- Department of Pediatrics, Beijing Anzhen Nanchong Hospital of Capital Medical University & Nanchong Central Hospital, Nanchong, Sichuan, China
Background: This study aimed to investigate the types and risk factors of airway clearance dysfunction (ACD) in children with severe pneumonia based on LASSO regression and multivariate Logistic regression analysis.
Methods: This study was approved by the Hospital Ethics Committee. A retrospective study was conducted on 147 children with severe pneumonia admitted between January 2024 and October 2024. Demographic and clinical characteristics were collected, and the incidence and types of ACD were analyzed. Patients with ACD were assigned to the ACD group, while those without ACD were assigned to the non-ACD group. LASSO regression and multivariate Logistic regression were used to identify risk factors for ACD in these children, and ROC curves were constructed to evaluate the predictive value of these factors for ACD.
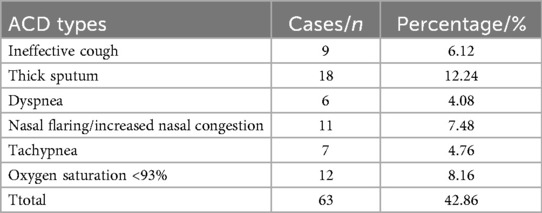
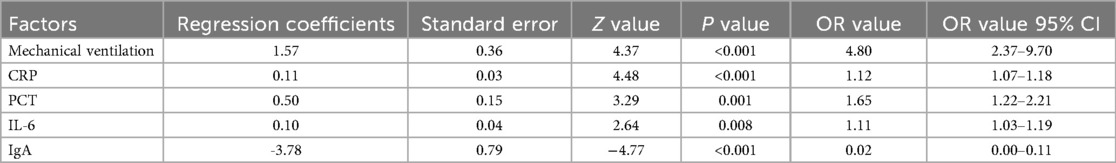
Results: A total of 63 cases of ACD were observed among children with severe pneumonia, including ineffective coughing, thick sputum, dyspnea, nasal flaring/ increased nasal congestion, tachypnea, and oxygen saturation <93%, with an incidence rate of 42.86%. Significant differences were found between the ACD group and non-ACD group in terms of age, mechanical ventilation, nutritional status, comorbid respiratory failure, comorbid heart failure, pulmonary rales, and pulmonary rhonchi (P < 0.05). Additionally, the ACD group had lower platelet count (PLT), immunoglobulin A (IgA), and immunoglobulin M (IgM) levels, while C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) levels were higher compared to the non-ACD group (P < 0.05). Variable screening was performed using the LASSO regression model, identifying three significant influencing factors. These were incorporated into a Logistic regression model, which revealed that mechanical ventilation, CRP, PCT, IL-6, and IgA were influencing factors for ACD in children with severe pneumonia. Based on these findings, an ROC curve was constructed, demonstrating that the combined prediction of mechanical ventilation, CRP, PCT, IL-6, and IgA for ACD in children with severe pneumonia achieved an AUC of 0.882, significantly higher than the AUC of any single indicator (P < 0.05).
Conclusion: Children with severe pneumonia are at risk of developing ACD, which may be influenced by mechanical ventilation, CRP, PCT, IL-6, and IgA levels. These five factors can be used to assess the risk of ACD in children with severe pneumonia. Accordingly, clinical measures should be developed to improve airway clearance ability and promote recovery in these patients.
Introduction
Severe pneumonia is a common critical emergency in pediatrics. Due to children's underdeveloped immune systems, poor cardiopulmonary function, and generally weaker physical condition, the morbidity and mortality rates of severe pneumonia are higher in children compared to other populations (1), posing a significant threat to their lives. According to World Health Organization statistics (2), severe pneumonia accounts for approximately 7%–13% of all pediatric pneumonia cases and is a major cause of death in children. Studies have shown (3) that patients with severe pneumonia often experience airway clearance dysfunction (ACD), which can exacerbate respiratory dysfunction and even lead to serious complications such as atelectasis and worsened infections, hindering rapid recovery. ACD refers to the inability to clear respiratory secretions or obstructions to maintain airway patency. Without timely and targeted intervention, it may result in hypoxia, respiratory failure, aggravated local ischemia in the heart and brain, and even immediate death (4). Relevant studies have found (5) that airway clearance is closely associated with the mucociliary clearance system and an effective cough mechanism. Cilia facilitate the transport of secretions from central to periphery airways through beating motions, while coughing expels secretions via high- velocity airflow. However, children with severe pneumonia often exhibit intense inflammatory responses that can alter mucus properties, leading to thickened secretions that are difficult to expel. This significantly impairs ciliary clearance and may cause weak or absent cough reflexes, ultimately resulting in secretion retention and an increased incidence of ACD (6, 7).
Currently, there are various clinically observed types of ACD in children with severe pneumonia, among which ineffective cough, thick sputum, and dyspnea are commonly seen, significantly impacting treatment and recovery. Ineffective cough, a major manifestation of ACD in these patients, serves as a clinical predictor of impaired airway clearance. It is often associated with weak cough reflexes or airway narrowing, preventing effective expulsion of secretions. Thick sputum is another contributing factor to ACD; its presence increases the risk of inadequate airway clearance and may even worsen airway secretions, further compromising respiratory function. Dyspnea, also a critical issue in ACD among severe pneumonia patients, may result from multiple factors such as retained airway secretions, airway blockage, and pulmonary infection. Additionally, excessive phlegm or increased mucus secretion in these patients can significantly exacerbate breathing difficulties (8, 9). If not effectively managed during hospitalization, ACD can lead to alveolar damage, atelectasis, pulmonary edema, and in severe cases, may progress to acute respiratory distress syndrome (ARDS) or multiple organ failure. These complications directly delay recovery and may even increase in-hospital mortality (10, 11).
