- 1Department of Biological Products Testing, Fujian Institute for Food and Drug Quality Control, Fuzhou, Fujian, China
- 2Department of Pharmacy, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
- 3Department of Business Management Section, Fujian Center For Drug Inspection, Fuzhou, Fujian, China
- 4Department of Clinical Pharmacy, Xiamen Haicang Hospital, Xiamen, Fujian, China
- 5Fujian Institute of Hematology, Fujian Provincial Key Laboratory on Hematology, Fujian Medical University Union Hospital, Fuzhou, Fujian, China
Background: Quadrivalent influenza vaccines (QIVs) are extensively administered to children for seasonal influenza prevention. However, comprehensive long-term evaluations of post-marketing safety remain limited.
Methods: We conducted a comprehensive 12-year pharmacovigilance analysis utilizing data from the Vaccine Adverse Event Reporting System (VAERS) for individuals under 18 years receiving six FDA-approved QIVs between 2013 and 2024, representing three distinct platforms: four egg-based inactivated vaccines (Afluria, Fluarix, FluLaval, Fluzone), one cell culture-based inactivated vaccine (Flucelvax), and one live-attenuated intranasal vaccine (FluMist). Disproportionality analyses using reporting odds ratios (ROR) and Bayesian information component (IC) were employed to identify safety signals. Safety signals were considered statistically significant when the lower bound of the 95% confidence interval for ROR exceeded 1.0 and the lower bound of the IC (IC025) exceeded 0.
Results: Of the 15,458 reported adverse events, 5.29% (95% CI: 4.95–5.65) were classified as serious, including 67 fatalities. Guillain-Barré syndrome (GBS) emerged as a statistically significant safety signal (42 cases; ROR = 1.71, 95% CI: 1.25–2.35; IC025 = 0.28). Among specific QIV products, Flucelvax demonstrated the lowest proportion of serious adverse events (2.35%). Notably, reporting volumes decreased by 42% during the COVID-19 pandemic.
Conclusion: This pharmacovigilance analysis demonstrates generally favorable safety profiles for pediatric QIVs, with cell culture formulations showing superior safety characteristics. The extremely low absolute risk of Guillain-Barré syndrome supports continued routine pediatric influenza vaccination while highlighting the importance of platform-specific safety monitoring.
1 Introduction
Annual influenza vaccination remains a cornerstone of pediatric preventive healthcare, with quadrivalent influenza vaccines (QIVs) now established as the global standard of care (1). The transition from trivalent to quadrivalent formulations between 2012 and 2015 aimed to expand immunological coverage by incorporating both influenza B virus lineages, thereby enhancing population-level protection (2, 3). Currently, six QIVs approved by the U.S. Food and Drug Administration (FDA) are available for pediatric use, including egg-based and cell culture-derived platforms, as well as live attenuated and inactivated vaccine formulations (4).
Although pre-licensure clinical trials are critical for establishing initial vaccine safety and immunogenicity profiles, they typically lack the statistical power to detect rare adverse events. Consequently, comprehensive post-marketing surveillance remains essential for monitoring vaccine safety (5). In the United States, the Vaccine Adverse Event Reporting System (VAERS), co-managed by the Centers for Disease Control and Prevention (CDC) and the FDA since 1990, serves as the principal passive surveillance platform for monitoring vaccine-related adverse events (6).
Previous pediatric influenza vaccine safety studies using VAERS have primarily focused on specific safety concerns or limited time periods. Notably, Vellozzi et al. examined VAERS reports for 2009 H1N1 monovalent vaccines, while studies such as those by McMahon et al. analyzed adverse events following inactivated influenza vaccination in children under 2 years using VAERS data from 1990 to 2003 (7, 8). More recent Vaccine Safety Datalink (VSD) studies by Tse et al. and Duffy et al. concentrated on febrile seizures following trivalent influenza vaccines in children aged 6–23 months (9, 10). However, these studies have several limitations: they either predate the widespread adoption of QIVs, focus on specific adverse events rather than comprehensive safety profiles, or examine limited age ranges or time periods. Our study uniquely provides the first comprehensive, longitudinal analysis spanning the entire post-QIV implementation period (2012–2024), comparing safety profiles across all FDA-approved pediatric QIV formulations using standardized methodology.
