- 1World Health Organization, Research for Health Department, Science Division, Geneva, Switzerland
- 2Pan American Health Organization - World Health Organization, Regional Office of the Americas, Duque de Caxias- Rio de Janeiro, Brazil
- 3icddr,b, 68 Shaheed Tajuddin Ahmed Saranai, Mokakhali, Dhaka, Bangladesh
- 4International Severe Acute Respiratory and emerging Infection Consortium, Pandemic Sciences Institute, University of Oxford, Oxford, United Kingdom
- 5Province Hospital, Surkhet, Nepal
- 6World Health Organization, Regional Office for South-East Asia, New Delhi, India
- 7World Health Organization, Regional Office for Africa, Brazzaville, Republic of Congo
- 8World Health Organization, Regional Office of the Eastern Mediterranean, Cairo, Egypt
- 9World Health Organization, Malaria and Neglected Tropical Disease Department, Geneva, Switzerland
Visceral leishmaniasis (VL) is a fatal disease if left untreated. Globally, at least 50% of VL cases are reported to be children younger than 15 years, with a higher incidence among males. VL is intrinsically associated with poverty and poor social determinants of health. Malnutrition and immune suppression are risk factors for severe VL, making children particularly vulnerable to this disease. Available treatment options vary depending on the eco-epidemiological context, but are in general suboptimal, especially for children. In 2023, the World Health Organization convened a paediatric drug optimization exercise (PADO) for VL, bringing together more than 60 experts globally in the field of VL to identify formulations of VL medicines to prioritize for development to address the specific needs of children. The group prioritized a 20 mg scored, dispersible tablet formulation of miltefosine and an oral solid dosage form of amphotericin B, acknowledging recent developments in allometric dosing for miltefosine and ongoing research for the development of oral amphotericin B. For miltefosine, this prompted an ongoing update of the WHO Prequalification Expression of interest to promote generic manufacturing. A compound with a new mechanism of action, LXE408, which is currently being investigated in Phase II, was included in the PADO watch list, signalling that paediatric investigations should start as soon as enough data are available from adult studies, not to delay access to latest available innovations for children with VL.
Introduction
Visceral leishmaniasis (VL), also known as Kala-azar, is a poverty-related parasitic vector-borne disease that is endemic in 80 countries in the world with an estimated 50,000–90,000 new cases per year (1). Its chronic nature, outbreak and high-mortality potential make it disproportionately endemic in low- and lower-middle-income countries, afflicting their poorest and most isolated populations. The disease is associated with malnutrition, population displacement, migration of non-immune people to endemic areas, poor housing, illiteracy, a weakened immune system, and lack of resources (2–4).
VL causes high disease burden in the three major eco-epidemiological hotspots – the Indian subcontinent, Brazil and Eastern Africa, which accounted for 6%, 14% and 74% of the global burden in 2023, respectively. With the success of the Kala-azar elimination programme in the Indian subcontinent, the main burden of VL has now shifted to Eastern Africa (5).
Treatment of VL depends on several factors, including the type of patients (new, relapse), the type of Leishmania parasite species, associated comorbid conditions, a person's immune status (e.g., people living with HIV) and other immune suppression conditions (1). As a result, treatment options vary across geographical regions (Supplementary Table S1).
Children under 15 years of age constitute approximately 50% of all VL cases. Malnutrition and immune suppression predispose to clinical disease, especially among children who are young and malnourished and act as a risk factor for severe VL (2, 4).
Except for miltefosine which is an oral drug, other antileishmanial drugs are given parenterally and require hospitalization or a visit to receive the injection, and the dosing is weight-based. Most VL drugs are available from a single supplier and are not registered in several endemic countries, with severe implications on costs and availability. Only liposomal amphotericin B (LAmB) is supplied through a donation programme in a few countries, while there is no donation of other antileishmanial drugs. Cold chain maintenance, storage and supply chain are also an issue for some of these medicines.
Currently available treatment options are particularly not suitable for children, which prompted the need to discuss how to focus limited resources on the most needed formulations for a population that is so severely affected by VL.
