- 1Department of Neonatology, Tianjin Central Hospital of Obstetrics and Gynecology, Tianjin, China
- 2Department of Neonatology, Nankai University Affiliated Maternity Hospital, Tianjin, China
- 3Tianjin Key Laboratory of Human Development and Reproductive Regulation, Tianjin Central Hospital of Obstetrics and Gynecology, Tianjin, China
Background: Bronchopulmonary dysplasia (BPD) is a common complication in extremely preterm infants (EPIs), and there is currently a lack of effective preventive strategies. Identifying risk factors may facilitate early interventions and improve outcomes.
Objective: To investigate risk factors for the occurrence and severity of BPD in EPIs and inform potential prevention strategies.
Methods: We conducted a retrospective analysis of medical records from EPIs admitted to the neonatal intensive care unit at Tianjin Central Hospital of Obstetrics and Gynecology between 2012 and 2024. BPD was diagnosed according to the 2018 revised criteria established by the National Institute of Child Health and Human Development. Multivariable logistic regression was used to identify independent risk factors.
Results: Among 468 EPIs, 136 (29.1%) developed BPD (mild: 14.1%, moderate: 7.1%, severe: 7.9%). Independent risk factors for BPD included prolonged invasive mechanical ventilation (IMV, OR = 1.10, 95% CI 1.03–1.17), frequent red blood cell transfusions (RBCTs, OR = 1.61, 95% CI 1.30–2.01), extended antibiotic exposure (OR = 1.03, 95% CI 1.01–1.06), and hemodynamically significant patent ductus arteriosus (hsPDA, OR = 2.27, 95% CI 1.22–4.20). Prolonged IMV (OR = 1.16, 95% CI 1.06–1.27) and higher fluid balance (FB) on postnatal day 7 (OR = 1.19, 95% CI 1.05–1.34) were independent risk factors for moderate-to-severe BPD, while higher birth weight (OR = 0.99, 95% CI 0.988–0.998) was found to be a protective factor. Whole blood transfusion was associated with an increased risk of BPD (OR = 4.48, 95% CI 1.92–10.43) and moderate-to-severe BPD (OR = 4.81, 95% CI 1.24–18.63) compared to packed RBCTs. In predicting moderate-to-severe BPD, the duration of IMV (cut-off: 6.5 days) and FB on postnatal day 7 (cut-off: −7.2) demonstrated significant predictive value.
Conclusions: In conclusion, the occurrence and severity of BPD in EPIs are influenced by prolonged IMV, frequent RBCTs, fluid overload, excessive antibiotic exposure, and hsPDA. Early interventions targeting modifiable factors, such as reducing IMV duration, maintaining an appropriate negative FB on postnatal day 7, and optimizing transfusion protocols, are critical to prevent moderate-to-severe BPD.
1 Introduction
Bronchopulmonary dysplasia (BPD) remains one of the most prevalent and serious complications affecting extremely preterm infants (EPIs), defined as those born at less than 28 weeks of gestational age (GA). Over the past few decades, advances in neonatal care particularly in high-resource settings have led to remarkable improvements in the survival of EPIs. However, this progress has been accompanied by a concerning rise in the incidence of BPD (1–3). BPD is associated with significant long-term morbidity, including cardiorespiratory dysfunction, impaired growth, and neurodevelopmental delays (4–6), with moderate-to-severe BPD imposing the highest burden. These sequelae often extend into adulthood, imposing substantial emotional and financial burdens on affected families and healthcare systems.
Numerous studies have investigated risk factors for BPD, however, the majority have focused on preterm infants with a GA <32 weeks and birth weight (BW) <1,500 g, particularly in the Chinese population. However, given that EPIs are at the highest risk for BPD, this study focuses exclusively on EPIs to evaluate the risk factors for BPD. By doing so, we aim to provide evidence-based insights that can guide targeted strategies for the prevention and management of BPD in this vulnerable population.
2 Materials and methods
2.1 Study population
This retrospective study included preterm infants with a GA <28 weeks admitted to the neonatal intensive care unit (NICU) at Tianjin Central Hospital of Obstetrics and Gynecology within 24 h of birth from January 2012 to December 2024. Exclusion criteria were: (1) length of hospital stay <14 days; (2) inter-hospital transfer; (3) congenital anomalies (e.g., complex congenital heart disease, chromosomal abnormalities, etc.); (4) death prior to 36 weeks postmenstrual age (PMA) due to non-persistent parenchymal lung disease and respiratory failure.
2.2 Data collection
General clinical data included: (1) Demographic factors: gestational age (GA), birth weight (BW), sex, multiple pregnancy (vs. singleton pregnancy), mode of delivery (cesarean vs. vaginal), and 5-minute Apgar score. (2) Maternal factors: maternal age, antenatal corticosteroid use, premature rupture of membranes (PROM), gestational diabetes mellitus (GDM), and hypertensive disorders of pregnancy (HDP). (3) Complications: pulmonary hemorrhage, sepsis (including early-onset and late-onset sepsis), necrotizing enterocolitis (NEC), hemodynamically significant patent ductus arteriosus (hsPDA), extrauterine growth restriction (EUGR), intraventricular hemorrhage (IVH) (grade 3/4 IVH vs. no IVH or grade 1/2 IVH), and retinopathy of prematurity (ROP). (4) Treatment-related factors: use of surfactant, ventilation modalities: invasive mechanical ventilation [IMV; categorized into high-frequency oscillatory ventilation [HFOV], conventional mechanical ventilation [CMV], and combined HFOV + CMV therapy] and non-invasive ventilation (NIV), red blood cell transfusions (RBCTs): frequency and product type (whole blood vs. packed red blood cells), duration of antibiotic therapy, duration of postnatal corticosteroid use, feeding method (breastfeeding vs. formula), fluid balance (FB) on postnatal days 3 and 7, achievement of caloric intake targets (>100 kcal/kg/day by week 1, >120 kcal/kg/day by week 4). (5) Outcomes: death or survival. Due to limited use of noninvasive positive pressure ventilation (NIPPV) and universal transition to continuous positive airway pressure (CPAP), NIPPV cases were classified as CPAP. All high-flow nasal cannula (HFNC) recipients had prior CPAP exposure and were designated as the CPAP + HFNC combination group. Data collection for variables potentially associated with BPD risk was completed prior to 36 weeks PMA.
Standardized interventions: Initial delivery room stabilization utilized a T-resuscitator, with CPAP as the primary intervention based on spontaneous breathing and heart rate assessment. All EPIs received prophylactic caffeine citrate (20 mg/kg loading dose, followed by 5–10 mg/kg/day maintenance).
