- 1Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
- 2Department of Pediatrics, The Affiliated Hospital of Jiangxi University of Chinese Medicine, Nanchang, Jiangxi, China
Objective: This network meta-analysis aims to explore the efficacy and safety of probiotics in children with asthma and attempts to determine which probiotics are most effective in improving outcomes in children with asthma by ranking methods.
Methods: A systematic search of Chinese and English databases, including China National Knowledge Infrastructure, Wanfang, VIP, PubMed, and Web of Science, was conducted from the establishment of the databases until July 2024 to screen for randomized controlled trials (RCTs) of probiotics in the treatment of childhood asthma. Lung function was used as the primary outcome measure, and secondary outcome measures included the total clinical response rate, recurrence rate, immune factors, cytokines, and Childhood Asthma Control Test (C-ACT) score. Data processing and analysis were performed using RevMan 5.4 and Stata 17.0 software.
Results: A total of 34 RCTs were included, involving 3,839 participants and 13 interventions. Our analysis showed that conventional treatment combined with probiotics improved outcome indicators in children with asthma better than conventional treatment. Conventional treatment combined with Bifidobacterium–Lactobacillus triplex live bacteria had the highest probability of being the optimal intervention in terms of increasing FEV1% and recurrence rate. Conventional treatment combined with Bifidobacterium adolescentis had the highest probability of being the optimal intervention in increasing FEV1. Conventional treatment combined with Lactobacillus tablets had the highest probability of being the optimal intervention in increasing peak expiratory flow. Conventional treatment combined with Bacillus subtilis diplex live bacteria had the highest probability of being the optimal intervention in improving the total clinical response rate. Conventional treatment combined with Bifidobacterium quadruplex live bacteria had the highest probability of being the optimal intervention in reducing IL-4 and IL-33. Conventional treatment combined with Bifidobacterium triplex live bacteria had the highest probability of being the optimal intervention in improving the C-ACT score.
Conclusion: Probiotics are effective in treating childhood asthma, and the therapeutic effect of conventional treatment combined with probiotics is superior to that of conventional treatment alone. Therefore, probiotics can be selected as appropriate in the clinical treatment of childhood asthma. However, the overall quality of the evidence was at most low or moderate, suggesting that the certainty of the evidence for probiotics in treating childhood asthma was low.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, PROSPERO CRD42024617940.
1 Background
Asthma is a heterogeneous disease characterized by chronic airway inflammation and airway hyperresponsiveness. Clinically, it is characterized by recurrent episodes of wheezing, coughing, shortness of breath, and chest tightness, which often occur or worsen at night and/or in the early hours of the morning. The cumulative incidence rates of childhood asthma in 1990, 2000, and 2010 were 1.09%, 1.97%, and 3.02% (1) based on epidemiological investigations. While asthma hospitalizations and deaths have declined in some countries, asthma, especially childhood asthma, still imposes an unacceptable burden (2). Beta-agonists and leukotriene antagonists are commonly used in clinical practice to treat childhood asthma, but they are less effective in regulating immune function, so childhood asthma is prone to relapse (3) and eventually develops into adult asthma (4).
With further research on the gut microbiota, researchers have found that probiotics can improve lung function in children with asthma, increase the total clinical response rate, improve quality of life, and reduce the recurrence rate (5) of asthma. The types of probiotics involved in these reports on treating childhood asthma are diverse. Currently, there is a lack of comparative studies on the efficacy of different interventions, and there is no mention of probiotic types, specific usage, or treatment regimens (6) in the relevant guideline for childhood asthma. As a result, clinicians often rely on subjective experience when making treatment decisions. Therefore, this study uses a network meta-analysis method to compare the effects of different probiotic interventions on children’s lung function, clinical efficacy, and reduction of recurrence rate, to provide evidence-based medical support for optimizing drug selection in clinical practice.
2 Data and analysis
2.1 Inclusion and exclusion criteria
The inclusion and exclusion criteria for this study were based on the PICOS strategy. Only randomized controlled trials (RCTs) reported in English or Chinese were included, with no geographical restrictions. Non-RCTs, animal experiments, case studies, expert opinions, and other types of studies were excluded.
This study included children with asthma aged 5–18 years. The age range of 5–18 years was selected to ensure diagnostic accuracy, as lung function testing is challenging in younger children, and to maintain homogeneity across studies. Intervention measures in the experimental group included probiotics (Bifidobacterium triplex live bacteria, Bifidobacterium quadruplex live bacteria, Bifidobacterium–Lactobacillus triplex live bacteria, Bifidobacterium adolescentis, Saccharomyces boulardii, Bacillus subtilis diplex live bacteria, Lactobacillus tablets, LF(LactobacillusBCRC 910259), LP(LactobacillusGMNL-133)) combined with conventional treatment. The control group received conventional treatment [standard treatment for childhood asthma based on contemporary guidelines, mainly including but not limited to: inhaled glucocorticoids (ICS), leukotriene receptor antagonists, short-acting/long-acting beta-2 agonists (SABA/LABA), anticholinergic drugs] or conventional treatment combined with placebo.
The exclusion criteria included (1) in cases of duplicate publications, only the earliest article (by publication date) is included, and if the same manuscript has been submitted to multiple publications, only the version with the most complete data is selected; (2) non-RCT and RCT studies not involving children; (3) reviews, discussions, empirical cases, animal experiments, etc.; (4) literature with missing data or where full text cannot be obtained; and (5) literature involving participants with coexisting organic diseases or other diseases.
2.2 Database and retrieval strategy
The system searched Chinese and English databases, including China National Knowledge Infrastructure, Wanfang, VIP, PubMed, and Web of Science. The search period was from the establishment of the database to 18 July 2024. Search terms include “Asthma,” “Cough-Variant Asthma,” “bronchial asthma,” “child*,” “Adolescence,” “probiotics,” “Bifidobacterium,” “Yeast fungus,” and “Bacillus subtilis double viable bacteria.”
2.3 Data screening and quality assessment
Two researchers (JS, MZ) independently conducted the literature search and screening using Zotero according to the established relevant criteria. They cross-checked the results, and any discrepancies were discussed with a third researcher until a consensus was reached.
Two researchers (DZ, YL) used the modified Jadad scale (7) and the Cochrane risk of bias tool (8) to assess the risk of deviation and quality of the included RCTs, and any disagreement was resolved by discussing with a third researcher.
2.4 Outcome measures
The primary outcome measures were lung function, including the ratio of forced expiratory volume in one second to forced vital capacity (FEV1/FVC), the percentage of forced expiratory volume in 1 s to the projected value (FEV1%), forced expiratory volume in 1 s (FEV1), and peak expiratory flow (PEF). The secondary outcome measures were (1) clinical total effective rate, (2) recurrence rate, (3) adverse reactions, (4) immune factors (IgA and IgE), (5) cytokines (IL-4 and IL-33), and (6) Childhood Asthma Control Test (C-ACT) score.
2.5 Data analysis
The network structure was star-shaped, and there were no direct comparisons, so this study relied on the transitivity hypothesis. We assessed transitivity by examining the following: (1) patient characteristics, all studies included children aged 5–18 years with mild-to-moderate persistent asthma; (2) conventional treatment principles, while specific drug regimens varied (ICS monotherapy, ICS + LABA, montelukast), all followed stepwise management principles per contemporaneous guidelines; and (3) study methodology, all were RCTs with similar outcome assessment methods.
2.5.1 Outcome measures
For binary variables, relative risk (RR) was used as the effect size, whereas for continuous variables, standardized mean difference (SMD) was used as the effect size. All SMDs were based on endpoint values after intervention, and the effect size was expressed as a 95% confidence interval (95% CI).
2.5.2 Analysis method
A network plot was plotted using Stata 17.0. All RCTs including one multi-arm trial (9) were included and analyzed as single units using multivariate meta-analysis models without manual splitting. For effect size calculation, Hedges’ g and its standard error (SE) were used for SMD. The comparative relationship between studies is presented visually through a network diagram. Subgroup analysis and meta-regression were conducted at high heterogeneity (I2 > 50%). The therapeutic effects of the intervention were ranked by the surface under the cumulative ranking curve (SUCRA), and a SUCRA difference of >15% with non-overlapping CI was considered a significant difference. Funnel plots were plotted to assess the small sample effect. The adequacy of the sample size was evaluated through assessment of the precision of effect estimates and confidence interval widths.
