- Department of Neonatology, The Second Hospital of Jilin University, Changchun, Jilin, China
This study explores the color Doppler ultrasonography features of congenital tuberculosis in preterm infants through a detailed analysis of a case from February 2025, including clinical, NGS, and sonographic data. The findings identify specific vascular signatures that facilitate early diagnosis, offering a valuable reference for clinicians encountering similar cases. High-frequency probe ultrasonography was used to characterize pulmonary lesions and differentiate tuberculosis from bronchopulmonary dysplasia (BPD) and neonatal respiratory distress syndrome (NRDS). A male infant born at gestational age of 28 weeks (birth weight: 1,180 g) presented with respiratory distress and cyanosis (Apgar score: 7 at 1 min). Initial ultrasonography revealed multiple confluent B-lines in both lungs. At 60 days postpartum, ultrasonography showed thickened pleura, scattered subpleural consolidations, atelectasis in the lower lobes, and hyperechoic fragments moving with respiration. First-line anti-TB regimen, isoniazid, rifampin, and pyrazinamide were administered and serial monitoring via color Doppler ultrasound and chest CT demonstrated favorable disease control. Main take-away lessons: Pulmonary color Doppler ultrasonography, combined with clinical history, can aid in the diagnosis of congenital tuberculosis, and congenital TB should be considered in preterm infants with refractory pneumonia. Additionally, NGS is a valuable tool for rapid pathogen identification.
1 Introduction
Congenital tuberculosis (TB) is a rare yet severe form of Mycobacterium tuberculosis (M. tuberculosis) infection. Fetuses may acquire the disease via transplacental hematogenous spread or through aspiration/ingestion of contaminated amniotic fluid during delivery (1).
According to 2022 global statistics, there were 10.6 million new TB cases worldwide (2). China reported 748,000 cases (7.1% of the global total), ranking third among the 30 high-TB-burden countries, with adult females accounting for 32% of cases (3). The WHO's 2021 Global TB Report estimated 9.9 million incident TB cases globally in 2020, including 1.09 million pediatric cases and approximately 200,000 child deaths (4). Currently, there is no systematic registry or large-scale cohort study specifically targeting congenital tuberculosis both domestically and internationally (5, 6). Relevant data are extremely scarce, mainly relying on case reports and regional retrospective studies. According to the WHO's Global Tuberculosis Report 2024, the estimated number of global tuberculosis cases was 10.8 million in 2023 (7). It is estimated that there were about 1.3 million cases among children and adolescents (8). Due to the challenges in accurate diagnosis, there is a significant gap in case detection and reporting, especially among children under 5 years old (9). In addition, about 50% of suspected cases were not reported in this age group (9). The global number of congenital tuberculosis cases is less than 12,500, equivalent to 0.3 per 100,000 live births (10).
Current TB diagnostic methods include acid-fast staining, molecular assays, and culture (11). Next-generation sequencing (NGS) (12, 13), an emerging molecular technique, demonstrates significant advantages in pathogen detection and antimicrobial resistance profiling—particularly for mycobacterial diseases (14). Additionally, color Doppler ultrasound applied in pulmonary TB reduces radiation exposure and alleviates patients' financial burdens (15).
This article presents a congenital TB case diagnosed through lung color Doppler ultrasound combined with NGS. We analyze sonographic features to improve diagnostic accuracy in preterm infants and discuss relevant literature.
2 Case description
2.1 Infant clinical data
A gestational age of 28-week male (1,180 g) was delivered via cesarean section due to maternal fever and preterm premature rupture of membranes (PPROM). At birth, he exhibited respiratory distress (Apgar: 7 at 1 min, 9 at 5 min). Initial chest x-ray showed reduced lung transparency; arterial blood gas analysis results revealed metabolic acidosis (pH 7.077, pCO₂ 67.5 mmHg, pO2 81.6 mmHg, Na+ concentration 134.0 mmol/L, K+ concentration 3.3 mmol/L, Ca2+ concentration 1.3 mmol/L, serum glucose levels 2.9 mmol/L, lactic acid 1.7 mmol/L, HCO3− 19.4 mmol/L, base excess −12.0 mmol/L, hemoglobin 172.0 g/L). He was diagnosed with NRDS, electrolyte imbalances, and metabolic acidosis. The infant's mother had a normal physical recovery after childbirth and underwent a pulmonary CT scan, which showed no abnormalities. She was breastfeeding the infant.