In light of this, the present study was conducted to early identify the types and risk factors of ACD in children with severe pneumonia. The objective is to elucidate the categories and underlying risk factors of ACD in pediatric severe pneumonia, thereby providing a scientific basis for developing more effective treatment strategies.
Methods
Research objects
This study has been reviewed by the ethical committee of the hospital. Using a retrospective research approach, 147 children with severe pneumonia were admitted between January 2024 and October 2024. Demographic and clinical characteristics were collected, and the incidence and type of ACD were analyzed. Patients who developed ACD were assigned to the ACD group, while those without ACD were assigned to the non-ACD group.
Inclusion and exclusion criteria
Inclusion criteria: (1) compliance with WHO guidelines for the management of severe pneumonia in children (12); (2) diagnosis confirmed by chest x-ray, CT, and other imaging examinations; (3) age <13 years; (4) complete medical record data and follow-up information; (5) normal cognition and no communication barriers.
Exclusion Criteria: (1) unstable vital signs or death during hospitalization; (2) basic diseases of blood system or immunodeficiency-based diseases; (3) presence of congenital diseases; (4) transferring to another hospital during the study or withdrawing on their own for any reason.
Patient demographics
Demographic and clinical characteristics of children with severe pneumonia were collected based on the medical record information management system, including age, gender, body mass index (BMI), body temperature, time from symptom onset to hospital visit, length of hospital stay, place of residence, congenital disease, mechanical ventilation, nutritional status, comorbid respiratory failure, comorbid heart failure, pulmonary rales, pulmonary rhonchi, use of sedative/analgesic drugs, and oxygen therapy modalities.
Laboratory indicators
On the day of testing, peripheral venous blood specimens (3 ml) were collected from the children and kept indoors for 15 minutes, then centrifuged at 3,000 r/min for 10 minutes. The supernatant serum was collected for subsequent analyses. White blood cell count (WBC) and platelet count (PLT) were measured using a D5-CRP fully automated hematology analyzer (Shenzhen Dimension Bio-Technology Co., Ltd.) with its matching reagents. C-reactive protein (CRP), procalcitonin (PCT), and interleukin-6 (IL-6) levels were determined by enzyme-linked immunosorbent assay (ELISA). Additionally, hemoglobin (Hb) and albumin (Alb) were quantified colorimetrically using an AU5800 fully automated biochemical analyzer (Beckman, USA).
Immunological indicators
On the day of testing, peripheral venous blood samples (3 ml) of children were collected. The samples were allowed to stand at room temperature for 15 minutes, followed by centrifugation at 3,000 r/min for 10 minutes to separate the serum. Levels of immunoglobulin G (IgG), immunoglobulin A (IgA), and immunoglobulin M (IgM) were measured in strict accordance with the immunoturbidimetric method and instructions of the assay kits (Tianjin Fremid Biomedical Technology Co., Ltd.).
Statistical analysis
Data analysis in this study was performed using SPSS 25.0 statistical software. Categorical data are presented as n (%), and were analyzed using the χ2 test. Measurement data were tested for normality with the Shapiro–Wilk test. Normal distributed data are expressed as mean ± standard deviation(SD), with comparison between two groups performed by independent samples t-test, and comparison between the two groups were conducted using independent samples t-test. Non- normally distributed data are expressed as [M (P25, P75)] and analyzed using the nonparametric Mann–Whitney U-test. LASSO regression and multivariate logistic regression were employed to analyze risk factors for ACD in children with severe pneumonia. Receiver operating characteristic (ROC) curves were constructed to evaluate the predictive value of these factors for ACD. A P < 0.05 was considered statistically significant.
Results
Types of ACD in children with severe pneumonia
All 147 children with severe pneumonia received broad-spectrum antibiotics for anti-infection treatment, along with antiviral therapy, anti-shock management, cough relief and bronchodilation, oxygen inhalation, and nutritional support after admission. The incidence and types of ACD were evaluated, including 9 cases of ineffective cough, 18 cases of thick sputum, 6 cases of dyspnea, 11 cases of nasal flaring/increased congestion, 7 cases of tachypnea, and 12 cases of oxygen saturation <93%. A total of 63 children were assigned to the ACD group, corresponding to an ACD incidence of 42.86%. The remaining 84 children without ACD were assigned to the non-ACD group (Table 1).
