- 1Morsani College of Medicine, University of South Florida, Tampa, FL, United States
- 2Department of Pediatrics, Walter Reed National Military Medical Center, Specialized Services University School of Medicine, Bethesda, MD, United States
- 3College of Arts and Sciences, University of South Florida, Tampa, FL, United States
- 4Department of Nursing, University of South Florida College of Nursing, Tampa, FL, United States
- 5Department of Neonatal Research, Inova Health Services, Falls Church, VA, United States
Introduction: Neonatal sepsis is a dysregulated immune response to bloodstream infection causing serious disease and death. Our review seeks to integrate the knowledge gained from studies of multiple molecular methods- such as genomics, metabolomics, transcriptomics, and the gut microbiome- in the setting of neonatal sepsis that may improve the diagnosis, classification, and treatment of the disease. Sepsis claims over 200,000 lives annually worldwide and remains a top 10 cause of infant mortality in the US. Diagnosis and treatment of neonatal sepsis remains a challenge as its mechanisms are poorly understood.
Methods: We conducted a scoping review of literature published between 2018 and 2024. Of 1,043 articles screened, 30 were included in the final review.
Results: The gut microbiome is associated with both pathogenicity and protection in the setting of neonatal sepsis, while expression levels of immune response and regulation help classify neonatal response to septic events. Metabolomic studies reveal possible biomarkers to detect, classify, and predict neonatal sepsis morbidity and mortality, and proteomic studies confirm mechanisms predicted by the other models.
Discussion: Studies using molecular methods foster greater understanding of neonatal sepsis and show promise to improve diagnosis, classification, and therapeutic intervention. Future research using multi-omic analyses may further elucidate the development and progression of inflammatory processes that occur as sepsis progresses.
1 Introduction
Neonatal sepsis is a leading cause of mortality among infants worldwide, accounting for roughly 227,000 deaths in 2019, of which only 740 deaths occurred in North America (1). A 2021 meta-analysis estimates a mortality rate of 17.6% with deaths disproportionately affecting middle and low-income countries (2). In the US, the CDC reports neonatal sepsis as a top 10 cause of infant mortality as of 2022 (3). Neonatal sepsis is classified as early-onset (EOS) or late-onset sepsis (LOS); EOS occurs within the first 72 h of life, typically resulting from exposure to the maternal vaginal tract during delivery. In contrast, LOS arises after 72 h and is generally acquired postnatally through environmental exposure, particularly through nosocomial transmission in neonatal intensive care units (NICUs).
The diagnosis, treatment, and prevention of neonatal sepsis continues to pose significant challenges. Although a positive blood culture remains the gold standard for diagnosis, its sensitivity is limited by factors such as inadequate blood volume (4), low bacterial load, and the fastidious nature of certain pathogens (5). As a result, false negative cultures are common (5) and low colony count bacteremia represents the majority of neonatal sepsis cases (6), remaining undetected in up to 60% of cases (7). In these patients, clinicians must make the diagnosis of sepsis on clinical signs and non-specific laboratory markers, most commonly neutrophil counts and acute phase reactants (8). Despite a 2016 international consensus redefining sepsis and septic shock as a “life-threatening organ dysfunction caused by a dysregulated host response to infection” (9), blood culture positivity remains central to both diagnostic criteria and therapeutic decision making. In fact, a review of 80 randomized control trials identifies positive blood culture as the most frequently used criterion for defining neonatal sepsis, followed by clinical and laboratory indicators (8).
Neonates primarily depend on an immature innate immune system for defense against pathogens, as their adaptive immunity is underdeveloped. Their reliance on innate immunity contributes to their heightened vulnerability to sepsis and the associated increased mortality among this patient population (10, 11). Several acute phase reactants such as C-reactive protein and procalcitonin have been studied for their diagnostic utility as biomarkers of sepsis (12). However, tests to determine levels of such biomarkers in blood often require serial measurements, as acute phase reactants increase in response to early inflammatory signals occurs gradually (12). Moreover, these biomarkers often lack specificity and may be elevated in response to non-infectious factors such as perinatal stress, delivery trauma, or neonatal surgery, limiting their diagnostic utility (12). Empiric antibiotic therapy remains the standard initial treatment when clinical signs of sepsis are present (13). For EOS, regimens typically include broad-spectrum antibiotics such as ampicillin and gentamicin, with a third-generation cephalosporin added if meningitis is suspected (13). In cases of LOS, similar broad coverage of gram-positive, gram-negative, and anaerobic organisms is generally initiated (13). Preventative strategies for EOS frequently involve maternal intrapartum antibiotic prophylaxis and, in some cases, neonatal antibiotics (13, 14). However, the widespread use of broad-spectrum antibiotics has contributed to the emergence of drug resistant pathogens in neonatal populations (15, 16). Furthermore, early life exposure to antibiotics, particularly within the first two years of life, has been associated with increased risks of long term adverse outcomes, including asthma, allergic disease, and obesity (17–19).
The need for prudent antibiotic stewardship, coupled with the inherent limitations of current diagnostic modalities in neonatal sepsis underscores the imperative to develop more precise diagnostic methodologies, targeted therapeutic strategies, and effective prevention measures. There also exists an increased need to elucidate the trajectory of disease progression, from bacteremia to sepsis and ultimately, septic shock or complications such as meningitis or encephalitis. Given the systemic nature of sepsis and its widespread physiological impact, molecular approaches to biomarker discovery hold considerable promise. Multilayered molecular analyses encompassing the microbiome, metabolome, transcriptome, and proteome offer a systems level perspective revealing complex biological interdependence. Although numerous systematic reviews have examined the individual contributions of omics technologies towards the enhancement of sepsis diagnostics and a broader understanding of pathophysiology, integrative multi-omics investigations remain limited (20–22). Two reviews published as recently as 2018 have addressed multi-omic approaches; however, they do not account for the most recent advancements in molecular diagnostic techniques (22, 23). We conducted a scoping review of molecular studies published over the past five years with the aim of updating and refining our understanding of the diagnostic and staging landscape of neonatal sepsis. This review seeks to address existing knowledge gaps by synthesizing recent findings related to the application of molecular methodologies in the diagnosis, classification and management of neonatal sepsis. Specifically, we sought to address the following research question: How can insights derived from recent non-animal, original research molecular studies be synthesized or compared against each other to inform the identification of biomarkers that enhance the diagnosis, classification, and therapeutic management of neonatal sepsis?
1.1 Background knowledge of molecular studies in neonatal sepsis
1.1.1 Microbiome
The neonatal microbiome has emerged as a critical area of research in early-life health and disease, with most studies to date focusing predominantly on the gastrointestinal tract, and to a lesser extent, the nasal microbiome. Understanding microbial colonization patterns and community dynamics in neonates is essential, given their potential influence on immune development, disease susceptibility, and clinical outcomes such as necrotizing enterocolitis (NEC) and sepsis. In a 2015 review, Gritz et al. (24) explored the association between neonatal gut dysbiosis and the development of NEC, with additional focus on the therapeutic potential of probiotics. While the review highlighted the significance of early microbial colonization patterns, it found no conclusive evidence supporting the efficacy of probiotics in reducing the incidence of either neonatal EOS or LOS (24).
Microbiome characterization in neonates typically relies on sequencing techniques. Two commonly employed approaches include 16S ribosomal RNA (rRNA) gene sequencing and shotgun metagenomic sequencing. In 16S rRNA sequencing, hypervariable regions of the bacterial 16S rRNA gene are selectively amplified from extracted DNA, passed through bioinformatics processing, and matched against curated microbial genome databases for taxonomic assignment. Shotgun metagenomic sequencing provides a more comprehensive approach by analyzing the entire genomic content of a sample, including bacterial, viral, fungal, and protozoan DNA. In this method, all extracted DNA is processed and subsequently annotated against genomic databases, enabling both taxonomic identification and functional profiling of microbial communities.
To quantify and compare microbial communities, several indices are available, each emphasizing different aspects of diversity. Alpha diversity refers to the richness and evenness of microbial species within a single sample. Indices measuring alpha diversity include the Shannon index, which measures both species richness and abundance, and the Inverse Simpson index, which places greater weight on the dominant species present. Beta diversity, in contrast, assesses compositional differences between microbial communities across distinct samples or groups. Key beta diversity metrics include the Bray-Curtis Dissimilarity, which reflects differences in species abundance, the Jaccard Index, which only considers the presence or absence of species, and the UniFrac, which incorporates phylogenetic relationships among taxa.
1.1.2 Genomics
Genomic analysis involves comprehensive profiling of the entire genome to identify genetic variants associated with disease susceptibility. This goal is commonly achieved through whole genome sequencing (WGS), which interrogates the complete DNA sequence, or whole exome sequencing (WES), which targets only the protein-coding regions of the genome. In the context of neonatal sepsis, genomic studies conducted prior to 2022 (25) predominantly aimed to identify genetic markers associated with an increased susceptibility to infection. These markers form the basis for polygenic risk scores (PRS), which may enable early identification of neonates at increased risk of sepsis and related complications.
The insights gained from genomic profiling hold promise for the future of personalized medicine, including potential applications in gene therapy and genome editing technologies. The emergence of personalized medicine may also offer a targeted alternative therapy to conventional antibiotic treatment. However, several limitations currently constrain the broader implementation of genomic analysis in neonatal care. These limitations include the high financial cost of sequencing technologies, the complexity and volume of genomic data, and significant ethical concerns related to data ownership, consent, and long-term privacy of genetic information.
1.1.3 Transcriptomics
Transcriptomics refers to the comprehensive analysis of the transcriptome—the complete set of RNA transcripts produced by the genome under specific physiological or pathological conditions. By profiling gene expression patterns, transcriptomic analysis provides insight into cellular function, disease states, and host responses to infection. Several methodologies are employed in transcriptome profiling, including microarray analysis, which quantifies predetermined messenger RNA (mRNA) sequences using complementary nucleotide probes. Other methodologies include bulk RNA sequencing, which measures average gene expression across a mixed population of cells, and single-cell RNA sequencing, which enables detection of gene expression at the level of individual cells and reveals heterogeneity within complex tissues (26).
Prior to 2018, transcriptomic applications in neonatal sepsis research focused on distinguishing neonates with sepsis from healthy controls to improve diagnostic precision (23). These studies aimed to identify host-derived transcriptional biomarkers that could differentiate between infectious and non-infectious inflammatory responses in early infancy. Unfortunateuly, widespread use of transcriptomic tools is limited by methodological and technical constraints. Microarrays are restricted to known gene sequences and are dependent on the availability of specific probes, necessitating prior knowledge of the organism's genome (23). RNA sequencing technologies, while more comprehensive, are hindered by the inherent fragility of RNA molecules, which require rapid sample processing and preservation (23). Furthermore, transcriptomic analyses generate large-scale datasets that demand robust bioinformatic infrastructure and computational resources for processing, storage, and interpretation (23).
1.1.4 Mirnomics
MicroRNAs (miRNA) are small, non-coding RNA molecules typically 18–25 nucleotides in length that bind to complementary sequences on mRNA transcripts, thereby inhibiting translation or promoting mRNA degradation. These regulatory molecules are found in both the cytoplasm and circulating blood, and are key modulators of immune signaling pathways and cellular stress responses.
MiRNA profiling is often considered a specialized subset of transcriptomics, with both fields employing similar analytical platforms, but can also be studied independently as miRNomics. Technologies commonly used include microarrays, quantitative reverse transcription PCR, and small RNA sequencing, which allows for comprehensive detection of miRNA without prior sequence knowledge. miRNAs demonstrate increased molecular stability relative to mRNAs, making them attractive biomarker candidates in conditions such as neonatal sepsis. However, challenges remain particularly with microarray-based detection, as it requires predefined sequence probes.
MiRNA-focused studies conducted prior to 2018 have identified multiple differentially expressed miRNAs during episodes of neonatal sepsis. Many of these miRNAs have been implicated in innate immune system modulation, suggesting their potential utility in elucidating the molecular mechanisms underlying neonatal immune responses, and in contributing to the development of sepsis-specific biomarkers (23).
1.1.5 Epigenomics
Epigenetics refers to the study of heritable changes in gene expression that occur without alterations to the underlying DNA sequence. These modifications regulate genomic activity and are influenced by a variety of endogenous and exogenous factors, including age, diet, pharmacologic agents, and environmental exposures. Epigenetic mechanisms include DNA methylation, histone modification, and regulation by non-coding RNAs, all of which contribute to chromatin remodeling and transcriptional control. Among these mechanisms, DNA methylation is the most extensively studied in the context of neonatal disease, as the process plays a key role in cellular differentiation, immune regulation, and disease susceptibility. The gold standard technique for methylation analysis involves bisulfite treatment, followed by DNA amplification and microarray hybridization to quantify levels of methylation with fluorescent signal intensity (27).
Epigenome-wide association studies have been conducted to investigate differential methylation patterns in neonates with and without sepsis (25). These studies have identified distinct epigenetic signatures associated with neonatal sepsis, suggesting aberrant DNA methylation in specific genes may serve as potential biomarkers for early detection or risk stratification in this vulnerable population.
1.1.6 Proteomics
Proteomics, the large-scale study of the complete set of proteins expressed by an organism, provides crucial insight into cellular function and disease processes. Methods include targeted assays, such as enzyme-linked immunosorbent assays (ELISA), to measure individual proteins or small panels with a high specificity. In contrast, comprehensive or untargeted proteomic analyses are typically conducted using mass spectrometry (MS), which allows for a broad assessment of protein abundance across the proteome.
Prior to 2018, proteomic studies in neonatal sepsis primarily aimed to construct biomarker panels to diagnose sepsis and distinguish it from related conditions, such as necrotizing enterocolitis. Research from this time showed untargeted MS-based approaches to identify proteins significantly increased the likelihood of discovering novel biomarkers. Despite this potential, incorporation of proteomic analysis into point-of-care testing remains challenging. Key limitations include the high cost of instrumentation and the labor-intensive nature of sample processing and data analysis. These factors currently hinder the routine clinical application of proteomic methods for rapid bedside diagnostics (23).