3 Results
3.1 Literature search results
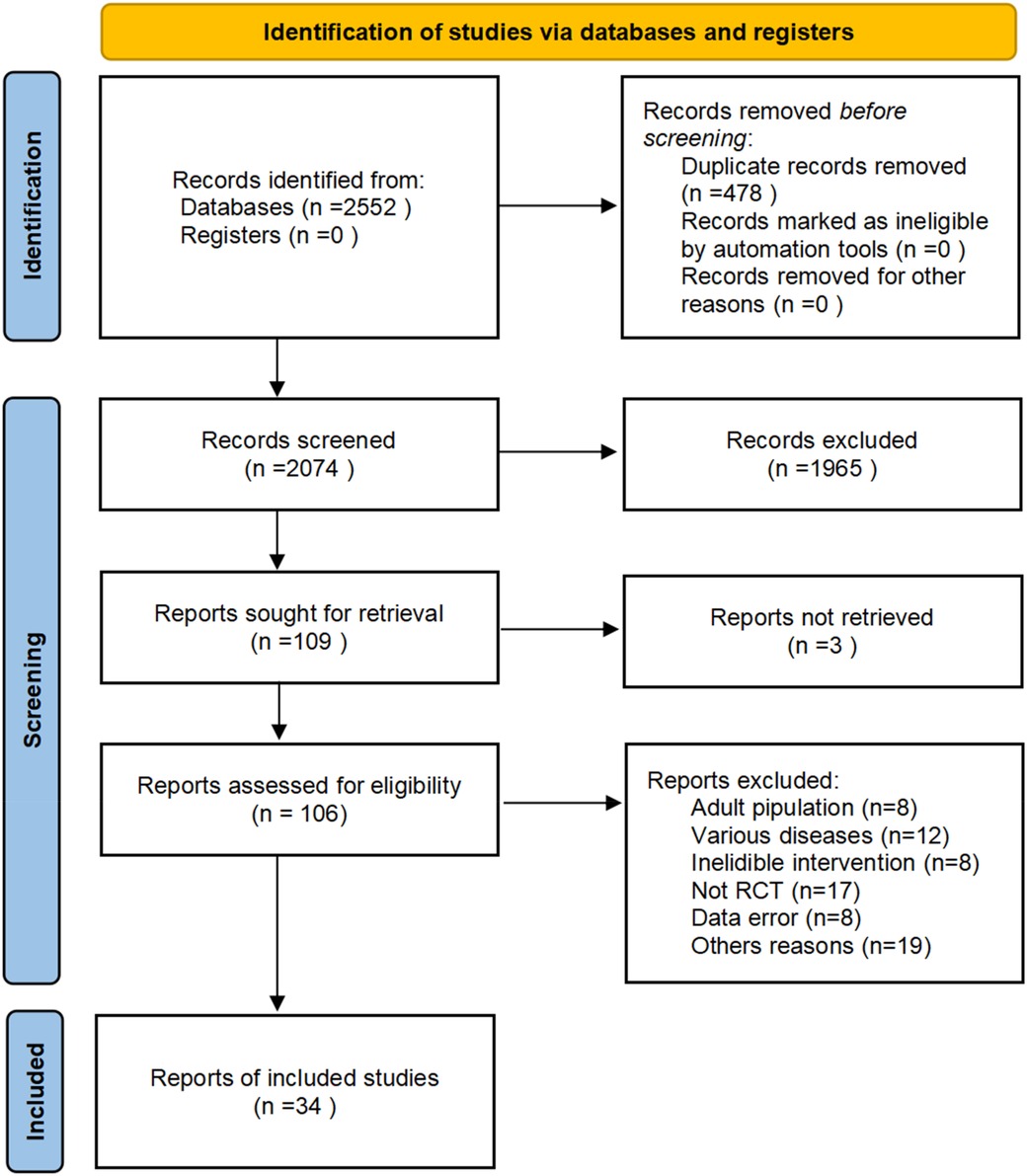
A total of 2,552 studies were retrieved from the database, and 478 duplicates were excluded. After screening for titles and abstracts, 109 records met the criteria for a full-text analysis. Among them, 72 studies were excluded by reading the full text. In total, 34 studies were included in this study (the literature screening process is shown in Figure 1).
3.2 Basic information and quality assessment of the included literature
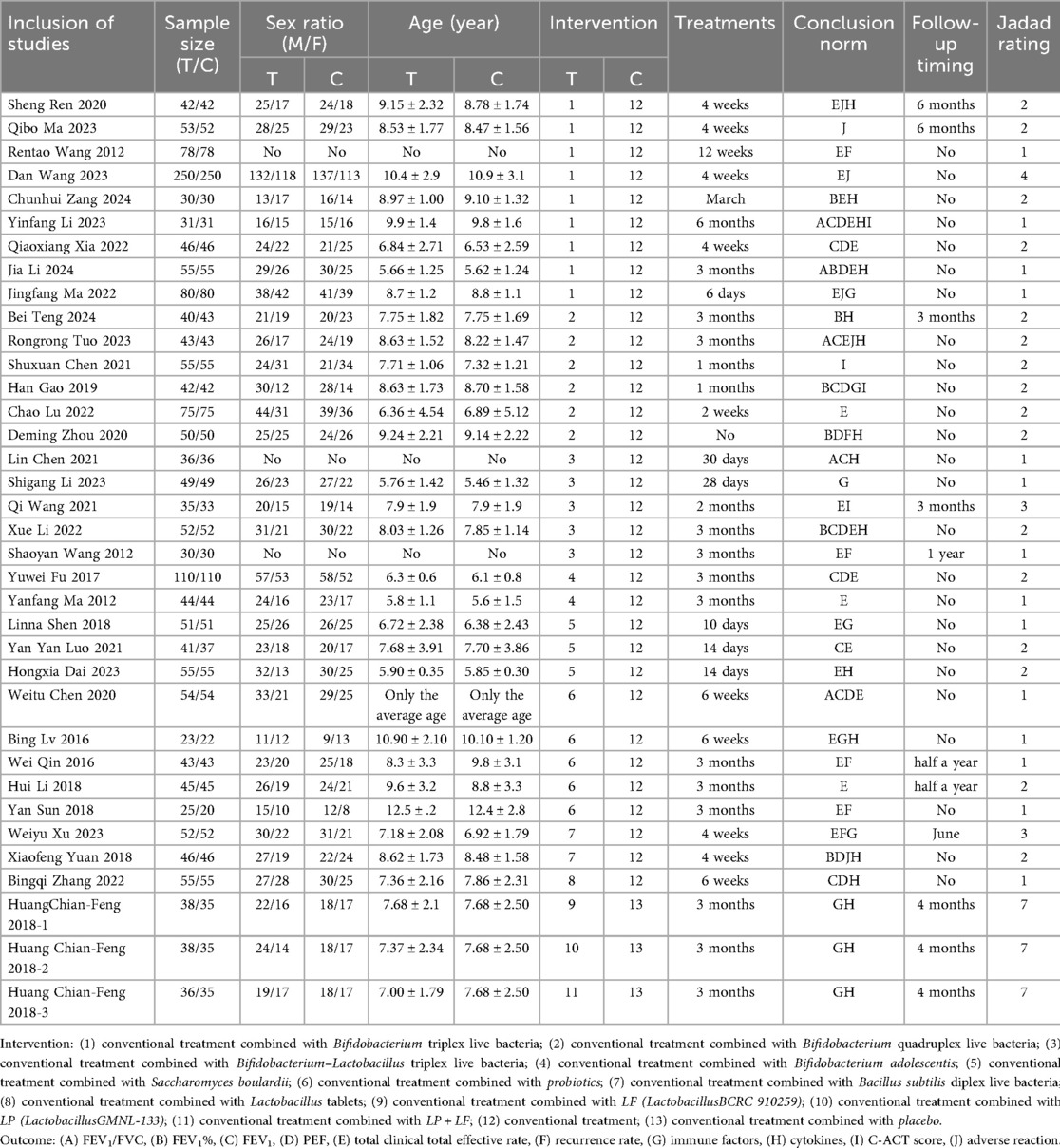
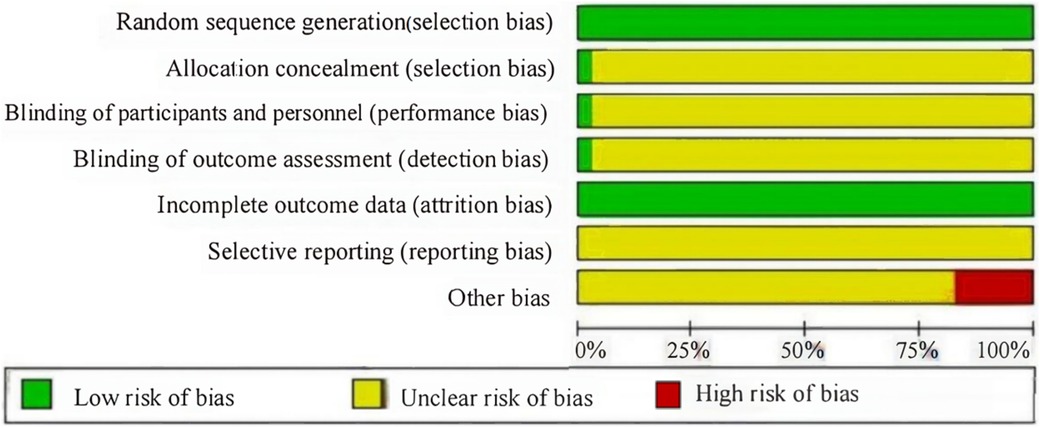
A total of 34 studies were included, 33 from a Chinese database and 1 from an English database. This study involved 3,839 participants and 13 interventions. The basic information of the included studies and the evaluation of literature quality (Jadad score) are presented in Table 1. The Cochrane risk of bias tool was used to evaluate the risk of deviation in the included studies (Figure 2). All the included studies were RCTs. Only one study discussed the use of allocation concealment and blinding, two studies discussed data dropout, the rest did not report data dropout or loss to follow-up, and six studies had small sample sizes and were at high risk of bias.