2.3 Diagnostic criteria
2.3.1 BPD
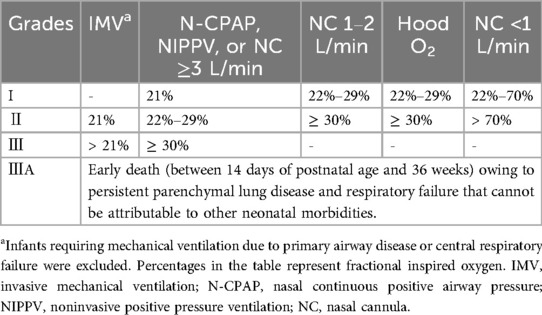
The diagnosis of BPD was based on the 2018 criteria established by the National Institute of Child Health and Human Development (NICHD) (7). Infants with a GA <32 weeks were diagnosed with BPD if they exhibited persistent radiographic evidence of parenchymal lung disease and required respiratory support for at least three consecutive days at 36 weeks PMA to maintain arterial oxygen saturation between 90% and 95%. BPD severity was classified into grades I, II, III, and IIIA based on the level of respiratory support and fractional inspired oxygen (FiO₂), as detailed in Table 1. For analysis, grade I was categorized as mild BPD, while grades II, III, and IIIA were grouped as moderate-to-severe BPD.
2.3.2 Other diagnostic criteria
FB was calculated by comparing daily weight with BW, in accordance with a previous study (8), using the following formula: FB = [(Daily Weight – BW)/BW] × 100. We calculated the FB on postnatal days 3 and 7. Hemodynamically significant patent ductus arteriosus (hsPDA) was defined using a combination of clinical and echocardiographic criteria (9). Clinical signs included the presence of a heart murmur, tachycardia, bounding pulses, hyperdynamic precordial impulse, wide pulse pressure, or worsening respiratory status. Echocardiographic and Doppler findings required for diagnosis included: (1) demonstration of left-to-right shunt; (2) left atrial-to-aortic root ratio >1.3; (3) ductal diameter >1.5 mm; and (4) disturbed diastolic flow in the main pulmonary artery. NEC was defined as Bell's stage II or higher (10). EUGR was identified if an infant's weight at 36 weeks PMA or at discharge was below the 10th percentile on the Fenton 2013 growth curves (11).
2.4 Statistical analysis
Data were analyzed using SPSS 29.0. Categorical variables were presented as n (%) and compared with Chi-square tests or continuity-corrected Chi-square tests. Bonferroni correction was applied for multiple comparisons. Normally distributed continuous variables were described using mean ± standard deviation (SD) and analyzed with independent t-tests. Non-normally distributed continuous variables were expressed as median (interquartile ranges, IQR) and compared using Mann–Whitney U-tests. Logistic regression was performed to identify risk factors for BPD and moderate-severe BPD. Variables considered clinically important and those for which a statistically significant difference (p < 0.10) was observed in the univariate analysis were included in the multivariate models, using Akaike information criterion (AIC) as the selection criterion in a stepwise forward selection strategy. The goodness of fit of the logistic regression models was assessed using the Hosmer-Lemeshow test and the Omnibus test. Predictive accuracy was evaluated using Cox and Snell's R2 and Nagelkerke's R2 (to quantify explained variance) and receiver operating characteristic (ROC) curves with area under the curve (AUC). Statistical significance was defined as p < 0.05.
3 Results
3.1 General characteristics
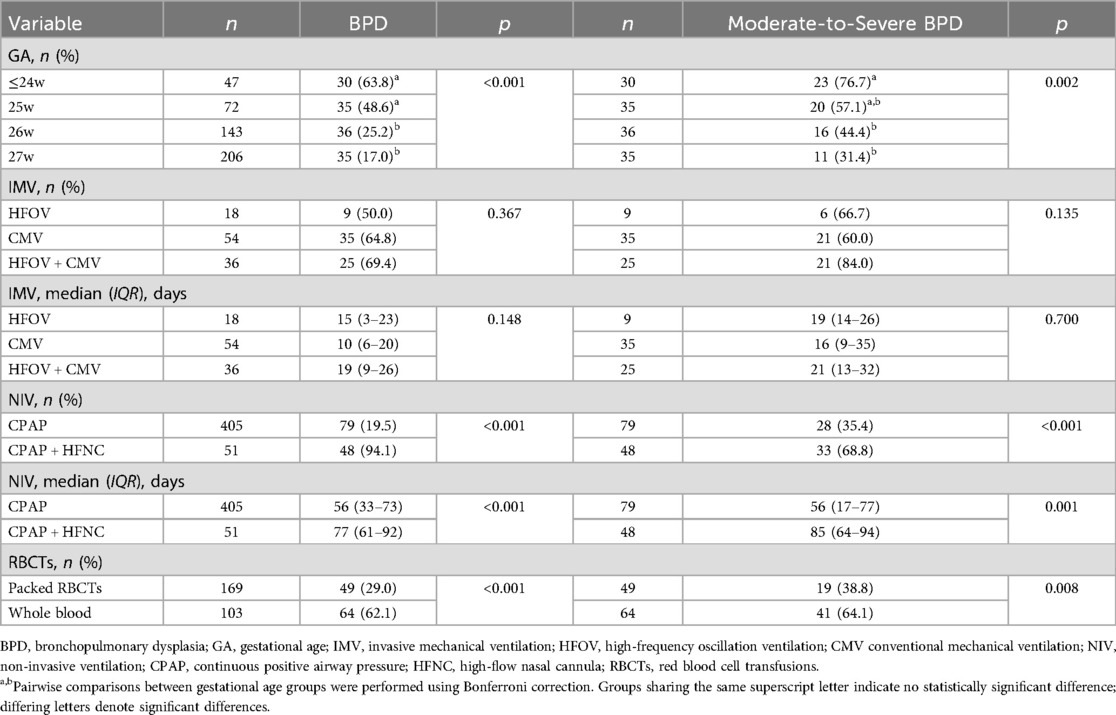
A total of 649 EPIs were admitted during the study period. After applying the exclusion criteria, 181 infants were excluded, leaving 468 infants for final analysis. Among them, 7 infants (1.5%) had a GA <24 weeks, 40 (8.5%) were 24 weeks, 72 (15.4%) were 25 weeks, 143 (30.6%) were 26 weeks, and 206 (44.0%) were 27 weeks, with a median GA of 26.6 weeks (IQR: 25.6–27.2). Regarding BW, 52 infants (11.1%) weighed <750 g, 261 (55.8%) weighed 750–1,000 g, and 155 (33.1%) weighed ≥1,000 g, with a median BW of 930 g (IQR: 820–1,030 g). There were 259 males (55.3%) and 209 females (44.7%).