1.1.7 Metabolomics
Metabolomics is the comprehensive study of small-molecule metabolites, such as amino acids, glucose, lipids, and fatty acids, that reflect the dynamic physiological state of an organism. In the context of neonatal sepsis, metabolomics aims to characterize the host's metabolic response to infection and potential interactions with the infectious agent. Metabolomic analyses are typically conducted using nuclear magnetic resonance (NMR) spectroscopy or MS, often in combination with liquid chromatography (LC) or gas chromatography (GC). These technologies allow for the detection and quantification of a wide range of metabolites in biological fluids, such as serum and urine.
Studies conducted prior to 2018 demonstrated significant differences in metabolic profiles between neonates with and without sepsis (23). Specifically, increased concentrations of glucose and lactate were observed in patients with sepsis, alongside altered regulation of key metabolic pathways. Several metabolites identified in these studies have shown promise as novel biomarkers for the early detection or stratification of neonatal sepsis. However, their translation into clinical diagnostics remains unrealized (23).
The primary limitations of metabolomic profiling are seen particularly when using MS-based approaches and include the high cost of instrumentation, technical complexity, and time-intensive nature of analysis (23). These barriers currently preclude widespread implementation in routine neonatal care, although ongoing technological advancements may improve accessibility and feasibility in the future.
2 Methods
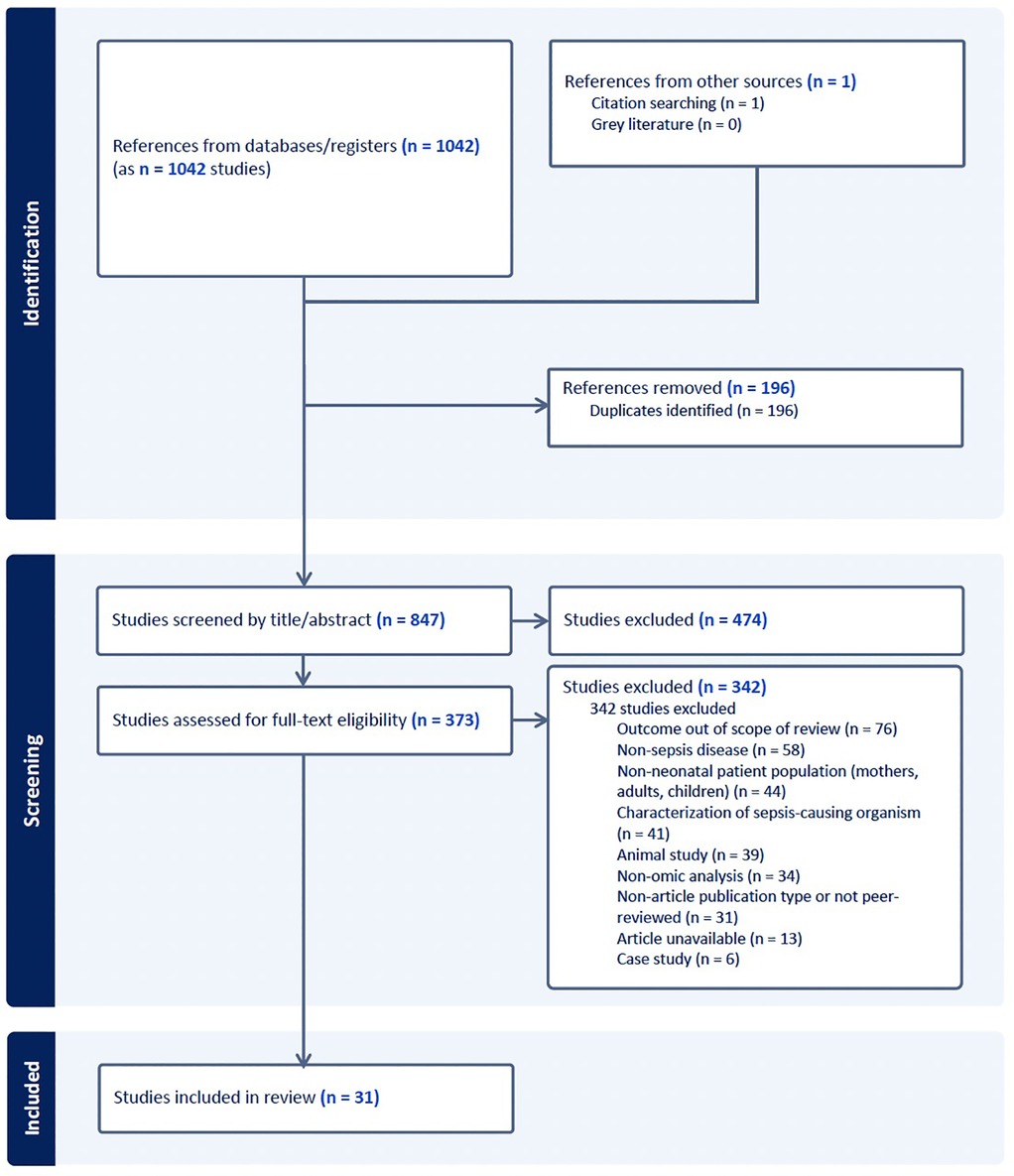
A systematic search of PubMed, EMBASE, and SCOPUS was conducted for articles published between January 2018 and June 2024 using the MeSH terms “neonatal sepsis”, “newborn”, “genomic*”, “proteomic*”, “transcriptomic*”, “microbiome”, “metabolomic*”, and “epigenomic*”. Equivalent controlled vocabulary terms were used for EMBASE and SCOPUS. Studies were included if they involved neonates aged 0–90 days with clinical suspicion or diagnosis of EOS or LOS, were published in English, and employed at least one molecular analysis technique as a primary methodology. Exclusion criteria included animal studies, non-original research, and dissertations. A total of 847 unique articles were identified, of which 31 met inclusion criteria for final review (Figure 1).
3 Results
3.1 Microbiome
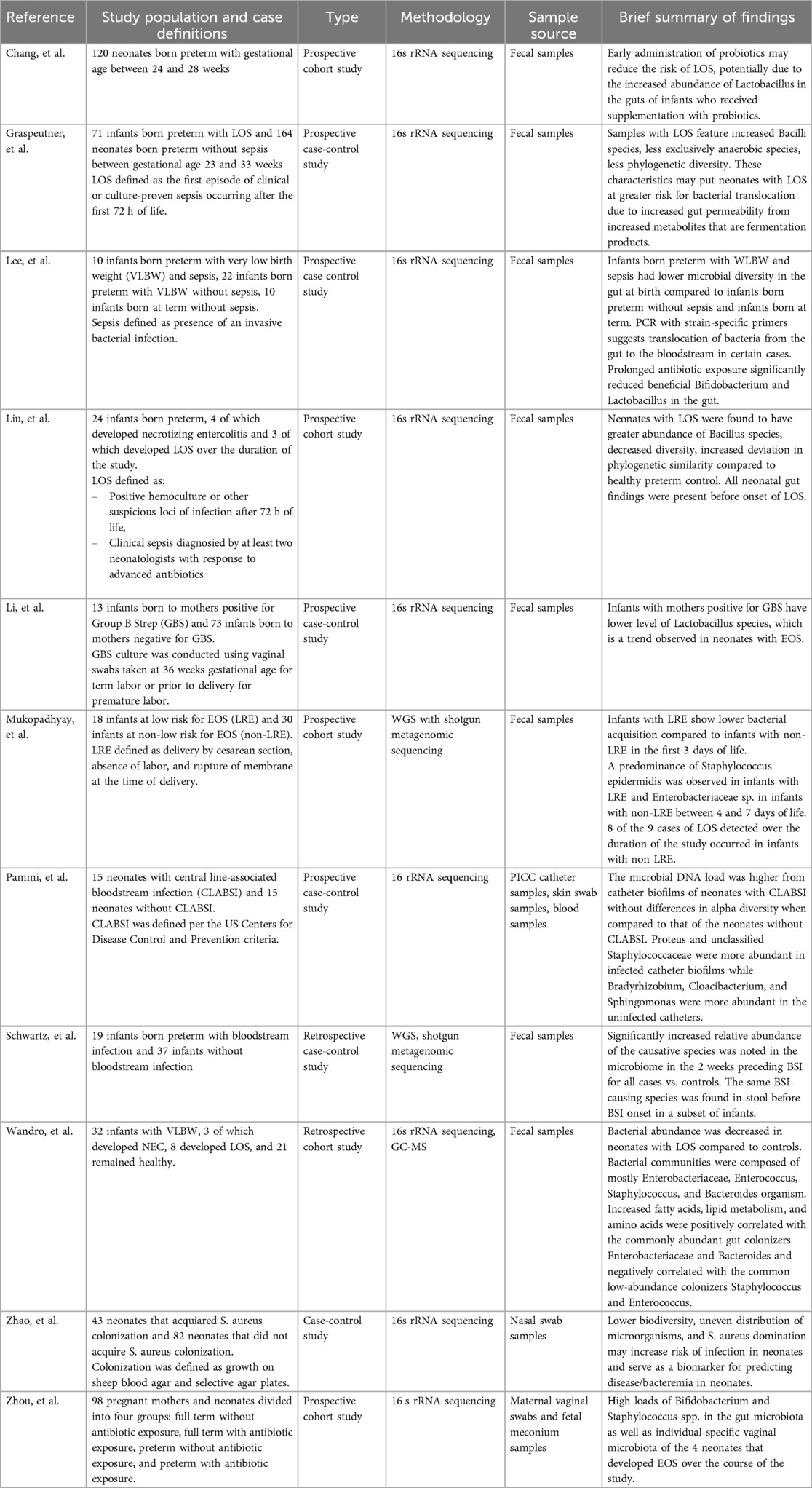
Eleven studies investigating the role of the microbiome in neonatal sepsis were identified, primarily targeting gut and nasal microbiota to explore the influence of microbial colonization patterns and antibiotic exposure on sepsis development (Table 1). All but one study collected microbiome samples both before and after sepsis onset, enabling analysis of dysbiosis preceding infection. The exception, conducted by Pammi et al. (28), analyzed microbial profiles on removed central line catheters and skin swabs from neonates with and without central line-associated bloodstream infections (CLABSI).
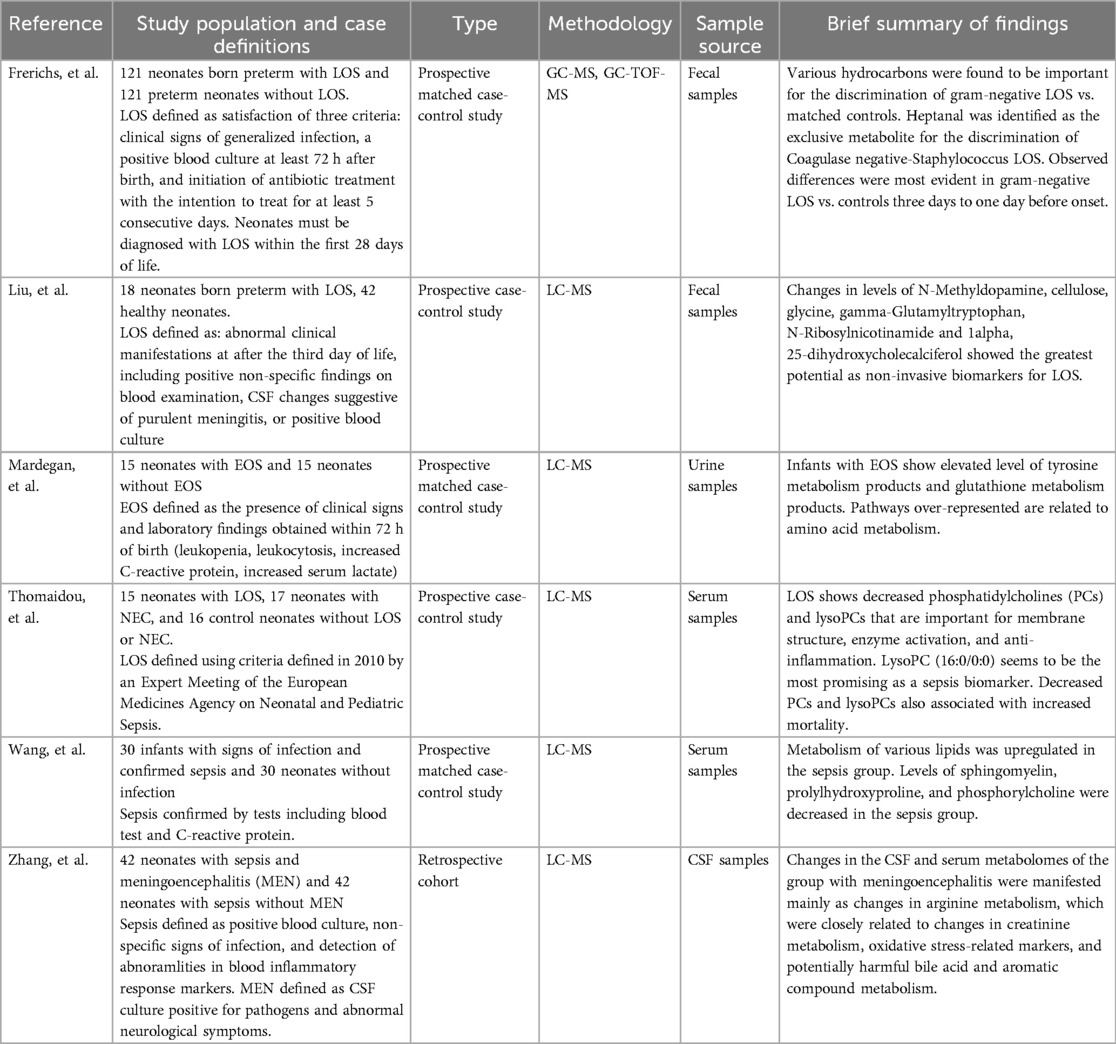
A common finding across multiple studies was a reduction in alpha diversity in neonates with sepsis (Table 1), although the specific bacteria noted to be present or absent varied by study. Lee et al. (29) reported significantly lower Shannon index values in infants born preterm with very low birth weight (VLBW) and sepsis compared to infants born preterm and term with VLBW but without sepsis from birth through 4–7 weeks of age. In contrast to infants born at term, whose microbiomes showed increased Firmicutes and Actinobacteria, healthy infants born preterm exhibited greater levels of Proteobacteria (29) (Table 2). Liu et al. (30) observed reduced alpha diversity during EOS or LOS, while Graspeuntner et al. (31) identified a predominance of Bacilli class species, including S. epidermidis and S. haemolyticus, in neonates with sepsis (Table 2). Similarly, Zhao et al. (32) found decreased diversity with a Staphylococcus aureus dominance in the nasal microbiome of infants with LOS in the week preceding bacteremia (Table 2). Wandro et al. (33) also reported a dominance of Staphylococcus in fecal samples from neonates who developed LOS (Table 2).