3.3 Network meta-analysis
3.3.1 Network diagrams of different interventions
The network diagram of conventional treatment combined with probiotics vs. conventional treatment is shown in Figure 3. Dots represent intervention measures, and arms represent comparative studies. The larger the dots, the larger the sample size included. The thicker the arms, the higher the contribution of the corresponding comparison studies (related to the number of references and sample size). Among them, the combination of conventional treatment and Bifidobacterium triplet live bacteria has the most direct comparisons with conventional treatment.
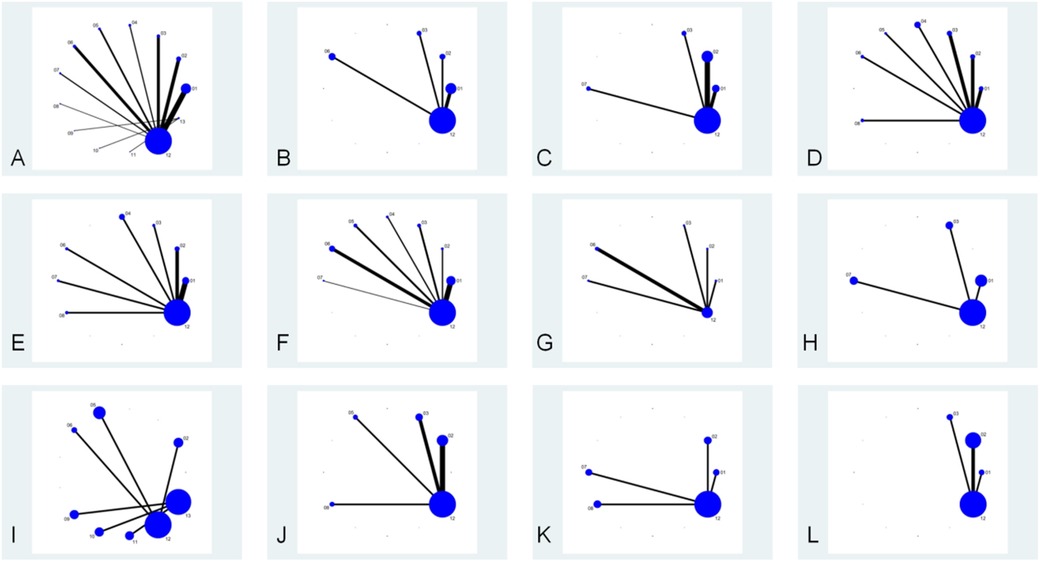
Figure 3. Network relationship diagram of each intervention, lung function, clinical effectiveness, recurrence rate, immune factors, cytokines, and C-ACT scores. The node “conventional treatment combined with probiotics” includes studies using probiotic supplements without specifying strain identity or commercial products. Each study was assigned to only one node based on intervention description clarity. (A) Each intervention; (B) FEV1/FVC; (C) FEV1%; (D) FEV1; (E) PEF; (F) total clinical effective rate; (G) recurrence rate; (H) IgA; (I) IgG; (J) IL-4; (K) IL-33; (L) C-ACT score. (1) Conventional treatment combined with Bifidobacterium triplex live bacteria; (2) conventional treatment combined with Bifidobacterium quadruplex live bacteria; (3) conventional treatment combined with Bifidobacterium–Lactobacillus triplex live bacteria; (4) conventional treatment combined with Bifidobacterium adolescentis; (5) conventional treatment combined with Saccharomyces boulardii; (6) conventional treatment combined with probiotics; (7) conventional treatment combined with Bacillus subtilis diplex live bacteria; (8) conventional treatment combined with Lactobacillus tablets; (9) conventional treatment combined with LF (LactobacillusBCRC 910259); (10) conventional treatment combined with LP (LactobacillusGMNL-133); (11) conventional treatment combined with LP + LF; (12) conventional treatment; (13) conventional treatment combined with placebo.
3.3.2 Inconsistency test
Due to the open network structure without closed loops, inconsistency testing was neither feasible nor necessary. We employed a consistency model throughout, assuming agreement between direct and indirect evidence.
3.4 Outcome measures
3.4.1 Primary outcome measures
3.4.1.1 FEV1/FVC
Five studies (10–14) were included in the analysis for the pooled estimation analysis of FEV1/FVC, and the network diagram (Figure 3) involved four interventions involving a total of 438 participants. Funnel plots were plotted, and Egger’s test was performed to assess publication bias for the metric. The funnel plots were visually asymmetrical, and the Egger’s test was performed with a P-value of 0.049, which is statistically significant but close to the critical value, suggesting the possibility of potential publication bias, considering the small sample effect and the higher possibility of publication bias in combination. The heterogeneity test for the forest plot (Figure 4) showed I2 = 97%, P = 0.000, suggesting high heterogeneity among the studies. Meta-regression and subgroup analyses were conducted on the included studies, and heterogeneity did not decrease. It is speculated that the sources of heterogeneity may be publication bias, small sample size, and low-quality literature. The random-effects model was used for statistical analysis. The combined SMD value in this study was 2.18, 95% CI (0.81, 3.55), and the difference was statistically significant, suggesting that probiotics are effective in enhancing FEV1/FVC function in children with asthma. In the SUCRA plot of the four interventions affecting FEV1/FVC (Figure 5), conventional treatment combined with probiotics has the largest area, indicating the highest probability of being the optimal intervention in improving the FEV1/FVC index in children with asthma.

Figure 4. Forest plots for lung function, clinical effectiveness, recurrence rate, immune factors, cytokines, and C-ACT scores. (A) FEV1/FVC; (B) FEV1%; (C) FEV1; (D) PEF; (E) total clinical effective rate; (F) recurrence rate; (G) IgA; (H) IgG; (I) IL-4; (J) IL-33; (K) C-ACT score.
