benefits of MSC transplantation in repairing injured tissues and modulating the inflammatory response to damage (121, 122). Importantly, due to the lack of HLA class II and co-stimulatory molecule expression, MSCs are immune-evasive, enabling MSC transplantation across histocompatibility barriers and the creation of off-the-shelf therapies, consisting of exogenous MSCs in culture (123).
One of the defining characteristics of MSCs is their immunomodulatory and anti-inflammatory properties (124). They can sense inflammatory signals via TLRs and secrete multiple anti-inflammatory molecules to support tissue regeneration, regulate immune responses to protect tissue from excessive damage, and promote the survival of tissue-derived precursor cells when injected in response to injury (125–137).
MSCs interact with and regulate the activity of innate and adaptive immune cells, inhibiting the proliferation of B, T, and natural killer (NK) cells through direct interactions and the release of soluble molecules, such as transforming growth factor (TGF)-β1, indoleamine 2,3-dioxygenase (IDO), and prostaglandin E2 (PGE2). Consequently, these molecules halt immune cells in the G0 stage to prevent their proliferation. Additionally, MSCs modulate T-cell activation by suppressing the production and secretion of inflammatory cytokines and preventing dendritic cells' activation and maturation (132, 138).
For this reason, MSCs are frequently used in patients to prevent heightened immune cell activation and to modulate the inflammatory response, promoting tissue regeneration in conditions like Crohn's disease and graft-versus-host disease (GvHD) following allogeneic hematopoietic stem cell transplantation (139, 140). MSCs have also been utilized clinically in regenerative medicine due to their ability to differentiate into bone and chondrocytes, thereby repairing cartilage and similar tissues (141). Recent findings increasingly suggest that the paracrine activity of MSCs is the primary mechanism underlying their therapeutic efficacy. Rather than relying solely on direct differentiation, MSCs promote tissue repair primarily through the secretion of paracrine factors that enhance the survival of injured cells and activate tissue-resident progenitor populations, thereby preventing the formation of non-functional scar tissue (142–145). Their regenerative potential is therefore attributed to modulation of the stem cell niche and secretion of bioactive molecules, including anti-inflammatory cytokines, immunoregulatory mediators, and growth factors such as insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and basic fibroblast growth factor (bFGF), all of which contribute to processes such as angiogenesis, cell survival, and proliferation (131, 134). A recent review by Che et al. emphasized the invaluable role of MSC-derived paracrine signaling in inflammatory bowel disease (IBD), highlighting how secreted bioactive molecules, such as HGF, VEGF, and epidermal growth factor (EGF), contribute to epithelial repair, angiogenesis, and mucosal regeneration (146). Furthermore, MSCs have been shown to enhance the expression of tight junction proteins (e.g., zonula occludens 1 (ZO-1), occludin), promote goblet cell function and mucosal healing, and stimulate the proliferation and differentiation of intestinal epithelial cells (146). Collectively, these findings suggest that MSCs also represent a promising therapeutic approach for NEC. In support of this hypothesis, several preclinical studies have demonstrated that MSC treatment significantly reduces the severity of NEC in rodent models (114, 115). The beneficial effects observed in these studies are thought to be mediated mainly by MSC-derived paracrine factors, including interleukin (IL)-6, IL-10, and TGF-β, which contribute to the resolution of inflammation and restoration of the intestinal barrier (147–149).
3.2 Bone marrow–derived mesenchymal stromal cells
McCulloh et al. have revealed that various types of stem cells exert a therapeutic effect by enhancing the intestinal barrier and decreasing intestinal permeability. However, it remains unclear whether the improved gut barrier function is influenced by common or unique pathways among different stem cell types (114).
BM-MSCs are a subpopulation of MSCs found in the medullary stroma of bone marrow. The characteristics of BM-MSCs are closely linked to the age and condition of the donors, as the quantity and differentiation ability decline with age (150). BM-MSCs derived from young and old donors (under 18 years and over 55 years) exhibit significant differences. The old BM-MSCs show increased expression of β-Gal, a hydrolase found in the lysosomes of aging cells, making it a marker of cellular senescence. Additionally, they display changes in cytoskeleton composition, which correlate with impairments in migration and the ability to respond to biological and mechanical signals (151). Even BM-MSCs from aged mice show a greater presence of cellular senescence markers, including the DNA double-strand break marker phosphorylated H2A histone family member X (γH2AX) and the DNA checkpoint response mediator p53-binding protein 1 (53BP1), compared to those from younger mice, which can enhance osteogenic activities and migration in mice instead (152). BM-MSCs release various paracrine factors that regulate fibrosis, proliferation, apoptosis, and angiogenesis in damaged tissues, thereby promoting the recovery of the injured area, modulating the immune system, and safeguarding cells from apoptosis (153). Their potential in treating neonatal diseases has been the subject of considerable research (154, 155). The administration of MSCs has been shown to be beneficial for inflammatory bowel disease. In animal models of colitis, MSC therapy not only reduced the Th1 cytokine response and enhanced the regulatory T cell (Treg) response. Furthermore, MSC therapy has been demonstrated to improve survival and quickly correct weight loss (156).
Kagia et al. have demonstrated that BM-MSCs can treat colitis in mice and improve overall survival compared to the control group. Additionally, colon health in the treated group was significantly better (157).
In a recent study, Abbuehl and colleagues found that freshly isolated murine BM-MSCs, unlike ex vivo expanded ones, can repair stromal niche damage after irradiation and enhance the engraftment of hematopoietic stem and progenitor cells (HSPCs) when co-transplanted intra-bone (158).
The administration of BM-MSCs has effectively reduced the incidence and severity of NEC in a murine model. Specifically, mice with NEC treated with BM-MSCs exhibited decreased levels of Caspase 3, a marker associated with apoptosis, and increased expression of ZO-1. ZO-1 is a protein found at the tight junctions of the intestinal barrier, and its elevated levels compared to the control group suggest that MSCs can help restore the proper functionality of the barrier. These findings indicate that BM-MSCs may have significant therapeutic potential for treating NEC in humans (15).
A study by Seok Lee and colleagues explored oral BM-MSC administration for premature infants to reduce invasive procedures. Administering BM-MSCs orally and intraperitoneally in neonatal mice reduced NEC-related histological injury after NEC induction. The TdT-mediated dUTP Nick-End labeling (TUNEL) assay assessed apoptosis levels, revealing decreased apoptosis in BM-MSC groups. Consequently, stem cell administration lowered TLR-4 expression (159). Previous studies have shown that stem cells can effectively combat NEC in mouse models, regardless of whether BM-MSCs are given before or after NEC onset. These findings suggest that BM-MSCs have potential for preventive and therapeutic uses, achieving notable results whether administered early or later. Additionally, the effects of BM-MSCs are similar whether given orally or intraperitoneally for NEC (159). Wang et al. argued that intraperitoneal injection is the best method for delivering stem cells to treat colitis, despite being an invasive procedure (160). The oral treatment administration is advantageous for vulnerable patients and deserves consideration (159).
Combining BM-MSCs with other factors can enhance their therapeutic potential in NEC prevention by improving their protective efficacy and modulating transcription factors that either suppress or increase the activity of BM-MSCs.
Heparin-binding EGF-like growth factor (HB-EGF) was initially recognized as a possible treatment for NEC almost twenty years ago (161). It safeguards intestinal injury by preventing injury to intestinal stem cells from damage, facilitating enterocyte proliferation and migration, enhancing gut barrier function, and minimizing intestinal apoptosis (162–165). Yang et al. investigated the potential for attaching HB-EGF to BM-MSCs to influence the activity of the stem cells. HB-EGF was found to enhance the proliferation and migration of BM-MSCs while simultaneously reducing their apoptosis. Furthermore, in a rat model of NEC, administering HB-EGF and BM-MSCs decreased the incidence of pathology and lowered levels of 70 kDa FITC-Dextran (FD70), a marker of intestinal permeability, compared to the control and stem cell-only groups (166).
A study by Chen et al. demonstrated that inhibiting the gene responsible for prolyl hydroxylase 2, which plays a role in activating hypoxia-related transcription factors, prompted BM-MSCs to enhance their paracrine effects by releasing protective factors such as TGF-β2. Additionally, the inhibition of prolyl hydroxylase 2 increases survival rates in rats with NEC treated with BM-MSCs by promoting epithelial regeneration (167).
The transplantation of MSCs in animal models of experimental NEC has proven feasible, safe, and effective. Notably, it has been reported that various types of stem cells can restore the integrity of the intestinal barrier (114). MSCs have been shown to protect against intestinal damage and reduce the incidence of NEC (168). Whether stem cell transplantation serves as a preventive or protective model for NEC is yet to be determined.
3.3 Alternative stem cell sources for treating NEC
Despite the importance of BM-MSCs as a potential therapy for treating NEC, several other stem cell types show characteristics that may be beneficial in managing intestinal diseases. Stem cells are typically classified based on their origin and differentiation potential.
Embryonic stem cells (ESCs), derived from the inner cell mass of blastocyst-stage embryos, represent the gold standard of pluripotency (169). They can differentiate into all three germ layers (ectoderm, mesoderm, and endoderm), thus giving rise to a broad range of specialized cell types, including neurons, muscle cells, and different tissue-specific cells (170–172). Furthermore, they hold potential for personalized cell-based therapies, where patient-specific pluripotent stem cells can generate replacement tissues or organs (173). However, their clinical application remains ethically controversial, due to their derivation from the human embryo (174). Amniotic fluid-derived stem cells (AF-SCs) represent a promising alternative. These cells exhibit both mesenchymal markers (CD29, CD44, CD90) and embryonic-like characteristics, such as the stage-specific embryonic antigen (SSEA-4) and the octamer-binding transcription factor 4 (Oct4) (175). Their pluripotency, combined with easier collection and culture methods compared to ESCs, makes them particularly attractive for clinical applications. In NEC models, AF-SCs have shown protective effects by enhancing intestinal barrier function through modulation of endoplasmic reticulum stress and upregulation of tight junction proteins like claudin-7 (112). Both enteral administration and intraperitoneal injection of AF-SCs have shown efficacy in experimental NEC models (68, 176).
The enteric nervous system (ENS), often referred to as the “second brain”, interacts with the diverse range of microbes that populate the gastrointestinal tract, forming critical linkages with intestinal microbiota, the immune system, and the endocrine system to maintain a stable intestinal environment (177–179). It undergoes significant damage during the NEC development process, thus increasing the interest in neural stem cells (NSCs) as a potential therapy for NEC.