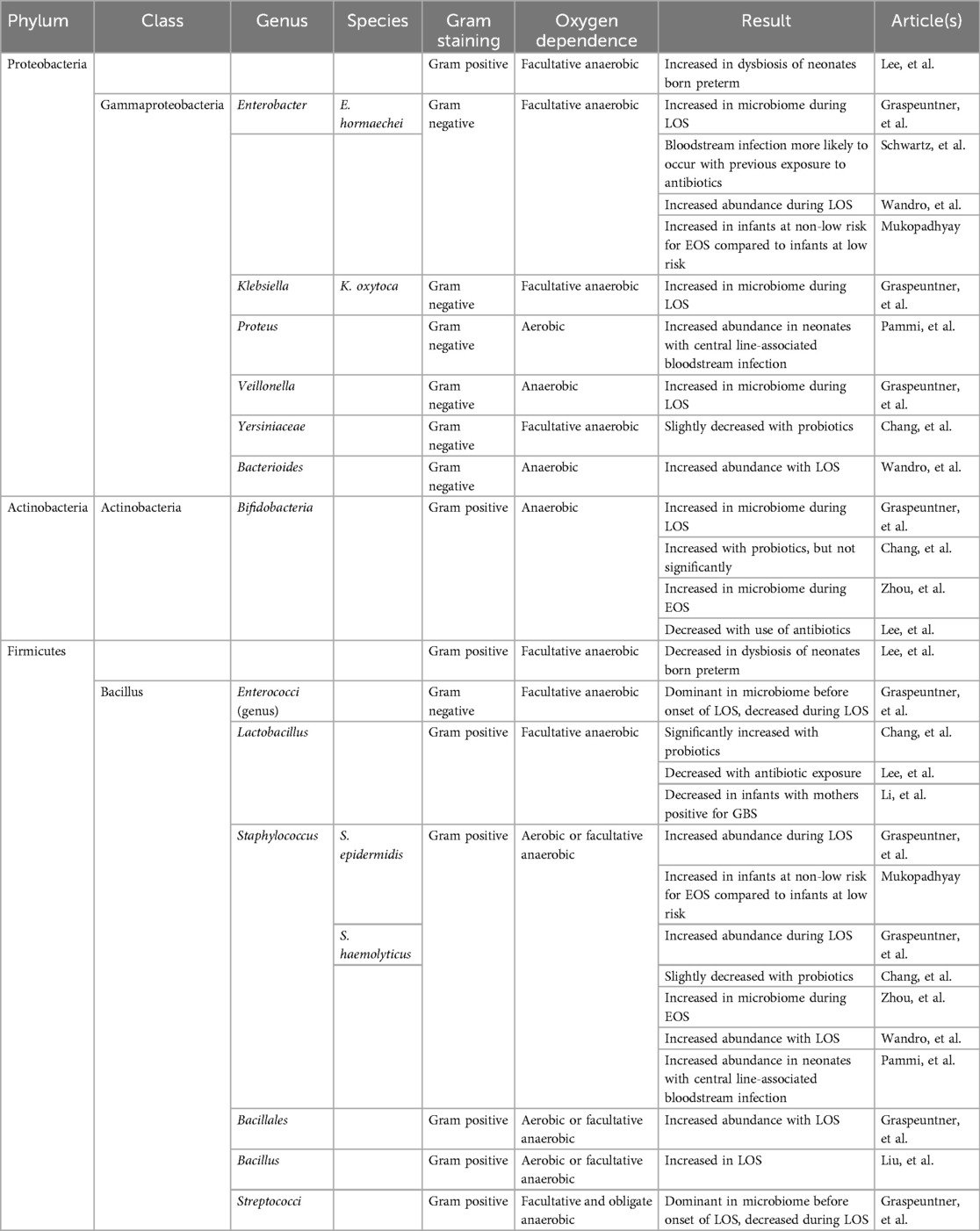
Table 2. Taxonomic classification of bacteria studied with relevant findings. Blank cells indicate lower levels of classification not studied by the authors.
Several studies assessed the impact of antibiotics and probiotics on the neonatal gut microbiome (Table 1). Chang et al. (34) found increased Lactobacillus and Bifidobacterium in infants exposed to probiotics, though only Lactobacillus remained significant after adjusting for gestational age. Conversely, use of antibiotics resulted in reductions of the two genera in neonates with WLBW and EOS or LOS, as reported by Lee et al. (29). In a similar vein, Zhou et al. (35) report mothers who received perinatal antibiotics gave birth to neonates with reduced Lactobacillus in their meconium samples. Li et al. (36) also noted lower Lactobacillus abundance in neonates born to Group B Streptococcus (GBS)-positive mothers. In regards to levels of Bifidobacterium, Wandro et al. (33) observed an absence in fecal samples of neonates born preterm with LOS, likely due to uniform antibiotic exposure.
Other findings on the impact of antibiotics include those reported by Mukhopadhyay et al. (37), namely a predominance of Enterobacteriaceae in fecal samples from infants with VLBW exposed to antibiotics during the first week of life, though the relationship with subsequent bacteremia was not specified. In contrast, Schwartz et al. (38) found that neonates with bloodstream infections caused by Enterobacteriaceae were more likely to have received antibiotics within 10 days of infection onset. Mukhopadhyay et al. (37) also noted an increasing trend in Enterobacteriaceae dominance over time, regardless of antibiotic exposure. However, no consistent shifts in relative abundance of the causative pathogens were observed in cases with LOS before or after antibiotic treatment, and infants generally remained clinically stable following discontinuation of antibiotics (37).
3.2 Genomics
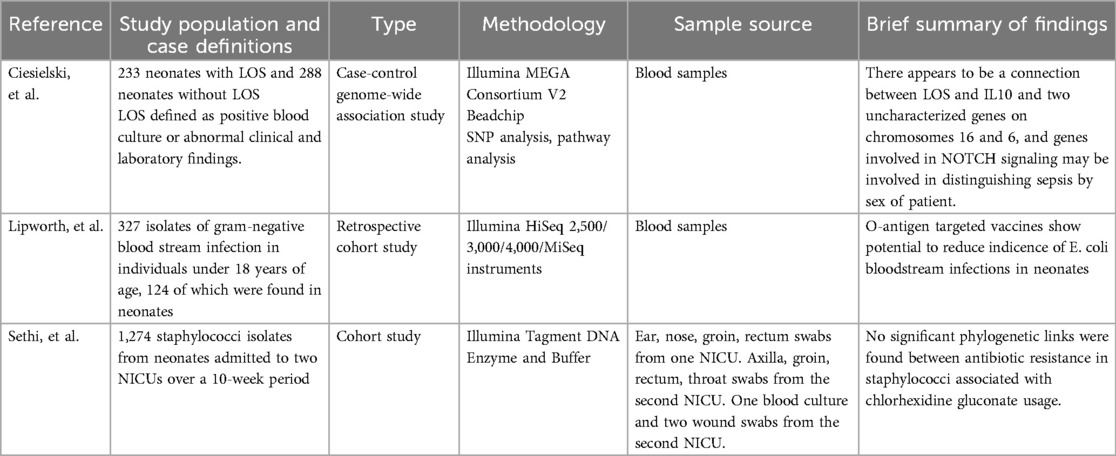
Three genomic studies published between 2018 and 2024 were included in the final review, each applying whole genome sequencing technologies with distinct objectives (Table 3). Two studies, conducted by Lipworth et al. (39) and Sethi et al. (40), focused on genomic characterization of bacterial isolates responsible for neonatal sepsis, while Ciesielski et al. (41) applied host genome sequencing to identify genetic variants associated with LOS in neonates.
In the study by Ciesielski et al. (41), single nucleotide polymorphism (SNP) analysis was used to explore host genetic susceptibility to LOS. Distinct autosomal and X-linked SNPs were identified in male and female neonates with no overlapping variants between sexes (41). Several of the SNPs associated with LOS were mapped to genes involved in NOTCH signaling, a pathway implicated in T-cell regulation and apoptosis. Furthermore, five SNPs associated with IL-10 and one with TNF-α were identified (41).
Lipworth et al. (39) conducted whole genome sequencing of Escherichia coli isolates from neonatal bloodstream infections. A notable finding was that a significant proportion of the isolates possessed O-antigen serotypes covered by the ExPEC-4V vaccine, currently under phase II clinical evaluation in adults (39).
Sethi et al. (40) investigated antiseptic susceptibility in neonatal sepsis isolates across hospitals in the UK and Germany. Using genomic sequencing, the study found higher minimum inhibitory concentrations (MICs) for chlorhexidine in UK isolates, where the antiseptic is more widely used, compared to German isolates (40). However, genomic analysis did not identify any significant associations between chlorhexidine or octenidine susceptibility and known resistance markers, including qac genes or NorA/NorB efflux pump genotypes (40).
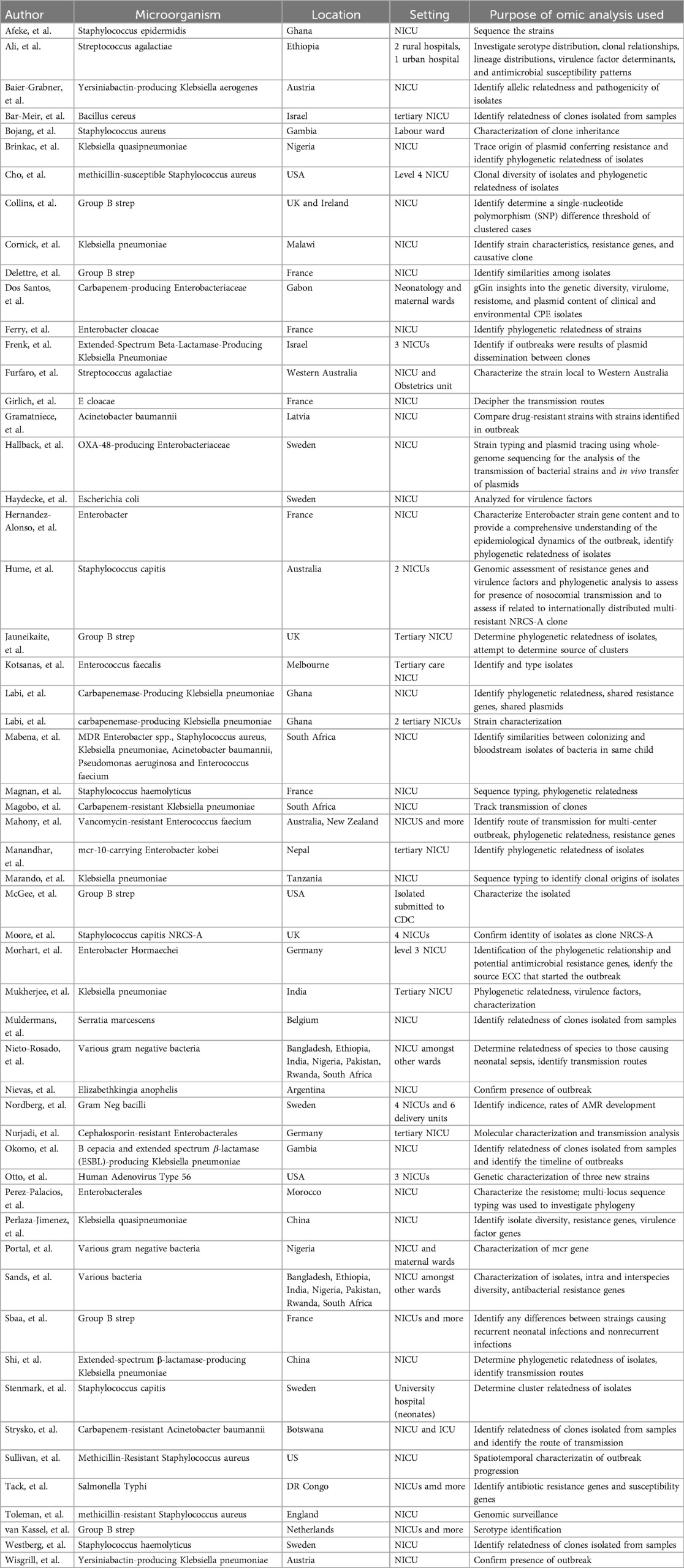
During the broader screening process, an additional 55 studies were identified that utilized genomic sequencing for epidemiological investigation of NICU outbreaks. These studies primarily focused on identifying transmission patterns and outbreak sources, and while highly valuable for infection control, they were excluded from the final review due to limited applicability to biomarker discovery. A summary of these studies is provided in Table 4 for reference.