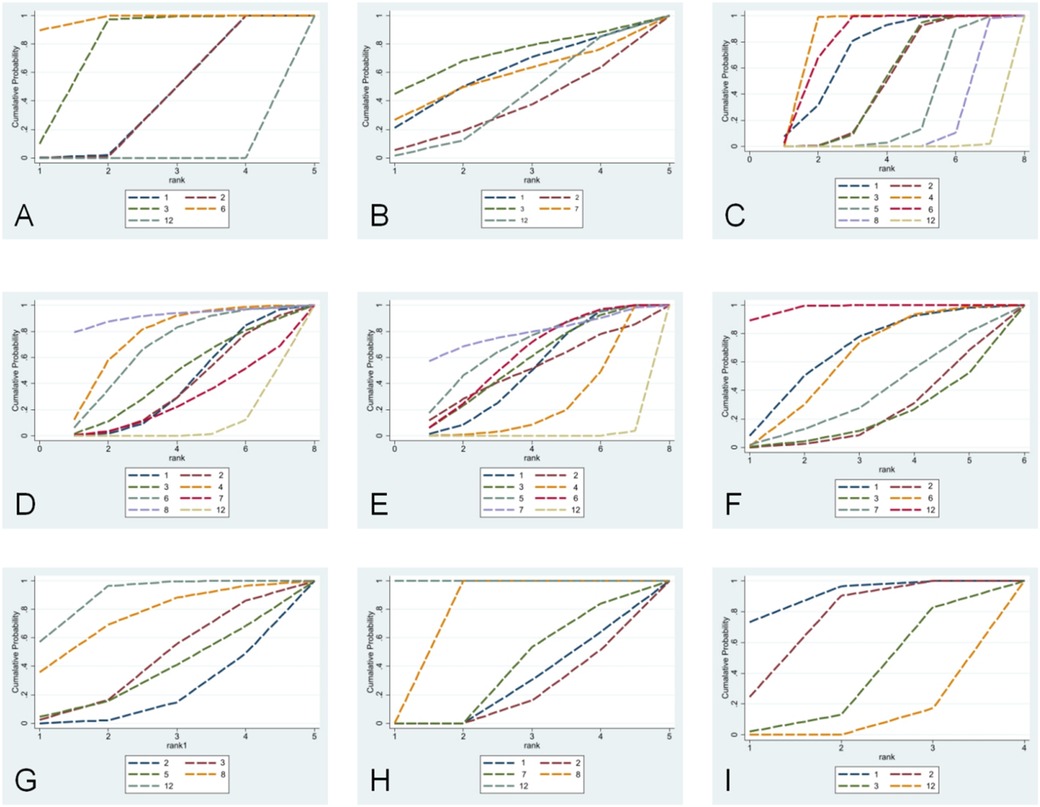
Figure 5. SUCRA plots for lung function, clinical effectiveness, recurrence rate, cytokines, and C-ACT scores. (A) FEV1/FVC; (B) FEV1%; (C) FEV1; (D) PEF; (E) total clinical effective rate; (F) recurrence rate; (G) IL-4; (H) IL-33; (I) C-ACT score. (1) Conventional treatment combined with Bifidobacterium triplex live bacteria; (2) conventional treatment combined with Bifidobacterium quadruplex live bacteria; (3) conventional treatment combined with Bifidobacterium–Lactobacillus triplex live bacteria; (4) conventional treatment combined with Bifidobacterium adolescentis; (5) conventional treatment combined with Saccharomyces boulardii; (6) conventional treatment combined with probiotics; (7) conventional treatment combined with Bacillus subtilis diplex live bacteria; (8) conventional treatment combined with Lactobacillus tablets; (9) conventional treatment combined with LF (LactobacillusBCRC 910259); (10) conventional treatment combined with LP (LactobacillusGMNL-133); (11) conventional treatment combined with LP + LF; (12) conventional treatment; (13) conventional treatment combined with placebo.
3.4.1.2 FEV1%
Six studies (11, 15–20) were included in the analysis for the pooled estimate analysis of FEV1%, and the network diagram (Figure 3) involved six interventions involving a total of 527 participants. Funnel plots were plotted, and Egger's test was performed to assess publication bias in the indicator. The funnel plots were significantly asymmetrical, suggesting a potential for publication bias. Egger's test was performed with a P-value of 0.058, which was not statistically significant, considering the marginal risk of publication bias in combination with the two. The heterogeneity test for the forest plot (Figure 4) showed I2 = 59.8%, P = 0.029, suggesting significant heterogeneity among the studies. Meta-regression and subgroup analysis of the included studies showed no decline in heterogeneity, suggesting that the sources of heterogeneity might be publication bias, small sample size, and low-quality literature. The random-effects model was used for statistical analysis, with a combined SMD value of 0.47, 95% CI (0.20, 0.75), and the difference was statistically significant, suggesting that probiotics are effective in improving FEV1% function in children with asthma. In the SUCRA ranking chart of the six interventions affecting FEV1% (Figure 5), conventional treatment combined with Bifidobacterium Lactobacillus triplex live bacteria has the largest area, indicating the highest probability of being the optimal intervention in improving the FEV1% index in children with asthma.
3.4.1.3 FEV1
Ten studies (10, 12–14, 17, 19, 21–24) reported FEV1, and the network diagram (Figure 3) involved eight interventions involving a total of 1,010 subjects. To assess publication bias for the indicator, funnel plots were plotted, and Egger's test was conducted. The funnel plots were symmetrically distributed, and Egger's test was performed with a P-value of 0.757, which was not statistically significant. Considering the lack of significant publication bias and the small sample effect, the results showed a lower risk of selective publication. The heterogeneity test for the forest plot (Figure 4) showed I2 = 88.7%, P = 0.000, suggesting significant heterogeneity among the studies. Meta-regression and subgroup analysis were conducted on the included studies, and the conclusion was that the number of strains (i.e., single strain and compound strain) was one of the sources of heterogeneity. After excluding the single strain, the heterogeneity test yielded I2 = 0.00%, P = 0.610. Two studies (14, 22) were identified as sources of heterogeneity. After excluding these two studies, the heterogeneity test yielded I2 = 0.00%, P = 0.510. Therefore, it is presumed that the sources of heterogeneity are these two studies and the number of strains. Using the random-effects model for statistical analysis, the combined SMD value was 0.99, 95% CI (0.59, 1.39), and the difference was statistically significant, suggesting that probiotics are effective in enhancing FEV1 function in children with asthma. In the SUCRA ranking chart of the eight interventions affecting FEV1 (Figure 5), conventional treatment combined with Bifidobacterium adolescentis has the largest area, indicating the highest probability of being the optimal intervention in improving FEV1 indicators in children with asthma.
3.4.1.4 PEF
Ten studies (10, 11, 14, 17–22, 24) reported PEF, and the network diagram (Figure 3) involved seven intervention measures, involving a total of 1,076 subjects. To assess publication bias for the indicator, funnel plots were plotted, and Egger's test was conducted. The funnel plots were relatively symmetrical in distribution, and Egger's test was performed with a P-value of 0.157, which was not statistically significant. No small sample effect was found. Considering the low risk of interference from publication bias, the results were relatively robust, and the reliability of the conclusion was guaranteed to some extent. The heterogeneity test for the forest plot (Figure 4) showed I2 = 96.8%, P = 0.000, suggesting significant heterogeneity among the studies. Meta-regression and subgroup analysis were conducted on the included studies, suggesting that the sources of heterogeneity might be publication bias, small sample sizes, and low-quality literature. Therefore, a random-effects model was used for statistical analysis. The combined SMD value was 1.76, 95% CI (0.74, 2.78), and the difference was statistically significant, suggesting that probiotics are effective in improving PEF function in children with asthma. In the SUCRA ranking chart of the seven interventions affecting PEF (Figure 4), conventional treatment combined with Lactobacillus tablets has the largest area, indicating the highest probability of being the optimal intervention in improving PEF indicators in children with asthma.
3.4.2 Secondary outcome measures
3.4.2.1 Clinical total effective rate
Twenty-four studies (5, 10–12, 14, 15, 19, 21–23, 25–38) reported the clinical total effective rate, and the network diagram (Figure 3) involved 11 interventions involving a total of 2,768 participants. To assess the publication bias of this indicator, funnel plots were plotted, and Egger's test was conducted. The funnel plots were asymmetrically distributed left and right, with a small portion close to the bottom of the funnel, suggesting a possible small sample effect and publication bias. Egger's test was performed with a P-value of 0.012, and the slope was statistically significant, considering the small sample effect and publication bias in combination with the two. The heterogeneity test for the forest plot (Figure 4) showed I2 = 0%, P = 1.000, suggesting that the heterogeneity among the studies was small. A fixed-effect model was used for statistical analysis. The combined RR value was 1.18, 95% CI (1.14, 1.21), and the difference was statistically significant, suggesting that probiotics were effective in the clinical total effective rate of treating childhood asthma. In the SUCRA ranking chart of 13 interventions affecting total clinical efficacy (Figure 5), conventional treatment combined with Bacillus subtilis diplex live bacteria has the largest area in the SUCRA chart, indicating the highest probability of being the optimal intervention in improving the total clinical efficacy index in children with asthma.