3.2 Incidence and clinical characteristics of BPD
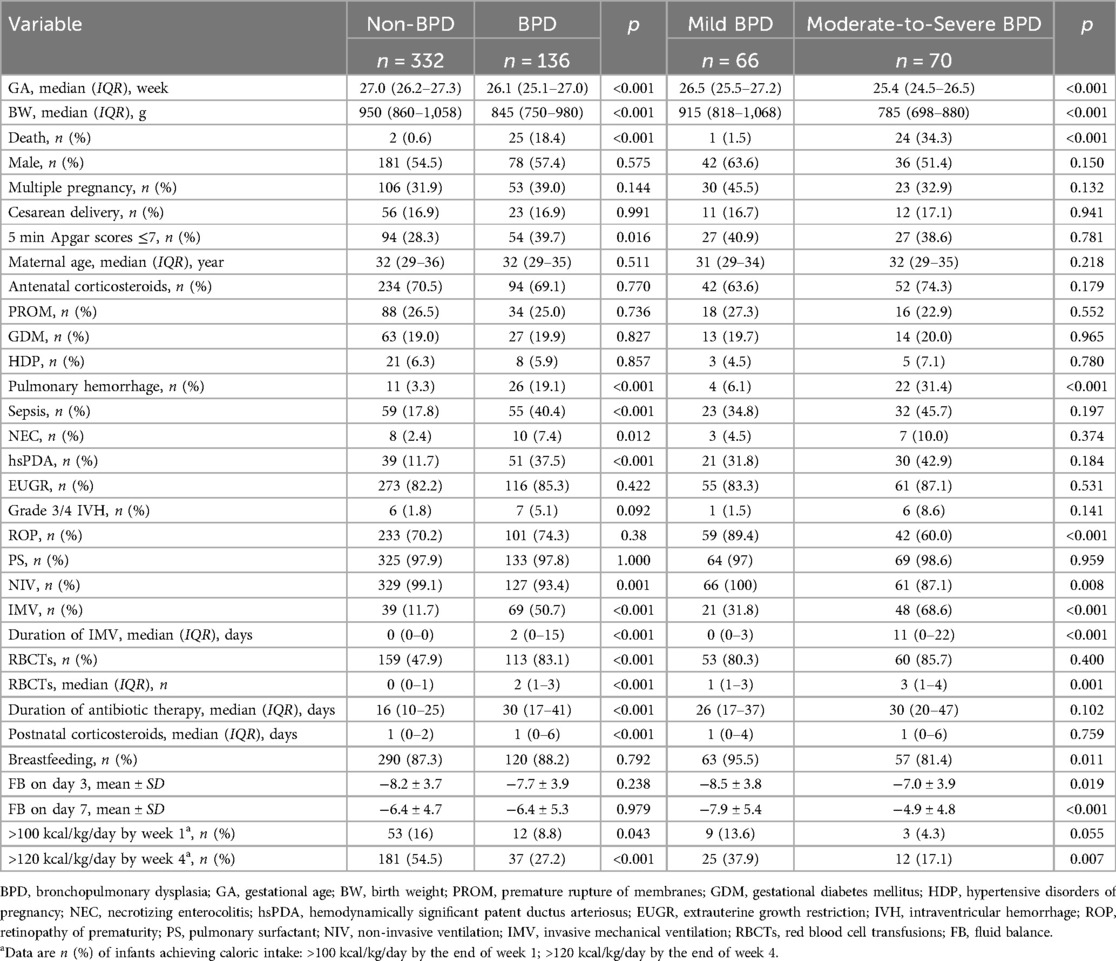
A total of 136 infants (29.1%) developed BPD, with 66 cases (14.1%) classified as grade I (mild), 33 (7.1%) as grade II, 20 (4.3%) as grade III, and 17 (3.6%) as grade IIIA. For analytical purposes, grades II, III, and IIIA were combined into a moderate-to-severe BPD category. Compared to 2012–2019, the 2020–2024 cohort exhibited stable survival rates (69.0% vs. 69.7%, p = 0.851) but demonstrated significantly elevated incidences of overall BPD (34.5% vs. 24.2%, p = 0.014), moderate-to-severe BPD (19.1% vs. 11.3%, p = 0.018), and a higher proportion of infants with GA <26 weeks (30.5% vs. 21.0%, p = 0.019). GA demonstrated a strong inverse relationship with both BPD and moderate-to-severe BPD incidence. Infants with GA <26 weeks, especially those ≤24 weeks, showed significantly higher BPD incidence (48.6–63.8%) and moderate-to-severe BPD rates (57.1–76.7%) compared to 26–27 week cohorts. Details are presented in Tables 2, 3.
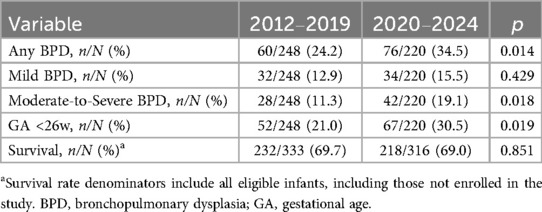
Table 2. Comparison of BPD incidence, gestational age distribution, and survival outcomes in EPIs: 2012–2019 vs. 2020–2024.
Compared to the non-BPD group, infants with BPD exhibited significantly lower GA and BW, lower rate of NIV, along with elevated rates of 5 min Apgar scores <7, mortality, need for and duration of IMV, transfusion frequency and number of RBCTs, duration of antibiotic use, and postnatal corticosteroid exposure. Additionally, the BPD group had higher incidences of pulmonary hemorrhage, sepsis, NEC, and hsPDA. In contrast, the BPD group had a lower proportion of infants achieving caloric intake targets: >100 kcal/kg/day by the end of week 1 and >120 kcal/kg/day by the end of week 4 of life (all p < 0.05). No significant differences were observed between the groups in terms of sex, multifetal pregnancy, mode of delivery, maternal age, maternal complications, antenatal corticosteroid use, feeding methods, use of surfactant, FB on postnatal days 3 and 7, EUGR, grade 3/4 IVH, or ROP (all p > 0.05). When comparing the moderate-to-severe BPD group to the mild BPD group, the former group had significantly lower GA and BW, higher mortality rates, lower rate of NIV, greater need for and duration of IMV, increased frequency and number of RBCTs, higher FB on postnatal days 3 and 7, and higher incidence of pulmonary hemorrhage. The moderate-to-severe BPD group also had lower rates of breastfeeding, lower incidences of ROP, and a lower proportion of infants achieving caloric intake target of >120 kcal/kg/day by the end of week 4 of life (all p < 0.05). See Table 4 for additional details.