Neural stem cells, also derived from amniotic fluid (AF-NSCs), express nestin, an intermediate filament protein that can be considered a marker of immature neural cells (180). Despite their slow growth rate when cultured in vitro, AF-NSCs have shown potential in treating NEC through Wnt-dependent mechanisms (112, 181, 182).
Neonatal enteric neural stem cells (N-ENSCs) are specialized stem cells found within the ENS that control the complex network of neurons in the gastrointestinal tract. N-ENSCs can differentiate into various neural cell types within the ENS, including neurons and glial cells (183). N-ENSCs have emerged as a promising avenue for research as they can potentially repair or regenerate the ENS's damaged neural and glial cells. This regenerative capacity offers hope for developing innovative treatments for NEC (184).
Beyond BM-MSCs, other MSC sources have also gained attention. Umbilical cord-derived mesenchymal stem cells (UC-MSCs) are multipotent stem cells that can be isolated in a non-invasive manner and exhibit regenerative properties comparable to BM-MSCs, making them a promising alternative for stem cell research and therapies (185). In NEC models, UC-MSCs can promote intestinal integrity by activating endothelial nitric oxide synthase and secreting hydrogen sulfide (108). Placental-derived MSCs (P-MSCs) have also been shown to be a good source of stem cells with potent anti-inflammatory effects (186), able to promote intestinal regeneration via the Wnt/β-catenin pathway (108).
These diverse stem cell populations, ranging from pluripotent ESCs to more specialized AF-SCs and neural stem cells, offer multiple avenues for regenerative medicine approaches, particularly for intestinal pathologies such as NEC. Their varying degrees of potency, combined with distinct mechanisms of action ranging from barrier reinforcement to neural regeneration, could expand the knowledge aimed at the development of novel cell-based therapies. Continuous research into stem cells could lead to a deeper understanding of their therapeutic potential and the resolution of associated ethical issues.
4 Mesenchymal stromal cells: the dark side
Preclinical studies involving MSCs in various diseases have generated significant interest and optimism among researchers and patients globally. However, translating these findings into clinical applications presents challenges due to ethical concerns, technical limitations, and potential adverse effects (106, 108).
One of the primary obstacles to the clinical use of adult MSCs is their isolation method. Harvesting MSCs, particularly from the bone marrow, often necessitates invasive procedures, posing a risk to donors and complicating broader clinical implementation (187). Furthermore, the intrinsic scarcity of MSCs exacerbates this challenge; for example, MSCs account for merely 0.001%–0.01% of total mononuclear cells in the bone marrow (188, 189). Therefore, extensive in vitro expansion is generally required to obtain clinically relevant cell numbers.
However, the in vitro expansion process introduces complications. MSCs expanded ex vivo may experience significant morphological changes, gene and protein expression profile modifications, and variations in their potential and physiological behaviors (190–192). Such changes can significantly affect the therapeutic use of MSCs and may even increase the risk of malignant transformation and tumor formation (18, 193). Indeed, while MSC-based therapies show significant promise for cancer treatment, evidence indicates that MSCs may contribute to tumor progression, local angiogenesis, metastasis, and drug resistance (194, 195).
A related issue is the poor engraftment and homing capacity of MSCs after administration. The delivery route significantly impacts therapeutic outcomes; for example, studies have shown that intravenous infusion can lead to the sequestration of MSCs in the capillaries of several organs, especially the lungs, liver, and spleen, which limits their effective distribution (16, 17, 196, 197). Furthermore, it is still poorly understood how MSCs behave and differentiate after being administered in vivo, with some studies suggesting that the surrounding microenvironment may trigger undesired differentiation or even pro-inflammatory behavior during the early inflammatory phase (19).
Nevertheless, inconsistencies in the isolation, expansion, and characterization of MSCs across laboratories continue to pose significant barriers. To tackle this issue, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) has established minimal criteria for defining MSCs, including plastic adherence, the expression of specific surface antigens, and the ability to differentiate into multiple cell lineages (14). Despite these guidelines, significant heterogeneity persists.
Published studies show that MSCs display greater variability in properties when subjected to plastic adherence and various culture media (198–200). Prolonged culture conditions further exacerbate this issue, often resulting in the loss of the MSCs' native supportive and anti-inflammatory functions (201). Genome-wide analyses have shown a dampening or downregulation of therapeutic gene expression profiles in cultured MSCs compared to their primary counterparts (202, 203). Additionally, ex vivo-expanded MSCs exhibit reduced expression of transcription factors crucial for producing paracrine factors, which diminishes their therapeutic potential. RNA sequencing data from human primary MSCs and their in vitro expanded counterparts have also indicated that primary MSCs maintain an enhanced hematopoietic supportive function (202).
Several strategies have been explored to address these challenges, including optimizing culture conditions by adjusting cytokine, glucose concentrations, and oxygen tension and employing three-dimensional culture systems such as mesenspheres (204). Alternatively, transcription factor-mediated reprogramming has been proposed for both murine and human MSCs to enhance their functional properties. This approach seeks to bolster the MSCs' anti-inflammatory response to in vitro stress and improve their hematopoietic supportive capacity (203, 205).
Ultimately, ethical and regulatory challenges persist, obstructing the clinical translation of MSC therapies. Issues regarding donor consent, cell ownership, manufacturing standards, and long-term safety monitoring must be addressed to guarantee responsible development and application (206–208).
5 Future direction: secretome and exosomes
Since MSCs primarily exert their effects through paracrine secretion, there has been a notable shift in MSC research toward the protective bioactive factors secreted by MSCs, such as the secretome, which has garnered considerable attention for its potential use in tissue repair and regeneration (209).
The secretome includes the factors and molecules secreted by stem cells into the extracellular space, such as soluble proteins, nucleic acids, lipids, and extracellular microvesicles. Based on their size and origin in the cell, these vesicles can be classified into three main categories: apoptotic bodies, microparticles, and exosomes (210).
The use of this cell-free therapeutic approach provides several advantages. Utilizing MSC-derived secretome as a lyophilized medical product could effectively alleviate concerns related to the infusion of ex vivo expanded cells as well as thawed and manipulated MSCs, presenting various biological and technological benefits. The secretome is favored over proliferating cells for safety reasons, including immune compatibility and tumorigenicity (210). The secretome derived from MSCs is also cost-effective because it eliminates the need for cell harvesting procedures (133).
A conditioned medium (CM) is a source of the secretome and vesicular elements used in regenerative therapies (210). The secretome's soluble components can be isolated through centrifugation, ion exchange chromatography, and filtration (211, 212). In the case of reprogrammed MSCs with enhanced paracrine activity, using the MSC secretome may alleviate any safety concerns associated with genetically modified cells.
The CM of MSCs is known to possess anti-inflammatory properties through various soluble molecules, such as TNF β1, IL-13, neurotrophin 3, ciliary neurotrophic factor, and IL-10 (213–215). Additionally, the CM also contains anti-apoptotic factors like bFGF and TGF, in addition to angiogenic factors, including VEGF and IGF-1 (21). Several studies have demonstrated the beneficial potential of CM in the context of various pathological conditions. For instance, CM derived from human cervical stem cells exhibited bactericidal activity against S. epidermidis and E. coli on infected corneal lenses (215). BM-MSCs-derived CM improved cortical neuron survival and neuronal connections in vitro and enhanced neurological recovery in a rat ischemia model. In particular, CM improved the survival of cortical neurons and facilitated the formation of neuronal connections in culture, while in vivo experiments revealed that the CM led to better neurological outcomes, suggesting its potential as a therapeutic option for neurological recovery (216). MSC secretome showed its efficiency in preventing and treating experimental NEC in mice and piglets by inhibiting TLR-4 signaling, reducing inflammation, and promoting intestinal remodeling and immune function, as shown by RNA sequencing (217). The TLR-4 pathway is well documented to contribute to the onset of pathology by facilitating the translocation of pathogenic bacteria, which subsequently elicits a pro-inflammatory response and leads to NEC development (218).
To proceed to administration in clinical trials, the secretome must be formulated into a standardized drug product that can be easily adopted by the clinical community (219). Several issues must be addressed, including the source, isolation methods, pharmaceutical quality controls, potency monitoring, toxicity, immunogenicity, administration route, and the definition of efficacy and long-term side effects (219, 220). Preclinical studies are necessary to identify the mechanisms of action of the components of the secretome. The diverse molecules carry various bioactive cargoes, including proteins, lipids, and metabolites, which can exert molecular and epigenetic effects on target cells. Additionally, the lack of standardized criteria for producing secretome presents a challenge. There is room for improvement in isolation methods to make more homogeneous preparations regarding particle number, potency, and purity (221).
Interest in the secretome for treating infant diseases like NEC has increased the focus on extracellular vesicles, especially exosomes (222). The term “exosome” refers to a distinct class of lipid membrane-bound extracellular vesicles, ranging in size from 40 to 150 nm in diameter and having a density between 1.09 and 1.18 g/ml (212). Many of the regenerative properties displayed by MSCs are mediated by secreted exosomes (223). Exosomes can deliver their contents to recipient cells through phagocytosis, fusion with the cell membrane, and receptor-ligand interactions (224). The lipid composition of these vesicles reflects their unique rigidity, and their function is to deliver bioactive lipids to recipient cells. Furthermore, exosomes contain genetic material, including messenger ribonucleic acid (mRNAs) and micro ribonucleic acid (miRNAs), which stimulate the degradation of their mRNA targets and circular ribonucleic acid (circRNAs) (225). Additionally, exosomes contain various cellular proteins, such as adhesion proteins, chaperones, and cytoskeletal proteins (226).
The recognition of exosomes as delivery vehicles for biological materials has prompted researchers to investigate their potential as therapeutic modalities, especially as drug carriers (227). Cell-derived vesicles provide several advantages over other drug delivery methods, including their natural composition, small size, and immune evasion capabilities that allow them to bypass the immune system (228). Extracellular vesicles can be loaded with exogenous cargo through several methods. The first method is electroporation, which uses an electric field from an electrode to create hydrophilic pores in the membrane, increasing membrane permeability and allowing substances with large molecular weights to pass through (229). Subsequently, transfection can be achieved by overexpressing a specific gene in the exosome donor cell or by treating a cell line with a drug of interest that will later be encapsulated in vesicles (230). The final technique is chemical-based exosome transfection, which uses commercially available transfection reagents to incorporate short interfering ribonucleic acid (siRNA) and deliver it to target cells via exosomes (231).