Demographic and clinical characteristics of children in the ACD and non-ACD groups
To compare the demographic and clinical characteristics between the two groups, the results showed that the mean age of the ACD group was 4.87 years, significantly younger than that of the non-ACD group (5.49 years; P < 0.05). The proportion of patients receiving mechanical ventilation in the ACD group was 68.25%, which was significantly higher than that in the non-ACD group (30.95%; P < 0.05). The prevalence of malnutrition in the ACD group was 73.02%, markedly higher than that in the non-ACD group (30.95%; P < 0.05). There were 41 cases of comorbid respiratory failure in the ACD group, compared to only 20 cases in the non-ACD group, indicating a significantly higher incidence in the ACD group (P < 0.05). Similarly, the ACD group had 45 cases of comorbid heart failure, significantly more than the 28 cases in the non-ACD group (P < 0.05). Pulmonary rales were observed in 61.90% of the ACD group, a higher proportion than in the non-ACD group (41.67%; P < 0.05). The incidence of pulmonary rhonchi in the ACD group was 69.84%, significantly higher than that in the non-ACD group (35.71%; P < 0.05). However, no significant differences were observed in BMI (ACD group: 22.75 vs. non-ACD group: 23.05; P > 0.05), mean body temperature (ACD group: 38.91°C vs. non-ACD group: 38.74°C; P > 0.05), time from symptom onset to hospital visit (ACD group: 5.27 days vs. non-ACD group: 5.09 days; P > 0.05), or length of hospital stay (ACD group: 11.41 days vs. non-ACD group: 10.97 days; P > 0.05). Additionally, no significant differences were found between the two groups in terms of gender, place of residence, congenital diseases, use of sedative/analgesic drugs, or oxygen therapy modalities (P > 0.05, Table 2).
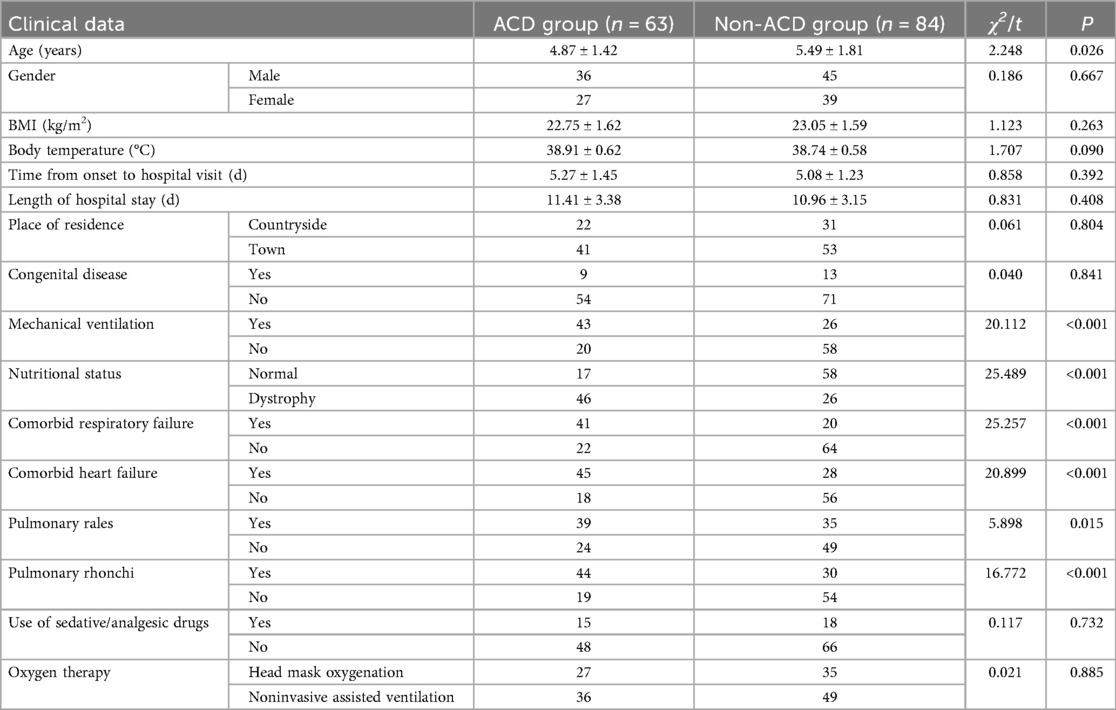
Table 2. Demographic data and clinical characteristics of children in the ACD and non-ACD groups (n = 147).
Comparison of laboratory indicators between the ACD and non-ACD groups
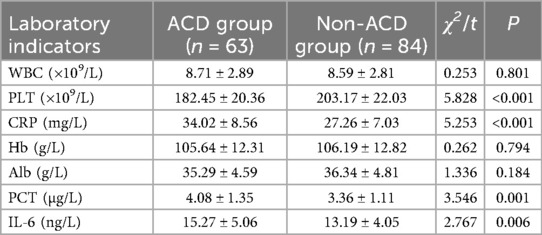
To compare the laboratory indicators between the two groups, the results showed that the PLT level in the ACD group was 182.45, significantly lower than that in the non-ACD group (203.17; P < 0.05). The CRP level in the ACD group was 34.02, markedly higher than that in the non-ACD group (27.26; P < 0.05). The PCT level in the ACD group was 4.08, higher than that in the non-ACD group (3.36; P < 0.05). The IL-6 level in the ACD group was 15.27, significantly elevated compared to the non-ACD group (13.19; P < 0.05).
However, no significant differences were observed in WBC (ACD group: 8.71 vs. non-ACD group: 8.59; P > 0.05), Hb level (ACD group: 105.64 vs. non-ACD group: 106.19; P > 0.05), or Alb level (ACD group: 35.29 vs. non-ACD group: 36.34; P > 0.05, Table 3).