2.2 Maternal clinical data
The mother had a history of infertility (suspected tubal TB) and fever (38°C) before delivery. The infant presented with elevated infection markers after birth. Antibacterial treatment proved ineffective and resulted in a prolonged clinical course. After 60 days of hospitalization, a detailed maternal medical history revealed that the mother had visited a gynecologist prior to pregnancy. Relevant imaging studies showed wire-like changes and rigidity in the fallopian tube lumen, which were considered indicative of tuberculosis-related damage. Since the disease was in an inactive phase at the time, a tuberculin test was not recommended. The mother was strongly suspected of having had pelvic tuberculosis in the past. Pathological examination of the placenta showed no abnormalities.
3 Diagnostic assessment & therapeutic intervention
3.1 Case presentation
Initial treatment included surfactant therapy, broad-spectrum antibiotics, and mechanical ventilation. However, the patient's condition failed to improve significantly. On day 20 of hospitalization, the patient developed a persistent fever. A chest x-ray revealed patchy infiltrates, raising the suspicion of hospital-acquired pneumonia. Despite escalating the antibiotic regimen, the clinical symptoms and radiographic findings showed no resolution.
3.2 Definitive diagnosis
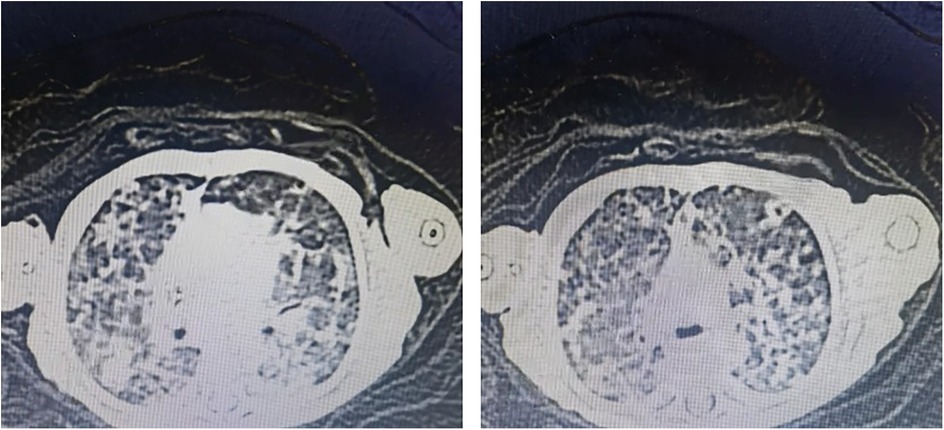
The infant had high levels of infection markers after birth, and the antibacterial treatment was not very effective, with a longer course of treatment. Blood specimen was used to perform NGS detection of M. tuberculosis complex at day 60. After the diagnosis of tuberculosis was confirmed, the antitubercular treatment was effective. CT revealed miliary TB (Figure 1).
3.3 Ultrasonography findings
Thickened, irregular pleura with “shred sign” (hyperechoic fragments). Subpleural consolidations with heterogeneous echotexture and vascularity. Multiple solid lesions of varying sizes were visible in the subpleural areas of both lungs, with the larger ones mainly located in the lung apices and bases, involving 2–3 intercostal spaces. The pleura was thickened at the sites of consolidation, with a thickness of about 2 mm. Blood flow signals were detected in the consolidated areas, with a resistance index (RI) of 0.86–0.88. Hepatosplenomegaly and renal calcifications were also identified on abdominal ultrasound (Figure 2).