Subgroup analysis revealed no statistically significant differences in either BPD incidence (overall or moderate-to-severe) or duration of IMV among the HFOV, CMV, and combined HFOV + CMV therapy groups (all p > 0.05). Regarding non-invasive ventilation use, the CPAP + HFNC combination group demonstrated significantly higher incidence rates of both BPD and moderate-to-severe BPD, along with a longer total duration of non-invasive ventilation therapy, compared to the CPAP group (all p < 0.05). Similarly, whole blood transfusion was associated with significantly greater BPD and moderate-to-severe BPD incidence vs. packed red blood cell transfusion (all p < 0.05). See Table 3 for details.
3.3 Analysis of risk factors for BPD and moderate-to-severe BPD
The potential risk factors and predictors included in the logistic regression models comprised: (1) Demographic factors: GA, BW, sex, multiple pregnancy (vs. singleton pregnancy), cesarean delivery (vs. vaginal), 5-min Apgar score. (2) Maternal factors: maternal age, antenatal corticosteroid use, PROM, GDM, HDP. (3) Complications: pulmonary hemorrhage, sepsis, NEC, hsPDA, EUGR, grade 3/4 IVH (vs. no IVH or grade 1/2 IVH). (4) Treatment-related factors: use of surfactant, duration of IMV, RBCTs frequency and product type (whole blood vs. packed red blood cell), duration of antibiotic therapy, duration of postnatal corticosteroid use, breastfeeding (vs. formula), FB on postnatal days 3 and 7, achievement of caloric intake targets (>100 kcal/kg/day by week 1, >120 kcal/kg/day by week 4).
The analysis revealed that prolonged duration of IMV, increased frequency of RBCTs, extended antibiotic therapy, and the presence of hsPDA were independent risk factors for BPD (all p < 0.05). Additionally, prolonged IMV and higher FB on postnatal day 7 were identified as independent risk factors for moderate-to-severe BPD, whereas higher BW was found to be protective against moderate-to-severe BPD (p < 0.05), as detailed in Table 5. When the transfusion cohort was stratified by blood product type (whole blood vs. packed red blood cells), multivariate analysis demonstrated that whole blood transfusion was associated with a significantly elevated risk of both BPD and moderate-to-severe BPD compared to packed red blood cell transfusion (p < 0.05). Comprehensive results are summarized in - Table 6.
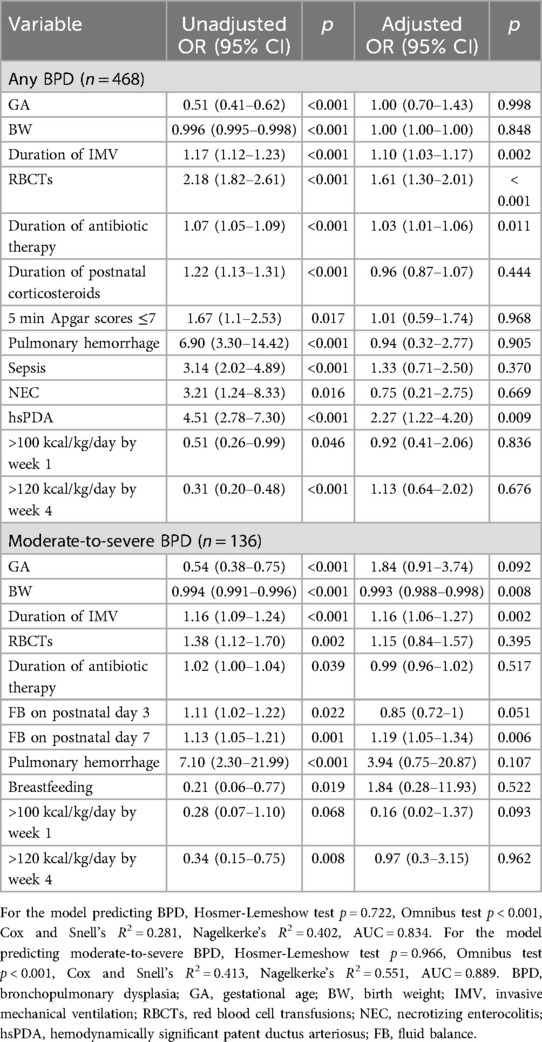
Table 5. Multivariate logistic regression analysis of risk factor for BPD and moderate-to-severe BPD.
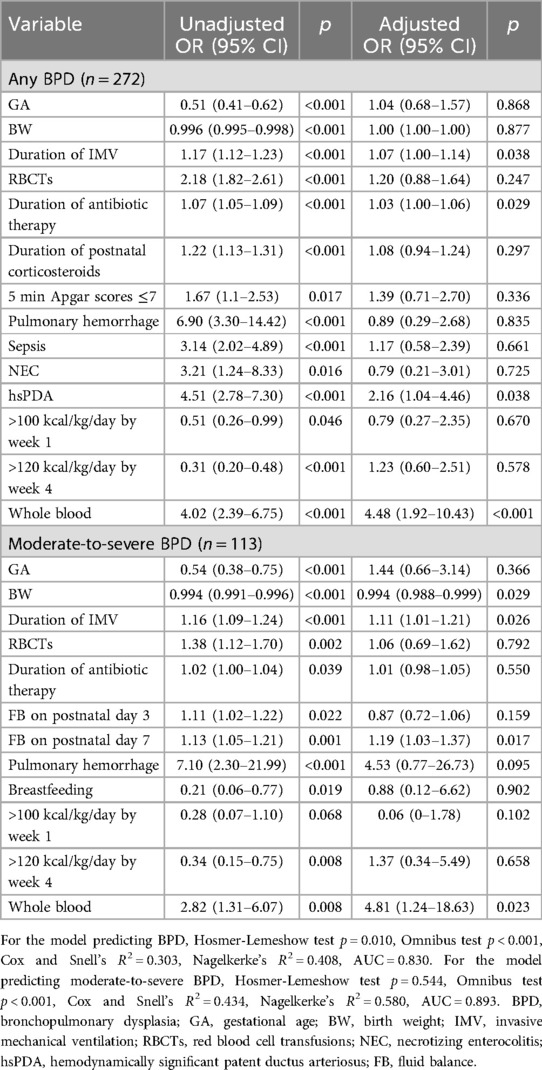
Table 6. Multivariate logistic regression analysis of risk factor for BPD and moderate-to-severe BPD in transfusion group.