The heterogeneity among QIV platforms necessitates platform-specific safety evaluation due to fundamental differences in manufacturing processes, antigen presentation, and immune activation patterns. Egg-based vaccines may contain residual egg proteins and ovalbumin that could trigger allergic reactions in sensitized individuals, despite extensive purification processes (11, 12). Cell culture-derived vaccines eliminate egg-related allergens and avoid potential egg-adaptation mutations that may affect immunogenicity, but may present different glycosylation patterns due to mammalian cell production systems (13, 14). Live-attenuated influenza vaccines (LAIV) employ temperature-sensitive, replication-competent viruses that replicate in the nasopharynx and may cause distinct adverse event patterns, including potential concerns about viral shedding and transmission risks to immunocompromised contacts (15, 16). Additionally, the different manufacturing processes, adjuvant systems, and preservatives used across platforms may contribute to varying reactogenicity profiles. Given these biological and manufacturing differences, platform-specific safety analysis is essential for identify potential differential risk signals that could inform clinical decision-making and regulatory guidance.
Guillain-Barré syndrome (GBS) has been consistently recognized as a rare but notable adverse event following influenza vaccination in adult populations, with an estimated excess risk ranging from 1 to 2 cases per million doses administered (17, 18). However, pediatric-specific GBS risk estimates are more limited and suggest potentially different risk profiles. Studies suggest that pediatric GBS following influenza vaccination occurs at lower rates than in adults, though precise estimates vary by study methodology and population (19, 20). Additionally, background GBS incidence rates differ substantially by age, with pediatric populations showing incidence rates of 0.62–0.69 per 100,000 person-years compared to higher adult rates that increase with age (21, 22). Despite these lower baseline rates, detailed characterization of GBS risk across different QIV formulations in pediatric populations remains limited, highlighting the need for platform-specific, pediatric surveillance data.
Additionally, the COVID-19 pandemic has disrupted routine pediatric immunization practices and healthcare utilization, potentially influencing both the frequency of vaccine-associated adverse events and their reporting patterns (23, 24).
This study provides a 12-year comprehensive analysis of post-marketing safety for all FDA-approved pediatric QIVs by leveraging data from VAERS. Our aim is to deliver a detailed comparative safety profile and address critical gaps in vaccine pharmacovigilance, examining both pre-pandemic and pandemic reporting periods.
2 Materials and methods
2.1 Data source and extraction
Adverse event reports were retrieved from the Vaccine Adverse Event Reporting System (VAERS) via the CDC WONDER interface, covering the period from January 1, 2013–December 31, 2024 (accessed March 2025). The dataset included patient demographics, vaccine identifiers, Medical Dictionary for Regulatory Activities (MedDRA) preferred terms, and serious event indicators (6).
2.2 Study population, inclusion criteria, and data handling
The study included reports for individuals younger than 18 years of age where an FDA-approved quadrivalent influenza vaccine (QIV) was coded as the primary suspected vaccine. Reports were systematically excluded if they (i) lacked age information, (ii) did not specify the QIV formulation, (iii) were flagged as duplicates through an automated pipeline (collapsing records with identical VAERS_ID and retaining only the most recent RECVDATE), or (iv) were incomplete, defined as lacking both vaccine type and any coded MedDRA preferred term. Data cleaning and handling of missing values were automated using scripted rules in R without manual adjudication or imputation; analyses requiring specific variables (e.g., sex, onset interval) were performed on complete-case subsets.