Extracellular vesicles from intestinal epithelial cells have been shown to activate wound repair mechanisms. Additionally, exosomes derived from these cells may affect antigen expression in the mucosal or systemic immune system through intercellular communication functions, thereby influencing NEC progression (167, 232, 233). Exosomes do not trigger human leukocyte antigen (HLA)-stem cell immune responses, making them less immunogenic than stem cells (234). The treatment of experimental NEC with exosomes derived from stem cells is as effective as treatment with stem cells themselves (111). Furthermore, exosomes from human umbilical cord mesenchymal stem cells reduced the severity of inflammatory bowel disease in mice by increasing IL-10 levels and decreasing TNF-α, IL-1β, and IL-6 in the colon tissues (109).
Research indicates that white matter injury observed in imaging studies contributes to adverse neurodevelopmental outcomes in children with NEC. Additionally, animal studies suggest that NEC-induced systemic inflammation may disrupt the blood-brain barrier (235, 236). In preclinical studies involving rodents, exosomes have been shown to traverse the blood-brain barrier; this has sparked interest in their therapeutic potential for NEC (237, 238). The paracrine effects of exosomes and their capacity to target brain injury present an intriguing therapeutic path for neuroprotection in NEC (239).
Rager et al. explored the protective role of exosomes derived from BM-MSCs against NEC. Administering them intraperitoneally to rat pups reduced NEC incidence and enhanced intestinal barrier function, showing no significant difference between the effects of exosomes and stem cells. This emphasizes that exosomes are likely the key mediators of the therapeutic effects of MSCs (240).
Figure 1 outlines the functions and mechanisms of action of NEC's BM-MSCs, secretomes, and exosomes.
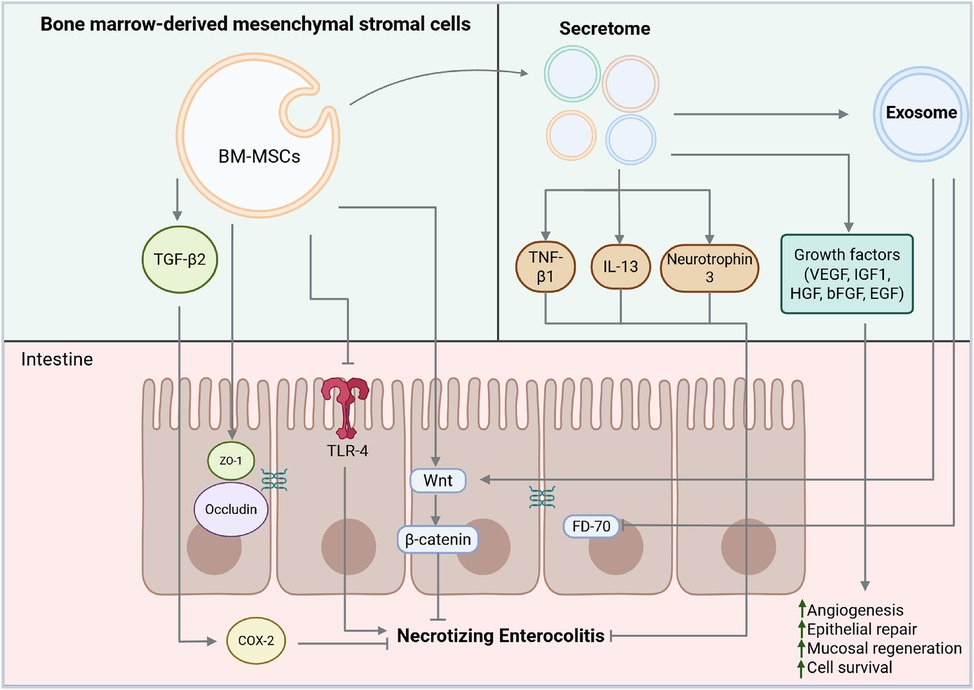
Figure 1. Functions and mechanisms of actions of bone marrow-derived mesenchymal stromal cells (BM-MSCs), secretomes, and exosomes in necrotizing enterocolitis (NEC). BM-MSCs act by activating transforming growth factor beta (TGF-β2), which, in a paracrine manner, triggers cyclooxygenase 2 (COX2), zonula occludens 1 (ZO-1) and occludin, thereby promoting the restoration of tight junctions. BM-MSCs impairs TLR4 pathway signaling thus interfering with NEC development. The secretome secretes a variety of bioactive molecules, including anti-inflammatory cytokines such as tumor necrosis factor β1 (TNF-β1), interleukin 13 (IL-13), and neurotrophin 3, which contribute to immune modulation. It also includes growth factors with potent regenerative and pro-angiogenetic properties, such as vascular endothelial growth factor (VEGF), insulin-like growth factor-1 (IGF-1), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and epidermal growth factor (EGF). All these factors collectively enhance angiogenesis, epithelial repair, mucosal regeneration, and cell survival. Exosomes contribute to NEC protection by modulating the Wnt/β-catenin signaling pathway and improving intestinal permeability and gut barrier function by reducing the levels of FD-70 (70 kDa FITC-Dextran). Created with BioRender.com.
6 Clinical translation prospects
Although MSCs have been investigated in several clinical trials, including those targeting rheumatic and autoimmune disorders, their large-scale clinical implementation remains complex (241, 242). In the context of necrotizing NEC, clinical translation is particularly challenging. A significant proportion of studies have explored the prophylactic use of MSCs in NEC, further amplifying the ethical concerns related to interventions in a highly vulnerable population such as preterm infants with multiple comorbidities. While cell-free strategies employing MSC-derived exosomes or secretomes offer a promising alternative by circumventing many of the inherent risks of cell-based therapies, their translational potential is currently limited by the lack of standardized protocols for their isolation, characterization, and quality control. Establishing universally accepted criteria for producing and validating these products is essential to reduce inter-study variability and ensure the safety and reproducibility required for future clinical application.
7 Conclusion
While the causes of NEC have been thoroughly studied, developing new therapeutic strategies has encountered several challenges because of the high incidence and mortality rates associated with this disease in preterm infants (98, 99). This highlights the ongoing need to examine additional aspects of NEC pathology. Due to their regenerative and anti-inflammatory properties, BM-MSCs are being reconsidered for treating neonatal diseases like NEC. In preclinical models, they secrete protective and reparative factors that reduce the incidence and severity of NEC. This mechanism includes the secretion of endocrine factors that aid tissue repair. While they enhance gut barrier function and lower intestinal permeability, whether these effects occur through shared or distinct molecular pathways remains unclear. Practical application addresses ethical, medical, and legal concerns (206).
Exosomes as therapeutic agents are promising candidates for developing novel strategies to treat NEC by reducing inflammation and intestinal permeability. Their ability to cross the blood-brain barrier and deliver therapeutic agents introduces a new concept for addressing NEC-related brain injury. Leveraging the therapeutic cargo and communication properties of exosomes enables us to explore cell-free therapy further while preserving the reparative potential of mesenchymal stromal cells (243). Despite their potential, the use of exosome-mediated therapy in NEC models remains limited. Therefore, further research is necessary to understand the safety, efficacy, and optimal administration methods for this treatment in NEC. Moreover, the type of stem cells, their origin, the various routes of administration, cell concentration, protocol duration, and starting point, combined with the marked variability in MSC isolation and culture methods, pose a substantial challenge for advancing this line of research in NEC. Standardization of these parameters is urgently needed to generate more robust and comparable results, ultimately facilitating their translation to the bedside. Enhancing our understanding of exosome biology in NEC could improve disease management and outcomes for premature infants globally.
Author contributions
AT: Conceptualization, Writing – review & editing, Validation, Methodology, Investigation, Data curation, Writing – original draft. MT: Methodology, Data curation, Writing – review & editing, Investigation, Writing – original draft, Validation, Conceptualization. LP: Data curation, Validation, Methodology, Conceptualization, Supervision, Project administration, Resources, Writing – original draft, Investigation, Writing – review & editing. GR: Writing – original draft, Resources, Writing – review & editing, Funding acquisition, Methodology, Validation, Data curation, Conceptualization, Investigation. SC: Conceptualization, Investigation, Validation, Methodology, Writing – review & editing. LR: Investigation, Validation, Writing – review & editing. CB: Writing – review & editing, Investigation, Validation. LS: Validation, Investigation, Writing – review & editing. CA: Validation, Writing – review & editing. MF: Validation, Writing – review & editing. SL: Writing – review & editing, Validation. ME: Validation, Writing – review & editing. FG: Data curation, Visualization, Project administration, Conceptualization, Resources, Methodology, Writing – review & editing, Investigation, Writing – original draft, Supervision, Validation. GC: Funding acquisition, Conceptualization, Investigation, Resources, Writing – review & editing, Supervision, Data curation, Writing – original draft, Project administration, Validation, Methodology, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was partially supported by the Italian Ministry of Health (Ricerca Corrente) and the grant Ricerca Finalizzata Giovani Ricercatori to GR (grant number GR-2021-12375473).
