- 1Department of Hematology and Oncology, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 2National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3Chongqing Key Laboratory of Child Infection and Immunity, Children’s Hospital of Chongqing Medical University, Chongqing, China
Background: Post-transplant infections are common complications, and the reasons are pre-transplant conditioning, time required for post-transplant immune reconstitution, and use of immunosuppressive agents. We aimed to analyze the risk factors for Epstein–Barr virus (EBV) reactivation and its progression to post-transplant lymphoproliferative disorder (PTLD) following allogeneic hematopoietic stem cell transplantation (allo-HSCT) in children and to determine the EBV PCR diagnostic threshold for PTLD.
Methods: We retrospectively analyzed clinical data of 309 patients who underwent allo-HSCT without engraftment failure at the Children's Hospital of Chongqing Medical University from January 1, 2016, to December 21, 2021. The occurrences of EBV reactivation and PTLD were also recorded. The risk factors for EBV reactivation and progression to PTLD were analyzed, and the diagnostic threshold for PTLD was determined using whole-blood EBV PCR.
Results: Among 309 pediatric patients, 256 experienced EBV reactivation within one year and 12 progressed to PTLD. Univariate and multivariate analyses indicated that ATG was the independent risk factor for EBV reactivation. Grade III-IV acute graft-vs.-host disease (aGVHD) was the risk factor for PTLD after EBV reactivation.
Conclusions: Post-transplant EBV reactivation is a common complication after allo-HSCT, but rarely progresses to PTLD. Identification of the risk factors for PTLD and regular monitoring of the EBV-DNA load play important roles in prevention and cure of PTLD after HSCT.
1 Introduction
Hematopoietic stem cell transplantation (HSCT) is a therapeutic procedure involving the transplantation of hematopoietic stem cells, derived from the bone marrow, peripheral blood, or umbilical cord blood of donors, for partial or entire reconstruction of a recipient's hematopoietic and immune systems. It is an effective treatment for malignant and nonmalignant hematologic diseases. However, due to factors such as pre-transplant conditioning, the time required for post-transplant immune reconstitution, and the use of immunosuppressive agents, infections are common complications of transplantation. Epstein–Barr Virus (EBV), a DNA virus belonging to the γ-herpesvirus family, infects over 90% of the global population (1). After initial infection, EBV establishes a latent infection in memory B cells. In the context of post-transplant immunosuppression, EBV reactivation is a common cause of infection. Moreover, EBV reactivation is associated with various clinical diseases, ranging from simple fever to post-transplant lymphoproliferative disorder (PTLD), characterized by uncontrolled tumor-like proliferation of lymphoid or plasma cells and a high mortality rate (2). In recent decades, the incidence of PTLD has increased due to the growing use of HSCT, introduction of new immunosuppressive agents and treatment regimens, enhanced understanding of PTLD, and improved diagnostic accuracy (3).
The reported incidence of post-transplant EBV reactivation ranges from 13% to 82%, (4–10) and that of post-transplant PTLD varies from 0.79% to 14% (6, 11–16). Previous studies have identified risk factors for EBV reactivation, including use of anti-thymocyte immunoglobulin (ATG), cytomegalovirus viremia, pre-transplant EBV seropositivity, and different types of transplantation (14, 16, 17). Risk factors for PTLD after EBV reactivation include T-cell depletion (ATG and ex vivo T-cell depletion), human leukocyte antigen (HLA)-mismatched transplantation, umbilical cord blood stem cell transplantation, splenectomy before transplantation, severe acute or chronic graft-vs.-host disease, mesenchymal stem cell infusion, concurrent cytomegalovirus (CMV) infection, age, and underlying diseases (2, 3, 7, 11, 13, 18).
Rituximab, a monoclonal anti-CD20 antibody, is effective in treating EBV reactivation and in reducing the risk and mortality of PTLD (12, 13). However, its use may delay B-cell reconstitution and increase the risk of post-transplant infections, and few patients with EBV reactivation progress to PTLD. Therefore, appropriate selection of patients for rituximab treatment is crucial. The sixth European Conference on Infections in Leukemia (ECIL-6) guidelines (2) recommend regular monitoring of the EBV DNA load in transplant recipients.
In this study, we aimed to determine the incidence and outcomes of post-transplant EBV reactivation and PTLD at our center, identify the risk factors, and determine the whole-blood EBV viral load threshold for preemptive rituximab treatment.
2 Materials and methods
2.1 Study participants
This retrospective study included patients aged <18 years who underwent allogeneic hematopoietic stem cell transplantation (allo-HSCT) at the Children's Hospital of Chongqing Medical University between 2016 and 2021 and achieved successful engraftment.
2.2 Definitions and diagnostics
HLA matched was defined as 10/10 matched, HLA mismatched was defined as ≤9/10 matched; EBVemia and CMVemia was defined as whole-blood PCR exceeding 400 copies/ml, EBV reactivation was defined as two consecutive EBV-DNA above 1,000 copies/ml post-transplantation (19). The diagnosis of EBV-associated PTLD was defined as proven or probable according to the published definition. Probable PTLD was defined as significant lymphadenopathy, hepatosplenomegaly, or other end-organ manifestations accompanied by significant EBV DNAemia and the absence of other documented cause. Proven PTLD was defined as the detection of EBV in tissue specimen accompanied by symptoms and/or signs from the affected organ.
2.3 EBV prevention, monitoring, and treatment
Before transplantation, the patients underwent serological tests for EBV antibody screening and whole blood PCR, and antiviral prophylaxis with acyclovir was administered. Routine whole-blood EBV PCR monitoring began first week after transplantation and was continued weekly until discharge. Monitoring was performed biweekly from discharging to 100 days post-transplantation, followed by monthly monitoring for up to one year after transplantation. If the PCR load was observed to be significantly increased in two consecutive measurements or if a high PCR load was observed with fever, immunosuppressive agents were reduced proportionally. If the viral load did not decrease after immunosuppression reduction, and the whole blood EBV viral loads in two consecutive measurements exceeded 106 copies/ml, preemptive rituximab treatment was considered. For patients clinically diagnosed with PTLD, rituximab was administered at 375 mg/m2 weekly for one to four weeks.
2.4 Statistical analysis
Data analysis was performed using SPSS [Computer Software]. Version 25.0. Armonk, NY: IBM Corp; 2017. The cumulative incidence of EBV infection was determined using the Kaplan–Meier method. Categorical data are presented as counts (percentages). Group comparisons were performed using the chi-squared test or Fisher's exact test. Nonparametric tests were conducted for between-group comparisons of continuous data. Variables with P values <0.05 in univariate analysis were included in the multivariate analysis, and logistic regression was employed to identify independent risk factors for EBV reactivation and progression to PTLD. Statistical significance was set at P < 0.05. Receiver operating characteristic (ROC) curves were used to determine the optimal cutoff point for EBV load for diagnosing PTLD.