Comparison of immunological indicators between the ACD and non-ACD groups
The immunological indicators were compared between the two groups. The results showed that the IgA level in the ACD group was 0.71, significantly lower than that in the non-ACD group (0.96; P < 0.05). The IgM level in the ACD group was 0.80, which was also lower than that in the non-ACD group (0.93; P < 0.05). However, no significant difference was observed in immunoglobulin G (IgG) levels between the ACD group (5.47) and the non-ACD group (5.58; P > 0.05, Figure 1).
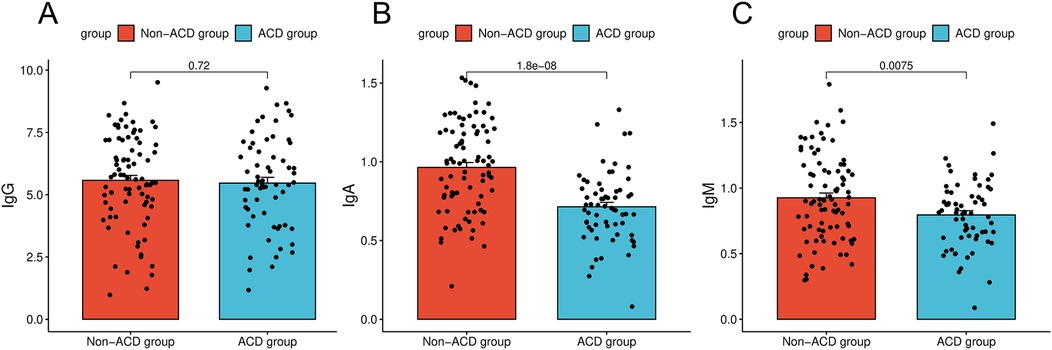
Figure 1. Comparison of immunologic indices between children in the ACD and non-ACD groups. (A) Comparison of immunologic indices between children in the ACD and IgG. (B) Comparison of immunologic indices between children in the ACD and IgA. (C) Comparison of immunologic indices between children in the ACD and IgM.
Screening of univariate factors influencing ACD using LASSO regression
With the occurrence of ACD in children with severe pneumonia as the dependent variable, the LASSO regression model was employed to screen potential influencing factors. Variables were selected from 13 candidate factors (age, mechanical ventilation, nutritional status, comorbid respiratory failure, comorbid heart failure, pulmonary rales, pulmonary rhonchi, PLT, CRP, PCT, IL-6, IgA, and IgM). The Ten-fold cross-validation was used to identify the optimal λ value. As the penalty coefficients λ varied, the coefficients of the independent variables were gradually compressed (Figure 2A). The λ value corresponding to the minimum cross-validation error (λ = 0.021) was ultimately selected as the optimal value (details in Figure 2B). This process identified five significant influencing factors: mechanical ventilation, CRP, PCT, IL-6, and IgA.
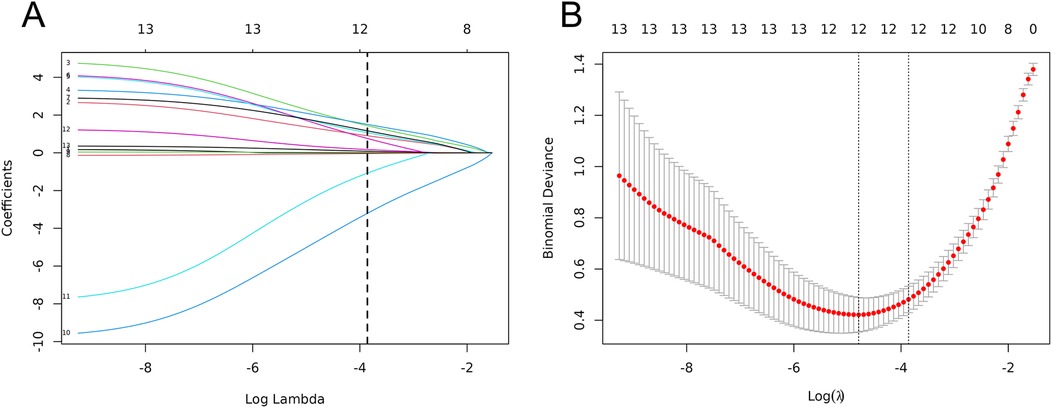
Figure 2. Clinical feature selection based on LASSO regression models. (A) LASSO coeffcient profles. (B) Three risk factors were selected using LASSO Cox regression analysis.
Construction of a predictive model for ACD in children with severe pneumonia using LASSO regression
The five factors selected by the LASSO regression model were incorporated into a Logistic regression model. Stepwise regression was then applied to identify influencing factors for ACD in children with severe pneumonia. The results demonstrated that mechanical ventilation (OR = 4.80), CRP (OR = 1.12), PCT (OR = 1.65), IL-6 (OR = 1.11), and IgA (OR = 0.02) were the independent influencing factors for ACD in children with severe pneumonia (Table 4).