Acknowledgments
Special thanks to Dr. Stefano Gatti, director of the Center for Preclinical Investigation of the Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thompson AM, Bizzarro MJ. Necrotizing enterocolitis in newborns: pathogenesis, prevention and management. Drugs. (2008) 68(9):1227–38. doi: 10.2165/00003495-200868090-00004
2. Berman L, Moss RL. Necrotizing enterocolitis: an update. Semin Fetal Neonatal Med. (2011) 16(3):145–50. doi: 10.1016/j.siny.2011.02.002
3. Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. (1990) 117(1 Pt 2):S6–13. doi: 10.1016/S0022-3476(05)81124-2
4. Bellodas Sanchez J, Kadrofske M. Necrotizing enterocolitis. Neurogastroenterol Motil. (2019) 31(3):e13569. doi: 10.1111/nmo.13569
5. Afrazi A, Sodhi CP, Richardson W, Neal M, Good M, Siggers R, et al. New insights into the pathogenesis and treatment of necrotizing enterocolitis: toll-like receptors and beyond. Pediatr Res. (2011) 69(3):183–8. doi: 10.1203/PDR.0b013e3182093280
6. Hug H, Mohajeri MH, La Fata G. Toll-like receptors: regulators of the immune response in the human gut. Nutrients. (2018) 10(2):203. doi: 10.3390/nu10020203
7. Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: an immature innate immune response. PLoS One. (2011) 6(3):e17776. doi: 10.1371/journal.pone.0017776
8. Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. (2012) 143(3):708–18.e5. doi: 10.1053/j.gastro.2012.05.053
9. Roberts AG, Younge N, Greenberg RG. Neonatal necrotizing enterocolitis: an update on pathophysiology, treatment, and prevention. Paediatr Drugs. (2024) 26(3):259–75. doi: 10.1007/s40272-024-00626-w
10. Chen Y, Chang KT, Lian DW, Lu H, Roy S, Laksmi NK, et al. The role of ischemia in necrotizing enterocolitis. J Pediatr Surg. (2016) 51(8):1255–61. doi: 10.1016/j.jpedsurg.2015.12.015
11. Downard CD, Grant SN, Matheson PJ, Guillaume AW, Debski R, Fallat ME, et al. Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg. (2011) 46(6):1023–8. doi: 10.1016/j.jpedsurg.2011.03.023
12. Goulet O, Sauvat F. Short bowel syndrome and intestinal transplantation in children. Curr Opin Clin Nutr Metab Care. (2006) 9(3):304–13. doi: 10.1097/01.mco.0000222116.68912.fc
13. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. (2005) 115(3):696–703. doi: 10.1542/peds.2004-0569
14. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. (2006) 8(4):315–7. doi: 10.1080/14653240600855905
15. Provitera L, Tomaselli A, Raffaeli G, Crippa S, Arribas C, Amodeo I, et al. Human bone marrow-derived mesenchymal stromal cells reduce the severity of experimental necrotizing enterocolitis in a concentration-dependent manner. Cells. (2023) 12(5):760. doi: 10.3390/cells12050760
16. Musiał-Wysocka A, Kot M, Majka M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transplant. (2019) 28(7):801–12. doi: 10.1177/0963689719837897
17. Grange C, Tapparo M, Bruno S, Chatterjee D, Quesenberry PJ, Tetta C, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med. (2014) 33(5):1055–63. doi: 10.3892/ijmm.2014.1663
18. Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. (2009) 69(13):5331–9. doi: 10.1158/0008-5472.CAN-08-4630
19. Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. (2020) 53(1):e12712. doi: 10.1111/cpr.12712
20. Chen W, Wang X, Yan X, Yu Z, Zhang J, Han S. The emerging role of exosomes in the pathogenesis, prognosis and treatment of necrotizing enterocolitis. Am J Transl Res. (2020) 12(11):7020–33.33312348
21. Wang Y, Long W, Cao Y, Li J, You L, Fan Y. Mesenchymal stem cell-derived secretomes for therapeutic potential of premature infant diseases. Biosci Rep. (2020) 40(5):BSR20200241. doi: 10.1042/BSR20200241
22. Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. (2006) 117(2):e137–42. doi: 10.1542/peds.2005-1543
23. Patole SK, de Klerk N. Impact of standardised feeding regimens on incidence of neonatal necrotising enterocolitis: a systematic review and meta-analysis of observational studies. Arch Dis Child Fetal Neonatal Ed. (2005) 90(2):F147–51. doi: 10.1136/adc.2004.059741
24. McCallie KR, Lee HC, Mayer O, Cohen RS, Hintz SR, Rhine WD. Improved outcomes with a standardized feeding protocol for very low birth weight infants. J Perinatol. (2011) 31(Suppl 1):S61–7. doi: 10.1038/jp.2010.185
25. Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. (1990) 336(8730):1519–23. doi: 10.1016/0140-6736(90)93304-8
26. Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. (2014) 4:CD002971. doi: 10.1002/14651858.CD002971.pub5
27. Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int J Biochem Cell Biol. (2013) 45(8):1730–47. doi: 10.1016/j.biocel.2013.04.028
28. Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. (2009) 123(1):58–66. doi: 10.1542/peds.2007-3423
29. Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. (2011) 159(3):392–7. doi: 10.1016/j.jpeds.2011.02.035
30. Roberts SA, Freed DL. Neonatal IgA secretion enhanced by breast feeding. Lancet. (1977) 310(8048):1131. doi: 10.1016/S0140-6736(77)90576-1
31. Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J Perinatol. (2007) 27(7):428–33. doi: 10.1038/sj.jp.7211758
32. Cassir N, Simeoni U, La Scola B. Gut microbiota and the pathogenesis of necrotizing enterocolitis in preterm neonates. Future Microbiol. (2016) 11(2):273–92. doi: 10.2217/fmb.15.136
33. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health. (2014) 9(3):584–671. doi: 10.1002/ebch.1976
34. Duchon J, Barbian ME, Denning PW. Necrotizing enterocolitis. Clin Perinatol. (2021) 48(2):229–50. doi: 10.1016/j.clp.2021.03.002
35. Llanos AR, Moss ME, Pinzòn MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol. (2002) 16(4):342–9. doi: 10.1046/j.1365-3016.2002.00445.x
36. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F182–9. doi: 10.1136/archdischild-2017-313880
37. Han SM, Hong CR, Knell J, Edwards EM, Morrow KA, Soll RF, et al. Trends in incidence and outcomes of necrotizing enterocolitis over the last 12 years: a multicenter cohort analysis. J Pediatr Surg. (2020) 55(6):998–1001. doi: 10.1016/j.jpedsurg.2020.02.046
38. Lambert DK, Christensen RD, Henry E, Besner GE, Baer VL, Wiedmeier SE, et al. Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol. (2007) 27(7):437–43. doi: 10.1038/sj.jp.7211738
39. Christensen RD, Lambert DK, Baer VL, Gordon PV. Necrotizing enterocolitis in term infants. Clin Perinatol. (2013) 40(1):69–78. doi: 10.1016/j.clp.2012.12.007
40. Anand RJ, Leaphart CL, Mollen KP, Hackam DJ. The role of the intestinal barrier in the pathogenesis of necrotizing enterocolitis. Shock. (2007) 27(2):124–33. doi: 10.1097/01.shk.0000239774.02904.65
41. Berseth CL. Gastrointestinal motility in the neonate. Clin Perinatol. (1996) 23(2):179–90. doi: 10.1016/S0095-5108(18)30237-9
42. Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. (1999) 80(3):F167–73. doi: 10.1136/fn.80.3.F167
43. Nowicki PT, Miller CE. Autoregulation in the developing postnatal intestinal circulation. Am J Physiol. (1988) 254(2 Pt 1):G189–93. doi: 10.1152/ajpgi.1988.254.2.G189
44. Nankervis CA, Nowicki PT. Role of nitric oxide in regulation of vascular resistance in postnatal intestine. Am J Physiol. (1995) 268(6 Pt 1):G949–58. doi: 10.1152/ajpgi.1995.268.6.G949
45. Yanowitz TD, Yao AC, Pettigrew KD, Werner JC, Oh W, Stonestreet BS. Postnatal hemodynamic changes in very-low-birthweight infants. J Appl Physiol (1985). (1999) 87(1):370–80. doi: 10.1152/jappl.1999.87.1.370
46. Snyder JD, Walker WA. Structure and function of intestinal mucin: developmental aspects. Int Arch Allergy Appl Immunol. (1987) 82(3-4):351–6. doi: 10.1159/000234225
47. Smith SD, Cardona MA, Wishnev SA, Kurkchubasche AG, Rowe MI. Unique characteristics of the neonatal intestinal mucosal barrier. J Pediatr Surg. (1992) 27(3):333–6; discussion 6–8. doi: 10.1016/0022-3468(92)90857-4
48. Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. (1999) 23(5 Suppl):S3–6. doi: 10.1177/014860719902300502
49. Nowicki PT, Nankervis CA, Miller CE. Effects of ischemia and reperfusion on intrinsic vascular regulation in the postnatal intestinal circulation. Pediatr Res. (1993) 33(4 Pt 1):400–4. doi: 10.1203/00006450-199304000-00017
50. Grand RJ, Watkins JB, Torti FM. Development of the human gastrointestinal tract. A review. Gastroenterology. (1976) 70(5 PT.1):790–810. doi: 10.1016/S0016-5085(76)80277-6
51. Correa-Rocha R, Perez A, Lorente R, Ferrando-Martinez S, Leal M, Gurbindo D, et al. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatr Res. (2012) 71(5):590–7. doi: 10.1038/pr.2012.6
52. Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest. (2016) 126(2):495–508. doi: 10.1172/JCI83356
53. Neu J, Weiss MD. Necrotizing enterocolitis: pathophysiology and prevention. JPEN J Parenter Enteral Nutr. (1999) 23(5 Suppl):S13–7. doi: 10.1177/014860719902300504
54. Mizrahi A, Barlow O, Berdon W, Blanc WA, Silverman WA. Necrotizing enterocolitis in premature infants. J Pediatr. (1965) 66:697–705. doi: 10.1016/S0022-3476(65)80003-8
55. Schmid KO. A specially severe form of enteritis in newborn, enterocolitis ulcerosa necroticans. I. Pathological anatomy. Osterr Z Kinderheilkd Kinderfuersorge. (1952) 8(2):114–35.13003087
56. Stiennon OA. Pneumatosis intestinals in the newborn. AMA Am J Dis Child. (1951) 81(5):651–63. doi: 10.1001/archpedi.1951.02040030664004
57. Palmer SR, Thomas SJ, Cooke RW, Low DC, Fysh WJ, Murphy JF, et al. Birthweight-specific risk factors for necrotising enterocolitis. J Epidemiol Community Health. (1987) 41(3):210–4. doi: 10.1136/jech.41.3.210
58. Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, et al. Discovery and validation of a new class of small molecule toll-like receptor 4 (TLR4) inhibitors. PLoS One. (2013) 8(6):e65779. doi: 10.1371/journal.pone.0065779