3 Results
3.1 Patient and transplantation characteristics
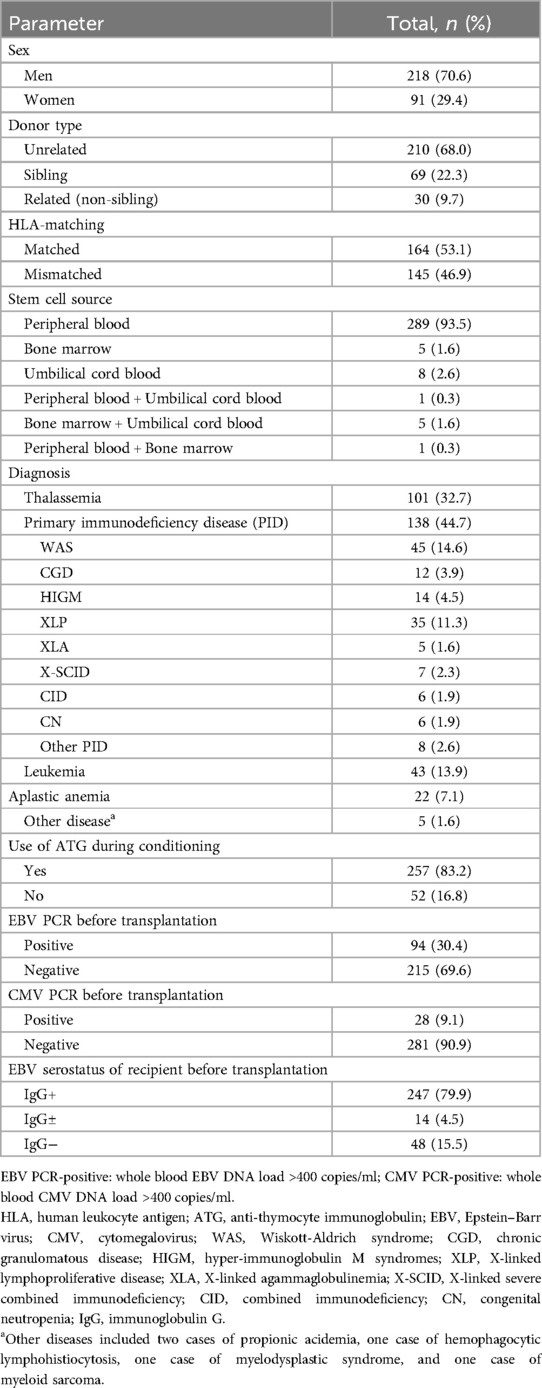
A total of 309 pediatric patients were included in the study, with a median age of three years (range, 0.3–14.8 years). The clinical characteristics of the 309 patients are summarized in Table 1. All patients receive a prophylactic regimen for GVHD based on cyclosporine, in combination with Mycophenolate Mofetil and/or methotrexate.
3.2 EBV reactivation after transplantation
Among the 309 patients, 256 (82.8%) experienced EBV reactivation within a year post-transplantation, and the median time to the first reactivation was 20 days (range, 3–244 days) after transplantation. The cumulative incidence in the first year is shown in Figure 1. The median initial EBV value at the time of reactivation was 7.18 × 103 copies/ml (range, 1.01 × 103–3.79 × 106 copies/ml). The median time to achievement of peak EBV-DNA levels was 55 days (range, 10–548 days), with a median peak value of 1.53 × 105 copies/ml (range, 2.14 × 103–7.38 × 109 copies/ml).
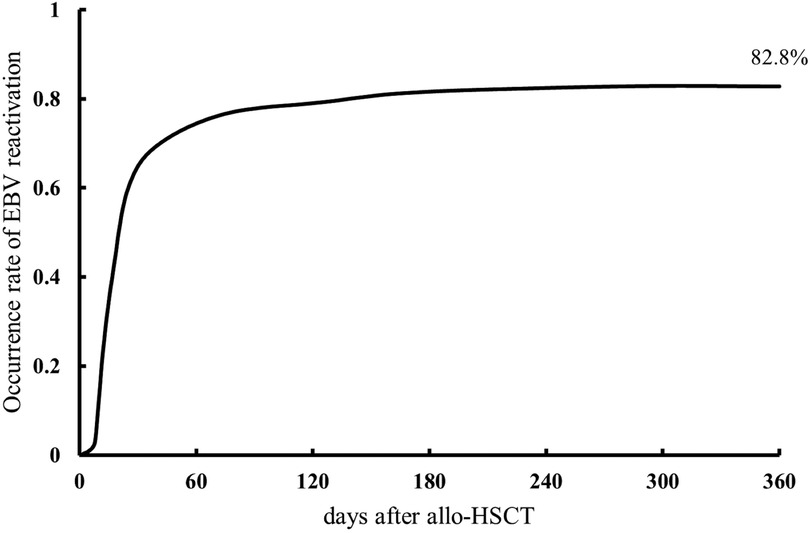
Figure 1. Cumulative incidence of Epstein–Barr virus (EBV) reactivation within one year of allogeneic hematopoietic stem cell transplantation.