3.3 Transcriptomics
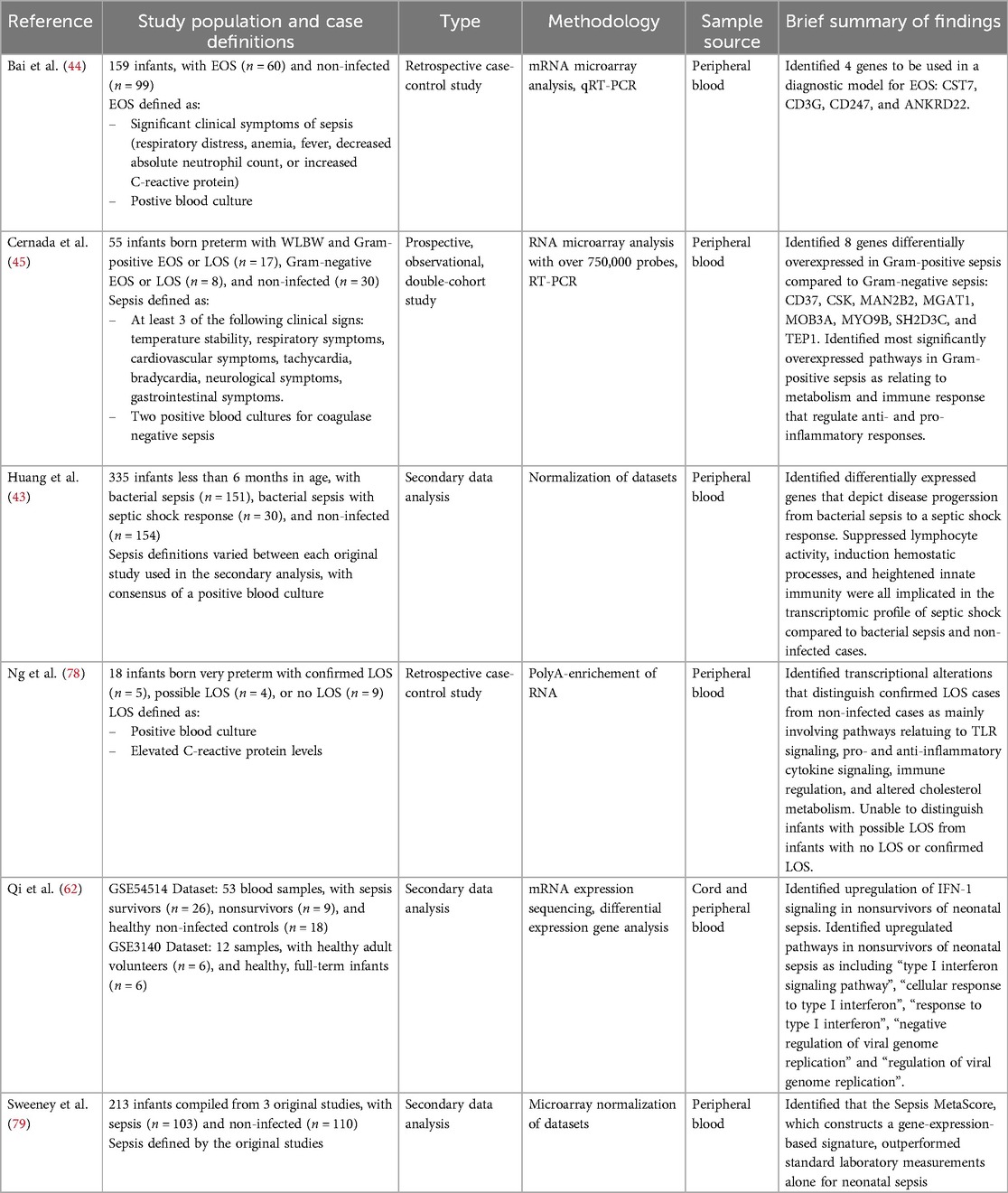
Six studies published between 2018 and 2024 utilized transcriptomic technologies as the primary method for investigating neonatal sepsis and were included in this review (Table 5). The studies demonstrated considerable heterogeneity in focus and design, including comparisons of neonates with EOS vs. LOS vs. neonates without sepsis (42), gram-positive vs. gram-negative infections (23), and varying clinical outcomes such as bacteremia vs. septic shock (43). No two studies directly evaluated the same neonatal subgroups, limiting direct comparisons across investigations.
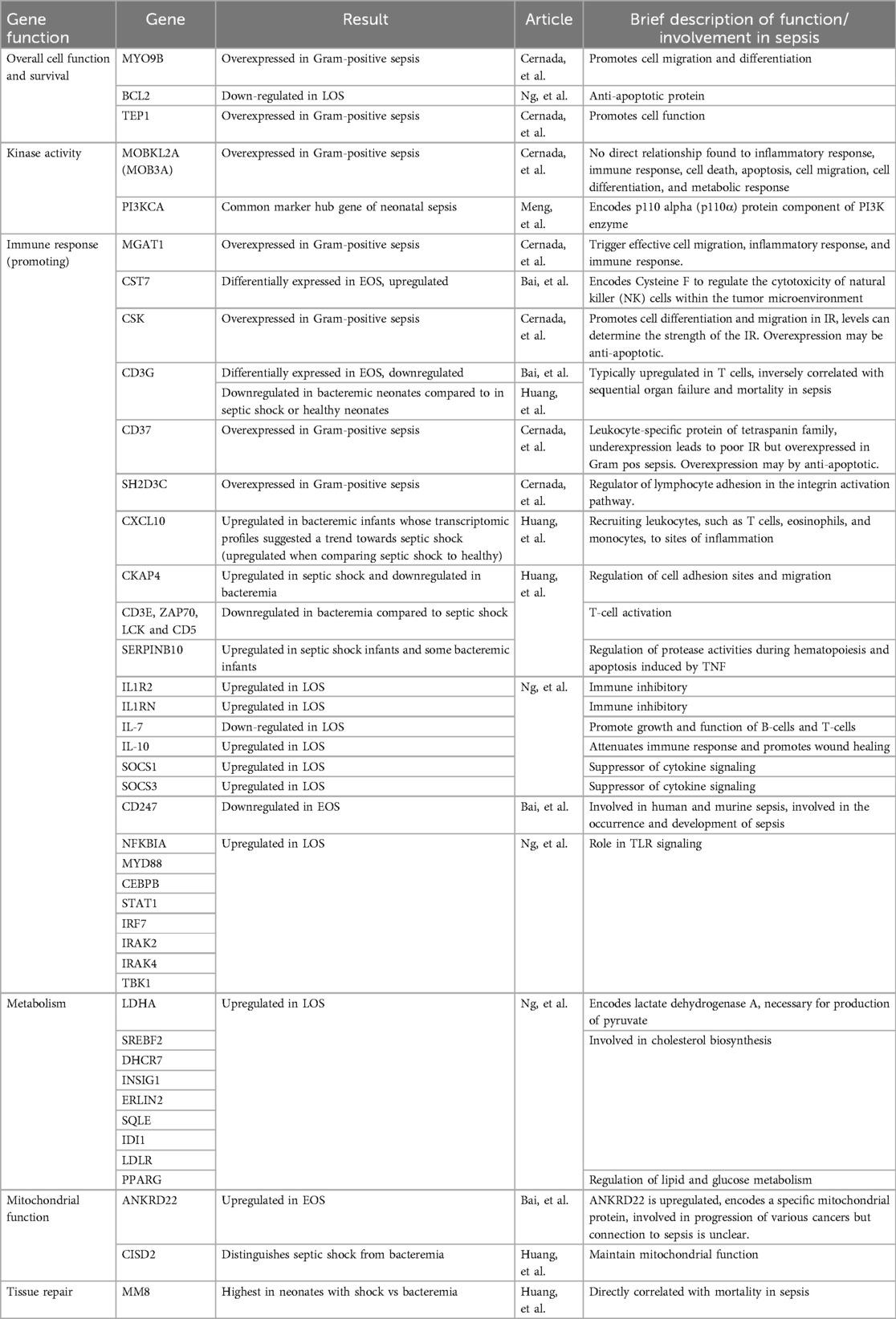
While the heterogeneity in patient populations and research aims limited the identification of consistently reported individual genes, CD3G emerged as a noteworthy exception (Table 6). This gene, which plays a key role in T-cell receptor signaling, was reported as downregulated in both Bai et al. (44) and Huang et al. (43). Bai et al. (44) highlighted CD3G as one of four differentially expressed genes distinguishing neonates with EOS from neonates without sepsis, while analysis by Huang et al. (43) revealed CD3G as a key gene differentiating neonates with septic shock from those with bacteremia.
Although overlap in specific genes was rare, several studies identified genes involved in similar immune pathways, suggesting convergent biological processes (Table 6). Cernada et al. (45) reported upregulation of CSK, a gene implicated in immune cell differentiation and migration, in gram-positive compared to gram-negative sepsis. Huang et al. (43) identified CXCL10, a chemokine known for recruiting immune cells to infection sites, as significantly upregulated in septic shock vs. bacteremia.
A cross-study comparison of two studies revealed a potential shared inflammatory pathway as the subject of investigation (Table 6). Huang et al. (43) reported increased expression of MM8, a gene associated with severe inflammatory responses, in neonates with septic shock. Ng et al. (23), examining cases with LOS, found upregulation of NF-κB1A, a downstream target activated by MM8. NF-κB1A mediates production of key inflammatory cytokines such as IL-6 and type I interferons.
3.4 Mirnomics
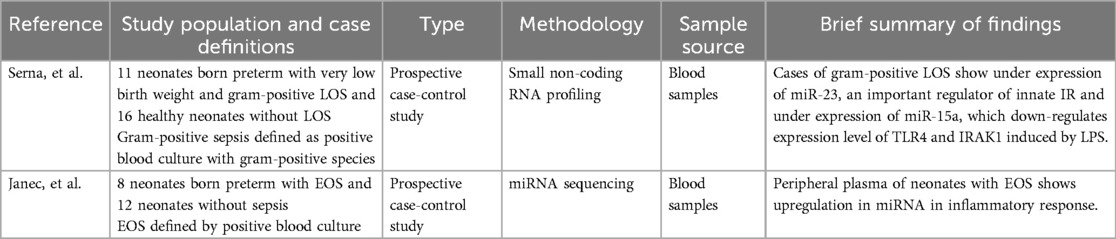
Two studies employing miRNA sequencing were selected for review, both of which used a prospective case-control design (Table 7). Serna et al. (46) investigated infants with VLBW and LOS, while Janec et al. (47) focused on neonates born preterm with EOS. Both studies identified differentially expressed miRNAs in neonates with sepsis that are known regulators of innate immune responses. Janec et al. (47) reported increased expression of miR-142-5p, miR-223-3p, and miR-148b-3p in neonates with sepsis. These miRNAs are associated with suppression of Toll-like receptor 4 (TLR4) signaling, a key pathway in the detection of bacterial pathogens. Serna et al. (46) found under expression of several immune-regulatory miRNAs in neonates with sepsis. These included miR-23, which modulates innate immunity through regulation of metalloproteinase 10; miR-17, which is involved in broader immune responses including T-cell function; and miR-15a, which suppresses TLR4 and IRAK1 expression in response to lipopolysaccharide (LPS) exposure.
3.5 Epigenomics
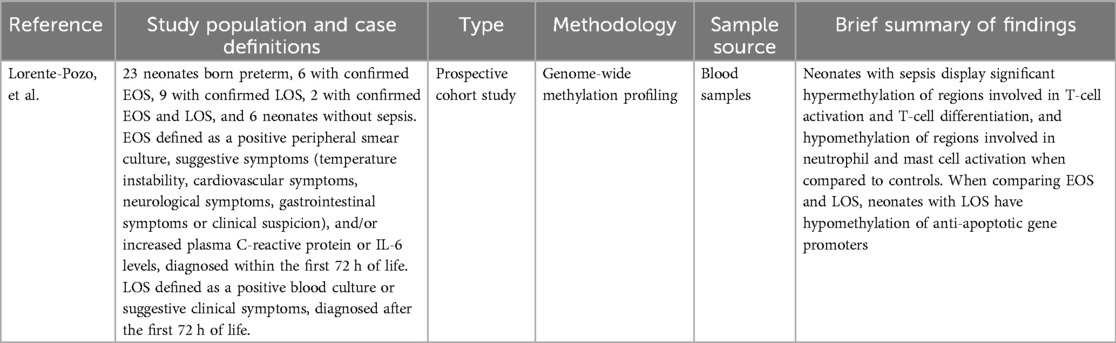
One study by Lorente-Pozo et al. (48) conducting epigenomic analysis was selected for review (Table 8). Neonates with sepsis show differential hypermethylation of the CD3G and CD3D gene promoters and hypomethylation of IL10 gene promoter compared to controls without sepsis. Greater changes in methylation were found when comparing neonates with LOS to controls than comparing neonates with EOS to controls. When comparing methylation in neonates with LOS to neonates with EOS, 4 significantly hypomethylated gene promoters were noted- DUSP22, PM20D1, MIR10A, and MIR886. Pathway analysis revealed significant hypermethylation of regions involved in T-cell activation and T-cell differentiation, and hypomethylation of regions involved in neutrophil and mast cell activation when comparing neonates with sepsis to controls.
3.6 Proteomics
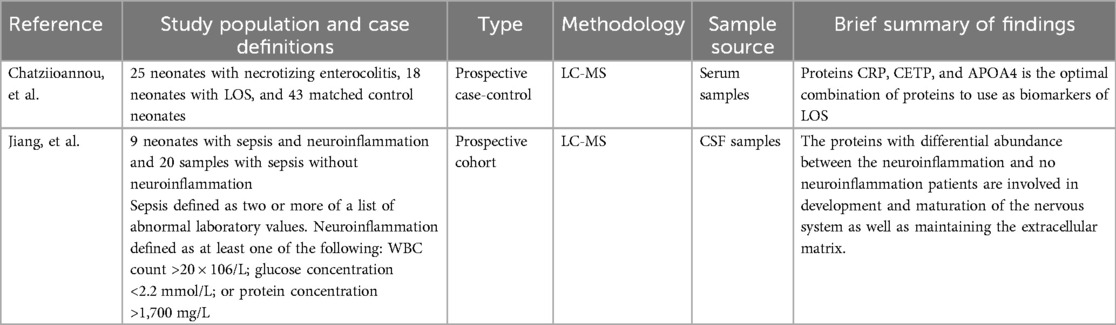
Two studies utilizing liquid chromatography–mass spectrometry (LC-MS) were selected for this review, each investigating different biological fluids to better understand protein expression in neonatal sepsis (Table 9). Chatziioannou et al. (49) conducted a prospective matched case-control study to evaluate the serum proteomic profile of neonates with LOS. Analysis of serum samples identified a panel of three proteins that showed potential as diagnostic biomarkers: apolipoprotein A-IV (APOA4), cholesteryl ester transfer protein (CETP), and C-reactive protein (CRP) (49). In contrast, Jiang et al. (50) explored cerebrospinal fluid (CSF) proteomic changes in a cohort of neonates with sepsis who also exhibited signs of neuroinflammation. Analysis of CSF samples revealed a consistent decrease in proteins essential for neuronal circuit formation and extracellular matrix (ECM) stability (50). Among these were matrix metalloproteinase-2 (MMP-2), an enzyme that degrades components of the ECM, and cystatin C, a protease inhibitor known to play a protective role in the central nervous system. Other proteins such as brevican, nidogen-1 and −2, and fibronectin, all important structural elements of the ECM, were also found to be reduced in neonates with neuroinflammatory responses (50).