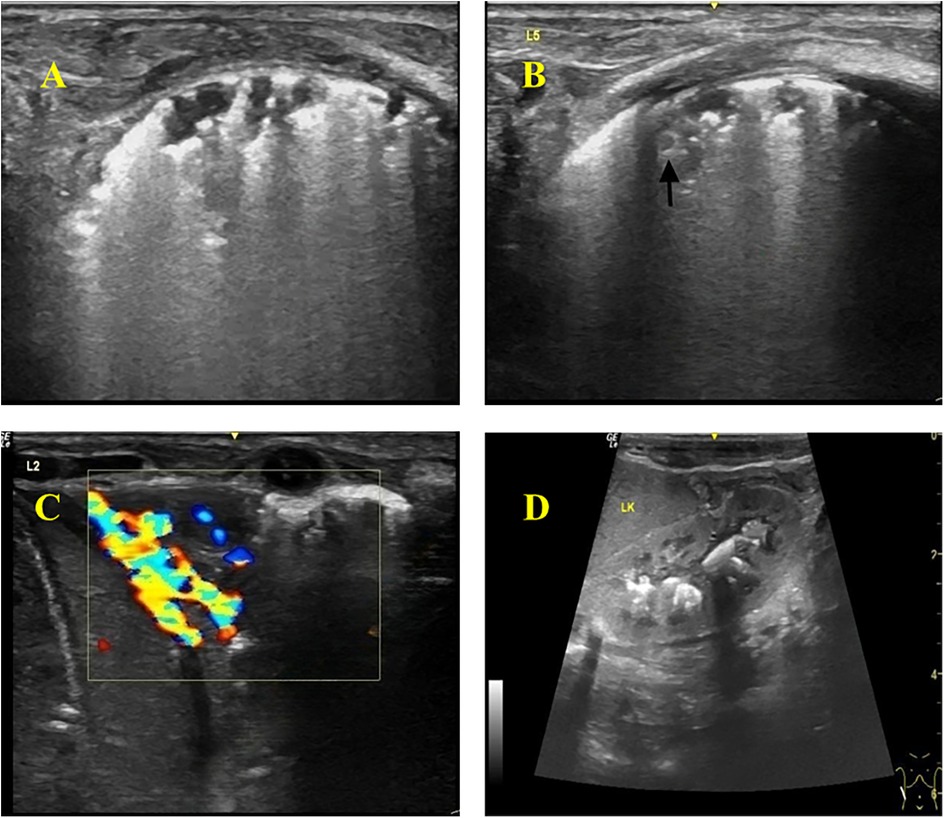
Figure 2. Color Doppler ultrasonography of the infant. (A) Thickened and hyperechoic pleural lines in both lungs, with partial discontinuity. (B) Multiple discontinuous small subpleural hypoechoic lesions (ragged cloth sign) with scattered hyperechoic patchy areas (fragmentation sign) at the margins. Nodular changes within some consolidated areas. (C) Wedge-shaped consolidation in the lower lobes of both lungs, with heterogeneous echogenicity and small hypoechoic areas, showing rich blood flow signals on Doppler. (D) Abdomen (Color Doppler Ultrasound): Hepatomegaly and splenomegaly. Calcifications visible in the renal pelvises and calyces of both kidneys.
The ultrasound settings used in this study. Machine: GE LOGIQ e; Transducer: L4-12t linear probe; Frequency: 12 MHz; Depth: 3–4 cm; Overall Gain: 72%; TGC: Set in a left-low–right-high pattern (approx. 45 dB on the right); Tissue Harmonic Imaging: Off; Compound Imaging: Off; Focal Zone: One zone positioned at the pleural line.