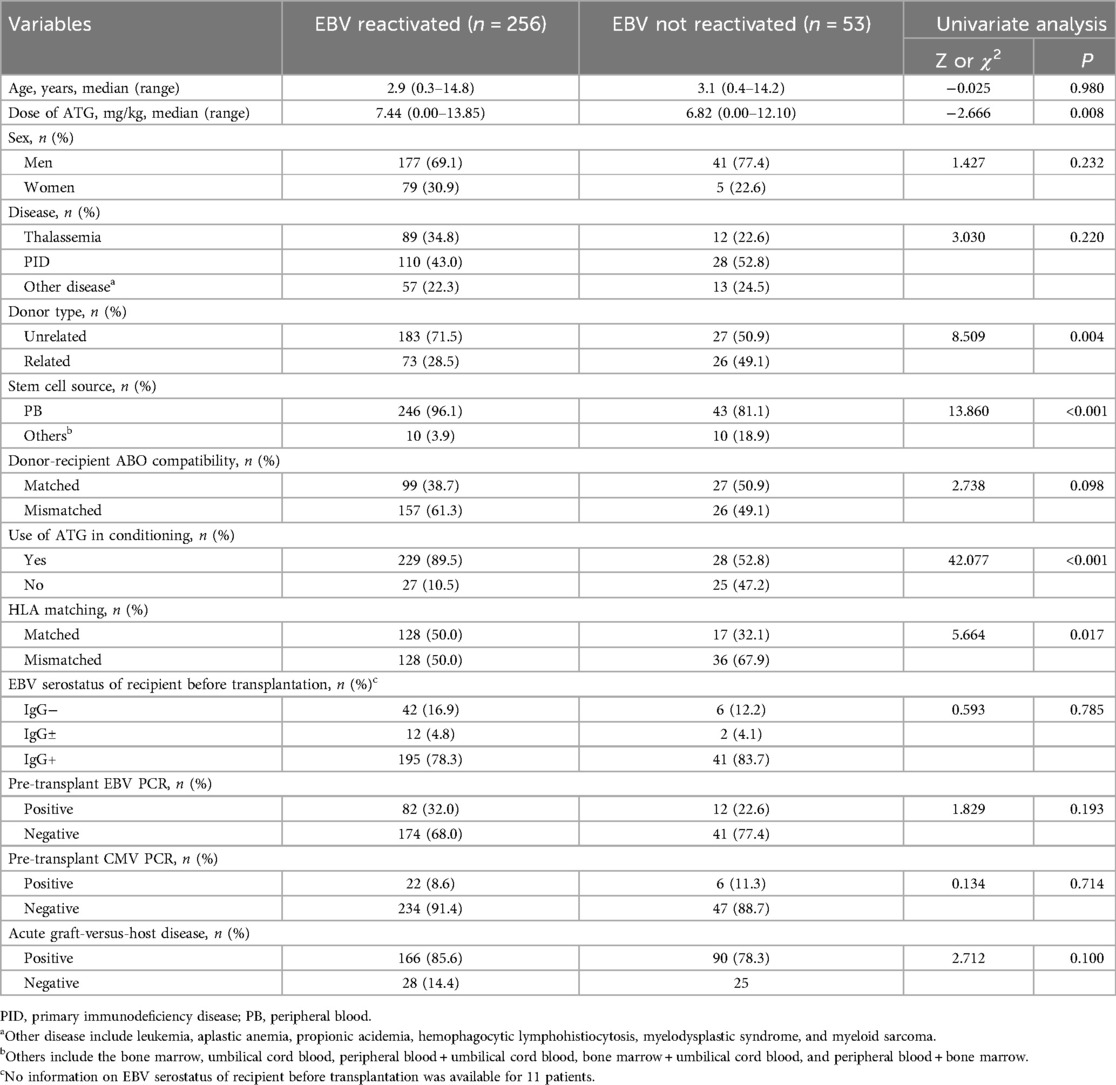
3.3 Analysis of risk factors for EBV reactivation
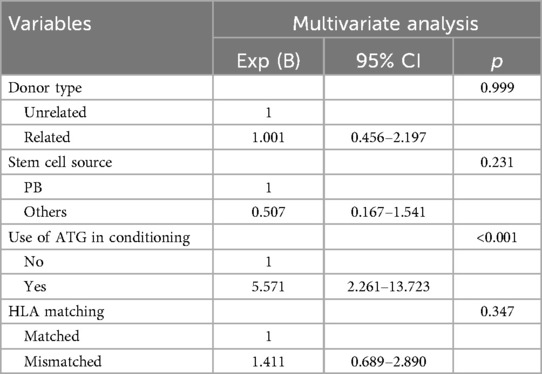
Univariate analysis identified risk factors for EBV reactivation, with variables exhibiting p-values < 0.05 advanced to multivariate analysis. As shown in Table 2, statistically significant variables in univariate analysis included donor type, stem cell source, ATG use in conditioning, HLA matching, and ATG dosage. In contrast, other conditioning agents—including busulfan, cyclophosphamide, fludarabine, acyclovir, thiotepa, and etoposide—showed no statistical significance. Given that ATG use and ATG dosage represent distinct data types for the same variable, both were included separately in the multivariate model. The final model with superior goodness-of-fit was selected using the Hosmer-Lemeshow test (Table 3), revealing ATG use (OR = 5.571, p < 0.001) as an independent risk factor.
3.4 Incidence of PTLD after EBV reactivation
Among the 256 post-transplant patients who exhibited EBV reactivation, 11 were diagnosed with probable PTLD, and 1 with proven PTLD. A summary of their clinical data is provided in the Supplementary Material. The median time to PTLD diagnosis post-transplantation was 66 days (range, 23–272 days). The whole-blood EBV PCR monitoring results for these 12 patients are depicted in Figure 2. The median time to EBV reactivation in the PTLD group was 20 days (range, 11–37 days), which was not significantly different from the non-PTLD group, with a median time of 20 days (range, 3–244 days; P = 0.444). Similarly, the median time to reach peak EBV load was not statistically different between the PTLD group (54 days; range, 18–271 days) and the non-PTLD group (55 days; range, 10–548 days; P = 0.986). However, there was a significant difference in the peak EBV levels detected by PCR between the PTLD and non-PTLD groups. The PTLD group exhibited peak EBV levels of 3.01 × 107 copies/ml (range, 6.99 × 104–7.38 × 109 copies/ml), whereas the non-PTLD group showed peak levels of 1.45 × 105 copies/ml (range, 2.14 × 103–4.66 × 108 copies/ml; P < 0.001). Additionally, there was a statistically significant difference in the blood lactic dehydrogenase levels at the time of peak EBV PCR between the non-PTLD and PTLD groups (Z = −2.702, P = 0.007).