3.7 Metabolomics
Six studies were selected that employed mass spectrometry-based metabolomic profiling to investigate neonatal sepsis (Table 10). All but one (51) utilized LC-MS as the primary analytical method, with a single study relying on gas chromatography coupled with time-of-flight mass spectrometry (GC-TOF-MS). These studies highlighted distinct alterations in the metabolic landscape of neonates with sepsis, revealing potential biomarkers and underlying pathways involved in disease progression.
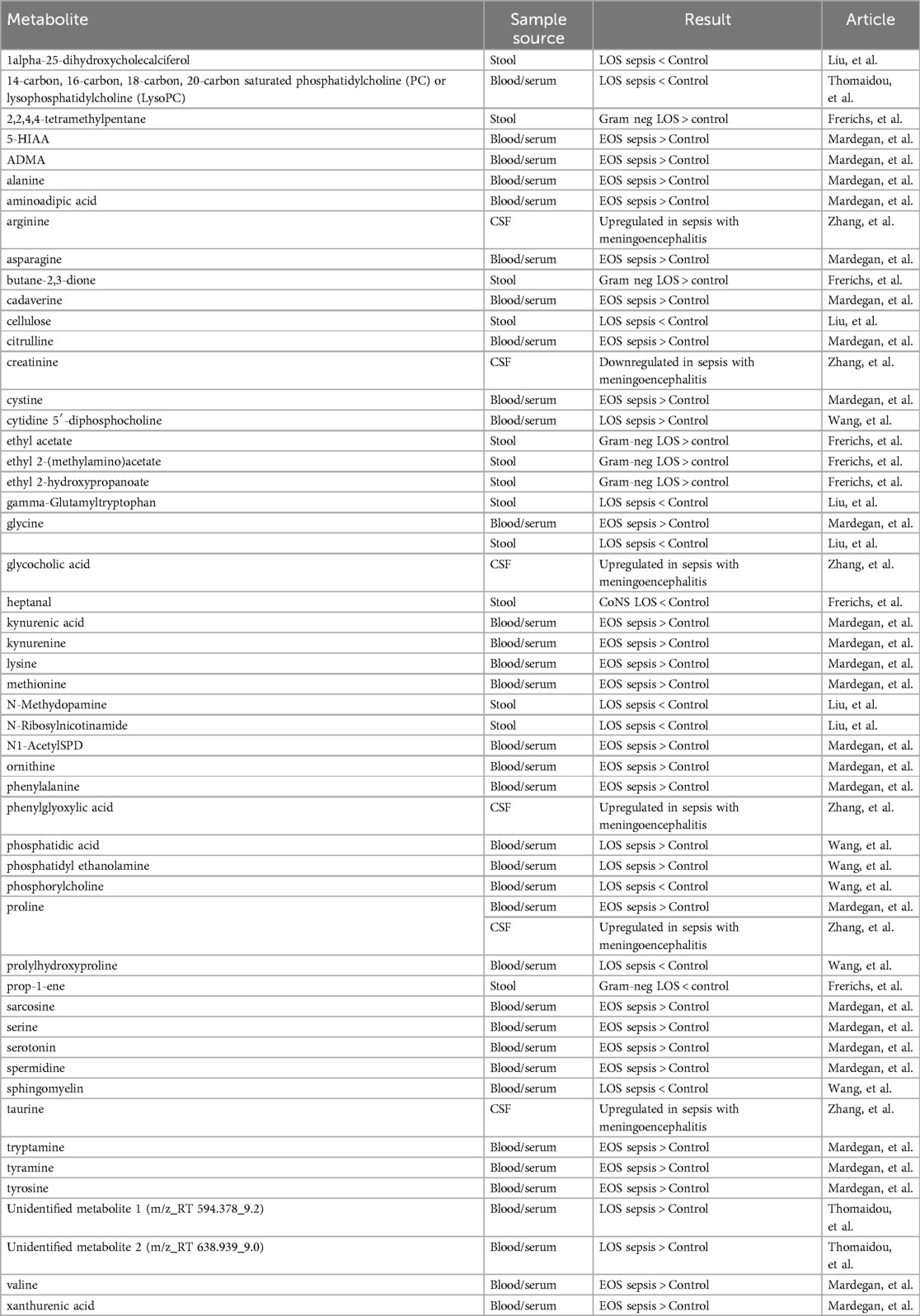
Alterations in lipid metabolism emerged as a common finding (Table 11). Both Thomaidou et al. (52) and Wang et al. (53) reported disruptions in lipid-related pathways in neonates with LOS. Thomaidou et al. (52) found reduced levels of phosphatidylcholine (PC) and lysophosphatidylcholine (lysoPC), with one specific lysoPC species, identified as 16:0/0:0, standing out as a strong biomarker candidate. Importantly, decreased levels of these lipids were also associated with increased mortality (52). Wang et al. (53) expanded upon these findings, reporting decreased sphingomyelin, prolyl hydroxyproline, and phosphorylcholine in cases with LOS. Interestingly, they also observed elevated levels of cytidine 5′-diphosphocholine (CDP-CHO), a precursor in PC biosynthesis (53).
Disruptions in amino acid metabolism were also consistently reported across studies. Mardegan et al. (54) and Wang et al. (53) both highlighted overrepresentation of the aminoacyl-tRNA biosynthesis pathway in neonates with sepsis. Mardegan et al. (54) specifically noted elevated taurine metabolism in EOS, a finding that was also mirrored by Zhang et al. (55), who observed increased taurine in the CSF of neonates with sepsis with meningoencephalitis (ME). Both Mardegan et al. (54) and Liu et al. (56) also reported increased glutathione metabolism in neonates with EOS and LOS.
Increased metabolism of aromatic compounds, long considered a biochemical signature of sepsis, was another consistent theme. CSF analysis of neonates with ME performed by Zhang et al. (55) revealed elevated levels of phenylglyoxylic acid, a metabolite derived from phenylacetic acid, which itself is a byproduct of phenylalanine catabolism (Table 10). Liu et al. (56) similarly reported increased glyoxylic acid metabolism in neonates with LOS. Mardegan et al. (54) identified enhanced phenylalanine metabolism and also reported increased catabolism of other aromatic amino acids such as tyrosine and tryptophan. Liu et al. (56) further identified an increase in gamma-glutamyl tryptophan, underscoring the systemic alteration of aromatic amino acid pathways in neonates with sepsis.
Lastly, changes in bile acid metabolism were noted in both Liu et al. and Zhang et al. Liu et al. (56) identified primary bile acid synthesis as one of thirteen significantly involved pathways in LOS, while Zhang et al. (55) reported elevated levels of glycocholic acid, a primary bile acid, in the CSF of neonates with sepsis and ME compared to those without neurological involvement (Table 10).
4 Discussion
4.1 Microbiome
Recent advancements in microbiome sequencing have significantly enhanced our understanding of the gut microbial landscape in neonates and its potential role in the pathogenesis of sepsis. The emerging consensus across multiple studies points to a consistent reduction in alpha diversity among neonates with sepsis, suggesting that early microbial imbalance may contribute to heightened vulnerability to infection. This trend was particularly evident in infants born preterm, where microbial diversity was markedly reduced compared their counterparts born at term. The predominance of Proteobacteria in healthy neonates born preterm contrasted with the Firmicutes and Actinobacteria dominance seen in infants born at term (29), suggesting that gestational age plays a critical role in shaping early microbial communities. Moreover, the enrichment of Bacilli class organisms, especially Staphylococcus species such as S. epidermidis and S. aureus, in both gut and nasal microbiomes prior to sepsis onset (31, 32), underscores their potential involvement in disease progression.
Analysis of alpha diversity also strengthens the hypothesis of endogenous microbial translocation as a plausible route of infection, as dominant Staphylococcus populations were found to precede the onset of bacteremia, observed in both fecal and nasal samples. Strain-specific PCR supports this notion by confirming the presence of S. epidermidis, Klebsiella pneumoniae, and Escherichia coli in the gut prior to bloodstream infection (29). These findings may substantiate the concept of bacterial translocation from the gastrointestinal tract, demonstrated with molecular precision.
Cross-comparison with metabolomic findings reinforce the idea of translocation from the neonatal gut as a source of bloodstream infection. Graspeuntner et al. (31) detected in neonates with sepsis elevated fecal levels of ethanol and formic acid, likely fermentation byproducts of Bacilli class organisms, which commonly reside in the gut.
Moving beyond analysis of gut microbiota composition, interpretation of use of antibiotics suggests a deleterious effect on the growth of beneficial organisms. Several studies highlighted the suppressive effects of perinatal and early life antibiotic administration on beneficial genera such as Lactobacillus and Bifidobacterium (29, 33–35). Notably, the absence or severe depletion of Bifidobacterium in neonates born preterm with sepsis (33), as well as the reduced abundance of Lactobacillus in neonates exposed to antibiotics or born to Group B Streptococcus-positive mothers (36), underscores the fragility of microbial populations in this vulnerable group. The inverse relationship between probiotic supplementation and these depletions further highlights the potential utility of microbial restoration strategies.
Despite the documented shifts in microbial profiles and diversity, the relationship between antibiotic exposure and the emergence of causative pathogens remains complex. While certain studies linked recent antibiotic use to subsequent bloodstream infections with Enterobacteriaceae (38), others did not observe clear changes in pathogen abundance before or after sepsis onset (37). Furthermore, the persistence of clinical stability following antibiotic discontinuation in several cases raises important questions regarding microbial resilience and the threshold at which dysbiosis translates into systemic infection.
While attention has recently turned to novel therapeutic avenues such as lantibiotics (57, 58), bacterially derived antimicrobial peptides with broad-spectrum efficacy, their clinical application in neonatal sepsis remains largely theoretical. Though promising in preclinical models, their role in modulating early-life microbiota or preventing sepsis has not yet been explored in neonatal cohorts.
Limitations of microbiome studies include collection of samples in relation to the timeline of disease progression. All studies noted collection of samples before and after either bloodstream infection or sepsis onset with the exception of one (31), in which samples were only collected after the detection of bloodstream infection. Timing of sample collection impacts the ability to associate dysbiotic changes with sepsis onset. Moreover, some studies chose to define sepsis as presence of clinical features along with a positive blood culture, while some studies defined cases solely by the presence of a positive bloodstream infection. Lastly, the use of maternal factors in certain studies as an independent variable of study makes comparison of results to those in which neonates are the primary subject difficult. Nonetheless, these studies underscore the complex interplay between microbial colonization patterns, antibiotic exposure, and the risk of neonatal sepsis. While the precise mechanistic links remain to be fully elucidated, these findings support continued exploration of microbiome-targeted interventions, such as probiotics and potentially lantibiotics, as part of a comprehensive strategy to mitigate sepsis risk in neonates, particularly those born preterm.
4.2 Genomics
Genomic research has recently expanded in scope, encompassing both pathogen and host related investigations. While still in its early stages compared to other omics approaches, the application of WGS to neonatal sepsis is beginning to uncover valuable insights into microbial virulence, host susceptibility, and antiseptic resistance patterns. The limited number of studies meeting inclusion criteria in this review reflects the early onset nature of this field but also emphasizes its growing relevance.
Host-focused genomic analysis, as conducted by Ciesielski et al. (41), reveals compelling evidence of sex-specific genetic susceptibility to LOS. The identification of distinct SNPs in male and female neonates, without overlap between the two, suggests potential differences in immune regulation or response pathways that may be inherently linked to sex chromosomes or sex dependent gene expression. Notably, several of the variants mapped to components of the NOTCH signaling pathway (41), which has known roles in T-cell development and apoptotic processes, both of which are critical to immune function. Additionally, SNPs in genes encoding IL-10 and TNF-α (41), two major cytokines involved in the neonatal inflammatory response, underscore the potential for inherited variation in immune signaling to shape infection outcomes. These findings provide a genomic framework for understanding individual variability in sepsis risk and open the door for future studies examining personalized approaches to sepsis prevention or treatment based on genetic profiling.
On the microbial side, the work by Lipworth et al. (39) adds an important dimension to pathogen genomics, specifically in relation to vaccine development. Their finding that a large subset of E. coli isolates from neonates wtih sepsis harbored O-antigen serotypes included in the ExPEC-4V vaccine formulation suggests that maternal immunization strategies, already being trialed in adults (59), may be repurposed to protect neonates against bloodstream infections. Continued clinical testing of the ExPEC-4V and other O-antigen vaccines in adult populations may yield results that could eventually be extended to neonates. If successful, this could provide a practical and highly targeted tool to reduce the burden of E. coli-mediated sepsis in this vulnerable group.
Though the focus of the study by Sethi et al. (40) differs, its findings also contribute to the broader goal of neonatal sepsis prevention. By identifying elevated chlorhexidine minimum inhibitory concentrations in UK isolates (40), where antiseptic use is more prevalent, the study highlights the potential for antiseptics to exert selective pressure on microbial populations. While no specific resistance genes were definitively associated with chlorhexidine or octenidine tolerance (40), the results imply that resistance may be developing through mechanisms not yet understood. This underscores the need for more prudent selection of antiseptics in neonatal intensive care settings to prevent the emergence of resistant strains. Preserving the effectiveness of these compounds could be critical to maintaining infection control measures and improving outcomes in neonates with sepsis.
Together, the findings from Lipworth et al. (39) and Sethi et al. (40) offer two complementary angles for advancing sepsis prevention: immunization to reduce infection risk and stewardship of antiseptic use to limit resistance. Both studies demonstrate how genomic tools can inform strategies that go beyond treatment to encompass proactive measures in infection control.
While the primary focus of this review was on biomarker and susceptibility research, it is worth noting the wealth of studies employing WGS for outbreak tracking in NICUs (Table 4). Though excluded from the core analysis due to their epidemiological nature, these studies reinforce the practical utility of genomics in infection control and could serve as a foundation for future biomarker discovery through strain specific virulence factor analysis.