2.3 Vaccine classification and characteristics
Six FDA-approved QIVs were evaluated:
2.3.1 Egg-based inactivated vaccines
Afluria Quadrivalent (CSL Seqirus; approved 2016).
Fluarix Quadrivalent (GSK; approved 2012).
FluLaval Quadrivalent (GSK; approved 2013).
Fluzone Quadrivalent (Sanofi Pasteur; approved 2013).
2.3.2 Cell culture-based inactivated vaccine
Flucelvax Quadrivalent (CSL Seqirus; approved 2016).
2.3.3 Live attenuated intranasal vaccine
FluMist Quadrivalent (AstraZeneca; approved 2012).
2.4 Outcome definitions
Serious adverse events (SAEs) were defined according to FDA criteria, including death, life-threatening conditions, hospitalization or prolonged hospitalization, persistent disability, and congenital anomalies (25). All adverse events were coded using MedDRA (version 27.0).
2.5 Statistical analysis
Categorical variables were summarized as frequencies and proportions, and continuous variables as medians with interquartile ranges (IQRs). Age was stratified into four groups: younger than 6 months, 6 months–5 years, 6–12 years, and 13–17 years.
Disproportionality analyses employed two complementary metrics: (i) reporting odds ratio (ROR), calculated as ROR = (a × d)/(b × c), where a = reports with the event and vaccine of interest, b = other events with the vaccine of interest, c = the event with other vaccines, and d = other events with other vaccines; and (ii) information component (IC), an empirical Bayesian shrinkage measure robust for sparse data, with IC025 indicating the lower 95% credibility bound. A safety signal was identified when both the lower 95% confidence interval of the ROR exceeded 1.0 and IC025>0. To account for multiple comparisons across MedDRA preferred terms, p-values from ROR analyses were adjusted using the Benjamini-Hochberg false discovery rate (FDR) procedure (q < 0.05) (26). All analyses were conducted using R statistical software (version 4.4.2).
2.6 Ethical statement
This study utilized publicly available and de-identified VAERS data; therefore, institutional review board (IRB) approval was not required. The analysis was conducted in accordance with the principles of the Declaration of Helsinki.
3 Results
3.1 Overall report characteristics
A total of 15,458 pediatric adverse event reports were included, with a median patient age of 7.3 years [interquartile range (IQR): 2.8–11.5 years]. The gender distribution was balanced, with females representing 47.5% of cases. Vaccine-specific report distribution was as follows: Fluzone (39.9%, n = 6,166), Fluarix (16.8%, n = 2,596), FluLaval (16.7%, n = 2,581), FluMist (15.4%, n = 2,373), Flucelvax (7.7%, n = 1,192), and Afluria (3.6%, n = 550).
3.2 Serious adverse events and mortality
Serious adverse events (SAEs) were reported in 818 cases (5.29%). The SAE proportions by vaccine were highest for Afluria (6.73%), followed by Fluarix (6.32%), FluMist (5.77%), Fluzone (5.71%), FluLaval (3.87%), and Flucelvax, which exhibited the lowest SAE rate (2.35%).
There were 67 reported deaths, corresponding to a crude mortality rate of 0.43%. Notably, 73% of fatalities occurred among children aged 6 months to 5 years, with 54% involving Fluzone. These associations are temporal, and causality should not be inferred from these reports given the limitations of passive surveillance data (27).
3.3 Age-Stratified safety patterns
An inverse relationship between age and SAE frequency was observed across all vaccines. Among children aged 6 months to 5 years, SAE rates ranged from 2.37% (Flucelvax) to 10.16% (Afluria). Adolescents aged 13–17 years showed substantially lower SAE rates, ranging from 1.78% to 6.73%. Table 1 presents the detailed age-specific distribution of serious adverse events following quadrivalent influenza vaccination.