3.4 Therapeutic interventions
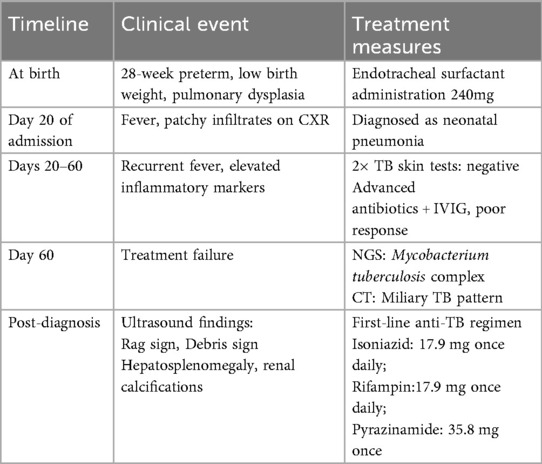
The management of congenital tuberculosis includes anti-tuberculosis pharmacotherapy, isolation measures, symptomatic/supportive care, and close monitoring of disease progression. Currently, pharmacotherapy remains the primary treatment for tuberculosis. The standard first-line regimen consists of isoniazid + rifampin + pyrazinamide, with a prolonged treatment course typically lasting 6–9 months for newly diagnosed patients (16, 17).
In this case, the infant was diagnosed with miliary tuberculosis and would ordinarily require transfer to a specialized TB hospital. However, as a 28-week preterm neonate with low body weight (at 60 days postnatal age) and ongoing ventilator dependence, transfer was considered unfeasible. Consequently, the patient received isolated treatment in our unit with the following regimen: Isoniazid: 17.9 mg once daily; Rifampin: 17.9 mg once daily; Pyrazinamide: 35.8 mg once daily; Serial monitoring via color Doppler ultrasound and chest CT demonstrated favorable disease control. It should be noted that although pelvic tuberculosis is non-infectious and newborns generally do not transmit tuberculosis, isolation measures were still implemented as a precaution based on the maternal pregnancy history and the infant's treatment response.
A timeline with relevant data from the episode of care is shown in Table 1.
4 Discussion
Mycobacterium tuberculosis is an aerobic bacterium, while the intrauterine environment is relatively hypoxic, which is unfavorable for its growth. After birth, Mycobacterium tuberculosis requires a certain incubation period before symptoms appear, typically manifesting within 2–3 weeks after delivery. Early symptoms include fever, tachypnea, apnea, weight loss, irritability, abdominal distension, feeding difficulties, and hepatosplenomegaly (18). Neonatal tuberculosis progresses rapidly, with a mortality rate as high as 40%–60%. Without timely diagnosis and treatment, the mortality rate increases further (19). Early diagnosis can significantly improve prognosis.
In this case, the infant's blood tests indicated elevated infection markers and recurrent fever. Despite treatment with high-dose antibiotics and intravenous immunoglobulin therapy, no significant improvement was observed. The infant underwent extensive diagnostic workup without a definitive diagnosis. Chest CT and Mycobacterium tuberculosis infection tests during hospitalization showed no obvious abnormalities, making it difficult to distinguish from traditional Neonatal Respiratory Distress Syndrome (NRDS). However, next-generation sequencing (NGS) and color Doppler ultrasound ultimately confirmed the diagnosis of miliary tuberculosis.
4.1 Characteristic color Doppler ultrasound findings in congenital tuberculosis
When Mycobacterium tuberculosis invades lung tissue, it triggers pathological reactions such as exudation, proliferation, and necrosis. The alveolar air spaces are replaced by pathological tissue, and when the pleura is involved, color Doppler ultrasound may reveal lung consolidation, subpleural nodules, pleural thickening, and pleural effusion (20).
In this case, the infant presented with recurrent fever and poor response to antibiotics. Chest x-ray showed prominent lung markings, prompting a lung color Doppler ultrasound, which revealed: (1) Abnormal pleural line: appeared rough, irregular, and hyperechoic, with posterior confluent or dense B-lines, some discontinuous with thin pleural segments. (2) Multiple irregular consolidations: subpleural hypoechoic consolidations of varying sizes and shapes were observed. Smaller lesions were highly irregular with fragmented hyperechoic borders, while larger wedge-shaped consolidations were seen in the apical and lower lobes, also with fragmented hyperechoic edges. The consolidations showed minimal aeration, heterogeneous echogenicity (patchy hypoechoic areas), and grade 2 blood flow signal. (3) Nodular changes: Nodular lesions with slightly hyperechoic rims were detected within subpleural consolidations.