59. Huda S, Chaudhery S, Ibrahim H, Pramanik A. Neonatal necrotizing enterocolitis: clinical challenges, pathophysiology and management. Pathophysiology. (2014) 21(1):3–12. doi: 10.1016/j.pathophys.2013.11.009
60. Huang K, Mukherjee S, DesMarais V, Albanese JM, Rafti E, Draghi Ii A, et al. Targeting the PXR-TLR4 signaling pathway to reduce intestinal inflammation in an experimental model of necrotizing enterocolitis. Pediatr Res. (2018) 83(5):1031–40. doi: 10.1038/pr.2018.14
61. Wu H, Guo K, Zhuo Z, Zeng R, Luo Y, Yang Q, et al. Current therapy option for necrotizing enterocolitis: practicalities and challenge. Front Pediatr. (2022) 10:954735. doi: 10.3389/fped.2022.954735
62. Dai S, Sodhi C, Cetin S, Richardson W, Branca M, Neal MD, et al. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of toll-like receptor-4 and increased cell-matrix adhesiveness. J Biol Chem. (2010) 285(7):4995–5002. doi: 10.1074/jbc.M109.067454
63. Yu R, Jiang S, Tao Y, Li P, Yin J, Zhou Q. Inhibition of HMGB1 improves necrotizing enterocolitis by inhibiting NLRP3 via TLR4 and NF-kappaB signaling pathways. J Cell Physiol. (2019) 234(8):13431–8. doi: 10.1002/jcp.28022
64. Nusse R, Brown A, Papkoff J, Scambler P, Shackleford G, McMahon A, et al. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. (1991) 64(2):231. doi: 10.1016/0092-8674(91)90633-A
65. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. (2012) 149(6):1192–205. doi: 10.1016/j.cell.2012.05.012
66. Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. (2004) 55(4):622–9. doi: 10.1203/01.PDR.0000113463.70435.74
67. Xi H, Wang S, Wang B, Hong X, Liu X, Li M, et al. The role of interaction between autophagy and apoptosis in tumorigenesis (review). Oncol Rep. (2022) 48(6):208. doi: 10.3892/or.2022.8423
68. Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S, et al. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. (2014) 63(2):300–9. doi: 10.1136/gutjnl-2012-303735
69. Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. (1997) 32(2):275–82. doi: 10.1016/S0022-3468(97)90194-9
70. Neal MD, Sodhi CP, Dyer M, Craig BT, Good M, Jia H, et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J Immunol. (2013) 190(7):3541–51. doi: 10.4049/jimmunol.1202264
71. Santos AJM, Lo YH, Mah AT, Kuo CJ. The intestinal stem cell niche: homeostasis and adaptations. Trends Cell Biol. (2018) 28(12):1062–78. doi: 10.1016/j.tcb.2018.08.001
72. Eaton S, Sebire N, Thyoka M, Pierro A. Histologic and immunohistochemical features associated with outcome in neonatal necrotizing enterocolitis. Eur J Pediatr Surg. (2014) 24(1):51–6. doi: 10.1055/s-0033-1349716
73. Patel RM, Ferguson J, McElroy SJ, Khashu M, Caplan MS. Defining necrotizing enterocolitis: current difficulties and future opportunities. Pediatr Res. (2020) 88(Suppl 1):10–5. doi: 10.1038/s41390-020-1074-4
74. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. (1987) 17(4):213–88. doi: 10.1016/0045-9380(87)90031-4
75. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
76. Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg. (2008) 17(2):98–109. doi: 10.1053/j.sempedsurg.2008.02.005
77. Kim JH, Sampath V, Canvasser J. Challenges in diagnosing necrotizing enterocolitis. Pediatr Res. (2020) 88(Suppl 1):16–20. doi: 10.1038/s41390-020-1090-4
78. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med. (2011) 364(3):255–64. doi: 10.1056/NEJMra1005408
79. Akre S, Sharma K, Chakole S, Wanjari MB. Gastrointestinal emergencies in neonates: a review article. Cureus. (2022) 14(10):e30538. doi: 10.7759/cureus.30538
80. D'Angelo G, Impellizzeri P, Marseglia L, Montalto AS, Russo T, Salamone I, et al. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital J Pediatr. (2018) 44(1):84. doi: 10.1186/s13052-018-0528-3
81. Buonomo C. The radiology of necrotizing enterocolitis. Radiol Clin North Am. (1999) 37(6):1187–98. doi: 10.1016/S0033-8389(05)70256-6
82. Daneman A, Woodward S, de Silva M. The radiology of neonatal necrotizing enterocolitis (NEC). A review of 47 cases and the literature. Pediatr Radiol. (1978) 7(2):70–7. doi: 10.1007/BF00975674
83. Kim JH. Role of abdominal US in diagnosis of NEC. Clin Perinatol. (2019) 46(1):119–27. doi: 10.1016/j.clp.2018.10.006
84. Muchantef K, Epelman M, Darge K, Kirpalani H, Laje P, Anupindi SA. Sonographic and radiographic imaging features of the neonate with necrotizing enterocolitis: correlating findings with outcomes. Pediatr Radiol. (2013) 43(11):1444–52. doi: 10.1007/s00247-013-2725-y
85. Bell RS, Graham CB, Stevenson JK. Roentgenologic and clinical manifestations of neonatal necrotizing enterocolitis. Experience with 43 cases. Am J Roentgenol Radium Ther Nucl Med. (1971) 112(1):123–34. doi: 10.2214/ajr.112.1.123
86. Hutter JJ Jr, Hathaway WE, Wayne ER. Hematologic abnormalities in severe neonatal necrotizing enterocolitis. J Pediatr. (1976) 88(6):1026–31. doi: 10.1016/S0022-3476(76)81069-4
87. Dominguez KM, Moss RL. Necrotizing enterocolitis. Clin Perinatol. (2012) 39(2):387–401. doi: 10.1016/j.clp.2012.04.011
88. Ten Barge JA, Vermeulen MJ, Simons SHP, van den Bosch GE. Pain management for necrotizing enterocolitis: getting the balance right. Pediatr Res. (2022) 92(5):1423–31. doi: 10.1038/s41390-022-01968-2
89. Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. (2006) 20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x
90. Carr BD, Gadepalli SK. Does surgical management Alter outcome in necrotizing enterocolitis? Clin Perinatol. (2019) 46(1):89–100. doi: 10.1016/j.clp.2018.09.008
91. Zani A, Pierro A. Necrotizing enterocolitis: controversies and challenges. F1000Res. (2015) 4:1373. doi: 10.12688/f1000research.6888.1
92. Kastenberg ZJ, Sylvester KG. The surgical management of necrotizing enterocolitis. Clin Perinatol. (2013) 40(1):135–48. doi: 10.1016/j.clp.2012.12.011
93. Blakely ML, Tyson JE, Lally KP, McDonald S, Stoll BJ, Stevenson DK, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics. (2006) 117(4):e680–7. doi: 10.1542/peds.2005-1273
94. Moss RL, Dimmitt RA, Barnhart DC, Sylvester KG, Brown RL, Powell DM, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. (2006) 354(21):2225–34. doi: 10.1056/NEJMoa054605
95. Rees CM, Eaton S, Kiely EM, Wade AM, McHugh K, Pierro A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. (2008) 248(1):44–51. doi: 10.1097/SLA.0b013e318176bf81
96. Hall NJ, Curry J, Drake DP, Spitz L, Kiely EM, Pierro A. Resection and primary anastomosis is a valid surgical option for infants with necrotizing enterocolitis who weigh less than 1000 g. Arch Surg. (2005) 140(12):1149–51. doi: 10.1001/archsurg.140.12.1149
97. Robinson JR, Rellinger EJ, Hatch LD, Weitkamp JH, Speck KE, Danko M, et al. Surgical necrotizing enterocolitis. Semin Perinatol. (2017) 41(1):70–9. doi: 10.1053/j.semperi.2016.09.020
98. Rich BS, Dolgin SE. Necrotizing enterocolitis. Pediatr Rev. (2017) 38(12):552–9. doi: 10.1542/pir.2017-0002
99. Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. (2009) 44(6):1072–5; discussion 5–6. doi: 10.1016/j.jpedsurg.2009.02.013
100. Hull MA, Fisher JG, Gutierrez IM, Jones BA, Kang KH, Kenny M, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg. (2014) 218(6):1148–55. doi: 10.1016/j.jamcollsurg.2013.11.015
101. Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD neonatal research network. Ann Surg. (2005) 241(6):984–9; discussion 9–94. doi: 10.1097/01.sla.0000164181.67862.7f
102. Guiducci S, Duci M, Moschino L, Meneghelli M, Fascetti Leon F, Bonadies L, et al. Providing the best parenteral nutrition before and after surgery for NEC: macro and micronutrients intakes. Nutrients. (2022) 14(5):919. doi: 10.3390/nu14050919
103. Teresa C, Antonella D, de Goyet J dV. New nutritional and therapeutical strategies of NEC. Curr Pediatr Rev. (2019) 15(2):92–105. doi: 10.2174/1573396315666190313164753
104. Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23(6):426–32. doi: 10.1016/j.siny.2018.08.005
105. Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. (2006) 3:CD004454. doi: 10.1002/14651858.CD004454.pub2
106. Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. (2019) 10(1):68. doi: 10.1186/s13287-019-1165-5
107. Bozdağ SC, Yüksel MK, Demirer T. Adult stem cells and medicine. Adv Exp Med Biol. (2018) 1079:17–36. doi: 10.1007/5584_2018_184
108. Di SJ, Wu SY, Liu TJ, Shi YY. Stem cell therapy as a promising strategy in necrotizing enterocolitis. Mol Med. (2022) 28(1):107. doi: 10.1186/s10020-022-00536-y
109. Umar S. Intestinal stem cells. Curr Gastroenterol Rep. (2010) 12(5):340–8. doi: 10.1007/s11894-010-0130-3
110. Salari V, Mengoni F, Del Gallo F, Bertini G, Fabene PF. The anti-inflammatory properties of mesenchymal stem cells in epilepsy: possible treatments and future perspectives. Int J Mol Sci. (2020) 21(24):9683. doi: 10.3390/ijms21249683
111. McCulloh CJ, Olson JK, Wang Y, Zhou Y, Tengberg NH, Deshpande S, et al. Treatment of experimental necrotizing enterocolitis with stem cell-derived exosomes. J Pediatr Surg. (2018) 53(6):1215–20. doi: 10.1016/j.jpedsurg.2018.02.086
112. Li B, Lee C, Chuslip S, Lee D, Biouss G, Wu R, et al. Intestinal epithelial tight junctions and permeability can be rescued through the regulation of endoplasmic reticulum stress by amniotic fluid stem cells during necrotizing enterocolitis. FASEB J. (2021) 35(1):e21265. doi: 10.1096/fj.202001426R
113. Li B, Lee C, O'Connell JS, Antounians L, Ganji N, Alganabi M, et al. Activation of wnt signaling by amniotic fluid stem cell-derived extracellular vesicles attenuates intestinal injury in experimental necrotizing enterocolitis. Cell Death Dis. (2020) 11(9):750. doi: 10.1038/s41419-020-02964-2