Comparison of the genomic studies is limited by the timing of sample collection, as all studies did not collect samples until the onset of bloodstream infection or sepsis diagnosis. Whereas one study solely defined sepsis criteria as presence of bloodstream infection, another included abnormal clinical and laboratory findings. The third study was of microbial focus and included all staphylococcal isolates admitted to two NICUs over a 10-week period. The heterogeneity of sepsis definition also limits the ability to draw parallels between findings of the studies.
Altogether, these genomic studies underscore the multifaceted utility of sequencing technologies in neonatal sepsis research. Whether through identifying host risk alleles, monitoring the impact of antimicrobial interventions, or informing future vaccine strategies, genomics is poised to become an integral component of precision-based neonatal care. As research in this area progresses, it may lead not only to improved understanding of disease pathophysiology but also to more effective, individualized approaches to sepsis prevention and management.
4.3 Transcriptomics
Transcriptomic technologies offer a dynamic snapshot of the host immune response to infection and have revealed promising gene-level insights into the pathophysiology of neonatal sepsis. The studies reviewed collectively highlight key immune pathways activated during infection, and although variability in study design limits direct comparisons, several recurring molecular signals emerge that may inform biomarker development and therapeutic interventions.
Among these signals, CD3G emerged as the most consistently reported gene across studies (43, 44). CD3G encodes the CD3-gamma subunit of the T-cell receptor complex, an essential component for T-cell antigen recognition and activation. Its downregulation in neonates with EOS relative to healthy controls (44) and in septic shock compared to bacteremia (43) suggests a broader role for T-cell dysfunction in sepsis progression. Moreover, these observations may represent a continuum of CD3G modulation along the clinical spectrum from healthy neonate to neonate with sepsis to septic shock. The observed initial CD3G suppression in EOS could contribute to a transient immunodeficient state, facilitating bacterial translocation into the bloodstream. As the neonate progresses through infection, upregulation of CD3G expression in septic shock may reflect a dysregulated immune response or compensatory overactivation, contributing to cytokine storm and tissue damage. Future studies examining CD3G expression longitudinally across sepsis stages, and specifically within neonates with bacteremia who do or do not progress to shock, may help establish its value as a predictive biomarker of disease severity.
Despite limited overlap in specific genes across studies, multiple investigations reported genes involved in similar immunologic pathways, reinforcing shared biological mechanisms of neonatal sepsis. For example, CXCL10, a chemokine that recruits T cells, natural killer cells, and monocytes, was found upregulated in neonates with bacteremia but not in those with septic shock (43). This suggests that more robust leukocyte recruitment may be protective, consistent with the improved outcomes observed in infants with bacteremia compared to those experiencing systemic decompensation. Similarly, CSK, identified by Cernada et al. (45) as upregulated in gram-positive sepsis, promotes immune cell migration and may exert anti-apoptotic effects. Together, these findings suggest a common immunoprotective role for leukocyte migration, particularly in less severe forms of sepsis or gram-positive infections. This aligns with clinical observations of better prognosis in bacteremia and gram-positive infections (60, 61) and suggests that modulation of immune cell trafficking could be an avenue for future therapeutic intervention.
In addition to specific immune-related genes and shared immune pathway involvement, inflammatory pathway activation also emerged as a recurrent theme. Huang et al. (43) reported upregulation of MM8 in neonates with septic shock, a gene known to activate NF-κB, a transcription factor central to inflammatory cytokine production. Complementarily, Ng et al. (23) observed increased expression of NF-κB1A, a downstream target of MM8, in neonates with LOS. This linkage between studies points to an MM8–NF-κB signaling axis as a potential driver of the exaggerated inflammatory response in severe sepsis. Further exploration of this pathway could clarify the mechanisms underpinning sepsis-associated organ dysfunction and identify novel targets for immunomodulation.
A fourth major theme involved IFN-1 signaling, highlighted in both Qi et al. and Ng et al. Qi et al. (62) found upregulation of IFN-1 pathways, including “cell response to type I interferon” and “negative regulation of viral genome replication”, in nonsurvivors of neonatal sepsis. Ng et al. (23) reported increased expression of genes that induce IFN-1 production in neonates with LOS, but this was not matched by elevated protein levels of IFN-α or IFN-β, suggesting post-transcriptional regulation or feedback inhibition. When examined together, these findings illustrate different stages of the IFN-1 signaling cascade, with Ng et al. (23) capturing the early transcriptional priming, and Qi et al. (62) identifying the functional consequences and associated mortality. This cross-study synthesis underscores the need for multi-omic validation to confirm findings at the protein and functional levels.
Beyond mechanistic insights, transcriptomics holds clinical promise for improving the diagnostic accuracy and risk stratification of neonatal sepsis. Several studies, including Huang et al. (43), proposed genes with potential predictive value for septic shock, though validation in larger, prospective cohorts is necessary. Combining transcriptomic biomarkers with proteomic, genomic, or metabolomic data may improve prediction models that distinguish neonates with sepsis at risk of deterioration from those likely to recover. Particularly, modular gene signatures, which capture specific immune responses such as T-cell suppression, leukocyte recruitment, or cytokine signaling, may be more effective than single gene markers in capturing the heterogeneity of neonatal sepsis.
A strength offered by the transcriptomic studies is the analysis of neonates through the stages of sepsis, from bloodstream infection to sepsis to shock. Such longitudinal analysis allows for association of transcriptomic disruption with specific points along the timeline of disease. However, two studies only collected samples after the onset of bloodstream infection or sepsis diagnosis, and three used data from public datasets, in which individual data was compiled from multiple studies, each with its own definition of sepsis.
In summary, transcriptomic profiling has uncovered key immune pathways involved in neonatal sepsis, including T-cell receptor signaling (CD3G), leukocyte chemotaxis (CXCL10, CSK), inflammatory activation (MM8, NF-κB1A), and IFN-1-mediated immune modulation. While further research is needed to translate these insights into clinical tools, the convergence of findings across independent studies strengthens the case for transcriptomics as a critical platform in the development of personalized sepsis diagnostics and interventions in the neonatal population.
4.4 Mirnomic
Although still an emerging field, miRNomic analysis offers valuable insights into the post-transcriptional regulation of immune responses during neonatal sepsis. The reviewed studies highlight the role of differentially expressed miRNAs in modulating key components of the innate immune system in neonates with sepsis. Despite differing in study populations and sepsis onset (EOS vs. LOS), both investigations converge on the importance of miRNAs in regulating TLR signaling pathways, which are central to neonatal immune defense against bacterial infections.
While Janec et al. (47) reported upregulation of miRNAs known to suppress elements of the TLR4 signaling cascade in neonates with EOS (63, 64), Serna et al. (49) observed downregulation of multiple immune-regulatory miRNAs in infants with VLBW and LOS. These included miR-23, miR-17, and miR-15a. miR-23 influences innate immunity via regulation of metalloproteinase 10, a protein involved in tissue remodeling and inflammatory responses (65). miR-17 has broader immunomodulatory functions, including impacts on T-cell proliferation and function (66), while miR-15a is known to suppress both TLR4 and interleukin-1 receptor-associated kinase 1, another key component of TLR signaling (67). The under expression of these miRNAs in LOS may lead to unchecked inflammatory signaling and exaggerated immune responses, which are commonly observed in neonates with sepsis. The contrasting expression patterns observed across EOS and LOS may indicate differences in host immune maturity, pathogen exposure, or temporal dynamics of sepsis development. It is also possible that miRNA profiles reflect divergent immune strategies, suppressing inflammation in EOS to prevent collateral tissue damage, vs. failing to adequately restrain inflammation in LOS, leading to immune dysregulation.
Despite contrasting findings, both studies highlight the regulatory influence of miRNAs on TLR4 signaling (40, 47), underscoring a shared molecular axis in sepsis pathophysiology. Given that overactivation and under-activation of TLR pathways can be detrimental in sepsis (68, 69), maintaining an optimal level of immune activation appears critical. Synthesis and comparison of the results from these two studies highlights the therapeutic potential of miRNAs as modulators of immune balance. For instance, restoring the expression of downregulated miRNAs in LOS or selectively inhibiting overexpressed miRNAs in EOS could offer a targeted approach to modulate immune responses without broadly suppressing immunity.
Conclusions drawn from the miRNA studies are strengthened by the shared use of blood samples as well as shared definition of sepsis by a positive blood culture. However, the small number of studies calls for more research to be done utilizing this molecular method. Fortunately, miRNAs show potential for future research, as they are stable in circulation and amenable to non-invasive sampling, making them attractive candidates for biomarker development. Their differential expression in neonates with and without sepsis, as demonstrated in both studies (40, 47), suggests that miRNA panels could support early diagnosis or stratification of sepsis severity. Future studies should validate these findings in larger cohorts and explore whether specific miRNA signatures are predictive of clinical outcomes such as progression to septic shock, need for intensive care interventions, or mortality.
4.5 Epigenomics
Genome-wide methylation analysis performed in epigenomic studies offers insights into changes in DNA expression that may provide an upstream explanation of changes in neonates with sepsis demonstrated by other omic analyses. The hypermethylation of the CD3G gene promoter in neonates with sepsis (48) aligns with transcriptomic analyses showing decreased expression of CD3G in neonates with EOS compared to neonates without sepsis (44) and in neonates with septic shock compared to neonates with bacteremia (43). Epigenomic analysis offers a potential explanation for the differential expression observed in transcriptomic analysis.
Results of epigenomic analysis may also underscore findings from other omic methods. When taken together, the hypomethylation of the IL10 promoter (48) in neonates with sepsis and the SNPs identified in genes encoding IL-10 in neonates with LOS compared to controls (41) suggest a multifactorial explanation of aberrant immune function that may place certain neonates at higher susceptibility of developing sepsis. Additionally, hypomethylation of the MIR886 promoter in LOS (48) may lead to increased expression of microRNA miR-886-5p seen in miRNomic analysis. miR-886-5p has been shown to downregulate BAX, inhibiting apoptosis (70), and the increase in expression may be another mechanism of aberrant immune function increasing susceptibility. Thus, epigenomic analysis may provide another pathway to study the disruption of apoptotic regulation associated with changes in NOTCH signaling observed on genomic analysis of neonates with LOS (41).
Cross-comparison of epigenomics with miRNomics may also provide insight into the disrupted immune regulation present in sepsis. Lorente-Pozo et al. (48) report hypomethylation of the promoter for MIR10A encoding the microRNA miR-10a, which has been shown to negatively correlate with production of pro-inflammatory cytokines (71). The hypomethylation of the MIR10A promoter in LOS (48) may work in tandem with the decreased expression of immune regulatory miRNAs observed in miRNomic analysis of neonates with LOS (46), ultimately creating a setting of extreme inflammation in sepsis. Epigenomic studies provide a way to approach neonatal sepsis pathogenesis from a multifactorial standpoint. However, the conclusions drawn here are severely limited by the fact that only one epigenomic study met criteria for inclusion in our review. That said, comparison with findings across other omics suggest potential relationships between transcriptomic, genomic, and miRNomic analyses. Future studies may aim to further elucidate these relationships.
4.6 Proteomics
Proteomic profiling has emerged as a powerful approach to uncover the molecular alterations associated with neonatal sepsis. By directly quantifying protein expression, proteomics can offer insight into both systemic inflammation and organ specific responses that may not be captured by genomic or transcriptomic methods.
Chatziioannou et al. (49) examined serum proteomes in neonates with LOS and identified three candidate biomarkers: APOA4, CETP, and CRP. These findings reflect key aspects of the host's inflammatory and metabolic responses to infection. The downregulation of APOA4 aligns with patterns previously observed in adult sepsis, where decreases in apolipoproteins are thought to reflect inflammation induced disruption of lipid transport and metabolism (49). Similarly, reduced CETP levels, known to correlate with worse outcomes in adult sepsis (49), suggest an impaired ability to manage lipid trafficking during the systemic inflammatory response. CRP, a well-established marker of inflammation, supports the validity of this proteomic panel and highlights the relevance of combining novel markers with conventional ones to enhance diagnostic accuracy.
In contrast, Jiang et al. (50) focused on CSF to investigate proteomic changes associated with neuroinflammation in neonates with sepsis. Their study revealed significant downregulation of proteins critical for maintaining neuronal structure and extracellular membrane (ECM) integrity (50). Among these were MMP-2, which regulates ECM remodeling, and cystatin C, a protective protease inhibitor involved in CNS homeostasis (50). The additional decreases in structural ECM proteins such as brevican, nidogen-1 and −2, and fibronectin suggest that neuroinflammatory damage in neonatal sepsis may involve degradation of the ECM (50), potentially contributing to long-term neurological complications.
Together, these studies emphasize the multifaceted nature of neonatal sepsis, wherein systemic inflammation, metabolic dysregulation, and neuroinflammation can all be reflected in proteomic changes. Importantly, the biological fluid selected for analysis, serum vs. CSF, yields insights into distinct compartments of disease activity. This supports the notion that a multi-compartmental approach to proteomic sampling may be valuable in fully characterizing neonatal sepsis, especially in cases with suspected or confirmed CNS involvement.
The identification of APOA4 and CETP as potential serum biomarkers for LOS (49) represents a promising step toward developing more precise diagnostic tools. Given that many laboratory tests evaluating for sepsis lack specificity or only become positive after clinical deterioration (72, 73), early detection using proteomic signatures may improve timely intervention and outcomes. Additionally, the downregulation of ECM related proteins in CSF points to possible mechanisms underlying sepsis-associated encephalopathy and may offer targets for therapeutic intervention or prognostic stratification.
Despite their contributions, both studies are limited by relatively small sample sizes and require validation in larger, diverse cohorts. Additionally, one study defined sepsis as the presents of two or more abnormal laboratory findings from a pre-determined list, while the other diagnosed sepsis by clinical and laboratory findings. Furthermore, proteomic data must ultimately be integrated with other omics approaches to obtain a comprehensive understanding of disease mechanisms.
4.7 Metabolomics
Metabolomic profiling has emerged as a tool for uncovering the biochemical underpinnings of neonatal sepsis, offering insight into the interplay between infection, inflammation, and systemic physiological responses. The studies reviewed consistently demonstrate that neonatal sepsis, whether early onset, late onset, or complicated by meningoencephalitis, is characterized by multifaceted disruptions in metabolic pathways. Key findings included alterations in lipid and amino acid metabolism, changes in aromatic compound catabolism, and disruptions in bile acid pathways, which collectively provide diagnostic and mechanistic insight into the disease.