Paediatric Drug Optimization (PADO) for visceral leishmaniasis
In June 2023, WHO convened a PAediatric Drug Optimization (PADO) (6) meeting for VL with 60 experts from five WHO regions, including researchers, funders, implementing partners, national programme managers, regulators and product development partnerships to review drugs and formulations currently used for VL and evaluate their appropriateness for children, review ongoing studies and efforts to develop paediatric formulations as well as VL drugs currently under investigation (7).
Meetings focusing on optimization of paediatric medicines have been successfully convened by WHO in several disease areas including HIV, tuberculosis, hepatitis C, COVID-19 and antibiotics, showing the impact that such a consensus-building process can have on focusing research and development efforts and resources.
The aim of the PADO-VL meeting was to reach a consensus on priority formulations of medicines for VL to be investigated and developed with a time horizon of 3–5 years (PADO priority list), identify promising candidates for investigation and development for children with a time horizon of 5–10 years (PADO watch list), as well as agree on a clear research agenda to support and enable future optimization work.
The PADO for VL meeting was part of a broader PADO exercise for neglected tropical diseases (NTDs) which focused on NTDs with the highest burden in the paediatric population, including schistosomiasis, human African trypanosomiasis, scabies and onchocerciasis (7).
The PADO-VL group reviewed each of the available antileishmanial drugs for their efficacy and use across different epidemiological regions, their available formulations, toxicity profiles and their suitability for children. The group noted that therapeutic efficacy study designs and their results in VL are complex with substantial variations in terms of inclusion and exclusion criteria, definitions for disease diagnosis and treatment outcomes. The drug trials mostly use linear dosing in children–therefore, generating potentially inadequate regimens. Adapted dosing regimens and formulations are generally lacking for children.
Except for miltefosine, antileishmanial drugs are administered parenterally: intramuscular injections are painful and often unsuited, given that many children with VL are severely malnourished and have little or no muscle mass, with a risk of muscle abscess and nerve damage; venous access for intravenous infusion is equally challenging – hence the risk of thrombophlebitis of vein. For miltefosine, acceptability in younger children who cannot swallow capsules is also poor, and capsules need protection from moisture, which is not ideal for use in tropical climates. In addition, oral miltefosine cannot be prescribed to women of childbearing age if pregnancy status cannot be verified and adequate contraception cannot be instituted during treatment and five months after the last dose, as the drug has a potential teratogenic effect – thus further limiting its use in adolescent girls. Gastrointestinal effects including vomiting and/or diarrhoea are common during miltefosine administration and may result in volume depletion (8).
Other combinations of available drugs have been studied in Africa and in Brazil, including in children, but target efficacy was not achieved for any of these regimens (9–11).
Discussion
PADO priority list for visceral leishmaniasis
To overcome low exposure and reduced efficacy in younger children at the conventional 2.5 mg/kg/day dose, recent trials investigated miltefosine given at allometric doses ranging from a 20 mg to 80 mg daily dose (divided into two daily administrations to reduce vomiting). This allometric dose regimen has shown high efficacy in children and in malnourished individuals with VL in Eastern Africa demonstrating that it leads to an equivalent exposure to the one observed in adults and it was reviewed in the context of a recent guideline development group convened by WHO (12–15). Considering future potential recommendations for allometric dosing of miltefosine, as well as the low acceptability and dose flexibility of currently available miltefosine formulations (i.e., 50 mg and 10 mg capsules), an age-appropriate formulation of oral miltefosine – 20 mg scored, dispersible tablets – was identified as a priority formulation to develop for children with visceral leishmaniasis in the short term (3–5 years) (Table 1). Dispersible tablets were preferred over other dosage forms such as oral liquids or syrups, after reflecting on well-known issues with such dosage forms related to procurement and supply, as well as dosing and stability (16). This formulation would also allow for doses in multiples of 20 mg and 10 mg, which would facilitate allometric dosing of miltefosine.

Table 1. Paediatric drug optimization (PADO) priority list, watch list and research priorities for visceral leishmaniasis.
The group acknowledged that miltefosine is not currently recommended for VL in the region of the Americas as trials showed 42% cure rate at 28 days of treatment and 68% at 42 days of treatment (17, 18). Moreover, reliable data on the efficacy of miltefosine in VL in the Mediterranean region are lacking. Nevertheless, a paediatric formulation of miltefosine was still considered a priority for further development, as it remains the only available oral treatment option for VL, which can be utilized in most endemic settings.