114. McCulloh CJ, Olson JK, Wang Y, Vu J, Gartner S, Besner GE. Evaluating the efficacy of different types of stem cells in preserving gut barrier function in necrotizing enterocolitis. J Surg Res. (2017) 214:278–85. doi: 10.1016/j.jss.2017.03.026
115. McCulloh CJ, Olson JK, Zhou Y, Wang Y, Besner GE. Stem cells and necrotizing enterocolitis: a direct comparison of the efficacy of multiple types of stem cells. J Pediatr Surg. (2017) 52(6):999–1005. doi: 10.1016/j.jpedsurg.2017.03.028
116. Li B, Lee C, Cadete M, O'Connell JS, Alganabi M, Lee D, et al. Amniotic fluid stem cell administration can prevent epithelial injury from necrotizing enterocolitis. Pediatr Res. (2022) 91(1):101–6. doi: 10.1038/s41390-021-01657-6
117. Tayman C, Uckan D, Kilic E, Ulus AT, Tonbul A, Murat Hirfanoglu I, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. (2011) 70(5):489–94. doi: 10.1203/PDR.0b013e31822d7ef2
118. Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, (ISCT) MCotISfCT. Immunological characterization of multipotent mesenchymal stromal cells--the international society for cellular therapy (ISCT) working proposal. Cytotherapy. (2013) 15(9):1054–61. doi: 10.1016/j.jcyt.2013.02.010
119. Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. (2010) 28(3):585–96. doi: 10.1002/stem.269
120. Maltais-Bilodeau C, Henckel E, Deguise M-O, Lesage F, Cobey KD, Ahmadzai N, et al. Cell-based therapies in preclinical models of necrotizing enterocolitis: a systematic review and meta-analysis. Stem Cells Transl Med. (2025) 14(2):szae102. doi: 10.1093/stcltm/szae102
121. Galderisi U, Peluso G, Di Bernardo G. Clinical trials based on mesenchymal stromal cells are exponentially increasing: where are we in recent years? Stem Cell Rev Rep. (2022) 18(1):23–36. doi: 10.1007/s12015-021-10231-w
122. Giovannelli L, Bari E, Jommi C, Tartara F, Armocida D, Garbossa D, et al. Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: risk-benefit profile and next steps for the market access. Bioact Mater. (2023) 29:16–35. doi: 10.1016/j.bioactmat.2023.06.013
123. Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. (2014) 32(3):252–60. doi: 10.1038/nbt.2816
124. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. (2008) 8(9):726–36. doi: 10.1038/nri2395
125. Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS One. (2010) 5(4):e10088. doi: 10.1371/journal.pone.0010088
126. Rashedi I, Gómez-Aristizábal A, Wang XH, Viswanathan S, Keating A. TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated treg induction via notch signaling. Stem Cells. (2017) 35(1):265–75. doi: 10.1002/stem.2485
127. Najar M, Krayem M, Meuleman N, Bron D, Lagneaux L. Mesenchymal stromal cells and toll-like receptor priming: a critical review. Immune Netw. (2017) 17(2):89–102. doi: 10.4110/in.2017.17.2.89
128. Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. (2008) 2(2):141–50. doi: 10.1016/j.stem.2007.11.014
129. Lim JY, Kim BS, Ryu DB, Kim TW, Park G, Min CK. The therapeutic efficacy of mesenchymal stromal cells on experimental colitis was improved by the IFN-gamma and poly(I:C) priming through promoting the expression of indoleamine 2,3-dioxygenase. Stem Cell Res Ther. (2021) 12(1):37. doi: 10.1186/s13287-020-02087-7
130. Burchfield JS, Iwasaki M, Koyanagi M, Urbich C, Rosenthal N, Zeiher AM, et al. Interleukin-10 from transplanted bone marrow mononuclear cells contributes to cardiac protection after myocardial infarction. Circ Res. (2008) 103(2):203–11. doi: 10.1161/CIRCRESAHA.108.178475
131. Shi Y, Su J, Roberts AI, Shou P, Rabson AB, Ren G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. (2012) 33(3):136–43. doi: 10.1016/j.it.2011.11.004
132. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. (2013) 13(4):392–402. doi: 10.1016/j.stem.2013.09.006
133. Klimczak A, Kozlowska U. Mesenchymal stromal cells and tissue-specific progenitor cells: their role in tissue homeostasis. Stem Cells Int. (2016) 2016:4285215. doi: 10.1155/2016/4285215
134. Sagaradze GD, Basalova NA, Efimenko AY, Tkachuk VA. Mesenchymal stromal cells as critical contributors to tissue regeneration. Front Cell Dev Biol. (2020) 8:576176. doi: 10.3389/fcell.2020.576176
135. Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. (2010) 17(6):952–61. doi: 10.1038/cdd.2009.187
136. Sémont A, Demarquay C, Bessout R, Durand C, Benderitter M, Mathieu N. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS One. (2013) 8(7):e70170. doi: 10.1371/journal.pone.0070170
137. Attar A, Bahmanzadegan Jahromi F, Kavousi S, Monabati A, Kazemi A. Mesenchymal stem cell transplantation after acute myocardial infarction: a meta-analysis of clinical trials. Stem Cell Res Ther. (2021) 12(1):600. doi: 10.1186/s13287-021-02667-1
138. Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. (2006) 36(10):2566–73. doi: 10.1002/eji.200636416
139. Vieujean S, Loly JP, Boutaffala L, Meunier P, Reenaers C, Briquet A, et al. Mesenchymal stem cell injection in Crohn’s disease strictures: a phase I–II clinical study. J Crohns Colitis. (2022) 16(3):506–10. doi: 10.1093/ecco-jcc/jjab154
140. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. (2008) 371(9624):1579–86. doi: 10.1016/S0140-6736(08)60690-X
141. Crippa S, Santi L, Bosotti R, Porro G, Bernardo ME. Bone marrow-derived mesenchymal stromal cells: a novel target to optimize hematopoietic stem cell transplantation protocols in hematological malignancies and rare genetic disorders. J Clin Med. (2019) 9(1):2. doi: 10.3390/jcm9010002
142. Harris VK, Stark J, Vyshkina T, Blackshear L, Joo G, Stefanova V, et al. Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis. EBioMedicine. (2018) 29:23–30. doi: 10.1016/j.ebiom.2018.02.002
143. Shichinohe H, Kawabori M, Iijima H, Teramoto T, Abumiya T, Nakayama N, et al. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): a study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. (2017) 17(1):179. doi: 10.1186/s12883-017-0955-6
144. Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. (2020) 11(5):349. doi: 10.1038/s41419-020-2542-9
145. Zhang S, Yang Y, Fan L, Zhang F, Li L. The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future. Ann Transl Med. (2020) 8(8):565. doi: 10.21037/atm.2020.03.218
146. Che Z, Ye Z, Zhang X, Lin B, Yang W, Liang Y, et al. Mesenchymal stem/stromal cells in the pathogenesis and regenerative therapy of inflammatory bowel diseases. Front Immunol. (2022) 13:952071. doi: 10.3389/fimmu.2022.952071
147. Gu C, Du W, Chai M, Jin Z, Zhou Y, Guo P, et al. Human umbilical cord-derived mesenchymal stem cells affect urea synthesis and the cell apoptosis of human induced hepatocytes by secreting IL-6 in a serum-free co-culture system. Biotechnol J. (2022) 17(1):e2100096. doi: 10.1002/biot.202100096
148. Tu C, Wang Z, Xiang E, Zhang Q, Zhang Y, Wu P, et al. Human umbilical cord mesenchymal stem cells promote macrophage PD-L1 expression and attenuate acute lung injury in mice. Curr Stem Cell Res Ther. (2022) 17(6):564–75. doi: 10.2174/1574888X17666220127110332
149. Barati S, Kashani IR, Tahmasebi F. The effects of mesenchymal stem cells transplantation on A1 neurotoxic reactive astrocyte and demyelination in the cuprizone model. J Mol Histol. (2022) 53(2):333–46. doi: 10.1007/s10735-021-10046-6
150. Cakouros D, Gronthos S. Epigenetic regulation of bone marrow stem cell aging: revealing epigenetic signatures associated with hematopoietic and mesenchymal stem cell aging. Aging Dis. (2019) 10(1):174–89. doi: 10.14336/AD.2017.1213
151. Zhang Y, Ravikumar M, Ling L, Nurcombe V, Cool SM. Age-related changes in the inflammatory status of human mesenchymal stem cells: implications for cell therapy. Stem Cell Rep. (2021) 16(4):694–707. doi: 10.1016/j.stemcr.2021.01.021
152. Chu D-T, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, et al. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. (2020) 21(3):708. doi: 10.3390/ijms21030708
153. Andrzejewska A, Lukomska B, Janowski M. Concise review: mesenchymal stem cells: from roots to boost. Stem Cells. (2019) 37(7):855–64. doi: 10.1002/stem.3016
154. Nitkin CR, Rajasingh J, Pisano C, Besner GE, Thébaud B, Sampath V. Stem cell therapy for preventing neonatal diseases in the 21st century: current understanding and challenges. Pediatr Res. (2020) 87(2):265–76. doi: 10.1038/s41390-019-0425-5
155. Liau LL, Al-Masawa ME, Koh B, Looi QH, Foo JB, Lee SH, et al. The potential of mesenchymal stromal cell as therapy in neonatal diseases. Front Pediatr. (2020) 8:591693. doi: 10.3389/fped.2020.591693
156. Liew A, O'Brien T, Egan L. Mesenchymal stromal cell therapy for Crohn’s disease. Dig Dis. (2014) 32(Suppl 1):50–60. doi: 10.1159/000367826
157. Kagia A, Tzetis M, Kanavakis E, Perrea D, Sfougataki I, Mertzanian A, et al. Therapeutic effects of mesenchymal stem cells derived from bone marrow, umbilical cord blood, and pluripotent stem cells in a mouse model of chemically induced inflammatory bowel disease. Inflammation. (2019) 42(5):1730–40. doi: 10.1007/s10753-019-01033-x
158. Abbuehl JP, Tatarova Z, Held W, Huelsken J. Long-term engraftment of primary bone marrow stromal cells repairs niche damage and improves hematopoietic stem cell transplantation. Cell Stem Cell. (2017) 21(2):241–55.e6. doi: 10.1016/j.stem.2017.07.004
159. Lee YS, Jun YH, Lee J. Oral administration of bone marrow-derived mesenchymal stem cells attenuates intestinal injury in necrotizing enterocolitis. Clin Exp Pediatr. (2024) 67(3):152–60. doi: 10.3345/cep.2023.01151
160. Wang M, Liang C, Hu H, Zhou L, Xu B, Wang X, et al. Intraperitoneal injection (IP), intravenous injection (IV) or anal injection (AI)? best way for mesenchymal stem cells transplantation for colitis. Sci Rep. (2016) 6(1):30696. doi: 10.1038/srep30696
161. Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. (2006) 41(1):144–9; discussion 9. doi: 10.1016/j.jpedsurg.2005.10.018
162. Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor–like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. (2006) 41(4):742–7. doi: 10.1016/j.jpedsurg.2005.12.020
163. Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. (2007) 42(1):214–20. doi: 10.1016/j.jpedsurg.2006.09.055
164. Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. (2009) 137(1):221–30. doi: 10.1053/j.gastro.2009.03.060
165. Chen CL, Yu X, James IO, Zhang HY, Yang J, Radulescu A, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. (2012) 92(3):331–44. doi: 10.1038/labinvest.2011.167
166. Yang J, Watkins D, Chen CL, Bhushan B, Zhou Y, Besner GE. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. (2012) 215(4):534–45. doi: 10.1016/j.jamcollsurg.2012.05.037
167. Chen H, Zhang H, Zheng Y, Min X, Luo Y, Zhou W, et al. Prolyl hydroxylase 2 silencing enhances the paracrine effects of mesenchymal stem cells on necrotizing enterocolitis in an NF-kappaB-dependent mechanism. Cell Death Dis. (2020) 11(3):188. doi: 10.1038/s41419-020-2378-3
168. Mesfin FM, Manohar K, Shelley WC, Brokaw JP, Liu J, Ma M, et al. Stem cells as a therapeutic avenue for active and long-term complications of necrotizing enterocolitis. Semin Pediatr Surg. (2023) 32(3):151311. doi: 10.1016/j.sempedsurg.2023.151311
169. Zeng R, Wang J, Zhuo Z, Luo Y, Sha W, Chen H. Stem cells and exosomes: promising candidates for necrotizing enterocolitis therapy. Stem Cell Res Ther. (2021) 12(1):323. doi: 10.1186/s13287-021-02389-4
170. Pekkanen-Mattila M, Pelto-Huikko M, Kujala V, Suuronen R, Skottman H, Aalto-Setälä K, et al. Spatial and temporal expression pattern of germ layer markers during human embryonic stem cell differentiation in embryoid bodies. Histochem Cell Biol. (2010) 133:595–606. doi: 10.1007/s00418-010-0689-7
171. Yilmaz A, Braverman-Gross C, Bialer-Tsypin A, Peretz M, Benvenisty N. Mapping gene circuits essential for germ layer differentiation via loss-of-function screens in haploid human embryonic stem cells. Cell Stem Cell. (2020) 27(4):679–91.e6. doi: 10.1016/j.stem.2020.06.023
172. Barad L, Schick R, Zeevi-Levin N, Itskovitz-Eldor J, Binah O. Human embryonic stem cells vs human induced pluripotent stem cells for cardiac repair. Can J Cardiol. (2014) 30(11):1279–87. doi: 10.1016/j.cjca.2014.06.023
173. Siddiqi S, Klomjit N, Jiang K, Conley SM, Zhu X, Saadiq IM, et al. Efficacy of human embryonic stem cells compared to adipose tissue-derived human mesenchymal stem/stromal cells for repair of murine post-stenotic kidneys. Stem Cell Rev Rep. (2023) 19(2):491–502. doi: 10.1007/s12015-022-10443-8
174. Varzideh F, Gambardella J, Kansakar U, Jankauskas SS, Santulli G. Molecular mechanisms underlying pluripotency and self-renewal of embryonic stem cells. Int J Mol Sci. (2023) 24(9):8386. doi: 10.3390/ijms24098386
175. Drucker NA, McCulloh CJ, Li B, Pierro A, Besner GE, Markel TA. Stem cell therapy in necrotizing enterocolitis: current state and future directions. Semin Pediatr Surg. (2018) 27(1):57–64. doi: 10.1053/j.sempedsurg.2017.11.011
176. Siggers J, Ostergaard MV, Siggers RH, Skovgaard K, Mølbak L, Thymann T, et al. Postnatal amniotic fluid intake reduces gut inflammatory responses and necrotizing enterocolitis in preterm neonates. Am J Physiol Gastrointest Liver Physiol. (2013) 304(10):G864–75. doi: 10.1152/ajpgi.00278.2012
177. Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. (2011) 12(8):453–66. doi: 10.1038/nrn3071
178. Sharkey KA, Mawe GM. The enteric nervous system. Physiol Rev. (2023) 103(2):1487–564. doi: 10.1152/physrev.00018.2022
179. Zizzo MG, Cavallaro G, Auteri M, Caldara G, Amodeo I, Mastropaolo M, et al. Postnatal development of the dopaminergic signaling involved in the modulation of intestinal motility in mice. Pediatr Res. (2016) 80(3):440–7. doi: 10.1038/pr.2016.91
180. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. (1990) 60(4):585–95. doi: 10.1016/0092-8674(90)90662-X
181. Balsamo F, Tian Y, Pierro A, Li B. Amniotic fluid stem cells: a novel treatment for necrotizing enterocolitis. Front Pediatr. (2022) 10:1020986. doi: 10.3389/fped.2022.1020986
182. Hu X, Zhang R, Liang H, An J, Yang Y, Huo J, et al. Comparison and investigation of exosomes from human amniotic fluid stem cells and human breast milk in alleviating neonatal necrotizing enterocolitis. Stem Cell Rev Rep. (2023) 19(3):754–66. doi: 10.1007/s12015-022-10470-5
183. Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. (2009) 136(7):2214–25.e3. doi: 10.1053/j.gastro.2009.02.048
184. Ganji N, Li B, Lee C, Pierro A. Necrotizing enterocolitis: recent advances in treatment with translational potential. Pediatr Surg Int. (2023) 39(1):205. doi: 10.1007/s00383-023-05476-0
185. Wegmeyer H, Bröske A-M, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. (2013) 22(19):2606–18. doi: 10.1089/scd.2013.0016
186. Damianos A, Xu K, Kalin GT, Kalinichenko VV. Placental tissue stem cells and their role in neonatal diseases. Semin Fetal Neonatal Med. (2022) 27(1):101322. doi: 10.1016/j.siny.2021.101322
187. Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, et al. Isolation and characterization of mesenchymal stromal cells from human umbilical cord and fetal placenta. J Vis Exp. (2017) (122):55224. doi: 10.3791/55224
188. Ingo DM, Redaelli D, Rossella V, Perini O, Santoleri L, Ciceri F, et al. Bone marrow-derived CD34. Cytotherapy. (2016) 18(12):1560–3. doi: 10.1016/j.jcyt.2016.08.011
189. Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. (2006) 24(2):462–71. doi: 10.1634/stemcells.2004-0331
190. Baranovskii DS, Klabukov ID, Arguchinskaya NV, Yakimova AO, Kisel AA, Yatsenko EM, et al. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. (2022) 9:7. doi: 10.21037/sci-2022-025
191. Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. (2014) 32(9):2430–42. doi: 10.1002/stem.1729
192. Naeem A, Gupta N, Naeem U, Khan MJ, Elrayess MA, Cui W, et al. A comparison of isolation and culture protocols for human amniotic mesenchymal stem cells. Cell Cycle. (2022) 21(15):1543–56. doi: 10.1080/15384101.2022.2060641
193. He L, Zhao F, Zheng Y, Wan Y, Song J. Loss of interactions between p53 and survivin gene in mesenchymal stem cells after spontaneous transformation in vitro. Int J Biochem Cell Biol. (2016) 75:74–84. doi: 10.1016/j.biocel.2016.03.018
194. Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. (2020) 60:225–37. doi: 10.1016/j.semcancer.2019.06.003
195. Li C, Zhao H, Wang B. Mesenchymal stem/stromal cells: developmental origin, tumorigenesis and translational cancer therapeutics. Transl Oncol. (2021) 14(1):100948. doi: 10.1016/j.tranon.2020.100948
196. Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. (2013) 2013:732742. doi: 10.1155/2013/732742
197. Eggenhofer E, Benseler V, Kroemer A, Popp F, Geissler E, Schlitt H, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. (2012) 3:32434. doi: 10.3389/fimmu.2012.00297
198. Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user’s guide. Stem Cells Dev. (2010) 19(10):1449–70. doi: 10.1089/scd.2010.0140
199. Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. (2001) 98(14):7841–5. doi: 10.1073/pnas.141221698
200. Selich A, Ha TC, Morgan M, Falk CS, von Kaisenberg C, Schambach A, et al. Cytokine selection of MSC clones with different functionality. Stem Cell Rep. (2019) 13(2):262–73. doi: 10.1016/j.stemcr.2019.06.001
201. Crippa S, Santi L, Berti M, De Ponti G, Bernardo ME. Role of ex vivo expanded mesenchymal stromal cells in determining hematopoietic stem cell transplantation outcome. Front Cell Dev Biol. (2021) 9:663316. doi: 10.3389/fcell.2021.663316
202. Ghazanfari R, Zacharaki D, Li H, Ching Lim H, Soneji S, Scheding S. Human primary bone marrow mesenchymal stromal cells and their in vitro progenies display distinct transcriptional profile signatures. Sci Rep. (2017) 7(1):10338. doi: 10.1038/s41598-017-09449-x
203. Nakahara F, Borger DK, Wei Q, Pinho S, Maryanovich M, Zahalka AH, et al. Engineering a haematopoietic stem cell niche by revitalizing mesenchymal stromal cells. Nat Cell Biol. (2019) 21(5):560–7. doi: 10.1038/s41556-019-0308-3
204. Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, et al. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. (2021) 78(2):447–67. doi: 10.1007/s00018-020-03600-0
205. Santi L, Beretta S, Berti M, Savoia EO, Passerini L, Mancino M, et al. Transcriptomic analysis of BM-MSCs identified EGR1 as a transcription factor to fully exploit their therapeutic potential. Biochim Biophys Acta Mol Cell Res. (2024) 1871(8):119818. doi: 10.1016/j.bbamcr.2024.119818
206. King NM, Perrin J. Ethical issues in stem cell research and therapy. Stem Cell Res Ther. (2014) 5(4):85. doi: 10.1186/scrt474
207. Kato K, Kimmelman J, Robert J, Sipp D, Sugarman J. Ethical and policy issues in the clinical translation of stem cells: report of a focus session at the ISSCR tenth annual meeting. Cell Stem Cell. (2012) 11(6):765–7. doi: 10.1016/j.stem.2012.11.004
208. Pera MF. Scientific considerations relating to the ethics of the use of human embryonic stem cells in research and medicine. Reprod Fertil Dev. (2001) 13(1):23–9. doi: 10.1071/RD00077
209. Maguire G. Stem cell therapy without the cells. Commun Integr Biol. (2013) 6(6):e26631. doi: 10.4161/cib.26631
210. Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. (2017) 18(9):1852. doi: 10.3390/ijms18091852
211. Vishnubhatla I, Corteling R, Stevanato L, Hicks C, Sinden J. The development of stem cell-derived exosomes as a cell-free regenerative medicine. J Circ Biomark. (2014) 3(1):2. doi: 10.5772/58597