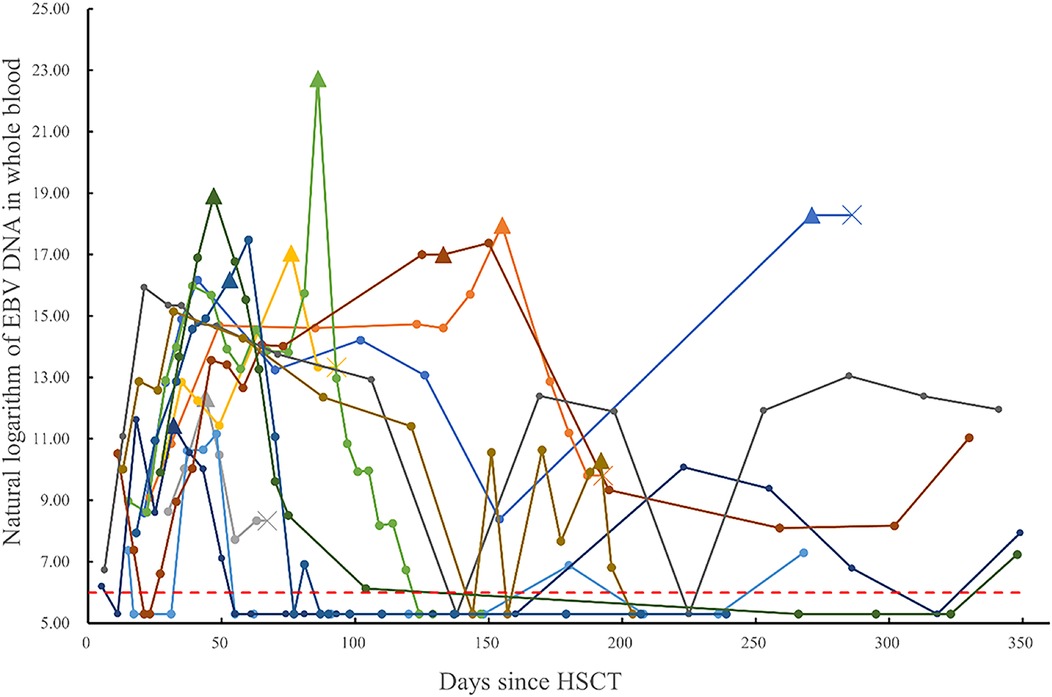
Figure 2. Monitoring of post-transplant whole blood EBV-DNA (natural logarithm) in patients diagnosed with PTLD (data points below the detection threshold are recorded as natural logarithm of 200 copies/ml. Different colored lines represent different patients, indicates the status of each monitoring point, ×indicates the death of the patient, ▴ represents the nearest monitoring point before Rituximab.
3.5 Analysis of risk factors for PTLD occurrence after EBV reactivation
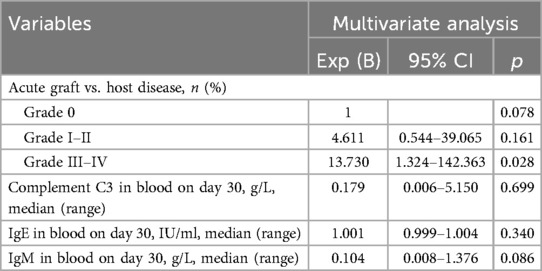
Outcomes of univariate and multivariate analyses for PTLD incidence are presented in Tables 4, 5, respectively. Variables not listed in Table 4—including alternative conditioning regimens, hematopoietic recovery status, GVHD prophylaxis, lymphocyte subpopulation counts, absolute lymphocyte counts, and serial measurements of immunoglobulins (IgM, IgA, IgG), complements (C3, C4), and blood LDH at reactivation—showed no significant association with PTLD in univariate analysis. Subsequent univariate analysis identified grade III–IV aGVHD, day-30 blood C3, day-30 IgE, day-30 IgM, and day-100 absolute/relative lymphocyte counts as significant risk factors (p < 0.05). Due to the strong correlation between CD19 + lymphocyte counts and rituximab use, we excluded patients receiving rituximab within 100 days post-transplant. This revealed no significant differences in day-100 CD19 + counts between groups. We therefore derived highest absolute/relative CD19 + counts within 100 days as new variables, but these likewise showed no intergroup differences. Multivariate analysis (Table 5) confirmed only grade III–IV aGVHD as an independent risk factor for PTLD following EBV reactivation (OR = 13.730, p = 0.028). In our study, 21 patients received rituximab, among whom 11 were diagnosed with PTLD. Six patients received 1–4 doses of rituximab at a median of 52 days (range, 31–171 days) due to significant EBVemia, while 3 patients were treated for autoimmune hemolytic anemia and 1 for immune thrombocytopenia.
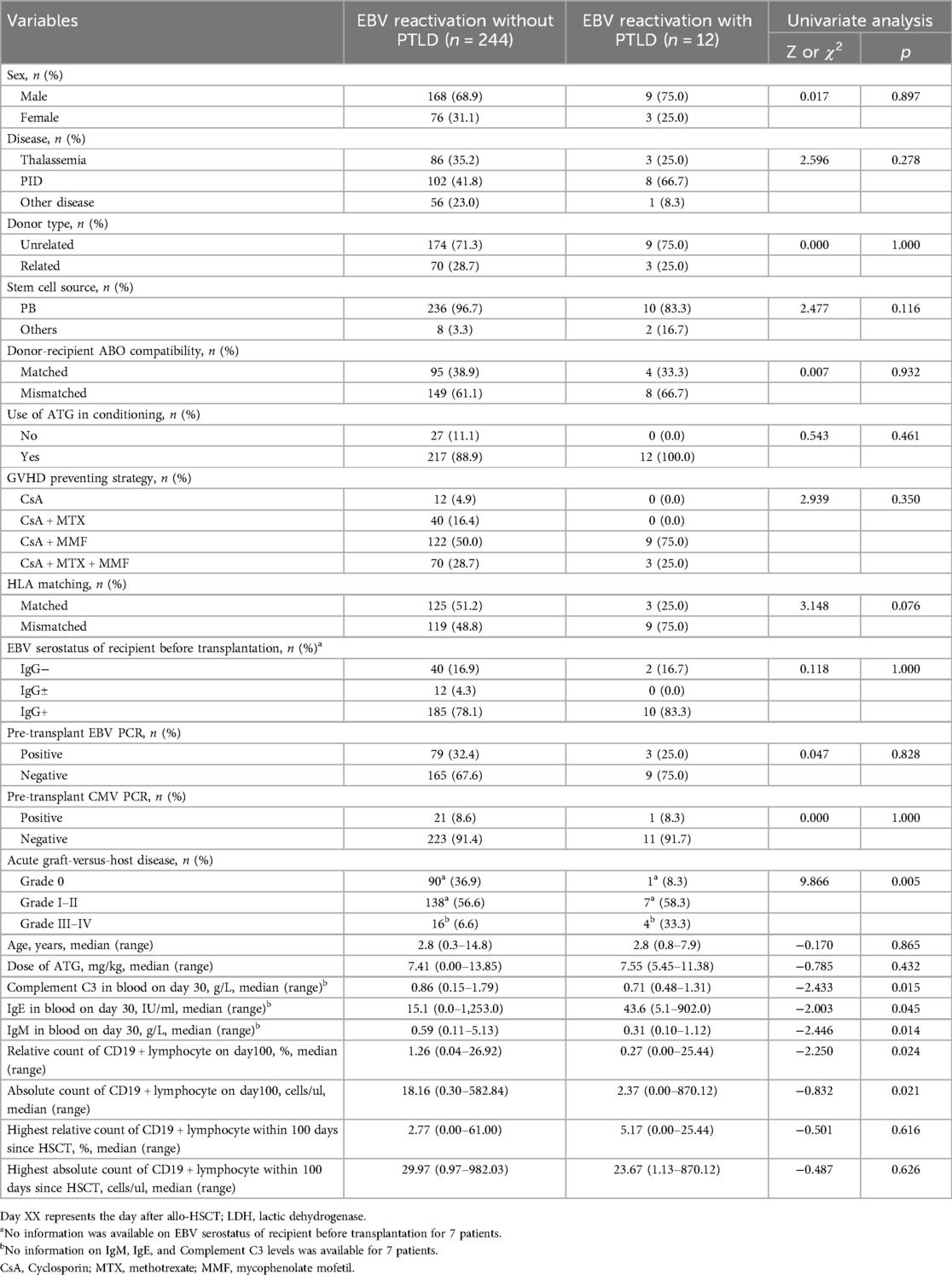
Table 4. Univariate analysis of risk factors for progression to post-transplant lymphoproliferative disorder (PTLD) after EBV reactivation.