3.4 Thresholds of duration of IMV and FB on postnatal day 7 in predicting moderate-to-severe BPD
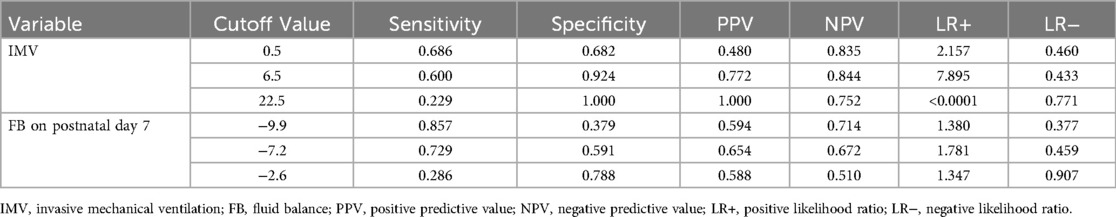
ROC curves were plotted to evaluate the predictive value of the duration of IMV and FB on postnatal day 7 for moderate-to-severe BPD. For IMV, the optimal cut-off value was 6.5 days, yielding an area under the curve (AUC) of 0.77 (95% CI: 0.69–0. 84, p < 0.0001), with sensitivity and specificity of 60.0% and 92.4%, respectively. For FB on postnatal day 7, a cut-off value of −7.2 demonstrated an AUC of 0.66 (95% CI: 0.56–0.75, p = 0.001), with sensitivity and specificity of 72.9% and 59.1%, respectively. See Figure 1 and Table 7 for additional details.
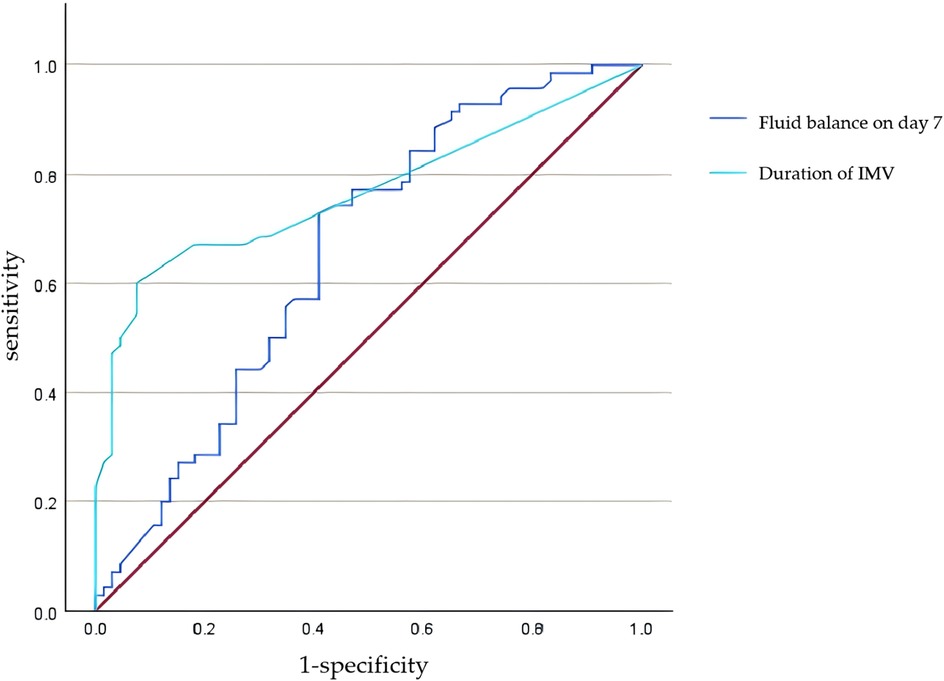
Figure 1. ROC curve for predicting moderate-to-severe BPD by duration of IMV and fluid balance on postnatal day 7.
4 Discussion
The incidence of BPD has shown a rising trend in recent years. In the United States, the incidence of BPD among infants with a GA <29 weeks ranged from 44.7% to 49.8% between 2008 and 2018 (1), while in Sweden, the incidence among infants with a GA <27 weeks was 65% to 68% between 2004 and 2016 (2). Data from 68 neonatal centers in China showed that the incidence of BPD in EPIs increased from 20.8% in 2010 to 40.7% in 2019 (3). A similar trend was observed at our center, where the BPD incidence rose from 24.2% during 2012–2019 to 34.5% in 2020–2024. This increase is likely associated with a higher proportion of preterm infants born at lower gestational ages. Both lower GA and lower BW are established as the strongest predictors of BPD (12), with disease incidence and severity exhibiting an inverse relationship to these factors. Our multivariate analysis confirmed increased BW as a protective factor against moderate-to-severe BPD, although a significant relationship between GA and BPD was not established. The overall BPD incidence at our center was lower than rates reported in developed countries (1, 2). This discrepancy may be explained by the predominance of infants born at 26 and 27 weeks GA in our cohort (constituting 74.6%), and a significant proportion of infants born at GA <26 weeks either died before 36 weeks PMA or experienced care withdrawal due to socioeconomic challenges. Notably, infants born at <24 weeks GA were particularly underrepresented (only 1.5%), limiting our ability to accurately reflect the true incidence within this highest-risk GA subgroup.
Multivariate analysis in this study identified prolonged IMV as an independent risk factor for both BPD and moderate-to-severe BPD, consistent with previous studies (13, 14). Ventilation-induced lung injury (VILI) is a known risk factor for BPD (15), with mechanisms including volutrauma, barotrauma, atelectrauma, oxygen toxicity, and biotrauma, which interact with patient-specific factors such as immature lungs, surfactant deficiency, non-uniform lung disease, and pulmonary inflammation to exacerbate lung injury. The optimal strategy to mitigate VILI and BPD risk is the use of non-invasive respiratory support. The American Academy of Pediatrics guidelines (16) recommend early application of continuous positive airway pressure at or soon after birth with subsequent selective surfactant therapy. However, avoiding IMV at all costs may expose some preterm infants to prolonged episodes of apnea or worsening respiratory distress syndrome, ultimately necessitating rescue invasive ventilation. Lung-protective ventilation strategies, such as volume-targeted ventilation, have been shown in a meta-analysis (17) to significantly reduce the composite outcome of death or BPD at 36 weeks PMA compared to pressure-controlled ventilation. This subgroup analysis revealed no significant differences in BPD incidence (overall or moderate-to-severe) or IMV duration among HFOV, CMV, or HFOV + CMV groups. In contrast, the CPAP + HFNC group exhibited significantly higher BPD and moderate-to-severe BPD rates and longer NIV duration vs. CPAP alone. This observation is likely attributable to the relatively limited respiratory support pressure provided by HFNC. As a well-established non-invasive modality, CPAP delivers more reliable alveolar recruitment, suggesting it remains a preferable clinical option over HFNC supplementation in this context.