The addition of this formulation of miltefosine to the PADO priority list prompted an ongoing update of the WHO Prequalification expression of interest to promote generic manufacturing of this medicine. Investigating the development of miltefosine dosage forms with reduced gastrointestinal side effects was noted as a key priority.
An oral formulation of amphotericin B that is cost-effective, safe, stable at tropical temperatures, accessible, and easy to administer, was also prioritized for development in the short term, with the characteristics – dosage form, strength – of this formulation pending upon results of ongoing studies (Table 1). In particular, a Phase II study exploring amphotericin B loaded in cochleates – to delay release in gastrointestinal media – for cryptococcal meningitis (19, 20) and Phase Ia, Ib studies to evaluate the safety, tolerability and PK of novel lipid-based self-emulsifying oral amphotericin B formulations including syrup and capsule formulations that have already proved stable at tropical temperatures (21–23). More data are needed to understand exposures with new oral formulations of amphotericin B to ensure that exposure is optimized to achieve optimal outcomes at non-toxic dosing regimens.
Potential co-formulations of VL medicines were also discussed as part of the PADO for VL meeting. However, the group agreed that standalone formulations of medicines for VL allow for greater flexibility in the combinations to use, which is particularly important considering that different treatment options are used in different geographic settings.
PADO watch list for visceral leishmaniasis
New treatments that are currently being studied for VL aim to move away from existing drugs to new effective, oral, safe and easy-to-use treatments. In particular, these compounds are being studied as oral, well-tolerated and safe treatments with improved efficacy that can be used at the primary health care centre level and are affordable. Such new treatments can benefit all clinical forms of leishmaniasis and also benefit people with PKDL and VL-HIV co-infected patients.
While most new chemical entities are still in Phase I, compound LXE408 is in a more advanced stage of development (Phase II). LXE408 is a kinetoplastid-selective proteasome inhibitor that has shown good tolerability with no specific safety concerns for its investigation in children. The paediatric formulation that is being explored is a minitablet. Considering this, LXE408 was included in the PADO watch list for VL, signalling that once enough data are available from adult studies to initiate paediatric investigations, this should be done promptly to ensure that children with VL benefit from the latest available innovations (Table 1).
As most compounds for VL are undergoing early clinical development, a preliminary conversation on preferred product characteristics was initiated during the meeting (Table 2). The group noted that the lowest age for a VL therapeutic indication should be 2 years of age, even though expanding the study population to children down to 6 months of age would be relevant, especially for settings where a substantial proportion of people in need for VL treatment are children less than 2 years of age such as Sudan and countries in other endemic geographies. It was also acknowledged that obtaining ethical approvals and including this age group in clinical trials – whether for VL or other NTDs – presents significant challenges (24). There was a general consensus that drug formulations to treat VL in children should be oral formulations that are acceptable for children across the paediatric age spectrum, and stable at room temperature – eliminating the need for refrigeration to facilitate procurement and streamline logistics at country level. Indeed, there is an expectation that cost-effective, orally administered formulations that are stable at room temperature will significantly facilitate access and simplify distribution. Furthermore, the introduction of such formulations should be accompanied by efforts to build the capacity of healthcare personnel and to strengthen pharmacovigilance systems.