3.6 Diagnostic value of whole-blood EBV PCR for PTLD
The ROC curves were analyzed to determine the optimal threshold for diagnosing PTLD based on the maximum EBV viral DNA load; the results are shown in Figure 3. This analysis demonstrated that when the whole-blood EBV load was 3.755 × 106 copies/ml, the area under the ROC curve was largest (0.864), with a sensitivity of 0.75 and specificity of 0.934.
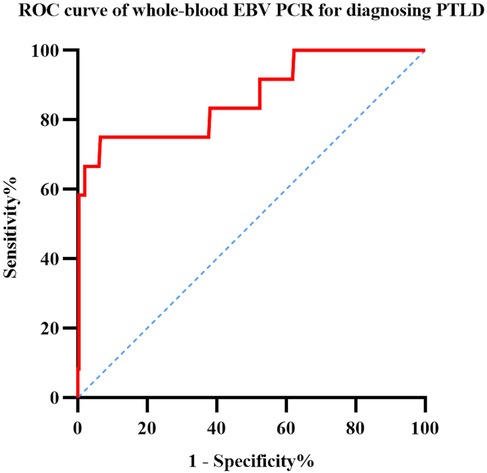
Figure 3. ROC curve analysis of sensitivity and specificity for diagnosing PTLD based on whole-blood EBV PCR.
3.7 The effect of ATG on T cell immune reconstitution after transplantation
In accordance with the application of ATG in conditioning regimens, participants were categorized into two distinct cohorts: the non-ATG usage group and the ATG group. A comparative analysis of T cell counts between these two groups was conducted on days 15, 30, 100, and 180, with the findings presented in Table 6. Notably, significant differences in the absolute counts of CD3+, CD8+, and CD4+ T cells were observed between the ATG group and the non-usage group on day 15. Subsequently, from day 30 to day 180, no significant differences were detected in the absolute counts of CD3+ T cells and CD8+ T cells between the two cohorts. In contrast, a statistically significant disparity in CD4+ T cell counts persisted between the groups throughout this period.
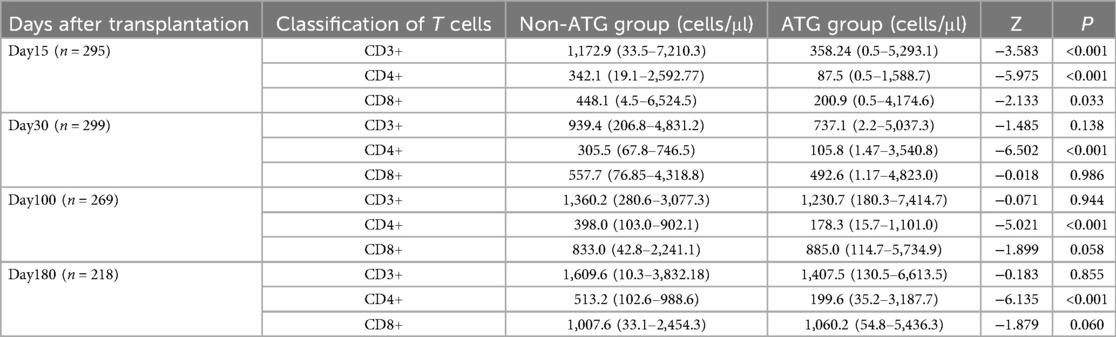
Table 6. The results of T cell reconstruction at different time after transplantation using ATG or not.
3.8 Analysis of dynamic parameters of whole-blood EBV load for predicting PTLD occurrence
The dynamic parameters of viral DNA, such as doubling time, have been shown to assist in the management of CMV infections in allogeneic HSCT (20). According to a study by Solano et al. (6), dynamic parameter analysis of the plasma EBV viral load might not predict the occurrence of PTLD. They speculated that whole-blood EBV viral load may have some significance. We assessed our center's whole-blood EBV monitoring results using the following formula: doubling time (dt) = (t2—t1) × log (2)/log (q2/q1), where q1 and t1 are the EBV DNA loads (copies/ml) at the time of the first positive PCR (in days), and q2 and t2 are the EBV DNA loads at the time of the second positive PCR. The time interval between t1 and t2 was less than 10 days, and an increase in the EBV DNA load by at least three or more times was considered relevant and used to evaluate dt. Among 289 EBVemia patients, excluding 13 children for whom calculations were not possible, a statistical difference was observed in the occurrence of EBV doubling between the PTLD and non-PTLD groups (χ2 = 5.735; P = 0.017), but no statistical differences were observed in the doubling time (dt) between the two groups (Z = −0.74; P = 0.459).