Previous studies have identified RBCTs as a risk factor for BPD (18), a finding corroborated by our study. The incidence of BPD increased with the number of transfusions, potentially explained by transfusion-related immunomodulation (TRIM), which may play a significant role in transfusion-associated diseases (19). Crawford found that early and repeated transfusions in very preterm infants led to changes in pro-inflammatory cytokines and endothelial activation markers, suggesting that TRIM may occur within the first few days of life (20). Iron and other inflammatory mediators in blood products can promote free radical generation, infection, and fibrosis (21), with oxidative stress and inflammation being key mechanisms in BPD pathogenesis. Additionally, transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) are the most common cardiopulmonary complications of transfusions (22), causing neutrophil activation, endothelial damage, protein leakage, increased hydrostatic pressure, or fluid extravasation, all of which contribute to pulmonary edema and ultimately lead to lung injury. Stainsby (23) observed that the incidence of adverse transfusion reactions increases progressively among adults, children, and infants, suggesting that neonates may be more susceptible to such adverse reactions. Bedford (24) found that preterm infants receiving multiple transfusions of non-leukoreduced whole blood developed anti-human leukocyte antigen antibodies, while those receiving leukoreduced blood did not. In our center, for infants requiring multiple transfusions within a short period, blood from a single donor is prioritized to minimize exposure to multiple donors. Multivariate analysis stratified by blood product type (whole blood vs. packed red blood cells) revealed that whole blood transfusion was associated with a higher risk of BPD and moderate-to-severe BPD compared to packed red blood cells. Since the whole blood transfused in our center was not leukoreduced, this suggests that TRIM may be related to the increased risk of BPD. Crawford (25) suggested that transfusing washed red blood cells might mitigate this response, and a prospective randomized controlled trial (26) is currently underway to evaluate the impact of washed vs. unwashed red blood cells on morbidity and mortality in EPIs. Recent guidelines (27), based on extensive randomized clinical trials, indicate that low-threshold transfusion does not increase short or long-term adverse outcomes, including BPD, and recommend restrictive transfusion strategy.
Previous studies have demonstrated that fluid overload or higher FB increases the risk of BPD (8, 28). Preterm infants typically undergo physiological diuresis postnatally, leading to a negative FB. However, fluid overload may induce pulmonary edema and inflammation, impairing the normal development of alveoli and pulmonary vasculature. Additionally, fluid overload can prolong the patency of PDA (29), while acute kidney injury (30) or medications such as indomethacin (31) may further compromise diuretic processes, exacerbating fluid retention and thereby contributing to lung injury and BPD progression. However, Diderholm (32), based on fluid intake, suggested that early fluid restriction did not reduce BPD incidence, a discrepancy potentially attributable to variations in study protocols. This study demonstrated that higher FB on postnatal day 7 was an independent risk factor for moderate-to-severe BPD, which is consistent with previous findings (8). Maintaining the FB on postnatal day 7 below −7.2 may be an effective preventive measure against BPD. However, since FB was assessed solely via weight changes rather than direct measurement of fluid intake or output, clinical practice should integrate GA, weight trends, and fluid intake and output data to refine individualized fluid management strategies. Prospective studies are warranted to validate the impact of fluid management on BPD outcomes.
Due to the immature immune systems of preterm infants, invasive procedures, and the use of medical devices, antibiotics are among the most commonly prescribed medications in the NICU (33). However, excessive antibiotic exposure may lead to increased antibiotic resistance and alter the gut microbiome (34). Previous studies have linked excessive antibiotic exposure to an elevated risk of adverse outcomes, including BPD, in preterm infants (35), a finding supported by our study. The gut-lung axis hypothesis provides a potential mechanism, suggesting that gut dysbiosis may disrupt the intestinal barrier, triggering inflammation, metabolic disturbances, and malnutrition, which in turn exacerbate pulmonary inflammation and injury, increasing the risk of BPD (36). The imbalance in the airway microbiota, influenced by factors such as antibiotic therapy and mechanical ventilation, is linked to the occurrence of BPD (37), while the gut and airway microbiomes interact bidirectionally through the gut-lung axis, contributing to abnormal inflammatory responses that play a critical role in the pathogenesis of BPD (37). Therefore, reducing unnecessary antibiotic exposure is critical. Fischer (38) demonstrated that a quality improvement project implementing standardized antibiotic therapy guidelines successfully reduced antibiotic utilization by 24.8%, with sustained effects over three years, highlighting the feasibility of reducing antibiotic exposure through education, ongoing feedback, and monitoring.
This study confirmed that hsPDA is a significant risk factor for BPD, consistent with existing research (39, 40). The persistent left-to-right shunt in hsPDA increases pulmonary blood flow, leading to pulmonary edema, reduced lung compliance (41), and even pulmonary hemorrhage (42), thereby increasing the need for mechanical ventilation and oxygen, which exacerbates the risk of VILI. Increased pulmonary blood flow also triggers pulmonary neutrophil margination and activation (43), further intensifying lung inflammation and contributing to BPD development. While it is logical to assume that closing the PDA would reduce BPD incidence, studies have shown that pharmacological or surgical interventions do not improve BPD rates and may even increase them (31, 44, 45). Huang (46) found that ibuprofen may inhibit vascular growth factor levels in preterm infants, potentially explaining the increased BPD risk. The optimal management of PDA remains controversial, indicating that its role in BPD pathogenesis may be more complex than previously thought.
To our knowledge, this is the largest single-center retrospective analysis of EPIs in China, providing critical insights through its large-scale longitudinal design. Multivariable logistic regression models were employed to adjust for confounding factors, and the independent predictive value of FB was rigorously validated through statistical analyses. Importantly, as a highly modifiable clinical parameter, FB may provide a direct intervention target for optimizing fluid management in preterm infants. Additionally, this study is the first to demonstrate that whole-blood transfusion was associated with a higher risk of BPD compared to traditional packed red blood cell transfusion. This study has several inherent limitations: (1) its retrospective design and single-center scope may introduce selection bias and limit generalizability, compounded by insufficient consideration of confounders such as socioeconomic status, treatment strategy variations, and evolving clinical protocols; (2) due to incomplete data on maternal infections, this factor was not included in the analysis; (3) only short-term outcomes were analyzed, and long-term follow-up is needed to evaluate the relationship between BPD and growth, cardiopulmonary, and neurodevelopmental outcomes.