Abdominal color Doppler ultrasound revealed hepatosplenomegaly and multiple hyperechoic foci in the renal pelvis, but no enlarged mediastinal lymph nodes.
While our study highlights the pivotal role of ultrasound in suggesting the diagnosis of congenital TB, it is crucial to acknowledge that a definitive diagnosis relies on a comprehensive approach rather than on microbiological tests alone. The integration of clinical symptomatology, detailed maternal history (including the type and activity of maternal TB disease), histopathological examination of the placenta, and developmental data of the infant is imperative, especially in cases where AFB smear, culture, or molecular tests are negative or unavailable. Our ultrasound findings should therefore be interpreted as a key component within this broader diagnostic framework, prompting clinicians to pursue a full array of investigations to confirm or exclude the disease.
4.2 Differential diagnosis of congenital tuberculosis vs. other neonatal lung diseases
Neonatal tuberculosis lacks specific clinical manifestations, particularly in preterm infants, where it can mimic NRDS or Bronchopulmonary Dysplasia (BPD), leading to delayed diagnosis and treatment. While chest x-ray has low specificity and sensitivity in early tuberculosis, chest CT provides more detailed imaging but has limitations in preterm infants.
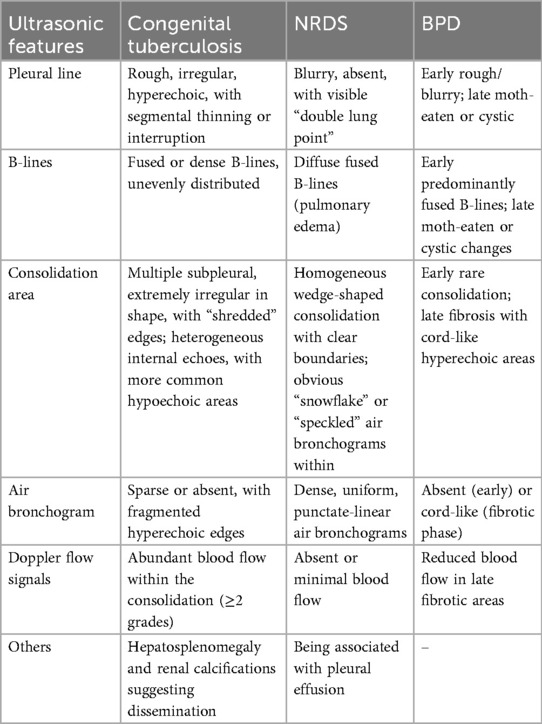
Bedside lung ultrasound has gained widespread use in neonatal lung disease diagnosis and can partially replace traditional x-rays. This infant, born at 28 weeks, received surfactant therapy and antibiotics but remained febrile. Lung ultrasound was performed to differentiate between NRDS and BPD (Table 2).
4.2.1 Differentiation from neonatal respiratory distress syndrome (NRDS)
NRDS is characterized by lung consolidation with air bronchograms, pleural line abnormalities (loss of A-lines), double lung points, and pleural effusion (21). It results from surfactant deficiency, leading to hyaline membrane formation and atelectasis (22).
In contrast, congenital tuberculosis consolidations arise from tuberculous exudation, proliferation, and necrosis. Key ultrasound differences: (1) NRDS: Dense, uniform air bronchograms (snowflake-like, dotted, or linear) with clear boundaries (23). (2) Tuberculosis: Irregular consolidations with fragmented borders, heterogeneous echogenicity, and minimal air bronchograms.