3.9 Follow-up of pediatric patients who underwent transplantation
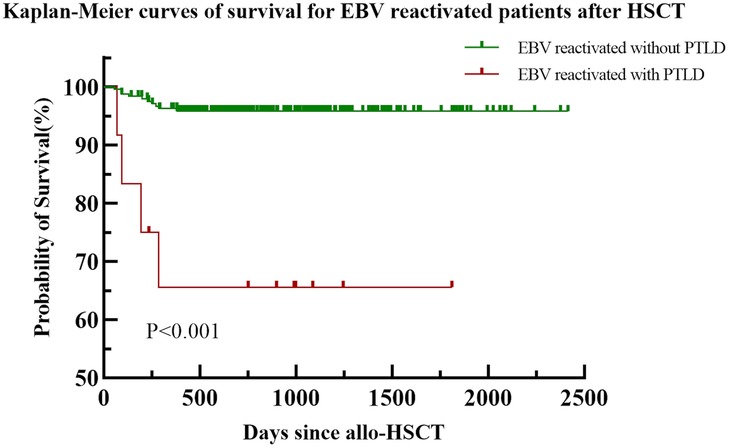
The median follow-up period of the 309 pediatric patients was 750 days (range, 53–2414 days). Sixteen pediatric patients died, with a median time to death of 196 days (range, 53–850 days). Common causes of death after transplantation included infection-related complications (11 cases; 68.75%), severe gastrointestinal GVHD (2 cases; 12.5%), intracranial hemorrhage (1 case; 6.25%), bronchiolitis obliterans (1 case; 6.25%), and immune cytopenia (1 case; 6.25%). The follow-up status of the 256 patients with EBV reactivation is shown in Figure 4, There is a significant statistical difference in survival with PTLD and without PTLD (P < 0.001). Among the 12 patients with PTLD, eight children survived, the clinical information of the four deceased children with PTLD is shown in Supplementary Material.
4 Discussion
In this study, we have reported the incidence of EBV reactivation and PTLD in pediatric recipients of allogeneic HSCT. We analyzed the risk factors for EBV reactivation in 309 pediatric patients and those for progression to PTLD in 256 patients with EBV reactivation after transplantation. We found that ATG was the independent risk factor for EBV reactivation after transplantation. The independent risk factor for PTLD after EBV reactivation was grade III–IV aGVHD. Using the whole-blood EBV DNA load for PTLD diagnosis, 3.755 × 106 copies/ml was determined to be the optimal threshold having the highest area under the ROC curve, indicating high specificity, which served as a suggestive threshold for initiating rituximab treatment at our center.
Our center observed a moderately elevated EBV reactivation rate compared to other transplant centers. This discrepancy may stem from inter-institutional variations in diagnostic criteria, specimen types (whole blood vs. serum/plasma), and detection thresholds. Whole blood EBV PCR demonstrates higher clinical sensitivity than plasma/serum testing—a critical methodological consideration (21, 22). Additionally, as delineated in established guidelines (2) and corroborated by other studies (14, 23, 24), as well as our own research findings, the use of anti-thymocyte globulin (ATG) emerges as an independent risk factor for EBV reactivation following transplantation. ATG administration results in the depletion of CD8+ T cells, which are pivotal in the suppression of viral reactivation. Our data reveal that the absolute count of CD8+ T cells on day 15 is markedly higher in patients who did not receive ATG as part of their conditioning regimen compared to those who did (Z = −2.133, P = 0.033). In conclusion, given that the majority of stem cell grafts at our center are derived from unrelated donors, the utilization rate of ATG is notably high at 83.2%. This contributes to the elevated incidence and rapid onset of EBV reactivation observed in our patient population.
The guidelines on PTLD (2) propose that T cell depletion in vivo or in vitro is a risk factor for PTLD. However, our research shows that the application of ATG has no significant impact on the progression of PTLD after EBV reactivation. Firstly, this may be due to the collinearity between EBV reactivation and PTLD after hematopoietic stem cell transplantation, which may affect the analysis of risk factors. Additionally, this may be due to the rapid reconstruction of CD8+ T cells after HSCT (25), which precedes EBV carcinogenic proliferation; The median occurrence time of PTLD is generally 2–4 months after transplantation (2). In our center, the median occurrence time of PTLD is 66 days after transplantation. Although our data shows a statistically significant difference in CD8+ T cell absolute counts between the ATG group and the non ATG group on day 15, there is no statistically significant difference in CD8+ T cell absolute count on day 30 between the two groups (Z = −0.018, P = 0.986), indicating that the use of ATG before the occurrence of PTLD has no significant effect on CD8+ T cell reconstruction. In addition, the use of ATG can reduce the incidence of acute and chronic GVHD (26), and the reduction of immunosuppressive therapy after transplantation is beneficial for immune monitoring and immune defense of the body after transplantation.
In our study, we observed a high incidence of EBV reactivation coupled with a surprisingly low rate of PTLD. Consequently, we conducted a thorough analysis of the factors associated with PTLD progression following EBV reactivation and synthesized these findings with the outcomes of post-transplant immune reconstitution. The development of grade III–IV aGVHD requires intensive immunosuppression, which may precipitate a proinflammatory cytokine storm capable of impairing targeted immune reconstitution and thus creating a conducive environment for PTLD development. Striking a balance between viral reactivation and the intensity of GVHD poses a significant therapeutic challenge. We noted that PTLD patients exhibit elevated LDH levels at the zenith of EBV-DNA load, a phenomenon attributed to the characteristic uncontrolled proliferation of highly motile malignant cells. While elevated LDH levels are a common feature in PTLD following solid organ transplantation (27); however, further research is required to determine the diagnostic value of elevated LDH levels for PTLD after HSCT.
A study by the Institute of Hematology at Peking University proposed that a low absolute count of CD8+ T cells on the 30th day is a risk factor for PTLD (28). However, in our study, the absolute and relative counts of lymphocytes on the 15th and 30th days did not show statistical significance in progressing to PTLD after EBV reactivation. This may be due to the fact that our transplant population is children, and due to differences in thymic function compared to adults, the influence of age on T cell reconstruction after hematopoietic stem cell transplantation cannot be ignored. Another study by the Institute of Hematology at Peking University (29) showed that the T cell subset of CD4-CD8- may interact with EBV reactivation. At 30, 90, and 180 days after haploid transplantation, the absolute count of CD4-CD8-T cells in EBV recipients was significantly lower than that in non EBV recipients. Burns’ study (30) showed that in patients with high EBV load, the proportion and quantity of CD27+ memory B cells significantly increased, expressing EBV latent transcripts with plasma cell phenotype associated with B cell growth and transformation, and frequently expressing proliferation marker Ki-67. EBV reactivation may drive the expansion of CD27+ B lymphoblasts with latent infection in peripheral blood. From the above research, it can be seen that the relationship between EBV reactivation, PTLD, and immune reconstitution is complex and not yet fully understood. Our study may require further research on the effects of immune reconstitution on EBV reactivation and progression to PTLD. Perhaps more accurate lymphocyte classification tests can yield richer results.