ROC curve analysis
ROC curves were constructed for the five factors—mechanical ventilation, CRP, PCT, IL-6, and IgA—to evaluate their predictive value for ACD in children with severe pneumonia. The results showed that the AUC values for predicting ACD using mechanical ventilation, CRP, PCT, IL-6, and IgA were 0.687, 0.737, 0.651, 0.628, and 0.751, respectively. In contrast, the combined predictive AUC reached 0.882, which was significantly higher than that of any single indicator (P < 0.05), indicating that the combination of these factors has high predictive value for ACD in children with severe pneumonia (Figure 3).
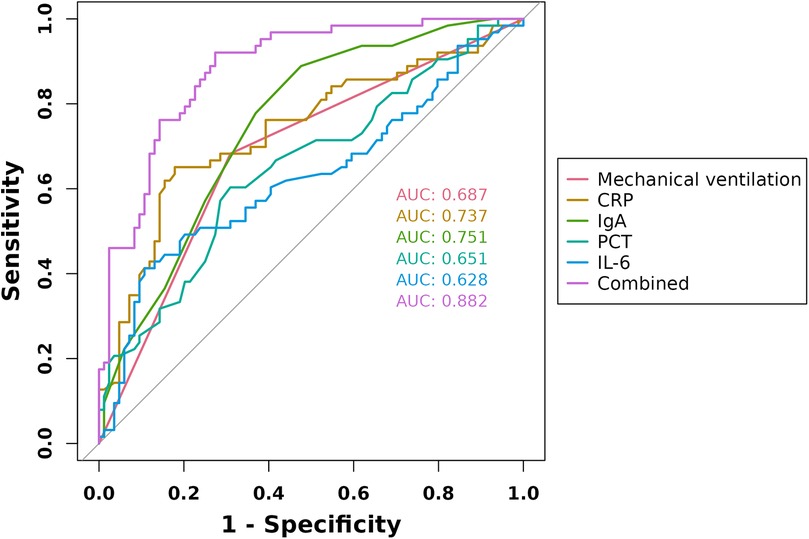
Figure 3. ROC curves of mechanical ventilation, CRP, PCT, IL-6, and IgA in predicting ACD in children with severe pneumonia.
Discussion
Studies have indicated (13) that 40%–60% of children with severe pneumonia are prone to producing large amounts of thick sputum, which not only increases the risk of airway obstruction but also elevates the likelihood of ACD. Severe pneumonia in children often involves respiratory system compromise, with clinical manifestations including cough, tachypnea, increased respiratory secretions, and may also be accompanied by severe dyspnea, nasal flaring, and cyanosis (14, 15). Additionally, due to their generally weaker physical condition, respiratory infections can lead to excessive secretions, making it difficult for children to effectively expectorate sputum on their own, thereby increasing the incidence of ACD and posing significant challenges in clinical management.
In this study, the types of ACD observed in children with severe pneumonia primarily included ineffective cough, thick sputum, dyspnea, nasal flaring/increased nasal congestion, tachypnea, and oxygen saturation <93%., Among these, thick sputum was the most common type, and the overall incidence of ACD reached 42.86%, which is consistent with clinical data. Severe pneumonia is often accompanied by varying degrees of inflammatory response, which can directly stimulate goblet cells and submucosal glands to overproduce mucin and alter mucus composition, thereby increasing its viscoelasticity and viscosity. Meanwhile, inflammatory mediators can damage airway ciliated epithelium, reducing the frequency and efficiency of coordinated ciliary beating. Moreover, young patients have underdeveloped organ function, which often leads to weak or ineffective coughing and an inability to generate sufficient airflow shear force to clear secretions, further contributing to the high incidence of ACD. Therefore, early recognition of ACD and implementation of targeted interventions are crucial.
In this study, it was observed that the children in the ACD group were younger than those in the non-ACD group, suggesting that younger children are more susceptible to ACD. This is primarily due to their immature respiratory system, relatively narrow airways, poor ciliary motility, and underdeveloped cough reflex, all of which increase the risk of ACD. Additionally, the nutritional status of ACD group was poorer than that of the non-ACD group. Malnutrition can compromise immune function, weaken respiratory muscle strength, impair mucosal repair capacity, adversely affect mucus composition and quality, and even lead to deteriorated pulmonary function and impaired respiratory muscle contraction. These factors can exacerbate infection and thereby increase the risk of ACD manifestations such as ineffective cough and thick sputum. The incidence of comorbid respiratory failure, heart failure, pulmonary rales, and pulmonary rhonchi was significantly higher in the ACD group than in the non-ACD group. This indicates that children with severe pneumonia who present with these complications are at a markedly elevated risk of developing ACD. Respiratory failure significantly impairs lung function, directly hinders gas exchange, and reduces cough efficacy, thereby compromising airway clearance. Heart failure can cause pulmonary congestion and edema, increasing the likelihood of lung pathology and ACD. Pulmonary rales and rhonchi are both signs of accumulated airway secretions, suggesting either excessive airway secretions or airway narrowing, which further raises the risk of impaired airway clearance.