This infant initially resembled NRDS, but lung ultrasound revealed atypical inflammatory signs. Despite broad-spectrum antibiotics, persistent fever prompted further investigation. Upon reviewing the mother's history, a suspected tubal tuberculosis infection was noted. NGS later confirmed MS infection.
4.2.2 Differentiation from bronchopulmonary dysplasia (BPD)
Early BPD lacks specific ultrasound findings, but late-stage BPD shows fibrosis and cystic changes (24). This infant, hospitalized for 3 months, required BPD exclusion.
BPD ultrasound findings: (1) Non-specific pleural line abnormalities (roughness, blurring, discontinuity). (2) Specific “moth-eaten” or cystic pleural changes in advanced stages (24).
Tuberculosis ultrasound findings: (1) Rough, irregular, hyperechoic pleural line (non-specific). (2) Multiple subpleural irregular consolidations (distinct from BPD).
Early BPD may mimic tuberculosis with confluent B-lines (pulmonary edema), but late-stage BPD's “moth-eaten” pleura helps differentiation.
5 Conclusion
Congenital tuberculosis lacks specific signs, and delayed treatment leads to high mortality. Bedside ultrasound, being radiation-free and portable, plays a crucial role in neonatal diagnosis. This report details color Doppler ultrasound features in a miliary tuberculosis case, highlighting subtle differences that may aid early diagnosis and improve outcomes. This study is a case report, LUS successfully visualized the lesion adjacent to the costophrenic angle; however, regions masked by bone could not be assessed. and more related cases are needed for multicenter prospective studies.
6 Key takeaways
(1) Congenital TB should be considered in preterm infants with refractory pneumonia.
(2) Pulmonary ultrasonography can differentiate TB from NRDS/BPD.
(3) NGS is a valuable tool for rapid pathogen identification.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Second Hospital of Jilin University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DZ: Data curation, Methodology, Writing – original draft, Formal analysis. WG: Data curation, Methodology, Writing – original draft. DM: Data curation, Methodology, Writing – original draft. YZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen S. Maternal and fetal infection with Mycobacterium tuberculosis. Arch Dis Child Fetal Neonatal Ed. (1997) 77(1):F77–8. doi: 10.1136/fn.77.1.f77a
2. Bagcchi S. WHO’s global tuberculosis report 2022. Lancet Microbe. (2023) 4(1):e20. doi: 10.1016/S2666-5247(22)00359-7
3. Lu CR, Tan WG, Lu PX, Wang HP. 2023 WHO global tuberculosis report: key data analysis of the world and China. Electron J Emerg Infect Dis. (2023) 8(6):73–8.
4. World Health Organization. Global Tuberculosis Report 2021. Geneva: World Health Organization (2021).
5. Chen J, Qiu Y, Wu W, Pan Y, Yang R, Li L, et al. Incomplete tuberculosis reporting and registration to the surveillance system in southwestern China of Yunnan province: an inventory survey. BMC Public Health. (2024) 24(1):1397. doi: 10.1186/s12889-024-18794-2
6. Antoine D, Abubakar I. Infectious Disease Surveillance. 2nd ed. Oxford: Wiley-Blackwell (2013). p. 234–47.
7. Chen Z, Wang T, Du JL, Sun L, Wang GR, Ni RZ, et al. Decoding the WHO global tuberculosis report 2024: a critical analysis of global and Chinese key data. Zoonoses. (2025) 5(1):1. doi: 10.15212/ZOONOSES-2024-0061
9. World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 5: Management of Tuberculosis in Children and Adolescents. Geneva: WHO (2022).
10. Peng W, Yang J, Liu E. Analysis of 170 cases of congenital TB reported in the literature between 1946 and 2009. Pediatr Pulmonol. (2011) 46(12):1215–24. doi: 10.1002/ppul.21490
11. Zhu QD, Wang Q, Song C, Zhao CY, Yang SX. Progress in high-throughput sequencing for TB diagnosis and drug resistance testing. Electron J Emerg Infect Dis. (2024) 9(05):78–82.