5 Conclusions
In summary, BPD is a multifactorial condition influenced by synergistic interactions of modifiable and non-modifiable risk factors. Independent risk factors for BPD include prolonged IMV, extended antibiotic exposure, frequent RBCTs, whole blood transfusion (compared to packed RBCTs), and hsPDA. For moderate-to-severe BPD, disease severity was independently associated with prolonged IMV, whole-blood transfusion, and higher FB on postnatal day 7, while higher birth weight was identified as a protective factor. Clinically, implementing stratified prevention strategies is critical: shortening duration of IMV through non-invasive ventilation support or lung-protective strategies (e.g., volume-targeted ventilation), maintaining an appropriate negative FB early, adopting restrictive transfusion protocols, and standardizing antibiotic stewardship. Future multicenter prospective studies with expanded demographic data collection and enhanced control of confounding variables are warranted to improve generalizability and analytical rigor, ultimately enabling precise prevention and management of bronchopulmonary dysplasia in very preterm infants.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by This study was approved by the Ethics Committee of Tianjin Central Hospital of Obstetrics and Gynecology (approval number: 2025KY033). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. WZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. FD: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. We acknowledge support from Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-039A).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA. (2022) 327(3):248–63. doi: 10.1001/jama.2021.23580
2. Löfberg L, Abrahamsson T, Björklund LJ, Hellström Westas L, Farooqi A, Domellöf M, et al. Respiratory support and bronchopulmonary dysplasia in infants born at 22–26 weeks gestation in Sweden, 2004–2007 and 2014–2016. Eur Respir J. (2025) 65(1):2401203. doi: 10.1183/13993003.01203-2024
3. Zhu Z, He Y, Yuan L, Chen L, Yu Y, Liu L, et al. Trends in bronchopulmonary dysplasia and respiratory support among extremely preterm infants in China over a decade. Pediatr Pulmonol. (2024) 59(2):399–407. doi: 10.1002/ppul.26761
4. Pulakka A, Risnes K, Metsälä J, Alenius S, Heikkilä K, Nilsen SM, et al. Preterm birth and asthma and COPD in adulthood: a nationwide register study from two Nordic countries. Eur Respir J. (2023) 61(6):2201763. doi: 10.1183/13993003.01763-2022
5. Hurst JR, Beckmann J, Ni Y, Bolton CE, McEniery CM, Cockcroft JR, et al. Respiratory and cardiovascular outcomes in survivors of extremely preterm birth at 19 years. Am J Respir Crit Care Med. (2020) 202(3):422–32. doi: 10.1164/rccm.202001-0016OC
6. Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. (2019) 200(6):751–9. doi: 10.1164/rccm.201812-2348OC
7. Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. (2018) 197:300–8. doi: 10.1016/j.jpeds.2018.01.043
8. Starr MC, Griffin R, Gist KM, Segar JL, Raina R, Guillet R, et al. Association of fluid balance with short- and long-term respiratory outcomes in extremely premature neonates: a secondary analysis of a randomized clinical trial. JAMA Network Open. (2022) 5(12):e2248826. doi: 10.1001/jamanetworkopen.2022.48826
9. Malviya MN, Ohlsson A, Shah SS. Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. (2013) 2013(3):Cd003951. doi: 10.1002/14651858.CD003951.pub3
10. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
11. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 g. J Pediatr. (1978) 92(4):529–34. doi: 10.1016/s0022-3476(78)80282-0
12. Lee SM, Sie L, Liu J, Profit J, Lee HC. Evaluation of trends in bronchopulmonary dysplasia and respiratory support practice for very low birth weight infants: a population-based cohort study. J Pediatr. (2022) 243:47–52.e2. doi: 10.1016/j.jpeds.2021.11.049
13. Nakashima T, Inoue H, Sakemi Y, Ochiai M, Yamashita H, Ohga S. Trends in bronchopulmonary dysplasia among extremely preterm infants in Japan, 2003–2016. J Pediatr. (2021) 230:119–25.e7. doi: 10.1016/j.jpeds.2020.11.041
14. Abushahin A, Hamad SG, Sabouni A, Alomar S, Sudarsanan A, Kammouh H, et al. Incidence and predictors of bronchopulmonary dysplasia development and severity among preterm infants born at 32 weeks of gestation or less. Cureus. (2024) 16(4):e59425. doi: 10.7759/cureus.59425
15. Kalikkot Thekkeveedu R, El-Saie A, Prakash V, Katakam L, Shivanna B. Ventilation-Induced lung injury (VILI) in neonates: evidence-based concepts and lung-protective strategies. J Clin Med. (2022) 11(3):557. doi: 10.3390/jcm11030557
16. Papile L-A, Baley JE, Benitz W, Cummings J, Eichenwald E, Kumar P, et al. Respiratory support in preterm infants at birth. Pediatrics. (2014) 133(1):171–4. doi: 10.1542/peds.2013-3442
17. Abiramalatha T, Ramaswamy VV, Bandyopadhyay T, Somanath SH, Shaik NB, Pullattayil AK, et al. Interventions to prevent bronchopulmonary dysplasia in preterm neonates: an Umbrella review of systematic reviews and meta-analyses. JAMA Pediatr. (2022) 176(5):502–16. doi: 10.1001/jamapediatrics.2021.6619
18. Bahr TM, Snow GL, Christensen TR, Davenport P, Henry E, Tweddell SM, et al. Can red blood cell and platelet transfusions have a pathogenic role in bronchopulmonary dysplasia? J Pediatr. (2024) 265:113836. doi: 10.1016/j.jpeds.2023.113836
19. Sparrow RL. Red blood cell storage and transfusion-related immunomodulation. Blood Transfus. (2010) 8 Suppl 3(Suppl 3):s26–30. doi: 10.2450/2010.005s
20. Crawford TM, Andersen CC, Stark MJ. Effect of repeat transfusion exposure on plasma cytokine and markers of endothelial activation in the extremely preterm neonate. Transfusion. (2020) 60(10):2217–24. doi: 10.1111/trf.15952
21. Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Med Hypotheses. (2006) 66(2):355–64. doi: 10.1016/j.mehy.2005.04.046
22. Vlaar APJ, Zwaginga JJ, Wiersum-Osselton JC. How I diagnose and treat cardiorespiratory complications of transfusion. Blood. (2025) 145(20):2283–92. doi: 10.1182/blood.2023022899