212. Kim DK, Nishida H, An SY, Shetty AK, Bartosh TJ, Prockop DJ. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. (2016) 113(1):170–5. doi: 10.1073/pnas.1522297113
213. Bermudez MA, Sendon-Lago J, Seoane S, Eiro N, Gonzalez F, Saa J, et al. Anti-inflammatory effect of conditioned medium from human uterine cervical stem cells in uveitis. Exp Eye Res. (2016) 149:84–92. doi: 10.1016/j.exer.2016.06.022
214. Zagoura DS, Roubelakis MG, Bitsika V, Trohatou O, Pappa KI, Kapelouzou A, et al. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut. (2012) 61(6):894–906. doi: 10.1136/gutjnl-2011-300908
215. Bermudez MA, Sendon-Lago J, Eiro N, Treviño M, Gonzalez F, Yebra-Pimentel E, et al. Corneal epithelial wound healing and bactericidal effect of conditioned medium from human uterine cervical stem cells. Invest Ophthalmol Vis Sci. (2015) 56(2):983–92. doi: 10.1167/iovs.14-15859
216. Tsai MJ, Tsai SK, Hu BR, Liou DY, Huang SL, Huang MC, et al. Recovery of neurological function of ischemic stroke by application of conditioned medium of bone marrow mesenchymal stem cells derived from normal and cerebral ischemia rats. J Biomed Sci. (2014) 21(1):5. doi: 10.1186/1423-0127-21-5
217. Sodhi CP, Ahmad R, Jia H, Fulton WB, Lopez C, Gonzalez Salazar AJ, et al. The administration of amnion-derived multipotent cell secretome ST266 protects against necrotizing enterocolitis in mice and piglets. Am J Physiol Gastrointest Liver Physiol. (2022) 323(3):G265–82. doi: 10.1152/ajpgi.00364.2021
218. Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. (2007) 179(7):4808–20. doi: 10.4049/jimmunol.179.7.4808
219. Albert C, Beladjine M, Tsapis N, Fattal E, Agnely F, Huang N. Pickering emulsions: preparation processes, key parameters governing their properties and potential for pharmaceutical applications. J Control Release. (2019) 309:302–32. doi: 10.1016/j.jconrel.2019.07.003
220. Kumar KN, Mallik S, Sarkar K. Role of freeze-drying in the presence of mannitol on the echogenicity of echogenic liposomes. J Acoust Soc Am. (2017) 142(6):3670. doi: 10.1121/1.5017607
221. Tung S, Delavogia E, Fernandez-Gonzalez A, Mitsialis SA, Kourembanas S. Harnessing the therapeutic potential of the stem cell secretome in neonatal diseases. Semin Perinatol. (2023) 47(3):151730. doi: 10.1016/j.semperi.2023.151730
222. Matei AC, Antounians L, Zani A. Extracellular vesicles as a potential therapy for neonatal conditions: state of the art and challenges in clinical translation. Pharmaceutics. (2019) 11(8):404. doi: 10.3390/pharmaceutics11080404
223. Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther. (2016) 16(4):489–506. doi: 10.1517/14712598.2016.1131976
224. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. (2014) 3:24641. doi: 10.3402/jev.v3.24641
225. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. (2014) 30:255–89. doi: 10.1146/annurev-cellbio-101512-122326
226. Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. (2009) 9(8):581–93. doi: 10.1038/nri2567
227. Kar R, Dhar R, Mukherjee S, Nag S, Gorai S, Mukerjee N, et al. Exosome-based smart drug delivery tool for cancer theranostics. ACS Biomater Sci Eng. (2023) 9(2):577–94. doi: 10.1021/acsbiomaterials.2c01329
228. Meng W, He C, Hao Y, Wang L, Li L, Zhu G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. (2020) 27(1):585–98. doi: 10.1080/10717544.2020.1748758
229. Faruqu FN, Xu L, Al-Jamal KT. Preparation of exosomes for siRNA delivery to cancer cells. J Vis Exp. (2018) (142):10-3791. doi: 10.3791/58814
230. Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A. (2015) 112(12):E1433–42. doi: 10.1073/pnas.1418401112
231. Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. (2019) 459(1–2):1–6. doi: 10.1007/s11010-019-03545-4
232. Leoni G, Neumann PA, Kamaly N, Quiros M, Nishio H, Jones HR, et al. Annexin A1-containing extracellular vesicles and polymeric nanoparticles promote epithelial wound repair. J Clin Invest. (2015) 125(3):1215–27. doi: 10.1172/JCI76693
233. Diaz-Garrido N, Cordero C, Olivo-Martinez Y, Badia J, Baldoma L. Cell-to-cell communication by host-released extracellular vesicles in the gut: implications in health and disease. Int J Mol Sci. (2021) 22(4):2213. doi: 10.3390/ijms22042213
234. Manchon E, Hirt N, Bouaziz JD, Jabrane-Ferrat N, Al-Daccak R. Stem cells-derived extracellular vesicles: potential therapeutics for wound healing in chronic inflammatory skin diseases. Int J Mol Sci. (2021) 22(6):3130. doi: 10.3390/ijms22063130
235. Garg PM, Paschal JL, Zhang M, Pippins M, Matthews A, Adams K, et al. Brain injury in preterm infants with surgical necrotizing enterocolitis: clinical and bowel pathological correlates. Pediatr Res. (2022) 91(5):1182–95. doi: 10.1038/s41390-021-01614-3
236. Brunse A, Abbaspour A, Sangild PT. Brain barrier disruption and region-specific neuronal degeneration during necrotizing enterocolitis in preterm pigs. Dev Neurosci. (2018) 40(3):198–208. doi: 10.1159/000488979
237. Arnhold S, Glüer S, Hartmann K, Raabe O, Addicks K, Wenisch S, et al. Amniotic-Fluid stem cells: growth dynamics and differentiation potential after a CD-117-based selection procedure. Stem Cells Int. (2011) 2011:715341. doi: 10.4061/2011/715341
238. Yang J, Watkins D, Chen CL, Zhang HY, Zhou Y, Velten M, et al. A technique for systemic mesenchymal stem cell transplantation in newborn rat pups. J Invest Surg. (2012) 25(6):405–14. doi: 10.3109/08941939.2012.661519
239. Xiong Y, Mahmood A, Chopp M. Emerging potential of exosomes for treatment of traumatic brain injury. Neural Regen Res. (2017) 12(1):19–22. doi: 10.4103/1673-5374.198966
240. Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. (2016) 51(6):942–7. doi: 10.1016/j.jpedsurg.2016.02.061
241. Li L, He Y, Zhao J, Yin H, Feng X, Fan X, et al. Mesenchymal stromal cell-based therapy: a promising approach for autoimmune diseases. Clin Rev Allergy Immunol. (2025) 68(1):21. doi: 10.1007/s12016-025-09030-9
242. Hetta HF, Elsaghir A, Sijercic VC, Ahmed AK, Gad SA, Zeleke MS, et al. Clinical progress in mesenchymal stem cell therapy: a focus on rheumatic diseases. Immun Inflamm Dis. (2025) 13(5):e70189. doi: 10.1002/iid3.70189
243. Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, et al. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int J Mol Sci. (2017) 18(7):1450. doi: 10.3390/ijms18071450
Glossary
53BP1 p53-binding protein 1
AF-NSCs amniotic fluid-derived neural stem cells
AF-SCs amniotic fluid-derived stem cells
ASCs adult stem cells
bFGF fibroblast growth factor
BM-MSCs bone marrow-derived mesenchymal stromal cells
C34 2-acetamidopyranoside
circRNAs circular ribonucleic acids
CM conditioned medium
DIC disseminated intravascular coagulation
EGF epidermal growth factor
ESCs embryonic stem cells
ENS enteric nervous system
FD70 70 kDa FITC-Dextran
GA gestational age
GIT gastrointestinal tract
GvHD graft-versus-host-disease
γH2AX phosphorylated H2A histone family member X
HB-EGF heparin-binding EGF-like growth factor
hESCs human embryonic stem cells
HGF hepatocyte growth factor
HLA human leukocyte antigen
HMGB1 high mobility group box 1
HSPC hematopoietic stem and progenitor cell
IBD inflammatory bowel disease
IDO indoleamine 2,3-dioxygenase
IGF-1 insulin-like growth factor
IL-1β interleukin-1β
IL-6 interleukin-6
IL-10 interleukin-10
ISCT international society for cellular therapy
LCA lithocholic acid
MMC migration motor complex
miRNAs microribonucleic acids
mRNAs messenger ribonucleic acids
MSCs mesenchymal stromal cells
NCSs neural stem cells
NEC necrotizing enterocolitis
N-ENSCs neonatal enteric neural stem cells
NF-kB nuclear factor-kB
NICUs neonatal intensive care units
NK natural killer
Oct4 octamer-binding transcription factor 4
PGE prostaglandin E2
P-MSCs placental-derived mesenchymal stem cells
PXR pregnane X receptor
siRNA short interfering ribonucleic acid
SSEA-4 stage-specific embryonic antigen 4
TGF transforming growth factor
Th1 T helper 1 cells
TLR-4 toll-like receptor 4
TPN total parenteral nutrition
Treg regulatory T cells
TUNEL TdT-mediated dUTP nick-end labeling
UC-MSCs umbilical cord-derived mesenchymal stem cells
VEGF vascular endothelial growth factor
VLBW very low birth weight
Wnt wingless and Int-1
ZO-1 zonula occludens 1
Keywords: necrotizing enterocolitis, bone marrow-derived mesenchymal stromal cells, toll-like receptor 4, exosomes, secretome
Citation: Tomaselli A, Tripodi M, Provitera L, Raffaeli G, Crippa S, Raymo L, Bronzoni CV, Santi L, Arribas C, Fumagalli M, Loukogeorgakis SP, Ester Bernardo M, Garrido F and Cavallaro G (2025) Bone marrow-derived mesenchymal stromal cells in necrotizing enterocolitis treatment: a narrative review. Front. Pediatr. 13:1624236. doi: 10.3389/fped.2025.1624236
Received: 7 May 2025; Accepted: 16 July 2025;
Published: 31 July 2025.
Edited by:
Kazumichi Fujioka, Kobe University, JapanReviewed by:
Xinyao Meng, Huazhong University of Science and Technology, ChinaYounggeon Jin, University of Maryland, United States
Copyright: © 2025 Tomaselli, Tripodi, Provitera, Raffaeli, Crippa, Raymo, Bronzoni, Santi, Arribas, Fumagalli, Loukogeorgakis, Ester Bernardo, Garrido and Cavallaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Livia Provitera, bGl2aWEucHJvdml0ZXJhQHBvbGljbGluaWNvLm1pLml0
†These authors contributed equally to this work.
 Andrea Tomaselli
Andrea Tomaselli Matteo Tripodi
Matteo Tripodi Livia Provitera
Livia Provitera Genny Raffaeli1,2
Genny Raffaeli1,2 Stefania Crippa
Stefania Crippa Carolina Vittoria Bronzoni
Carolina Vittoria Bronzoni Ludovica Santi
Ludovica Santi Monica Fumagalli
Monica Fumagalli Maria Ester Bernardo
Maria Ester Bernardo Felipe Garrido
Felipe Garrido Giacomo Cavallaro
Giacomo Cavallaro