3.4.2.2 Recurrence rate
Six studies (18, 25, 30, 35, 37, 38) reported recurrence rates, and the network diagram (Figure 3) involved six interventions involving a total of 544 subjects. To assess the publication bias of the indicator, funnel plots were plotted, and Egger's test was conducted. The funnel plots were asymmetrically distributed, mostly near the bottom of the funnel, suggesting a possible small sample effect. Egger's test was performed with a P-value of 0.069, which was not statistically significant, considering the marginal risk of the small sample effect in combination with the two. Subgroup analysis by follow-up duration (0–6 months vs. 6–12 months) showed that follow-up time was not a source of heterogeneity. The heterogeneity test for the forest plot (Figure 4) showed I2 = 0.00%, P = 0.528, suggesting that the heterogeneity among the studies was small. A fixed-effect model was used for statistical analysis. The combined RR value was 0.31, 95% CI (0.21, 0.48), and the difference was statistically significant, suggesting that probiotics are effective in reducing the recurrence rate of asthma in children. In the SUCRA ranking chart of the six interventions affecting the clinical total effective rate (Figure 5), conventional treatment combined with Bifidobacterium–Lactobacillus–Enterococcus triple probiotic has the smallest area, indicating the highest probability of being the optimal intervention in reducing the recurrence rate of asthma in children.
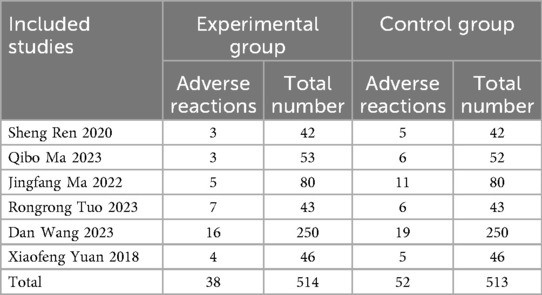
3.4.2.3 Adverse reactions
Six studies (3, 5, 12, 20, 26, 27) reported adverse reaction rates, with no statistically significant difference. The combination of probiotics did not increase the risk of adverse events compared to conventional treatment alone. Adverse reactions are shown in Table 2 (P > 0.05).
3.4.2.4 Immune factors IgA and IgE
Three studies (27, 38, 39) reported IgA, and the network diagram (Figure 3) involved four interventions involving a total of 362 subjects. The heterogeneity test for the forest plot (Figure 4) showed I2 = 97.9%, P = 0.000, suggesting significant heterogeneity among the studies. Due to the small sample size, meta-regression and subgroup analysis were not conducted, and the random-effects model was used for statistical analysis. The combined SMD value was 2.11, 95% CI (0.29, 3.92), and the difference was statistically significant. That is, conventional treatment combined with probiotics was better than conventional treatment in improving IgA.
Four studies (9, 17, 32, 34) reported IgE, and the network diagram (Figure 3) involved four interventions involving a total of 372 subjects. The heterogeneity test for the forest plot (Figure 4) showed I2 = 95.2%, P < 0.001, suggesting significant heterogeneity among the studies. Due to the small sample size, meta-regression and subgroup analysis were not conducted, and the random-effects model was used for statistical analysis. The combined SMD value was −1.53, 95% CI (−2.69, −0.37), and the difference was statistically significant. Conventional treatment combined with probiotics was better than conventional treatment in improving IgE.
3.4.2.5 Cytokines IL-4, IL-33
Seven studies (12, 13, 16, 18, 19, 24, 33) reported IL-4. The network diagram (Figure 3) involved five intervention measures and a total of 665 subjects. Egger's test was performed on the data, with a P-value of 0.008, which was statistically significant, considering publication bias and small sample effects. The heterogeneity test for the forest plot (Figure 4) showed I2 = 95.4%, P = 0.000, suggesting significant heterogeneity among the studies. Meta-regression and subgroup analysis were conducted on the included studies, and the conclusion was that the Jadad score was one of the sources of heterogeneity. It was speculated that the sources of heterogeneity might be publication bias, small sample sizes, and low-quality literature. Random-effects models were used for statistical analysis. The combined SMD value was −1.87, 95% CI (−2.84, −0.9), and the difference was statistically significant, suggesting that conventional treatment combined with probiotics was better than conventional treatment in reducing IL-4. In the SUCRA ranking chart of the effects of IL-4 among the five interventions (Figure 5), conventional treatment combined with Bifidobacterium quadruplex live bacteria has the smallest area, indicating the highest probability of being the optimal intervention in improving IL-4 indicators in children with asthma.
Four studies (5, 18, 20, 24) reported IL-33, and the network diagram (Figure 3) involved five interventions involving a total of 386 subjects. Egger's test was performed with a P-value of 0.016, which was statistically significant, considering publication bias and small sample effect. The heterogeneity test for the forest plot (Figure 4) showed I2 = 95.4%, P = 0.000, suggesting significant heterogeneity among studies. Due to the small sample size, meta-regression and subgroup analysis were not conducted, and the random-effects model was used for statistical analysis. The combined SMD value was −1.94, 95% CI (−2.95, −0.93), and the difference was statistically significant. That is, conventional treatment combined with probiotics was better than conventional treatment in reducing IL-33. In the SUCRA ranking chart of the five interventions affecting IL-33 (Figure 5), conventional treatment combined with Bifidobacterium quadruplex live bacteria has the smallest area, indicating the highest probability of being the optimal intervention in improving IL-33 indicators in children with asthma.
3.4.2.6 C-ACT score
Four studies (10, 17, 29, 40) reported C-ACT scores, with network diagrams (Figure 3) involving four interventions involving a total of 318 subjects. The heterogeneity test for the forest plot (Figure 4) showed I2 = 73.3%, P = 0.011, suggesting significant heterogeneity among the studies. Due to the small sample size, meta-regression and subgroup analysis were not conducted, and the random-effects model was used for statistical analysis. The combined SMD value was 1.16, 95% CI (0.69, 1.63), and the difference was statistically significant. That is, conventional treatment combined with probiotics significantly improved C-ACT scores compared to conventional treatment alone. In the SUCRA ranking chart of the four interventions affecting the C-ACT score (Figure 5), conventional treatment combined with Bifidobacterium triplex live bacteria has the largest area, indicating the highest probability of being the optimal intervention in improving the C-ACT index in children with asthma.
4 Discussion
The Global Burden of Disease Study 2016 estimates that more than 339 million people worldwide have asthma, and the age-standardized prevalence has increased by 3.6% since 2006. First asthma often appears in early childhood, with global prevalence rates of childhood wheezing and adolescent wheezing being 11.7% and 14.1% respectively, increasing by an average of 0.13% and 0.06% (41) annually. Professor David Strachan believes that too little exposure of children to environmental bacteria can lead to an increased (41) risk of allergies and asthma. Based on this hypothesis, probiotics have been proposed for the prevention and treatment (42) of allergic diseases such as asthma. This network meta-analysis focused on clinical outcomes rather than mechanistic pathways, as the included RCTs primarily reported clinical endpoints. While our analysis cannot establish strain-specific mechanisms from clinical outcome data alone, published mechanistic studies suggest different mechanisms for various strains. For example, Bifidobacterium can stimulate the Th1/Th2 balance and upregulate the secretion of IFN-γ, IL-4, and IL-12 in the spleen. Lactobacillus plantarum can reduce the number of innate immune cells in the lungs and the levels of IL-6 and TNF-α in bronchoalveolar lavage fluid and induce immunosuppressive Treg responses (43) in the lungs.