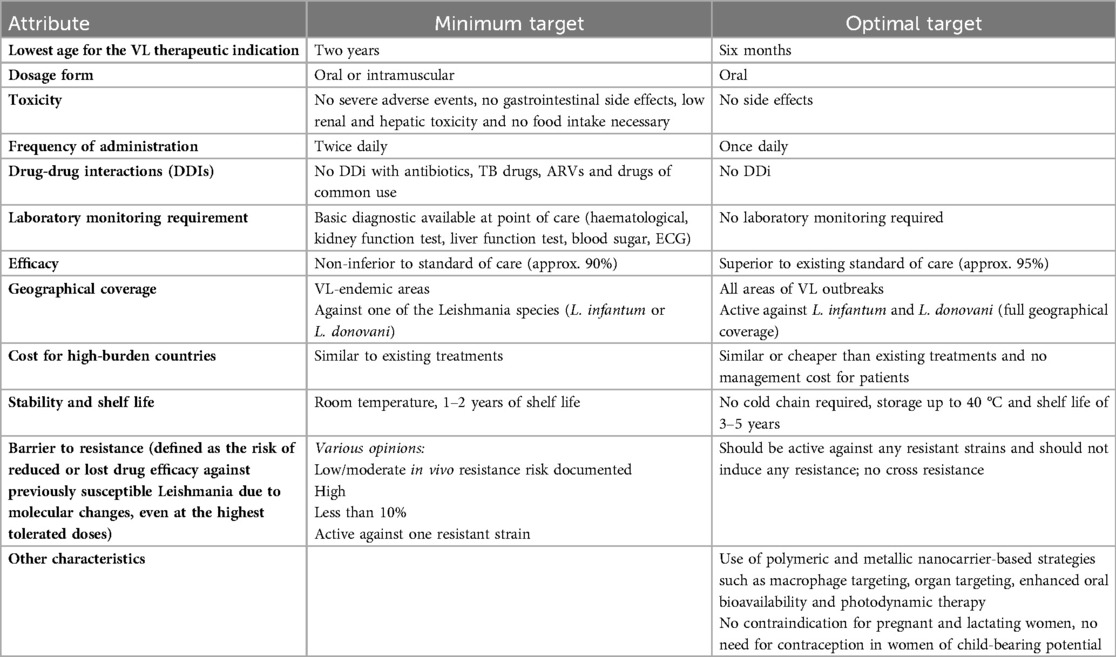
Table 2. First draft of proposed preferred product characteristics for medicines used in children with VL.
Research priorities
Following the priority-setting exercise, the PADO-VL group discussed research gaps for VL medicines for children to address with priority, which are summarized in Table 1.
Considering the high rates of gastrointestinal side effects of miltefosine and lack of regular food intake in endemic settings, the group noted the importance of investigating formulations with reduced gastrointestinal side effects. The absence of any side effects was also one of the key features noted in the PPC (Table 2). The development of simplified weight-band-based dosing tables for the proposed allometric dosing of miltefosine was also noted, with considerations around harmonizing weight bands with those used for other diseases that are common in VL high-burden settings.
Establishing specific exposure-response relationships for VL in relation to the target exposure for children was noted as a key priority to optimize dosing strategies for children. These studies, conducted only for miltefosine in specific settings, should be undertaken in all regions where VL is endemic to account for regional specificities and their effect on medicine PK.
Conclusions
Due to limited financial incentives, few new drugs are being developed for NTDs. In particular, children with NTDs are often left behind in accessing the latest available innovations that are suitable for them. This is particularly concerning given that several NTDs, such as VL, have a high burden in this vulnerable population. Research conducted in 2022 showed that, overall, less than half of WHO-recommended medicines for NTDs are approved for children, highlighting the urgent need to increase research activity for NTDs for children, giving priority to diseases that represent a significant burden and lack adequate treatment options (25).
The PADO for VL was the first ever exercise conducted to determine a clear set of priorities for children with VL by building consensus from various actors in the VL community. This will help researchers, developers and other stakeholders to focus efforts and mobilize funding around the identified priorities to ensure that children with VL have access to the latest available innovations that are suitable for them. The addition of a paediatric formulation of miltefosine to the WHO Prequalification expression of interest will promote generic production of this key formulation.
The PADO priorities will be monitored and, if needed, reviewed and revised in the future to guarantee alignment of the priorities to the latest evidence from ongoing and planned studies and development programmes.
This work represents a critical first step in prioritizing paediatric formulations for visceral leishmaniasis, contributing to a broader, coordinated effort to accelerate the development and equitable introduction of optimal treatments for children in resource-limited settings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
TM: Writing – review & editing, Conceptualization, Writing – original draft. AM-E: Writing – review & editing. DM: Writing – review & editing, Writing – original draft. PO: Writing – review & editing, Writing – original draft. KP: Writing – original draft, Writing – review & editing. MP: Writing – original draft, Writing – review & editing. SB: Writing – review & editing. AY: Writing – review & editing. AB: Writing – review & editing. SW: Writing – review & editing. SJ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1635252/full#supplementary-material
References
1. World Health Organization. Control of the leishmaniases: WHO TRS N°949. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010. Available online at: https://www.who.int/publications/i/item/WHO-TRS-949 (Accessed April 20, 2024).