12. Xu ZA, Pu FF, Feng J, Xia P. Progress in high-throughput sequencing for managing osteoarticular tuberculosis. Chin J Antituberc. (2025) 47(02):224–30.
13. Shi CL, Han P, Tang PJ, Chen MM, Ye ZJ, Wu MY, et al. Clinical metagenomic sequencing for diagnosis of pulmonary tuberculosis. J Infect. (2020) 81(4):567–74. doi: 10.1016/j.jinf.2020.08.004
14. Shin DJ, Andini N, Hsieh K, Yang S, Wang TH. Emerging analytical techniques for rapid pathogen identification and susceptibility testing. Annu Rev Anal Chem (Palo Alto Calif). (2019) 12(1):41–67. doi: 10.1146/annurev-anchem-061318-115529
15. Zhang J. Role of lung ultrasound in diagnosing pulmonary Tuberculosis. J Clin Resarch. (2025) 42(03):474–6.
16. Li JY, Hu HY, Lei J, Gong XT. Comparative efficacy of moxifloxacin+rifampin+pyrazinamide+ethambutol vs isoniazid+rifampin+pyrazinamide+ethambutol chemotherapy regimens in treatment-naïve pulmonary Tuberculosis patients: impact on CT lesion resolution and sputum culture conversion rates. J Clin Intern Med. (2024) 41(09):640–2.
17. Artawan Eka Putra IWG, Purnama Dewi NPE, Probandari AN, Notobroto HB, Wahyuni C. The implementation of comprehensive health education to improve household Contacts’ participation in early detection of tuberculosis. Health Educ Behav. (2023) 50(1):136–43. doi: 10.1177/10901981211001829
18. Lu WC. Two cases of congenital tuberculosis in neonates. Chin Birth Health Heredity. (2015) 23(07):67–8.
19. Di Comite A, Esposito S, Villani A, Stronati M, Italian Pediatric TB Study Group. How to manage neonatal tuberculosis. J Perinatol. (2016) 36(2):80–5. doi: 10.1038/jp.2015.99
20. Heuvelings CC, Bélard S, Andronikou S, Jamieson-Luff N, Grobusch MP, Zar HJ. Chest ultrasound findings in children with suspected pulmonary tuberculosis. Pediatr Pulmonol. (2019) 54(4):463–4. doi: 10.1002/ppul.24230
21. Liu J. Utility of lung ultrasound in the diagnosis of neonatal respiratory distress syndrome. Chin J Appl Clin Pediatr. (2014) 29(18):1438–40.
22. Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. (2023) 120(1):3–23. doi: 10.1159/000528914
23. Liu J, Cao HY, Cheng XY. Ultrasonography for Neonatal Pulmonary Diseases. 2nd ed. Zhengzhou: Henan Science and Technology Press (2019). p. 4–207.
Keywords: Mycobacterium tuberculosis, congenital tuberculosis, color Doppler ultrasound, neonatal respiratory distress syndrome (NRDS), preterm infant
Citation: Zhou D, Guo W, Ma D and Zhang Y (2025) Case Report: Pulmonary color Doppler ultrasonography features of congenital tuberculosis in a preterm infant. Front. Pediatr. 13:1639412. doi: 10.3389/fped.2025.1639412
Received: 2 June 2025; Accepted: 9 October 2025;
Published: 23 October 2025.
Edited by:
Hong Ren, Shanghai Children's Medical Center, ChinaReviewed by:
Clemax Couto Sant'Anna, Federal University of Rio de Janeiro, BrazilJonas Wolf, Moinhos de Vento Hospital, Brazil
Copyright: © 2025 Zhou, Guo, Ma and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunfeng Zhang, emhhbmd5dW5mQGpsdS5lZHUuY24=
 Di Zhou
Di Zhou Wanxu Guo
Wanxu Guo Yunfeng Zhang
Yunfeng Zhang