Lipid metabolism was the most commonly affected pathway across studies (52, 53). Reductions in PC, lysoPC, and sphingomyelin were reported in neonates with LOS. These lipids play critical roles as structural components of cellular membranes but also as enzyme activators and anti-inflammatory mediators (74, 75). The decrease in lysoPC and PC reported by Thomaidou et al. (52) aligns with Wang et al.'s (53) observation of reduced sphingomyelin, another essential membrane lipid. Notably, sphingomyelin was highlighted as a strong predictor of sepsis mortality (53), further supporting its clinical relevance. While Wang et al. (53) also reported an increase in total PC levels and its precursor CDP-CHO, this finding may reflect differential use of CDP-CHO in other biosynthetic or stress response pathways during LOS. Together, these results reinforce the notion that disrupted lipid metabolism is a defining feature of neonatal sepsis and may provide targets for early detection and risk stratification.
Amino acid metabolism was similarly affected, particularly those pathways related to stress response and immune modulation. Taurine metabolism was notably upregulated in both blood and CSF, with Zhang et al. (55) and Mardegan et al. (54) reporting elevated levels in neonates with sepsis and those with ME. Taurine is a well-known antioxidant with protective effects on mitochondria, suggesting that its upregulation may be a response to sepsis induced oxidative stress. However, the overproduction of antioxidants, as seen in neonates with ME (55), raises the possibility that excessive antioxidant activity may also contribute to pathological processes such as neuroinflammation. This interpretation is supported by the concurrent upregulation of glutathione metabolism in both EOS and LOS (54, 56), further indicating a system wide oxidative stress response in sepsis, regardless of timing.
Another theme across studies was the alteration of aromatic amino acid metabolism. Phenylglyoxylic acid, a metabolite of phenylacetic acid and a downstream product of phenylalanine catabolism, was found to be elevated in neonates with ME (55). Phenylalanine has been shown to stimulate pro-inflammatory cytokine release (76, 77), supporting a link between elevated phenylglyoxylic acid levels and heightened inflammatory responses in sepsis. Liu et al. (56) similarly reported increased glyoxylic acid metabolism, and Mardegan et al. (54) identified enhanced breakdown of aromatic amino acids including phenylalanine, tyrosine, and tryptophan. Interestingly, not all derivatives of these pathways are purely proinflammatory. Gamma-glutamyl tryptophan, for instance, has been described as possessing anti-inflammatory and immune supportive properties (56), highlighting the dual and complex roles of these metabolites. These observations suggest that neonatal sepsis involves an interplay between pro inflammatory and anti-inflammatory mechanisms, rather than a unidirectional immune activation.
Disruptions in bile acid metabolism were also noted, providing a possible link between gut health and systemic inflammatory responses. Elevated glycocholic acid levels in the CSF of neonates with ME (55) and upregulation of primary bile acid synthesis pathways in LOS (56) point to potential gut-liver-brain axis involvement in sepsis. Bile acids are known to modulate inflammation and regulate the gut microbiota, and their dysregulation may contribute to the leaky gut phenomenon proposed by Graspeuntner et al. (31). However, it is important to note that samples in these studies were collected after clinical suspicion or onset of sepsis, limiting the ability to determine whether bile acid changes are a cause or consequence of infection. Nevertheless, given that bile acids facilitate lipid absorption, disruptions in bile acid metabolism may lie upstream of the lipid metabolic changes described earlier, suggesting that multiple metabolomic findings may be linked through interconnected pathways.
Many of the reported metabolic changes were consistent across both EOS and LOS, indicating shared pathophysiologic mechanisms and limiting the utility of some biomarkers in distinguishing onset stage. However, several metabolites, particularly those measured in CSF, showed potential to differentiate between sepsis with and without neurologic complications such as ME. For example, increased taurine and phenylglyoxylic acid in ME cases (55) suggest that metabolic profiling may help predict complications of sepsis and guide neuroprotective interventions.
Metabolomics provides compelling evidence that neonatal sepsis is characterized by widespread alterations in lipid, amino acid, and bile acid metabolism. These findings support a complex, multi system model of disease involving proinflammatory, anti-inflammatory, oxidative stress, and potential organ specific vulnerabilities.
All metabolomic studies included a positive blood culture as a requirement for sepsis diagnosis, and none relied solely on a positive culture to make the diagnosis. However, only two studies collected samples before suspicion or confirmed diagnosis of sepsis; the rest collected the first sample upon diagnosis of sepsis. Future studies should explore longitudinal changes in metabolite profiles, incorporate pre-symptomatic sampling when feasible, and integrate findings with other omics data to construct a holistic view of sepsis pathogenesis. Ultimately, these efforts may lead to improved diagnostic tools and targeted therapies that reduce sepsis related morbidity and mortality in neonates.
5 Conclusion
The expanding field of molecular research has advanced our understanding of neonatal sepsis to offer novel insights into its diagnosis, pathogenesis, and potential avenues for prevention and treatment. Each branch of molecular science contributes uniquely to the broader picture. Transcriptomic studies enhance diagnostic precision and risk stratification by identifying gene expression signatures associated with sepsis severity and outcomes, such as shock. Microbiome analyses highlight the role of gut dysbiosis in sepsis susceptibility, shedding light on how early microbial colonization, antibiotic exposure, and probiotic use may influence infection risk. Metabolomic studies continue to uncover promising biomarkers for early diagnosis and detection of sepsis-related complications, while proteomic investigations corroborate these findings by identifying altered protein expression patterns with prognostic value. Genomic studies offer potential for personalized prevention strategies, particularly in identifying inherited vulnerabilities, and often reinforce key findings from transcriptomic and metabolomic work. Meanwhile, miRNomic research provides mechanistic insight into post-transcriptional regulation of immune responses, further refining our understanding of neonatal host-pathogen interactions.
Looking ahead, future molecular research methods should focus on distinguishing mechanisms leading to EOS from mechanisms contributing to LOS, an area where current biomarkers lack sufficient specificity. Additionally, greater attention is needed to delineate the cascade of inflammatory processes that unfold during disease progression and to identify biomarkers predictive of long-term complications, such as neuroinflammation. Integration of multi-omics approaches holds particular promise for constructing a comprehensive systems-level model of neonatal sepsis. While significant progress has been made, molecular science still has much to offer in transforming neonatal sepsis care, from more accurate and timely diagnosis to targeted interventions and personalized therapies that improve outcomes for this vulnerable population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CL: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. SZ: Writing – review & editing. SAS: Data curation, Investigation, Writing – original draft. DA: Data curation, Investigation, Writing – original draft. SS: Data curation, Investigation, Writing – original draft. PS: Data curation, Investigation, Writing – original draft. SP: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This manuscript is supported by NIGMS R21GM150136, The Scientific Value of Premature Infant Biospecimens Collection.
Acknowledgments
We thank Dr. Ardis Hanson for her guidance in conducting the literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li J, Shen L, Qian K. Global, regional, and national incidence and mortality of neonatal sepsis and other neonatal infections, 1990–2019. Front Public Health. (2023) 11:1139832. doi: 10.3389/fpubh.2023.1139832
2. Fleischmann C, Reichert F, Cassini A, Horner R, Harder T, Markwart R, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. (2021) 106:8. doi: 10.1136/archdischild-2020-320217
3. Ely DM, Driscoll AK. Infant mortality in United States: provisional data from the 2022 period linked birth/infant death file. In: Gregory ECW, editor. NCHS Vital Statistics Rapid Release Reports. Hyattsville (MD): National Center for Health Statistics (US) (2023). p. 33.
4. Zea-Vera A, Ochoa TJ. Challenges in the diagnosis and management of neonatal sepsis. J Trop Pediatr. (2015) 61:1. doi: 10.1093/tropej/fmu079
5. Oeser C, Pond M, Butcher P, Bedford Russell A, Henneke P, Laing K, et al. PCR For the detection of pathogens in neonatal early onset sepsis. PLoS One. (2020) 15:1. doi: 10.1371/journal.pone.0226817
6. Thaver D, Zaidi AKM. Burden of neonatal infections in developing countries: a review of evidence from community-based studies. Pediatr Infect Dis J. (2009) 28:1. doi: 10.1097/INF.0b013e3181958755
7. Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. (1996) 129:2. doi: 10.1016/s0022-3476(96)70254-8
8. Hayes R, Hartnett J, Semova G, Murray C, Murphy K, Carroll L, et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr Res. (2023) 93:5. doi: 10.1038/s41390-021-01749-3
9. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. (2016) 315:8. doi: 10.1001/jama.2016.0287
10. Hensler E, Petros H, Gray CC, Chung CS, Ayala A, Fallon EA. The neonatal innate immune response to sepsis: checkpoint proteins as novel mediators of this response and as possible therapeutic/diagnostic levers. Front Immunol. (2022) 13:940930. doi: 10.3389/fimmu.2022.940930
11. Raymond SL, Stortz JA, Mira JC, Larson SD, Wynn JL, Moldawer LL. Immunological defects in neonatal sepsis and potential therapeutic approaches. Front Pediatr. (2017) 5:14. doi: 10.3389/fped.2017.00014
12. Delanghe JR, Speeckaert MM. Translational research and biomarkers in neonatal sepsis. Clin Chim Acta. (2015) 451:Pt A. doi: 10.1016/j.cca.2015.01.031
13. Ershad M, Mostafa A, Dela Cruz M, Vearrier D. Neonatal sepsis. Curr Emerg Hosp Med Rep. (2019) 7:3. doi: 10.1007/s40138-019-00188-z
14. Boscarino G, Romano R, Iotti C, Tegoni F, Perrone S, Esposito S. An overview of antibiotic therapy for early- and late-onset neonatal sepsis: current strategies and future prospects. Antibiotics. (2024) 13:3. doi: 10.3390/antibiotics13030250
15. Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol. (2012) 36:6. doi: 10.1053/j.semperi.2012.06.005
16. Cantey JB, Patel SJ. Antimicrobial stewardship in the NICU. Infect Dis Clin North Am. (2014) 28:2. doi: 10.1016/j.idc.2014.01.005
17. Duong QA, Pittet LF, Curtis N, Zimmermann P. Antibiotic exposure and adverse long-term health outcomes in children: a systematic review and meta-analysis. J Infect. (2022) 85:3. doi: 10.1016/j.jinf.2022.01.005
18. Aversa Z, Atkinson EJ, Schafer MJ, Theiler RN, Rocca WA, Blaser MJ, et al. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin Proc. (2021) 96:1. doi: 10.1016/j.mayocp.2020.07.019
19. Zven SE, Susi A, Mitre E, Nylund CM. Association between use of multiple classes of antibiotic in infancy and allergic disease in childhood. JAMA Pediatr. (2020) 174:2. doi: 10.1001/jamapediatrics.2019.4794
20. Miao H, Chen S, Ding R. Evaluation of the molecular mechanisms of sepsis using proteomics. Front Immunol. (2021) 12:733537. doi: 10.3389/fimmu.2021.733537
21. Bjerkhaug AU, Granslo HN, Klingenberg C. Metabolic responses in neonatal sepsis—a systematic review of human metabolomic studies. Acta Paediatr. (2021) 110:8. doi: 10.1111/apa.15874
22. Conti MG, Angelidou A, Diray-Arce J, Smolen KK, Lasky-Su J, De Curtis M, et al. Immunometabolic approaches to prevent, detect, and treat neonatal sepsis. Pediatr Res. (2020) 87:2. doi: 10.1038/s41390-019-0647-6
23. Ng S, Strunk T, Jiang P, Muk T, Sangild PT, Currie A. Precision medicine for neonatal sepsis. Front Mol Biosci. (2018) 5:70. doi: 10.3389/fmolb.2018.00070
24. Gritz EC, Bhandari V. The human neonatal gut microbiome: a brief review. Front Pediatr. (2015) 5:3. doi: 10.3389/fped.2015.00017
25. Dai W, Zhou W. A narrative review of precision medicine in neonatal sepsis: genetic and epigenetic factors associated with disease susceptibility. Transl Pediatr. (2023) 12:4. doi: 10.21037/tp-22-369
26. Lowe R, Shirley N, Bleackley M, Dolan S, Shafee T. Transcriptomics technologies. PLoS Comput Biol. (2017) 13:5. doi: 10.1371/journal.pcbi.1005457