Studies have shown that mechanical ventilation, an invasive procedure used to support respiratory function through an artificial airway, can disrupt the natural defense mechanisms of the respiratory tract. It impedes ciliary movement, reduces mucus clearance, and promotes the accumulation of airway secretions, thereby worsening ACD (16, 17). Consistent with this, the current study found a significantly higher rate of mechanical ventilation in the ACD group, confirming it as a risk factor for ACD in children with severe pneumonia. Analysis indicates that mechanical ventilation requires endotracheal intubation or tracheotomy, reflecting the critical condition of the children and pronounced respiratory symptoms. Prolonged mechanical ventilation can impair spontaneous breathing, promote excessive secretions retention, weaken the cough reflex, and diminish the autonomous cleaning ability of the airways, ultimately increasing the incidence of ACD (18, 19). In light of these findings, clinical practice should emphasize enhanced airway management for mechanically ventilated children with severe pneumonia, including timely clearance of respiratory secretions, to maintain airway patency.
Typically, children with severe pneumonia release large amounts of inflammatory mediators and cytokines, making them highly significant in assessing the severity of the disease (20). Changes in PLT often reflect the intensity of the inflammatory response. An elevated PLT level indicates a serious inflammatory reaction or abnormal coagulation, which is commonly observed in children with severe pneumonia. CRP, a nonspecific acute-phase inflammatory marker, is associated with the degree of systemic inflammation and tissue damage. Its elevated expression in children with severe pneumonia suggests active inflammation and a higher risk of pulmonary complications. PCT, a prohormone of calcitonin without hormonal activity, increases significantly in response to severe stimuli such as bacterial infection, making it a key indicator for clinical identification of bacterial infections. IL-6, secreted by various cells including T lymphocytes, fibroblasts, B lymphocytes, and monocytes/macrophages, plays a critical role in inflammatory and immune responses, serving as an important marker for early diagnosis and warning of infectious diseases (21). In this study, the ACD group exhibited lower PLT levels but significantly higher CRP, PCT, and IL-6 levels compared to the non-ACD group. This suggests that PLT and CRP expression may influence the occurrence of ACD, with CRP, PCT, and IL-6 being particularly significant factors contributing to ACD in children with severe pneumonia. Although PLT was significantly lower in the ACD group, it was not selected in the final LASSO model, possibly due to its complex relationship with inflammation and coagulation, which may have been overshadowed by more direct inflammatory markers such as CRP and PCT, or due to multicollinearity. Elevated CRP levels are closely related to airway mucosal damage and impaired mucociliary clearance. As CRP increases, severe damage to the airway mucosa, dysfunction of ciliary clearance, and epithelial cell shedding may occur, leading to the formation of mucus plugs that obstruct the airways. Additionally, elevated CRP can contribute to the formation of plastic mucus, further increasing the risk of airway obstruction and ACD. Similarly, elevated PCT levels in children with severe pneumonia often indicate severe infection, which may be accompanied by intense inflammatory responses and tissue damage, resulting in increased mucus secretion, impaired mucociliary function, and ultimately ACD. As a pro-inflammatory cytokine, IL-6 is often elevated in severe pneumonia, reflecting exacerbated airway inflammation, increased mucus production, and impaired ciliary function, all of which significantly raise the incidence of ACD (22, 23). Therefore, close clinical monitoring of CRP, PCT, and IL-6 levels is essential. Regular assessment of these markers can help identify the risk of ACD in children with severe pneumonia at an early stage, enabling timely intervention.
It has been found (24) that children with severe pneumonia are often associated with immune dysfunction, while the immune function of children directly affects the severity of this disease. Immune dysfunction is manifested as abnormalities in cellular and humoral immunity, and several studies have shown that compared with children with simple pneumonia, children with severe pneumonia have relatively lower levels of IgA and IgM, with their condition worsening as the levels of indicators decrease (25). Among them, IgM is the earliest type of antibody produced in the immune response, which activates the complement system and enhances phagocytosis of pathogens, and in children with severe pneumonia, the changes of its level mainly reflect the acute phase characteristics of pathogen infection; while IgA, as a key factor of mucosal local immunity, tends to be distributed on the mucosal surfaces of respiratory and digestive tracts, which can play the role of anti-infection and protective barrier. The reduced IgA expression in children with severe pneumonia indicates that their respiratory mucosal defense ability is attenuated, and children are highly susceptible to the attack of pathogens (26, 27). From the results of this study, the levels of IgA and IgM were significantly lower in ACD group than in non-ACD group, and especially IgA as a key factor in the development of ACD in children with severe pneumonia. It has been reported (28) that IgA is the main protective antibodies of respiratory mucosa, which can directly inhibit the adhesion of pathogens on respiratory epithelial cells, thus avoiding pathogens from colonizing and infecting. When the level of its expression decreases, the ability of body to resist the pathogens is clearly weakened and even results in recurrent infections and intensification of inflammatory reactions, which in turn causes ACD. In addition, reduced IgA levels are closely related to immune dysfunction in children with severe pneumonia, and once their IgA expression is abnormal, their respiratory mucosal barrier function is directly affected (29, 30) In response, clinical attention should be paid to the monitoring of IgA levels in children with severe pneumonia, with timely and effective measures been taken for intervention to improve airway clearance in children with severe pneumonia.
However, this study has certain limitations. For instance, as a single-center study with a small sample size, the limited number of participants may introduce selection bias, potentially affecting the representativeness and reliability of the findings. Additionally, the classification of ACD types in this study may differ from criteria used in other research, leading to limited comparability across studies. Therefore, future clinical efforts should focus on conducting multicenter, large-sample prospective studies to comprehensively understand the epidemiological characteristics, risk factors and prognosis of ACD in children with severe pneumonia. It is also essential to establish a unified and quantifiable classification standard for ACD types in this population to enhance the comparability and scientific rigor of research outcomes.