In clinical practice, it is necessary not only to follow the principles of relevant guidelines and diagnosis and treatment norms to improve the accuracy (44) of diagnosis of childhood asthma but also to seek safer and more effective treatment regimens. Although probiotics are often used to treat childhood asthma, there are currently no standard and effective probiotic usage regimens, and there is a lack of comparative studies on the efficacy of probiotic treatment regimens. How to select more effective probiotic usage regimens for different clinical conditions is a concern in clinical practice.
This study included only one English study that met the criteria [the single English study (9) showed consistent effect directions with Chinese studies, though formal statistical comparison was not possible], and the quality assessment showed an overall low risk of bias and a moderate risk of bias. The analysis suggests that probiotics combined with conventional treatment can significantly improve lung function in children with asthma, increase the clinical total effective rate, reduce the recurrence rate, and may be effective by increasing IgA (45), reducing IgE, IL-4 (46), and IL-33 levels. Although some studies (47) have reported that probiotics have no statistical significance in improving FEV1, PEF, and C-ACT scores, which may be related to the small number of studies included, the non-exclusion of comorbidities and young children, this study believes that the combination of probiotics is effective. At the same time, given the favorable safety profile demonstrated (no increase in adverse events) and efficacy shown, probiotics may be considered as adjunctive therapy in childhood asthma management.
This meta-analysis found issues of publication bias and small sample effect in indicators such as pulmonary function and total clinical effective rate (funnel plot and Egger's test). Publication bias may arise from the difficulty in publishing negative results and the preference for positive results, which undermines data symmetry. Small sample studies tend to overestimate the true effect, and the combination of these increases the risk of “false positives.” The field should vigorously promote large sample sizes, encourage the public release of negative results, and reduce bias throughout the entire process from research design to dissemination. Despite the bias limitations of this analysis, the core conclusions still provide a reference for clinical and scientific research, and more high-quality studies are needed in the future to verify them.
For highly heterogeneous outcome measures, we conducted subgroup analyses and meta-regression on intervention measures (i.e., strain types), strain quantities (single strains or complex strains), treatment courses, manufacturers, and Jadad scores, but did not analyze the dosage of probiotics. The reasons are as follows: (1) weak evidence base (dose range 1–650 × 106 colony-forming units without established thresholds), making forced categorization prone to bias; (2) unclear dose–response relationships; (3) strain type and other factors having stronger confounding effects than dosage; and (4) studies focusing on strain–disease associations rather than dose optimization. The analysis did not identify the source of heterogeneity. It is presumed to be related to small sample size, publication bias, and low-quality literature. Therefore, given the high heterogeneity context, the pooled effect sizes provided should be considered exploratory estimates pending validation through high-quality RCTs. Due to the high risk of bias and heterogeneity, the quality of evidence suggesting probiotics improve FEV1/FVC, FEV1%, PEF, cytokines, and immune factors is low. The quality of evidence that probiotics improve FEV1, clinical total effective rate, and recurrence rate is moderate.
The validity of our indirect comparisons depends critically on the transitivity assumption. While we maintained consistency in age range (5–18 years) and disease severity (mild-to-moderate persistent asthma), variations in background conventional therapy may modify probiotic effects, representing a key limitation. (1) We strictly limited the subjects included in the study to children with asthma aged 5–18 years, ensuring comparability between studies in this dimension and meeting the transitivity requirement. (2) Although there was no uniform grading, we carefully examined the baseline characteristics of all included studies, which included children with mild-to-moderate persistent asthma. Therefore, there was good consistency in the characteristics of the study population in terms of disease severity. (3) There were differences in specific drug regimens among the studies, but these differences reflected the conventional range of practice for stepwise treatment of childhood asthma in the real world, and the core treatment principles were consistent. Therefore, we believe that good consistency was maintained among the studies on key patient characteristics, supporting the transmissibility hypothesis. The findings of this study emphasize the effect of probiotics as an add-on therapy to conventional treatment, and the background differences of conventional treatment should be taken into account when interpreting, with conclusions made cautiously.
Based on the SUCRA ranking analysis, conventional treatment combined with Bifidobacterium–Lactobacillus triplex live bacteria has the highest probability of being the optimal intervention in terms of increasing FEV1% and reducing recurrence rate. Studies (48, 49) suggest that it may promote asthma recovery by suppressing inflammatory markers such as serum chemokine-like factor-1 and nerve growth factor, and by enhancing dendritic cell (DC) and T-cell activity to regulate immune function. Conventional treatment combined with Bifidobacterium adolescentis has the highest probability of being the optimal intervention in increasing FEV1. This may be related to its upregulation of CD86 expression to promote DC maturation and stimulate DC secretion of IL-12 and IFN-γ, thereby altering Th2 dominant differentiation and correcting Th1/Th2 imbalance (50). Conventional treatment combined with Lactobacillus tablets has the highest probability of being the optimal intervention in increasing PEF. Animal studies (51) support lactic acid bacteria in improving asthma responses by inducing IL-10 production, downregulating Th1/Th2 responses, and inhibiting eosinophilic inflammation. Conventional treatment combined with Bacillus subtilis diplex live bacteria has the highest probability of being the optimal intervention in terms of improving the clinical total effective rate. Conventional treatment combined with Bifidobacterium quadruplex live bacteria has the highest probability of being the optimal intervention in reducing IL-4 and IL-33 levels. Conventional treatment combined with Bifidobacterium triplex live bacteria has the highest probability of being the optimal intervention in improving the C-ACT score. It is notable that, with the exception of a few outcome measures such as IgA and FEV1, most SUCRA ranking differences were not statistically significant (95% CI overlap rate >75%). Therefore, when making clinical decisions, the uncertainty reflected by the probability ranking results and the range of confidence intervals should be taken into account.
4.1 Comparison with other network meta-analyses
Over the past decade or so, studies on probiotics for childhood asthma have mainly focused on preventive effects and the exploration (52–54) of mechanisms of action, and there is no meta-analysis similar to this one. Western studies have focused more on early life intervention to prevent asthma development (developmental origins hypothesis), while the included studies mainly addressed clinical management of children with diagnosed asthma (primarily Chinese children). Due to the scarcity of eligible Western studies, the results cannot be directly extrapolated to Western populations at present.
4.2 Strengths and limitations of this study
4.2.1 Strengths
This study innovatively compares the regimens and efficacy of different types of probiotics in the treatment of childhood asthma, providing evidence-based medical support for standardizing the application of probiotics in the treatment of this disease. Excluding children under 5 years ensured diagnostic accuracy and internal validity, though this limits applicability to early childhood when probiotic interventions may have greater immunomodulatory potential. To ensure homogeneity and internal effectiveness of the included studies, we strictly limited the age range to 5–18 years. Asthma phenotypes, diagnostic criteria, and outcome measurement methods were relatively more consistent in children within this age group. Including groups with a wide age range, such as infants and adolescents, would introduce uncontrollable sources of heterogeneity, making the interpretation of the combined effect size ambiguous or even misleading. The 5–18 age group represents an important and large subgroup in asthma management, and its treatment effect assessment is equally instructive for clinical practice. Our study fills the evidence gap for this specific age group. The reliability of the study method was demonstrated through meta-regression and subgroup analyses of interventions, strain counts, etc.
4.2.2 Limitations
Although this study included studies with a sample size of more than 30 cases, which was somewhat credible, there were still limitations. (1) The predominance of Chinese studies (33/34) limits generalizability to Western populations. Our comprehensive search strategy included major English databases (PubMed, Web of Science); the low yield of English studies reflects the current evidence landscape rather than selective searching. (2) Future systematic reviews should prioritize inclusion of international studies to enable robust cross-cultural comparisons. (3) Differences in dosing time, dose–course, follow-up time, and evaluation indicators among different studies led to high heterogeneity in the meta-analysis. The total sample size of the included studies was small. (4) Excluding children <5 years ensures diagnostic accuracy but limits applicability to early childhood, when probiotic interventions may have greater immunomodulatory potential. There is an urgent need for well-designed RCTs in the future to use age-appropriate validation indicators to evaluate the efficacy of probiotics for asthma (or recurrent wheezing) in infants and young children.