Due to the serious impact of PTLD on the survival prognosis of transplanted children, early identification and diagnosis are very important. Compared with pathological biopsy, monitoring the EBV-DNA load after transplantation is relatively easy and less traumatic. However, currently there is no unified test standard, and the recommended treatment thresholds vary from center to center. However, the combination of rituximab treatment and immunosuppressive therapy is currently the first-line treatment for PTLD, and the prognosis of about 70% of PTLD patients can be improved (2). Based on the results of ROC curve analysis, we recommend rituximab intervention if the whole-blood EBV load reaches 3.755 × 106 copies/ml; similar to that observed in Solano's study (6) on the plasma EBV load, our findings showed that the doubling time of the whole-blood EBV load was not a significant predictive factor for PTLD.
Our study was limited by its single-center retrospective design. Further multicenter prospective studies are needed to validate the generalizability and reproducibility of the results. Multiple studies identify donor-recipient EBV serological mismatch as a significant risk factor for PTLD, with the EBV-seronegative recipient/EBV-seropositive donor combination conferring the highest risk (2, 11, 31), Mechanistically, donor EBV IgG positivity reflects latent infection, while recipient EBV IgG negativity indicates absent protective antibodies. Under post-transplant immunosuppression, this seromismatch potentiates uncontrolled EBV proliferation. However, our dataset lacked donor EBV serostatus because routine EBV serological testing was not included in donor screening protocols by the China Marrow Donor Program during the study period.
In conclusion, our study is limited by its retrospective a single-center design, but it helps to identify the patients have the risk for progression to PTLD after EBV reactivation since the incidence rate of PTLD is not high. While no consensus has been reached regarding the optimal sample type or EBV PCR threshold for initiating rituximab treatment. Our study gives the threshold of whole-blood EBV-DNA for rituximab treatment. Considering the high mortality of PTLD, it could be of great interest to study the risk factors for PTLD and threshold for rituximab treatment in a larger and multi-center cohort with unified standards of EBV PCR. Finally, regular monitoring of EBV PCR plays an important role after hematopoietic stem cell transplantation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of Children’s Hospital, Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LJ: Data curation, Investigation, Writing – original draft. HY: Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. YM: Writing – review & editing. LZ: Writing – review & editing. XL: Writing – review & editing. XG: Writing – review & editing. JY: Writing – review & editing. YD: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by funds from the Program for Youth Innovation in Future Medicine, Chongqing Medical University (grant No. W0132) and Chongqing Natural Science Foundation (CSTB2023NSCQ-MSX0212).
Acknowledgments
We thank the medical staff of the Department of Hematology and Oncology, Children’s Hospital of Chongqing Medical University, for their support with data collection for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1627990/full#supplementary-material
References
1. Chang CM, Yu KJ, Mbulaiteye SM, Hildesheim A, Bhatia K. The extent of genetic diversity of Epstein–Barr virus and its geographic and disease patterns: a need for reappraisal. Virus Res. (2009) 143(2):209–21. doi: 10.1016/j.virusres.2009.07.005
2. Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, et al. Management of Epstein–Barr virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: sixth European conference on infections in leukemia (ECIL-6) guidelines. Haematologica. (2016) 101(7):803–11. doi: 10.3324/haematol.2016.144428
3. Fujimoto A, Hiramoto N, Yamasaki S, Inamoto Y, Uchida N, Maeda T, et al. Risk factors and predictive scoring system for post-transplant lymphoproliferative disorder after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25(7):1441–9. doi: 10.1016/j.bbmt.2019.02.016
4. Kołodziejczak M, Gil L, de la Camara R, Styczyński J. Impact of donor and recipient Epstein–Barr virus serostatus on outcomes of allogeneic hematopoietic cell transplantation: a systematic review and meta-analysis. Ann Hematol. (2021) 100(3):763–77. doi: 10.1007/s00277-021-04428-9
5. Kang HM, Kim SK, Lee JW, Chung NG, Cho B. Efficacy of low dose antithymocyte globulin on overall survival, relapse rate, and infectious complications following allogeneic peripheral blood stem cell transplantation for leukemia in children. Bone Marrow Transplant. (2021) 56(4):890–9. doi: 10.1038/s41409-020-01121-9
6. Solano C, Mateo EM, Pérez A, Talaya A, Terol MJ, Albert E, et al. Epstein–Barr virus DNA load kinetics analysis in allogeneic hematopoietic stem cell transplant recipients: is it of any clinical usefulness? J Clin Virol. (2017) 97:26–32. doi: 10.1016/j.jcv.2017.10.016
7. Wareham NE, Mocroft A, Sengeløv H, Da Cunha-Bang C, Gustafsson F, Heilmann C, et al. The value of EBV DNA in early detection of post-transplant lymphoproliferative disorders among solid organ and hematopoietic stem cell transplant recipients. J Cancer Res Clin Oncol. (2018) 144(8):1569–80. doi: 10.1007/s00432-018-2674-9
8. Wang H, Zhang TT, Qi JQ, Chu TT, Miao M, Qiu HY, et al. Incidence, risk factors, and clinical significance of Epstein–Barr virus reactivation in myelodysplastic syndrome after allogeneic haematopoietic stem cell transplantation. Ann Hematol. (2019) 98(4):987–96. doi: 10.1007/s00277-019-03603-3
9. Ru Y, Zhang X, Song T, Ding Y, Zhu Z, Fan Y, et al. Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation: multifactorial impact on transplant outcomes. Bone Marrow Transplant. (2020) 55(9):1754–62. doi: 10.1038/s41409-020-0831-7
10. Kalra A, Roessner C, Jupp J, Williamson T, Tellier R, Chaudhry A, et al. Epstein-Barr virus DNAemia monitoring for the management of post-transplant lymphoproliferative disorder. Cytotherapy. (2018) 20(5):706–14. doi: 10.1016/j.jcyt.2018.02.367