2. Leishmaniasis fact sheet. Geneva: World Health Organization (2024). https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (Accessed February 2025).
3. Dye C, Williams BG. Malnutrition, age and the risk of parasitic disease: visceral leishmaniasis revisited. Proc Biol Sci. (1993) 254(1339):33–9. doi: 10.1098/rspb.1993.0123
4. Cerf BJ, Jones TC, Badaro R, Sampaio D, Teixeira R, Johnson WD. Malnutrition as a risk factor for severe visceral leishmaniasis. J Infect Dis. (1987) 156(6):1030–33. doi: 10.1093/infdis/156.6.1030
5. Jain S, Madjou S, Farah Virrey AJ, Maia-Elkhoury AN, Valadas S, Warasavithana S, et al. Global Leishmaniasis Surveillance Updates 2023: 3 Years of the NTD Road Map. Geneva: World Health Organization (2024). p. 653–69. Available online at: https://iris.who.int/bitstream/handle/10665/379491/WER9945-653-669.pdf?sequence=1 (Accessed August 21, 2025).
6. Paediatric drug optimization standard procedure. Geneva: World Health Organization (2021). Licence: CC BY-NC-SA 3.0 IGO. Available at: Available online at: https://www.who.int/publications/i/item/9789240039520 (Accessed February 10, 2025).
7. Paediatric drug optimization for neglected tropical diseases: meeting report, September 2023. Geneva: World Health Organization (2023). Licence: CC BY-NC-SA 3.0 IGO. Available online at: https://www.who.int/publications-detail-redirect/9789240085176 (Accessed February 10, 2025).
8. WHO guidelines for the Treatment of Visceral Leishmaniasis in HIV co-infected patients in East Africa and South-East Asia. Geneva: World Health Organization (2022). Licence: CC BY-NC-SA 3.0 IGO.
9. Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. (2002) 347(22):1739–46. doi: 10.1056/NEJMoa021556
10. Bhattacharya SK, Jha TK, Sundar S, Thakur CP, Engel J, Sindermann H, et al. Efficacy and tolerability of miltefosine for childhood visceral leishmaniasis in India. Clin Infect Dis. (2004) 38(2):217–21. doi: 10.1086/380638
11. Singh UK, Prasad R, Mishra OP, Jayswal BP. Miltefosine in children with visceral leishmaniasis: a prospective, multicentric, cross-sectional study. Indian J Pediatr. (2006) 73(12):1077–80. doi: 10.1007/BF02763048
12. Mazariegos Herrera A, Karlsson MO, Svensson EM, Dorlo TPC. Weight-band based simplification of oral allometric miltefosine dosing in pediatric patients with visceral leishmaniasis in Eastern Africa. Poster. PAGE. Thessaloniki, Greece (2025).