23. Stainsby D, Jones H, Wells AW, Gibson B, Cohen H. Adverse outcomes of blood transfusion in children: analysis of UK reports to the serious hazards of transfusion scheme 1996–2005. Br J Haematol. (2008) 141(1):73–9. doi: 10.1111/j.1365-2141.2008.07022.x
24. Bedford Russell AR, Rivers RP, Davey N. The development of anti-HLA antibodies in multiply transfused preterm infants. Arch Dis Child. (1993) 68(1 Spec No):49–51. doi: 10.1136/adc.68.1_spec_no.49
25. Crawford TM, Andersen CC, Hodyl NA, Robertson SA, Stark MJ. Effect of washed versus unwashed red blood cells on transfusion-related immune responses in preterm newborns. Clin Transl Immunol. (2022) 11(3):e1377. doi: 10.1002/cti2.1377
26. Stark MJ, Collins CT, Andersen CC, Crawford TM, Sullivan TR, Bednarz J, et al. Study protocol of the WashT trial: transfusion with washed versus unwashed red blood cells to reduce morbidity and mortality in infants born less than 28 weeks’ gestation—a multicentre, blinded, parallel group, randomised controlled trial. BMJ Open. (2023) 13(7):e070272. doi: 10.1136/bmjopen-2022-070272
27. Deschmann E, Dame C, Sola-Visner MC, Fustolo-Gunnink SF, Guyatt GH, Patel RM, et al. Clinical practice guideline for red blood cell transfusion thresholds in very preterm neonates. JAMA Netw Open. (2024) 7(6):e2417431. doi: 10.1001/jamanetworkopen.2024.17431
28. Li YJ, Zhu XF, Liu JH, Yi XQ, He H. Influence of early fluid overload on bronchopulmonary dysplasia in very low-birth-weight infants. Front Pediatr. (2022) 10:980179. doi: 10.3389/fped.2022.980179
29. Valentine GC, Perez KM, Wood TR, Mayock DE, Comstock BA, Puia-Dumitrescu M, et al. Postnatal maximal weight loss, fluid administration, and outcomes in extremely preterm newborns. J Perinatol. (2022) 42(8):1008–16. doi: 10.1038/s41372-022-01369-7
30. Starr MC, Boohaker L, Eldredge LC, Menon S, Griffin R, Mayock DE, et al. Acute kidney injury and bronchopulmonary dysplasia in premature neonates born less than 32 weeks’ gestation. Am J Perinatol. (2020) 37(3):341–8. doi: 10.1055/s-0039-3400311
31. Schmidt B, Roberts RS, Fanaroff A, Davis P, Kirpalani HM, Nwaesei C, et al. Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the trial of indomethacin prophylaxis in preterms (TIPP). J Pediatr. (2006) 148(6):730–4. doi: 10.1016/j.jpeds.2006.01.047
32. Diderholm B, Normann E, Ahlsson F, Sindelar R, Ågren J. The impact of restricted versus liberal early fluid volumes on plasma sodium, weight change, and short-term outcomes in extremely preterm infants. Nutrients. (2022) 14(4):795. doi: 10.3390/nu14040795
33. Krzyżaniak N, Pawłowska I, Bajorek B. Review of drug utilization patterns in NICUs worldwide. J Clin Pharm Ther. (2016) 41(6):612–20. doi: 10.1111/jcpt.12440
34. Thänert R, Schwartz DJ, Keen EC, Hall-Moore C, Wang B, Shaikh N, et al. Clinical sequelae of gut microbiome development and disruption in hospitalized preterm infants. Cell Host Microbe. (2024) 32(10):1822–37.e5. doi: 10.1016/j.chom.2024.07.027
35. Vatne A, Hapnes N, Stensvold HJ, Dalen I, Guthe HJ, Støen R, et al. Early empirical antibiotics and adverse clinical outcomes in infants born very preterm: a population-based cohort. J Pediatr. (2023) 253:107–14.e5. doi: 10.1016/j.jpeds.2022.09.029
36. Yang K, He S, Dong W. Gut microbiota and bronchopulmonary dysplasia. Pediatr Pulmonol. (2021) 56(8):2460–70. doi: 10.1002/ppul.25508
37. Colombo SFG, Nava C, Castoldi F, Fabiano V, Meneghin F, Lista G, et al. Preterm infants’ airway microbiome: a scoping review of the current evidence. Nutrients. (2024) 16(4):465. doi: 10.3390/nu16040465
38. Fischer AM, Mitchell JL, Stanley KC, Javed MJ. A quality improvement project to reduce antibiotic exposure in premature neonates. Hosp Pediatr. (2023) 13(5):435–48. doi: 10.1542/hpeds.2022-006644
39. Huang J, Shen W, Wu F, Mao J, Liu L, Chang Y, et al. Risk factors for severe bronchopulmonary dysplasia in a Chinese cohort of very preterm infants. Saudi Med J. (2024) 45(4):369–78. doi: 10.15537/smj.2024.45.4.20230741
40. Chesi E, Rossi K, Ancora G, Baraldi C, Corradi M, Di Dio F, et al. Patent ductus arteriosus (also non-hemodynamically significant) correlates with poor outcomes in very low birth weight infants. A multicenter cohort study. PLoS One. (2024) 19(7):e0306769. doi: 10.1371/journal.pone.0306769
41. Gerhardt T, Bancalari E. Lung compliance in newborns with patent ductus arteriosus before and after surgical ligation. Biol Neonate. (1980) 38(1–2):96–105. doi: 10.1159/000241348
42. Jung JK, Kim EY, Heo JS, Park KH, Choi BM. Analysis of perinatal risk factors for massive pulmonary hemorrhage in very low birth weight infant: a nationwide large cohort database. Early Hum Dev. (2024) 191:105977. doi: 10.1016/j.earlhumdev.2024.105977
43. Varsila E, Hallman M, Venge P, Andersson S. Closure of patent ductus arteriosus decreases pulmonary myeloperoxidase in premature infants with respiratory distress syndrome. Biol Neonate. (1995) 67(3):167–71. doi: 10.1159/000244159
44. Hundscheid T, Onland W, Kooi EMW, Vijlbrief DC, de Vries WB, Dijkman KP, et al. Expectant management or early ibuprofen for patent ductus arteriosus. N Engl J Med. (2023) 388(11):980–90. doi: 10.1056/NEJMoa2207418
45. Weisz DE, More K, McNamara PJ, Shah PS. PDA ligation and health outcomes: a meta-analysis. Pediatrics. (2014) 133(4):e1024–46. doi: 10.1542/peds.2013-3431
Keywords: extremely premature infants, bronchopulmonary dysplasia, invasive mechanical ventilation, transfusion, fluid balance, antibiotics, patent ductus arteriosus
Citation: Liu X, Zhang W and Ding F (2025) Predictors of bronchopulmonary dysplasia occurrence and severity in extremely preterm infants. Front. Pediatr. 13:1629279. doi: 10.3389/fped.2025.1629279
Received: 15 May 2025; Accepted: 28 July 2025;
Published: 12 August 2025.
Edited by:
Shahana Perveen, Cohen Children's Medical Center, United StatesReviewed by:
Prem Chandra, Hamad Medical Corporation, QatarJoanna Beachy, Baystate Medical Center, United States
Copyright: © 2025 Liu, Zhang and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangrui Ding, eW91bmdiZWFyQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Xuejing Liu
Xuejing Liu Wanxian Zhang
Wanxian Zhang Fangrui Ding
Fangrui Ding