In addition, 12 studies used multi-strain probiotic formulations analyzed as combined interventions, as individual strain contributions could not be separated. Most included studies used compound probiotic preparations where synergistic or antagonistic effects between strains remain unexplored, making it difficult to attribute efficacy to specific strains. Future studies need to strengthen strain-specific design and delve deeper into the mechanisms and clinical effects of individual strains to provide more precise guidance for application. Four interventions were supported by single studies only, limiting confidence in their rankings. Interventions supported by ≤2 studies require confirmatory trials before clinical recommendations.
The observed differences in SUCRA rankings may reflect the combined effects of strain properties, dosing, duration, and patient factors rather than strain-specific mechanisms alone. Direct mechanistic comparisons require specifically designed trials with biomarker assessments.
Some of the randomized controlled studies published domestically failed to describe in detail the random allocation method, allocation concealment, and blinding or had problems with the research methods themselves, which to some extent affected the methodological quality of the studies and posed a risk of bias in the results. Therefore, higher-quality, standardized design, large-sample, RCTs are still needed to evaluate the actual efficacy of probiotics to provide better evidence for probiotics in the treatment of childhood asthma.
4.3 Prospects for the future
Traditional Chinese medicine has unique advantages in treating childhood asthma (such as personalized herbal medicine, simple and well-tolerated external treatment methods), and related research is increasing. However, no RCTs of probiotics combined with traditional Chinese medicine or external treatment of traditional Chinese medicine for childhood asthma were found in this search, and the combined effect remains unknown and awaits exploration. This study confirmed the effectiveness of probiotics as an adjunctive treatment for childhood asthma and provided a direction for strain selection. Future research should focus on the following areas. (1) Fill the evidence gap: There is an urgent need to conduct high-quality RCTs to evaluate the efficacy of probiotics in infantile asthma/recurrent wheezing using age-appropriate indicators. (2) In-depth mechanism and precision: Dose-standardization studies should be conducted to clarify strain-specific dose-effect relationships. (3) Exploring combination options: further research should investigate probiotics and traditional Chinese medicine (internal/external) combination therapy to provide a more comprehensive and effective treatment strategy for childhood asthma by advancing research to a deeper and more precise level.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
JS: Writing – original draft. MZ: Writing – review & editing, Supervision, Visualization. YL: Writing – review & editing. DZ: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Jiangxi, China (grant number 20224BAB216095) and the National Natural Science Foundation of China (grant number 82305314).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chinese Medical Association Pediatric Society Respiratory Group, Editorial Board of Chinese Journal of Pediatrics. Guidelines for the diagnosis and prevention of bronchial asthma in children (2016 edition). Chin J Pediatr. (2016) 54(3):167–81. doi: 10.3760/cma.j.issn.0578-1310.2016.03.003
2. Global Initiative for Asthma. Global strategy for asthma management and prevention. Am J Surg. (2024).
3. Qibo M, Feng Z, Chong Z, Tao G. Effectiveness of enteric probiotic supplementation in the treatment of children with bronchial asthma and its mechanism of action. Hebei Med. (2023) 29(4):595–600. doi: 10.3969/j.issn.1006-6233.2023.04.014
4. Yanping H. Analysis of current status and influencing factors of asthma control in children. Chin J Mod Drug Appl. (2024) 18(17):39–42. doi: 10.14164/j.cnki.cn11-5581/r.2024.17.009
5. Sheng R, Meimei X, Giang Z, Wenbing D. Effects of intestinal probiotics adjuvant therapy on immune function, intestinal flora and recurrence rate in children with bronchial asthma. Chin Gen Pract J. (2020) 23(S1):72–5.
6. Society of Microecology, Chinese Preventive Medical Association. Evidence-based guideline for pediatric clinical application of probiotics (2023). Chin J Pract Pediatr. (2024) 39(1):1–15. doi: 10.19538/j.ek2024010601
7. Palys KE, Berger VW. A note on the Jadad score as an efficient tool for measuring trial quality. J Gastrointest Surg. (2013) 17(6):1170–1. doi: 10.1007/s11605-012-2106-0
8. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
9. Huang CF, Chie WC, Wang IJ. Efficacy of Lactobacillus administration in school-age children with asthma: a randomized, placebo-controlled trial. Nutrients. (2018) 10(11):1678. doi: 10.3390/nu10111678
10. Yinfang LI, Xia K, Hongmei W. Effects of probiotics on inflammatory factors and oxidative stress markers in children with allergic asthma. J Pediatr Pharm. (2023) 29(10):45–8. doi: 10.13407/j.cnki.jpp.1672-108X.2023.010.013
11. Jia L, Jie Z, Junhua Z. Clinical effects of probiotics combined with salmeterol ticarcoson in the treatment of bronchial asthma. Mater Child Health Care China. (2024) 39(9):1616–9. doi: 10.19829/j.zgfybj.issn.1001-4411.2024.09.017
12. Rongrong T, Feng'e Y, Yuling H, Xiaohong L. Effects of Bifidobacterium mixture as supplementary therapy on clinical symptoms and macrophages chemotaxis in children with bronchial asthma. Hainan Med J. (2023) 34(6):822–6. doi: 10.3969/j.issn.1003-6350.2023.06.014
13. Lin C, Lina L, Lulu C. Effects of Bifidobacterium bifidum on pulmonary function and serum IFN-γ and IL-4 in children with bronchial asthma. J Math Med. (2021) 34(7):1007–9. doi: 10.3969/j.issn.1004-4337.2021.07.023
14. Weitu C, Dongmei H. Clinical effects of budesonide combined with probiotics in the treatment of pediatric bronchial asthma. J Baotou Med Coll. (2020) 36(4):46–7. doi: 10.16833/j.cnki.jbmc.2020.04.015
15. Chunhui Z, Shiyun L, Wen Z, Linying X, Ying C, Shuyu W. The efficacy of probiotics and vitamin D assisted montelukast sodium in the treatment of obese children with asthma and its effects on Th17/Treg, T-beta/GATA-3 expression, and leptin. Chin J Diff Complicated Cases. (2024) 23(2):154–9. doi: 10.3969/j.issn.1671-6450.2024.02.006
16. Bei T, Yi W, Lingjian M, Lin L. Expression of M2 macrophages in children with asthma and study of probiotic-assisted treatment to pediatric asthma. J Xuzhou Med Univ. (2024) 44(4):302–6. doi: 10.3969/j.issn.2096-3882.2024.04.012
17. Han G, Hongmei M, Bin W, Yuge X, Kaikai Q. Interventional effect of tetralogy of viable Bifidobacterium tablets assisted ICS on childhood asthma. Acta Acad Med Weifang. (2019) 41(2):96–9. doi: 10.16846/j.issn.1004-3101.2019.02.005
18. DeMing Z. Analysis of clinical effect of probiotics on bronchial asthma in children. Syst Med. (2020) 5(9):105–7, 113. doi: 10.19368/j.cnki.2096-1782.2020.09.105
19. Xue L. Observation on the effect of Bifidobacterium bifidum combined with budesonide and montelukast sodium in the treatment of bronchial asthma. J Med Theory Pract. (2022) 35(2):292–4. doi: 10.19381/j.issn.1001-7585.2022.02.048
20. Xiaofeng Y, Xia T, Hongjun L. Effects of probiotics intervention on respiratory mechanical parameters, induced adhesions factor and interleukin in children with bronchial asthma. Clini Med J. (2018) 16(7):65–9. doi: 10.3969/j.issn.1672-3384.2018.07.015
21. Qiaoxiang X, Xiaoyun L, Jinping F. Efficacy of probiotics combined with budesonide in children with bronchial asthma and the effect on peripheral blood CD4+ and CD8+ cell levels. Chin J Microecol. (2022) 34(4):451–4. doi: 10.13381/j.cnki.cjm.202204014
22. Yuwei F, Xiaonan W, Lei S. Observation on the efficacy of Bifidobacterium adolescentis combined with montelukast in the treatment of childhood asthma. Harbin Med J. (2017) 37(6):534–5. doi: 10.3969/j.issn.1001-8131.2017.06.015
23. Qing C, Yanyan L. Probiotic adjuvant treatment of pediatric asthma and effects on S100β protein, TLR4 and inflammatory immune factors. Chine J Lung Dis. (2021) 14(5):608–10. doi: 10.3877/cma.j.issn.1674-6902.2021.05.015
24. Bingqi Z, Yanjuan H. Effects of ipratropium bromide aerosol inhalation combined with probiotic supplementation on airway remodeling and immune function in children with bronchial asthma. J Med Theory Pract. (2022) 35(11):1910–1, 1930. doi: 10.19381/j.issn.1001-7585.2022.11.046
25. Rentao W, Dun S, Xingchu H, Jing W. Efficacy of Bifidobacterium terreus enterosoluble capsules in the adjuvant treatment of asthma in children. Chin J Mod Drug Appl. (2012) 6(2):54–5. doi: 10.14164/j.cnki.cn11-5581/r.2012.02.101
26. Dan W, Jiandi L, Yinfeng W. Effects of bifidobacterium triple live powder adjuvant therapy on induced sputum adhesion factor index and serum AT-III, C3, CD5l in children with bronchial asthma. Zhejiang Pract Med. (2023) 28(6):492–6. doi: 10.16794/j.cnki.cn33-1207/r.2023.06.018
27. Jingfang M, Tian X. Effect of Bifidobacterium on immune function and symptom control of children with lgE-mediated allergic asthma. Chinese Science and Technology Journal Database (citation edition). Med Health. (2022) (2):95–8.