In summary, children with severe pneumonia are at risk of developing ACD, which may be influenced by factors such as mechanical ventilation, CRP, PCT, IL-6, and IgA levels. These five factors can be used to assess the risk of ACD in this population. If ACD is confirmed early through evaluation of these markers, clinical management should be promptly adjusted. Treatment strategies may include nebulized inhalation of mucolytic agents such as N-acetylcysteine to reduce sputum viscosity, supplementation with immunonutrients or intravenous immunoglobulin for children with low IgA levels, enhanced airway humidification, high-frequency oscillatory sputum expulsion, and daily assessment of weaning criteria to shorten mechanical ventilation duration, thereby reducing ventilator-induced lung injury.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
XD: Writing – original draft, Resources, Project administration, Data curation, Writing – review & editing, Supervision. JB: Software, Writing – original draft, Methodology, Investigation, Conceptualization. RL: Validation, Writing – original draft, Software, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issue please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cao Y, Zhao L, Miao H. [Risk factors for progression to severe pneumonia in children visiting the emergency department with pneumonia]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2023) 35(5):528–32.37308236
2. Choo S, Lee YY, Lee E. Clinical significance of respiratory virus coinfection in children with Mycoplasma pneumoniae pneumonia. BMC Pulm Med. (2022) 22(1):212. doi: 10.1186/s12890-022-02005-y
3. Collaro AJ, McElrea MS, Marchant JM, Chatfield MD, Sondergeld P, Perret JL, et al. The effect of early childhood respiratory infections and pneumonia on lifelong lung function: a systematic review. Lancet Child Adolesc Health. (2023) 7(6):429–40. doi: 10.1016/S2352-4642(23)00030-5
4. Deng F, Cao H, Liang X, Li Q, Yang Y, Zhao Z, et al. Analysis of cytokine levels, cytological findings, and MP-DNA level in bronchoalveolar lavage fluid of children with Mycoplasma pneumoniae pneumonia. Immun Inflamm Dis. (2023) 11(5):e849. doi: 10.1002/iid3.849
5. Gebre M, Haile K, Duke T, Faruk MT, Kamal M, Kabir MF, et al. Effectiveness of bubble continuous positive airway pressure for treatment of children aged 1–59 months with severe pneumonia and hypoxaemia in Ethiopia: a pragmatic cluster-randomised controlled trial. Lancet Glob Health. (2024) 12(5):e804–e14. doi: 10.1016/S2214-109X(24)00032-9
6. Huang X, Gu H, Wu R, Chen L, Lv T, Jiang X, et al. Chest imaging classification in Mycoplasma pneumoniae pneumonia is associated with its clinical features and outcomes. Respir Med. (2024) 221:107480. doi: 10.1016/j.rmed.2023.107480
7. Zhao ZW, Cui XW, Zhao MC, Yao JP. A clinical investigation of hospitalized children with congenital heart disease with severe pneumonia during 2016–2020. Asian J Surg. (2023) 46(4):1890–1. doi: 10.1016/j.asjsur.2022.10.085
8. Jullien S, Richard-Greenblatt M, Ngai M, Lhadon T, Sharma R, Dema K, et al. Performance of host-response biomarkers to risk-stratify children with pneumonia in Bhutan. J Infect. (2022) 85(6):634–43. doi: 10.1016/j.jinf.2022.10.010
9. Lee KL, Lee CM, Yang TL, Yen TY, Chang LY, Chen JM, et al. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010–2019. J Formos Med Assoc. (2021) 120(1 Pt 1):281–91. doi: 10.1016/j.jfma.2020.08.018
10. Li YT, Zhang J, Wang MZ, Ma YM, Zhi K, Dai FL, et al. Changes in coagulation markers in children with Mycoplasma pneumoniae pneumonia and their predictive value for Mycoplasma severity. Ital J Pediatr. (2023) 49(1):143. doi: 10.1186/s13052-023-01545-1
11. Zhong L, Lin J, Dai J. Risk factors for the development of bronchiolitis obliterans in children with severe adenovirus pneumonia: a retrospective study with dose-response analysis. J Med Virol. (2020) 92(12):3093–9. doi: 10.1002/jmv.25703
12. Liang Y, Wu J, Chen G, Du Y, Yan Y, Xie S, et al. Risk factor analysis and prediction model construction for severe adenovirus pneumonia in children. Ital J Pediatr. (2024) 50(1):210. doi: 10.1186/s13052-024-01771-1
13. Ling Y, Zhang T, Guo W, Zhu Z, Tian J, Cai C, et al. Identify clinical factors related to Mycoplasma pneumoniae pneumonia with hypoxia in children. BMC Infect Dis. (2020) 20(1):534. doi: 10.1186/s12879-020-05270-6