11. Uhlin M, Wikell H, Sundin M, Blennow O, Maeurer M, Ringden O, et al. Risk factors for Epstein–Barr virus-related post-transplant lymphoproliferative disease after allogeneic hematopoietic stem cell transplantation. Haematologica. (2014) 99(2):346–52. doi: 10.3324/haematol.2013.087338
12. Stocker N, Labopin M, Boussen I, Paccoud O, Bonnin A, Malard F, et al. Pre-emptive rituximab treatment for Epstein–Barr virus reactivation after allogeneic hematopoietic stem cell transplantation is a worthwhile strategy in high-risk recipients: a comparative study for immune recovery and clinical outcomes. Bone Marrow Transplant. (2020) 55(3):586–94. doi: 10.1038/s41409-019-0699-6
13. Kinch A, Hallböök H, Arvidson J, Sällström K, Bondeson K, Pauksens K. Long-term outcome of Epstein–Barr virus DNAemia and PTLD with the use of preemptive rituximab following allogeneic HSCT. Leuk Lymphoma. (2018) 59(5):1172–9. doi: 10.1080/10428194.2017.1365860
14. Gao XN, Lin J, Wang LJ, Li F, Li HH, Wang SH, et al. Risk factors and clinical outcomes of Epstein–Barr virus DNAemia and post-transplant lymphoproliferative disorders after haploidentical and matched-sibling PBSCT in patients with hematologic malignancies. Ann Hematol. (2019) 98(9):2163–77. doi: 10.1007/s00277-019-03742-7
15. Salas MQ, Prem S, Remberger M, Lam W, Kim DDH, Michelis FV, et al. High incidence but low mortality of EBV-reactivation and PTLD after alloHCT using ATG and PTCy for GVHD prophylaxis. Leuk Lymphoma. (2020) 61(13):3198–208. doi: 10.1080/10428194.2020.1797010
16. Lindsay J, Othman J, Yong MK, Ritchie D, Chee L, Tay K, et al. Dynamics of Epstein–Barr virus on post-transplant lymphoproliferative disorders after antithymocyte globulin-conditioned allogeneic hematopoietic cell transplant. Transplant Infect Dis. (2021) 23(5):e13719. doi: 10.1111/tid.13719
17. Kania SP, Silva JMF, Charles OJ, Booth J, Cheung SYA, Yates JWT, et al. Epstein–Barr virus reactivation after paediatric haematopoietic stem cell transplantation: risk factors and sensitivity analysis of mathematical model. Front Immunol. (2022) 13:903063. doi: 10.3389/fimmu.2022.903063
18. Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. (2009) 113(20):4992–5001. doi: 10.1182/blood-2008-09-178046
19. Liu J, Bian Z, Wang X, Xu LP, Fu Q, Wang C, et al. Inverse correlation of Vδ2(+) T-cell recovery with EBV reactivation after haematopoietic stem cell transplantation. Br J Haematol. (2018) 180(2):276–85. doi: 10.1111/bjh.15037
20. Giménez E, Muñoz-Cobo B, Solano C, Amat P, Navarro D. Early kinetics of plasma cytomegalovirus DNA load in allogeneic stem cell transplant recipients in the era of highly sensitive real-time PCR assays: does it have any clinical value? J Clin Microbiol. (2014) 52(2):654–6. doi: 10.1128/JCM.02571-13
21. Rzepka M, Depka D, Gospodarek-Komkowska E, Bogiel T. Diagnostic value of whole-blood and plasma samples in Epstein–Barr virus infections. Diagnostics (Basel). (2023) 13(3):476. doi: 10.3390/diagnostics13030476
22. Gao W, Wu J, Han Y, Fu X. Study on diagnostic value of results of EB virus nucleic acid quantification in whole blood lymphocyte and serum in children with EB virus infection. Cytokine. (2022) 155:155902. doi: 10.1016/j.cyto.2022.155902
23. Massoud R, Gagelmann N, Fritzsche-Friedland U, Zeck G, Heidenreich S, Wolschke C, et al. Comparison of immune reconstitution between anti-T-lymphocyte globulin and posttransplant cyclophosphamide as acute graft-versus-host disease prophylaxis in allogeneic myeloablative peripheral blood stem cell transplantation. Haematologica. (2022) 107(4):857–67. doi: 10.3324/haematol.2020.271445
24. Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. (2016) 17(2):164–73. doi: 10.1016/S1470-2045(15)00462-3
25. Minculescu L, Marquart HV, Ryder LP, Andersen NS, Schjoedt I, Friis LS, et al. Improved overall survival, relapse-free-survival, and less graft-vs.-host-disease in patients with high immune reconstitution of TCR gamma Delta cells 2 months after allogeneic stem cell transplantation. Front Immunol. (2019) 10:1997. doi: 10.3389/fimmu.2019.01997
26. Chang YJ, Wu DP, Lai YR, Liu QF, Sun YQ, Hu J, et al. Antithymocyte globulin for matched sibling donor transplantation in patients with hematologic malignancies: a multicenter, open-label, randomized controlled study. J Clin Oncol. (2020) 38(29):3367–76. doi: 10.1200/JCO.20.00150
27. Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. (2009) 27(20):3354–62. doi: 10.1200/JCO.2008.20.0857
28. Xu LP, Zhang CL, Mo XD, Zhang XH, Chen H, Han W, et al. Epstein–Barr virus-related post-transplantation lymphoproliferative disorder after unmanipulated human leukocyte antigen haploidentical hematopoietic stem cell transplantation: incidence, risk factors, treatment, and clinical outcomes. Biol Blood Marrow Transplant. (2015) 21(12):2185–91. doi: 10.1016/j.bbmt.2015.07.035
29. Bian Z, Liu J, Xu LP, Chang YJ, Wang Y, Zhang XH, et al. Association of Epstein–Barr virus reactivation with the recovery of CD4/CD8 double-negative T lymphocytes after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. (2017) 52(2):264–9. doi: 10.1038/bmt.2016.238
30. Burns DM, Tierney R, Shannon-Lowe C, Croudace J, Inman C, Abbotts B, et al. Memory B-cell reconstitution following allogeneic hematopoietic stem cell transplantation is an EBV-associated transformation event. Blood. (2015) 126(25):2665–75. doi: 10.1182/blood-2015-08-665000
Keywords: allogeneic hematopoietic stem cell transplantation, pediatric, Epstein–Barr virus (EBV), reactivation, post-transplant lymphoproliferative disorder (PTLD)
Citation: Jia L, Yang H, Zhou Y, Meng Y, Zhang L, Lei X, Guan X, Yu J and Dou Y (2025) Analysis of risk factors for Epstein–Barr virus reactivation and progression to post-transplant lymphoproliferative disorder in pediatric patients undergoing allogeneic hematopoietic stem cell transplantation. Front. Pediatr. 13:1627990. doi: 10.3389/fped.2025.1627990
Received: 13 May 2025; Accepted: 1 July 2025;
Published: 17 July 2025.
Edited by:
Ren Lin, Southern Medical University, ChinaReviewed by:
Dao Wang, First Affiliated Hospital of Zhengzhou University, ChinaXu-Ying Pei, Peking University People’s Hospital, China
Copyright: © 2025 Jia, Yang, Zhou, Meng, Zhang, Lei, Guan, Yu and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Dou, ZG91eWluZzUyM0BhbGl5dW4uY29t
 Lanzhou Jia
Lanzhou Jia Hui Yang1,2,3
Hui Yang1,2,3 Ya Zhou
Ya Zhou Jie Yu
Jie Yu Ying Dou
Ying Dou