13. Musa AM, Mbui J, Mohammed R, Olobo J, Ritmeijer K, Alcoba G, et al. Paromomycin and miltefosine combination as an alternative to treat patients with visceral leishmaniasis in Eastern Africa: a randomized, controlled, multicountry trial. Clin Infect Dis. (2022) 76(3):e1177–85. doi: 10.1093/cid/ciac643
14. Younis BM, Musa AM, Monnerat S, Monnerat S, Saeed MA, Khalil EAG, et al. Safety and efficacy of paromomycin/miltefosine/liposomal amphotericin B combinations for the treatment of post-kala-azar dermal leishmaniasis in Sudan: a phase II, open label, randomized, parallel arm study. PLoS Negl Trop Dis. (2023) 17(11):e0011780. doi: 10.1371/journal.pntd.0011780
15. Sundar S, Pandey K, Mondal D, Madhukar M, Topno RK, Kumar A, et al. A phase II, non-comparative randomised trial of two treatments involving liposomal amphotericin B and miltefosine for post-kala-azar dermal leishmaniasis in India and Bangladesh. PLoS Negl Trop Dis. (2024) 18(6):e0012242. doi: 10.1371/journal.pntd.0012242
16. Gerrard SE, Walsh J, Bowers N, Salunke S, Hershenson S. Innovations in pediatric drug formulations and administration technologies for low resource settings. Pharmaceutics. (2019) 11(10):518. doi: 10.3390/pharmaceutics11100518
17. Pan American Health Organization. Guideline for the Treatment of Leishmaniasis in the Americas. 2nd edn. Washington, DC: PAHO (2022). doi: 10.37774/9789275125038
18. Carnielli JBT, Monti-Rocha E, Costa DL, Molina Sesana A, Pansini LNN, Segatto M, et al. Natural resistance of leishmania infantum to miltefosine contributes to the low efficacy in the treatment of visceral leishmaniasis in Brazil. Am J Trop Med Hyg. (2019) 101(4):789–94. doi: 10.4269/ajtmh.18-0949
19. Boulware D. Amphotericin B, an oral lipid nanocrystal for cryptococcal meningitis, Phase 2 results: David Boulware, ECCMID 2023 [video]. Stockport: touchINFECTIOUS DISEASES (2023).
20. Encochleated Oral Amphotericin for Cryptococcal Meningitis Trial 3 (EnACT3). Washington, DC: National Library of Medicine (2023).
21. Wasan E, Mandava T, Crespo-Moran P, Nagy A, Wasan KM. Review of novel oral amphotericin B formulations for the treatment of parasitic infections. Pharmaceutics. (2022) 14(11):2316. doi: 10.3390/pharmaceutics14112316
22. Wasan EK, Gershkovich P, Zhao J, Zhu X, Werbovetz K, Tidwell RR, et al. A novel tropically stable oral amphotericin B formulation (iCo-010) exhibits efficacy against visceral leishmaniasis in a murine model. PLoS Negl Trop Dis. (2010) 4(12):e913. doi: 10.1371/journal.pntd.0000913
23. Wasan KM, Wasan EK, Gershkovich P, Zhu X, Tidwell RR, Webovetz KA, et al. Highly effective oral amphotericin B formulation against murine visceral leishmaniasis. J Infect Dis. (2009) 200(3):357–60. doi: 10.1086/600105
24. N'Goran E, Aka NAD, Ouattara M, Huber E, Bezuidenhout D, Kourany-Lefoll E. Challenges and lessons from conducting a paediatric clinical trial in sub-saharan Africa: the case of the praziquantel oral dispersible tablets phase II study in côte d’Ivoire. Adv Parasitol. (2019) 103:75–89. doi: 10.1016/bs.apar.2018.09.002
Keywords: visceral leishmaniasis, Kala-azar, paediatrics, miltefosine, amphotericin B
Citation: Masini T, Maia-Elkhoury ANS, Mondal D, Olliaro P, Paudel KN, Penazzato M, Branco Valadas SYO, Yajima A, Beshah A, Warusavithana S and Jain S (2025) Optimization and prioritization of paediatric drugs for visceral leishmaniasis. Front. Pediatr. 13:1635252. doi: 10.3389/fped.2025.1635252
Received: 13 June 2025; Accepted: 31 July 2025;
Published: 4 September 2025.
Edited by:
Catherine M. T. Sherwin, University of Western Australia, AustraliaReviewed by:
Vishal Kumar Singh, Banaras Hindu University, IndiaAmrita Kar, SASTRA University, India
Valdir Sabbaga Amato, University of São Paulo, Brazil
Copyright: © 2025 Masini, Maia-Elkhoury, Mondal, Olliaro, Paudel, Penazzato, Branco Valadas, Yajima, Beshah, Warusavithana and Jain. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saurabh Jain, amFpbnNhdUB3aG8uaW50
 Tiziana Masini
Tiziana Masini Ana Nilce Silveira Maia-Elkhoury2
Ana Nilce Silveira Maia-Elkhoury2 Dinesh Mondal
Dinesh Mondal Piero Olliaro
Piero Olliaro Khechar N. Paudel
Khechar N. Paudel Saurabh Jain
Saurabh Jain