27. Zuo T, Tycko B, Liu TM, Lin HJL, Huang THM. Methods in DNA methylation profiling. Epigenomics. (2009) 1:2. doi: 10.2217/epi.09.31
28. Pammi M, Thapa S, Balderas M, Runge JK, Venkatachalam A, Luna RA. Microbiome signatures in neonatal central line associated bloodstream infections. PLoS One. (2020) 15:1. doi: 10.1371/journal.pone.0227967
29. Lee CC, Feng Y, Yeh YM, Lien R, Chen CL, Zhou YL, et al. Gut dysbiosis, bacterial colonization and translocation, and neonatal sepsis in very-low-birth-weight preterm infants. Front Microbiol. (2021) 12:746111. doi: 10.3389/fmicb.2021.746111
30. Liu J, Li Y, Feng Y, Pan L, Xie Z, Yan Z, et al. Patterned progression of gut microbiota associated with necrotizing enterocolitis and late onset sepsis in preterm infants: a prospective study in a Chinese neonatal intensive care unit. PeerJ. (2019) 7:e7310. doi: 10.7717/peerj.7310
31. Graspeuntner S, Waschina S, Künzel S, Twisselmann N, Rausch TK, Cloppenborg-Schmidt K, et al. Gut dysbiosis with Bacilli dominance and accumulation of fermentation products precedes late-onset sepsis in preterm infants. Clin Infect Dis. (2019) 69:2. doi: 10.1093/cid/ciy882
32. Zhao N, Khamash DF, Koh H, Voskertchian A, Egbert E, Mongodin EF, et al. Low diversity in nasal microbiome associated with Staphylococcus aureus colonization and bloodstream infections in hospitalized neonates. Open Forum Infect Dis. (2021) 8:10. doi: 10.1093/ofid/ofab475
33. Wandro S, Osborne S, Enriquez C, Bixby C, Arrieta A, Whiteson K. The microbiome and metabolome of preterm infant stool are personalized and not driven by health outcomes, including necrotizing enterocolitis and late-onset sepsis. mSphere. (2018) 3:3. doi: 10.1128/mSphere.00104-18
34. Chang CM, Tsai MH, Liao WC, Yang PH, Li SW, Chu SM, et al. Effects of probiotics on gut microbiomes of extremely preterm infants in the neonatal intensive care unit: a prospective cohort study. Nutrients. (2022) 14:15. doi: 10.3390/nu14153239
35. Zhou P, Zhou Y, Liu B, Jin Z, Zhuang X, Dai W, et al. Perinatal antibiotic exposure affects the transmission between maternal and neonatal microbiota and is associated with early-onset sepsis. mSphere. (2020) 5:1. doi: 10.1128/mSphere.00984-19
36. Li YF, Gong XL, Chen SX, Wang K, Jiang YH. Deviations in the gut microbiota of neonates affected by maternal group B Streptococcus colonization. BMC Microbiol. (2021) 21:1. doi: 10.1186/s12866-021-02204-3
37. Mukhopadhyay S, Lee JJ, Hartman E, Woodford E, Dhudasia MB, Mattei LM, et al. Preterm infants at low risk for early-onset sepsis differ in early fecal microbiome assembly. Gut Microbes. (2022) 14:1. doi: 10.1080/19490976.2022.2154091
38. Schwartz DJ, Shalon N, Wardenburg K, DeVeaux A, Wallace MA, Hall-Moore C, et al. Gut pathogen colonization precedes bloodstream infection in the neonatal intensive care unit. Sci Transl Med. (2023) 15:694. doi: 10.1126/scitranslmed.adg5562
39. Lipworth S, Vihta KD, Davies T, Wright S, Tabirao M, Chau K, et al. Molecular epidemiology and antimicrobial resistance phenotype of paediatric bloodstream infections caused by gram-negative bacteria. Commun Med. (2022) 2:1. doi: 10.1038/s43856-022-00161-0
40. Sethi DK, Felgate H, Diaz M, Faust K, Kiy C, Clarke P, et al. Chlorhexidine gluconate usage is associated with antiseptic tolerance in staphylococci from the neonatal intensive care unit. JAC Antimicrob Resist. (2021) 3:4. doi: 10.1093/jacamr/dlab173
41. Ciesielski TH, Zhang X, Tacconelli A, Lutsar I, De Cabre VM, Roilides E, et al. Late-onset neonatal sepsis: genetic differences by sex and involvement of the NOTCH pathway. Pediatr Res. (2023) 93:4. doi: 10.1038/s41390-022-02114-8
42. Meng Y, Cai XH, Wang L. Potential genes and pathways of neonatal sepsis based on functional gene set enrichment analyses. Comput Math Methods Med. (2018) 2018:6708520. doi: 10.1155/2018/6708520
43. Huang SSY, Toufiq M, Eghtesady P, Van Panhuys N, Garand M. The molecular landscape of sepsis severity in infants: enhanced coagulation, innate immunity, and T cell repression. Front Immunol. (2024) 15:1281111. doi: 10.3389/fimmu.2024.1281111
44. Bai Y, Zhao N, Zhang Z, Jia Y, Zhang G, Dong G. Identification and validation of a novel four-gene diagnostic model for neonatal early-onset sepsis with bacterial infection. Eur J Pediatr. (2022) 182:3. doi: 10.1007/s00431-022-04753-9
45. Cernada M, Pinilla-González A, Kuligowski J, Morales JM, Lorente-Pozo S, Piñeiro-Ramos JD, et al. Transcriptome profiles discriminate between gram-positive and gram-negative sepsis in preterm neonates. Pediatr Res. (2022) 91:3. doi: 10.1038/s41390-021-01444-3
46. Serna E, Parra-Llorca A, Panadero J, Vento M, Cernada M. miRNomic signature in very low birth-weight neonates discriminates late-onset gram-positive sepsis from controls. Diagnostics. (2021) 11:8. doi: 10.3390/diagnostics11081389
47. Janec P, Mojžíšek M, Pánek M, Haluzík M, Živný J, Janota J. Early-onset neonatal sepsis: inflammatory biomarkers and MicroRNA as potential diagnostic tools in preterm newborns. Folia Biol. (2023) 69:5–6. doi: 10.14712/fb2023069050173
48. Lorente-Pozo S, Navarrete P, Garzón MJ, Lara-Cantón I, Beltrán-García J, Osca-Verdegal R, et al. DNA methylation analysis to unravel altered genetic pathways underlying early onset and late onset neonatal sepsis. A pilot study. Front Immunol. (2021) 12:622599. doi: 10.3389/fimmu.2021.622599
49. Chatziioannou AC, Wolters JC, Sarafidis K, Thomaidou A, Agakidis C, Govorukhina N, et al. Targeted LC-MS/MS for the evaluation of proteomics biomarkers in the blood of neonates with necrotizing enterocolitis and late-onset sepsis. Anal Bioanal Chem. (2018) 410:27. doi: 10.1007/s00216-018-1320-3
50. Jiang PP, Peng SS, Pankratova S, Luo P, Zhou P, Chen Y. Proteins involved in synaptic plasticity are downregulated in the cerebrospinal fluid of infants with clinical sepsis complicated by neuroinflammation. Front Cell Neurosci. (2022) 16:887212. doi: 10.3389/fncel.2022.887212
51. Frerichs NM, El Manouni El Hassani S, Deianova N, Van Weissenbruch MM, Van Kaam AH, Vijlbrief DC, et al. Fecal volatile metabolomics predict gram-negative late-onset sepsis in preterm infants: a nationwide case-control study. Microorganisms. (2023) 11:3. doi: 10.3390/microorganisms11030572
52. Thomaidou A, Deda O, Begou O, Lioupi A, Kontou A, Gika H, et al. A prospective, case-control study of serum metabolomics in neonates with late-onset sepsis and necrotizing enterocolitis. J Clin Med. (2022) 11:18. doi: 10.3390/jcm11185270
53. Wang L, Cha X, Zhang Z, Qian J. Discrimination of serum metabolomics profiles in infants with sepsis, based on liquid chromatography-mass spectrometer. BMC Infect Dis. (2023) 23:1. doi: 10.1186/s12879-023-07983-w
54. Mardegan V, Giordano G, Stocchero M, Pirillo P, Poloniato G, Donadel E, et al. Untargeted and targeted metabolomic profiling of preterm newborns with EarlyOnset sepsis: a case-control study. Metabolites. (2021) 11:2. doi: 10.3390/metabo11020115
55. Zhang P, Wang Z, Qiu H, Zhou W, Wang M, Cheng G. Machine learning applied to serum and cerebrospinal fluid metabolomes revealed altered arginine metabolism in neonatal sepsis with meningoencephalitis. Comput Struct Biotechnol J. (2021) 19:3284–92. doi: 10.1016/j.csbj.2021.05.024
56. Liu J, Zhang L, Li D, Yu X, Gao Y, Zhou Y. Intestinal metabolomics in premature infants with late-onset sepsis. Sci Rep. (2024) 14:1. doi: 10.1038/s41598-024-55398-7
57. Zhang ZJ, Wu C, Moreira R, Dorantes D, Pappas T, Sundararajan A, et al. Activity of gut-derived nisin-like lantibiotics against human gut pathogens and commensals. ACS Chem Biol. (2024) 19:2. doi: 10.1021/acschembio.3c00577
58. Van Staden ADP, Van Zyl WF, Trindade M, Dicks LMT, Smith C. Therapeutic application of lantibiotics and other lanthipeptides: old and new findings. Appl Environ Microbiol. (2021) 87:14. doi: 10.1128/AEM.00186-21
59. Frenck RW, Ervin J, Chu L, Abbanat D, Spiessens B, Go O, et al. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect Dis. (2019) 6:631–40. doi: 10.1016/S1473-3099(18)30803-X
60. Song WS, Park HW, Oh MY, Jo JY, Kim CY, Lee JJ, et al. Neonatal sepsis-causing bacterial pathogens and outcome of trends of their antimicrobial susceptibility a 20-year period at a neonatal intensive care unit. Clin Exp Pediatr. (2022) 65:7. doi: 10.3345/cep.2021.00668
61. Bhargava A, Gur R, Kaur H, Agarwal P, Maheshwari M, Roy RD. Risk factors and outcome analysis of gram-positive and gram-negative neonatal sepsis: a case-control study. Can J Infect Control. (2017) 32:98–103.
62. Qi Y, Chen X, Wu N, Ma C, Cui X, Liu Z. Identification of risk factors for sepsis-associated mortality by gene expression profiling analysis. Mol Med Report. (2018) 17:4. doi: 10.3892/mmr.2018.8491
63. Wang J, Bai X, Song Q, Fan F, Hu Z, Cheng G, et al. miR-223 inhibits lipid deposition and inflammation by suppressing toll-like receptor 4 signaling in macrophages. Int J Mol Sci. (2015) 16:10. doi: 10.3390/ijms161024965
64. Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, et al. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol. (2010) 185:12. doi: 10.4049/jimmunol.1001573
65. Zhang W, Lu F, Xie Y, Lin Y, Zhao T, Tao S, et al. miR-23b negatively regulates sepsis-induced inflammatory responses by targeting ADAM10 in human THP-1 monocytes. Mediators Inflamm. (2019) 2019:5306541. doi: 10.1155/2019/5306541
66. Li H, Gupta S, Du WW, Yang BB. MicroRNA-17 inhibits tumor growth by stimulating T-cell mediated host immune response. Oncoscience. (2014) 1:7. doi: 10.18632/oncoscience.69
67. Wang X, Wang X, Liu X, Wang X, Xu J, Hou S, et al. miR-15a/16 are upreuglated in the serum of neonatal sepsis patients and inhibit the LPS-induced inflammatory pathway. Int J Clin Exp Med. (2015) 8:5683–90.26131152
68. Alves MJ, Browe BM, Carolina Rodrigues Dias A, Torres JM, Zaza G, Bangudi S, et al. Metabolic trade-offs in neonatal sepsis triggered by TLR4 and TLR1/2 ligands result in unique dysfunctions in neural breathing circuits. Brain Behav Immun. (2024) 119:333–50. doi: 10.1016/j.bbi.2024.03.027
69. Dias ML, O’Connor KM, Dempsey EM, O’Halloran KD, McDonald FB. Targeting the Toll-like receptor pathway as a therapeutic strategy for neonatal infection. Am J Physiol Regul Integr Comp Physiol. (2021) 321:6. doi: 10.1152/ajpregu.00307.2020
70. Li JH, Xiao X, Zhang YN, Wang YM, Feng LM, Wu YM, et al. MicroRNA miR-886-5p inhibits apoptosis by down-regulating Bax expression in human cervical carcinoma cells. Gynecol Oncol. (2011) 120:1. doi: 10.1016/j.ygyno.2010.09.009
71. Zheng G, Qiu G, Ge M, Meng J, Zhang G, Wang J, et al. miR-10a in peripheral blood mononuclear cells is a biomarker for sepsis and has anti-inflammatory function. Mediators Inflamm. (2020) 2020:4370983. doi: 10.1155/2020/4370983
72. Molloy EJ, Wynn JL, Bliss J, Koenig JM, Keij FM, McGovern M, et al. Neonatal sepsis: need for consensus definition, collaboration and core outcomes. Pediatr Res. (2020) 88:1. doi: 10.1038/s41390-020-0850-5
73. Celik IH, Hanna M, Canpolat FE, Pammi M. Diagnosis of neonatal sepsis: the past, present and future. Pediatr Res. (2022) 91:2. doi: 10.1038/s41390-021-01696-z
74. Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. (2009) 158:4. doi: 10.1111/j.1476-5381.2009.00281.x
75. Treede I, Braun A, Sparla R, Kühnel M, Giese T, Turner JR, et al. Anti-inflammatory effects of phosphatidylcholine. J Biol Chem. (2007) 282:37. doi: 10.1074/jbc.M704408200
76. Tang Y, Yu Y, Li R, Tao Z, Zhang L, Wang X, et al. Phenylalanine promotes alveolar macrophage pyroptosis via the activation of CaSR in ARDS. Front Immunol. (2023) 12:14. doi: 10.3389/fimmu.2023.1114129
77. Jiang M, Gong QY, Lai SS, Cheng ZX, Chen ZG, Zheng J, et al. Phenylalanine enhances innate immune response to clear ceftazidime-resistant Vibrio alginolyticus in Danio rerio. Fish Shellfish Immunol. (2019) 84:912–9. doi: 10.1016/j.fsi.2018.10.071
78. Ng S, Strunk T, Lee A, Gill E, Falsafi R, Woodman T, et al. Whole blood transcriptional responses of very preterm infants during late-onset sepsis. PLoS One. (2020) 15:6. doi: 10.1371/journal.pone.0233841
Keywords: genomics, metabolomics, transcriptomics, microbiome, multiomic, neonatal sepsis, neonates
Citation: Lee CY, Zven SE, Sathya SA, Abukhalaf D, Sahoo S, Samal P and Prescott SM (2025) Using molecular methods to diagnose, classify, and treat neonatal sepsis: a scoping review. Front. Pediatr. 13:1625449. doi: 10.3389/fped.2025.1625449
Received: 9 May 2025; Accepted: 13 August 2025;
Published: 25 August 2025.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Andrew Franklin, NorthShore University HealthSystem, United StatesNidhi Sharma, Sawai Man Singh Hospital, India
Copyright: © 2025 Lee, Zven, Sathya, Abukhalaf, Sahoo, Samal and Prescott. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie M. Prescott, c3RlcGhhbmllLnByZXNjb3R0QGlub3ZhLm9yZw==
†These authors have contributed equally to this work
 Catherine Y. Lee
Catherine Y. Lee Sidney E. Zven2
Sidney E. Zven2 Shreyas A. Sathya
Shreyas A. Sathya Danielle Abukhalaf
Danielle Abukhalaf Sneha Sahoo
Sneha Sahoo Pratyusha Samal
Pratyusha Samal Stephanie M. Prescott
Stephanie M. Prescott