14. Luo XQ, Luo J, Wang CJ, Luo ZX, Tian DY, Xie XH. Clinical features of severe Mycoplasma pneumoniae pneumonia with pulmonary complications in childhood: a retrospective study. Pediatr Pulmonol. (2023) 58(10):2815–22. doi: 10.1002/ppul.26593
15. Zhang Y, Lin J, Shi Q, Li C, Liu J, Dai J. Diagnostic accuracy of time to first positivity of blood cultures for predicting severe clinical outcomes in children with pneumonia-related bacteremia. J Investig Med. (2020) 68(7):1241–9. doi: 10.1136/jim-2020-001473
16. Poddighe D, Demirkaya E, Sazonov V, Romano M. Mycoplasma pneumoniae infections and primary immune deficiencies. Int J Clin Pract. (2022) 2022:6343818. doi: 10.1155/2022/6343818
17. Rahman AE, Hossain AT, Chisti MJ, Dockrell DH, Nair H, El Arifeen S, et al. Hypoxaemia prevalence and its adverse clinical outcomes among children hospitalised with WHO-defined severe pneumonia in Bangladesh. J Glob Health. (2021) 11:04053. doi: 10.7189/jogh.11.04053
18. Ramgopal S, Cotter JM, Navanandan N, Shah SS, Ruddy RM, Ambroggio L, et al. Viral detection is associated with severe disease in children with suspected community-acquired pneumonia. Pediatr Emerg Care. (2023) 39(7):465–9. doi: 10.1097/PEC.0000000000002982
19. Su DQ, Huang HL, Zhuo ZQ. Pathogen distribution and bacterial resistance in children with severe pneumonia: a single-center retrospective study. Medicine (Baltimore). (2021) 100(35):e27128. doi: 10.1097/MD.0000000000027128
20. Tolla HS, Woyessa DB, Balkew RB, Asemere YA, Fekadu ZF, Belete AB, et al. Decentralizing oxygen availability and use at primary care level for children under-five with severe pneumonia, at 12 health centers in Ethiopia: a pre-post non-experimental study. BMC Health Serv Res. (2022) 22(1):676. doi: 10.1186/s12913-022-08003-4
21. Tran XD, Hoang VT, Goumballa N, Vu TN, Tran TK, Pham TD, et al. Viral and bacterial microorganisms in Vietnamese children with severe and non-severe pneumonia. Sci Rep. (2024) 14(1):120. doi: 10.1038/s41598-023-50657-5
22. Tran-Quang K, Nguyen-Thi-Dieu T, Tran-Do H, Pham-Hung V, Nguyen-Vu T, Tran-Xuan B, et al. Antibiotic resistance of Streptococcus pneumoniae in Vietnamese children with severe pneumonia: a cross-sectional study. Front Public Health. (2023) 11:1110903. doi: 10.3389/fpubh.2023.1110903
23. Zhang X, Sun R, Jia W, Li P, Song C. A new dynamic nomogram for predicting the risk of severe Mycoplasma pneumoniae pneumonia in children. Sci Rep. (2024) 14(1):8260. doi: 10.1038/s41598-024-58784-3
24. Wang H, Xu WH, Liu JR, Peng Y, Peng XX, Wen XH, et al. [Clinical phenotyping of severe Mycoplasma pneumoniae pneumonia in children]. Zhonghua Er Ke Za Zhi. (2024) 62(7):669–75.38955686
25. Wilkes C, Bava M, Graham HR. Duke T, group ARIR. What are the risk factors for death among children with pneumonia in low- and middle-income countries? A systematic review. J Glob Health. (2023) 13:05003. doi: 10.7189/jogh.13.05003
26. Wu L, Zhang J, Zhang HM, Wang CY. Study on red blood cell distribution width in children with severe Mycoplasma pneumoniae pneumonia. Biomark Med. (2024) 18(2):69–77. doi: 10.2217/bmm-2023-0671
27. Yi X, Jia W, Li W, Jia C, Song C. Diagnostic value of cytokines in severe childhood Mycoplasma pneumoniae pneumonia combined with adenovirus infection. Ital J Pediatr. (2024) 50(1):92. doi: 10.1186/s13052-024-01661-6
28. Xu M, Li Y, Shi Y, Liu H, Tong X, Ma L, et al. Molecular epidemiology of Mycoplasma pneumoniae pneumonia in children, Wuhan, 2020–2022. BMC Microbiol. (2024) 24(1):23. doi: 10.1186/s12866-024-03180-0
29. Xu S, Tang Y, Li M, Zhang L, Su Y, Wang Y, et al. Clinical characteristics of Chinese children with EV-D68-associated pneumonia: a single-center retrospective analysis. Pediatr Pulmonol. (2024) 59(12):3660–6. doi: 10.1002/ppul.27285
Keywords: children with severe pneumonia, airway clearance dysfunction, mechanical ventilation, c-Reactive protein, ineffective cough
Citation: Ding X, Bai J and Liu R (2025) Risk factors for airway clearance dysfunction in children with severe pneumonia: a retrospective study of LASSO model. Front. Pediatr. 13:1638103. doi: 10.3389/fped.2025.1638103
Received: 30 May 2025; Accepted: 8 September 2025;
Published: 29 October 2025.
Edited by:
Bülent Taner Karadağ, Marmara University, TürkiyeReviewed by:
Vladimir Pohanka, Retired, Liptovská Teolička, SlovakiaChristian Bohringer, UC Davis Medical Center, United States
Changjing Zhuge, Beijing University of Technology, China
Copyright: © 2025 Ding, Bai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Ding, MTM4OTA3OTkwMDVAMTYzLmNvbQ==
 Xiaoyan Ding
Xiaoyan Ding Jing Bai
Jing Bai