28. Chao L, Rong D, Zhenxing Z. Microecological preparations improving immune function in children with allergic asthma. Chin J Microecol. (2022) 34(1):57–61.
29. Qi W, Shuo L, Wenfang Y, Peng Z. Observation on the curative effect of lung-bowel therapy on childhood asthma of type of lung-spleen qi deficiency in the chronic persistent phase. Mod J Integr Trad Chin West Med. (2021) 30(14):1492–6. doi: 10.3969/j.issn.1008-8849.2021.14.003
30. Shaoyan W. Analysis of the efficacy of microecological preparations in the adjuvant treatment of bronchial asthma in children. J Pract Med Tech. (2012) 19(6):637–8. doi: 10.3969/j.issn.1671-5098.2012.06.052
31. Yanfang M, Huini P, Haiyan M. Efficacy of Bifidobacterium adolescentis combined with montelukast in the treatment of childhood asthma. Contemp Med. (2012) 18(18):140–1. doi: 10.3969/j.issn.1001-8131.2017.06.015
32. Linna S, Haibin W, Mingxing R, Guochang X. Effect of budesonide combined with yeast on total lgE and efficacy observation in pediatric bronchial asthma patients. Guizhou Med J. (2018) 42(4):437–8. doi: 10.3969/j.issn.1000-744X.2018.04.021
33. HongXia D. Effect of probiotics on children with bronchial asthma and S100 β effects of protein, TLR4 and inflammatory immune factors. Med Forum. (2023) 27(34):31–3. doi: 10.19435/j.1672-1721.2023.34.010
34. Bing L, Guilan W, Xuejun C, Huanrong L, Xinan L. Clinical study on cytokines balance of Th1/Th2 and treatment of bronchial asthma in children with combination therapy of probiotics and budesonide powder for inhalation. Clin Med. (2016) 36(9):11–4.
35. Wei Q, Hui Z, Yuhua Z. Clinica1.efectofprobioticsin treating children with bronchial asthma. China Contemp Med. (2016) 23(8):89–91.
36. Hui L. Observation on the efficacy of probiotics in the treatment of bronchial asthma in children. China Pharm. (2018) 27(0):55. doi: 10.3969/j.issn.1006-4931.2018.Z1.044
37. Yan S, Xinggang Z, Yanjun G. Immune mechanism and prognosis of 45 patients with bronchial asthma treated with probiotics. Chin J Pract Pediatr. (2018) 18(17):39–42.
38. Weiyu X, Xiaoyan Z, Sumin Y. Analysis of the effect of adjuvant therapy with Bacillus subtilis bacillus granules in children with bronchial asthma. J Med Theory Pract. (2023) 36(15):2613–5. doi: 10.19381/j.issn.1001-7585.2023.15.035
39. Shigang L, Weihong C, Rui L. Effects of probiotic-assisted therapy on the level of immune indicators in children with bronchial asthma. Life Sci Instrum. (2023) 21(S2):52–4. doi: 10.11967/202301246
40. Shuxuan C. Effect of Bifidobacterium bifidum tetragonum tablets in the treatment of bronchial asthma in children and its effect on inflammatory factors. Healthful Friend. (2021) (24):101.
41. Chiu CJ, Huang MT. Asthma in the precision medicine era: biologics and probiotics. Int J Mol Sci. (2021) 22(9):4528. doi: 10.3390/ijms22094528
42. Di J, Chenxiao B, Ou C. Systematic review of the preventive and therapeutic effects of probiotics on asthma. Acta Acad Med Sin. (2020) 42(2):178–89. doi: 10.3881/j.issn.1000-503X.11346
43. Chunxi L, Haiyue L, Yanxia L, Jianbing P, Jin S. The gut microbiota and respiratory diseases: new evidence. J Immunol Res. (2020):2340670. doi: 10.1155/2020/2340670
44. Jianguo H. Review of and reflections on the current status of childhood asthma diagnosis and treatment in China. J Sichuan Univ. (2021) 52(5):725–8. doi: 10.12182/20210960201
45. Smits HH, Gloudemans AK, van Nimwegen M. Cholera toxin B suppresses allergic inflammation through induction of secretory IgA. Mucosal Immunol. (2009) 2(4):331–9. doi: 10.1038/mi.2009.16
46. Joeris T, Müller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. (2017) 10(4):845–64. doi: 10.1038/mi.2017.22
47. Lin J, Zhang Y, He C, Dai J. Probiotics supplementation in children with asthma: a systematic review and meta-analysis. J Paediatr Child Health. (2018) 54(9):953–61. doi: 10.1111/jpc.14126
48. Jian H. Efficacy of budesonide suspension combined with Bifidobacterium and Lactobacillus triple viable tablets in the treatment of bronchial asthma and its influence on the serum levels of NGF and CKLF-1. J Clin Rational Drug Use. (2022) 15(1):78–81. doi: 10.15887/j.cnki.13-1389/r.2022.01.025
49. Shuli L. Effect of budesonide suspension combined with Bifidobacterium and Lactobacillus triple viable tablets on lung function and serum levels of NGF and CKLF-1 in children with bronchial asthma. China Licensed Pharmacist. (2018) 15(12):24–6. doi: 10.3969/j.issn.2096-3327.2018.12.007
50. Xiongwei Y, Hongling M, Heping W, Ying Z, Yuejie Z. Effects of Bifidobacterium adolescentis on the function of dendritic cells of the children with allergic asthma. Chin J Allergy Clin Immunol. (2009) 3(2):91–4. doi: 10.3969/j.issn.1673-8705.2009.02.003
51. Li L, Baodan Y, Jun X. Immunomodulatory effects of lactic acid bacteria on a mouse model of asthma sensitized by dust mites. J Med Postgrad. (2006) (6):515–20, 591. doi: 10.16571/j.cnki.1008-8199.2006.06.010
52. Azad MB, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, et al. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. (2013) 347:f6471. doi: 10.1136/bmj.f6471
53. Yongdong Y, Ting W, Jie H. Current status of probiotics for prevention and treatment of allergic diseases in children. J Clin Pediatr. (2022) 40(8):573–9. doi: 10.12372/jcp.2022.22e0133
Keywords: probiotics, conventional treatment, network meta-analysis, childhood, asthma
Citation: Sun J, Zhu M, Liu Y and Zhang D (2025) A network meta-analysis of probiotics in the treatment of childhood asthma. Front. Pediatr. 13:1637284. doi: 10.3389/fped.2025.1637284
Received: 29 May 2025; Accepted: 26 August 2025;
Published: 25 September 2025;
Corrected: 30 September 2025.
Edited by:
Ke Chen, University of Electronic Science and Technology of China, ChinaReviewed by:
José J. Leija-Martínez, Autonomous University of San Luis Potosí, MexicoXin Sun, Air Force Medical University, China
Copyright: © 2025 Sun, Zhu, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Di Zhang, emhhbmdkaUBqeHV0Y20uZWR1LmNu; Ying Liu, NTk1MzE3OTA2QHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Jiajia Sun
Jiajia Sun Meiyi Zhu1,†
Meiyi Zhu1,†