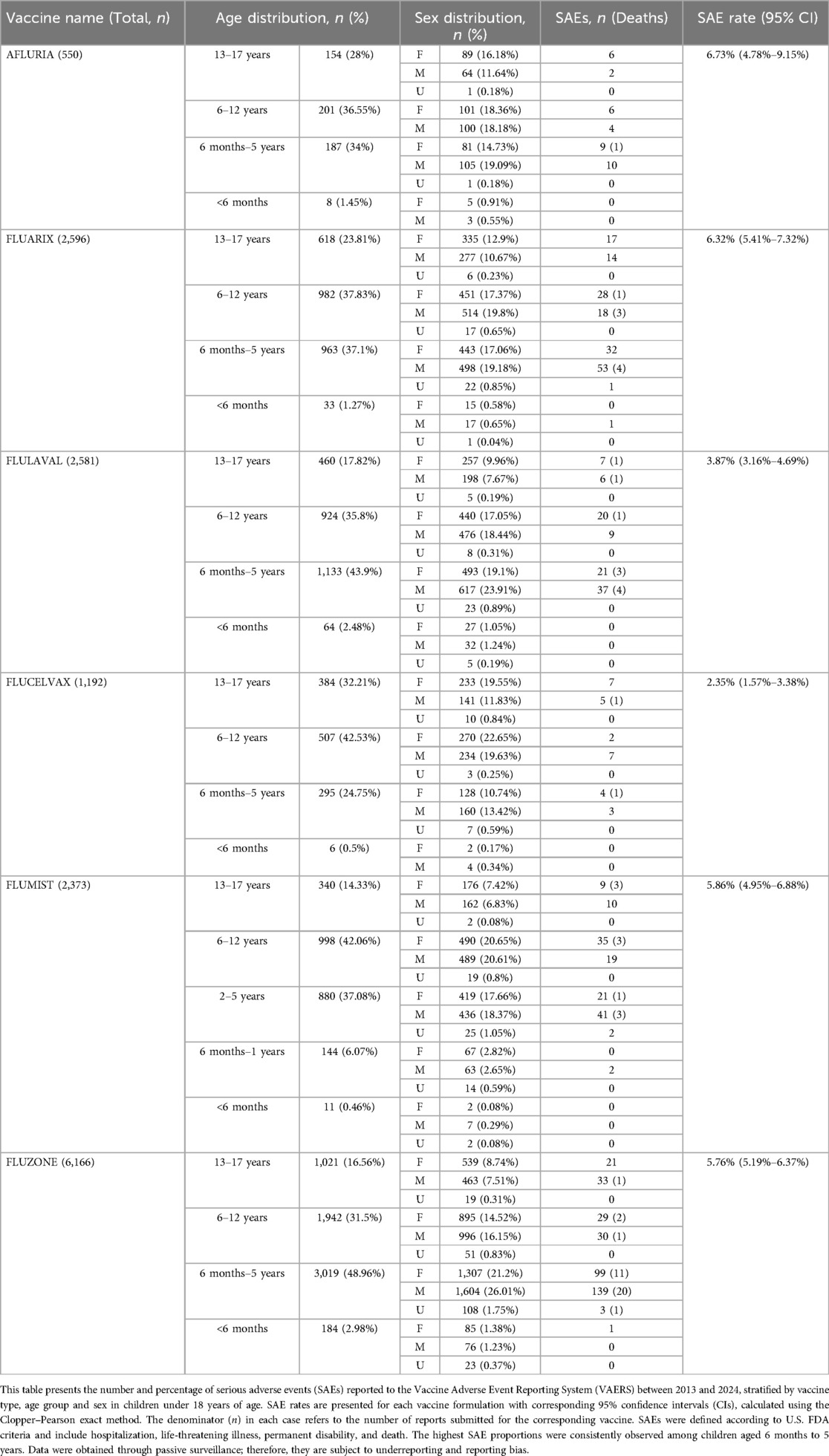
Table 1. Age-specific distribution of serious adverse events (SAEs) following quadrivalent influenza vaccination in children aged <18 years (2013–2024).
3.4 Disproportionality signal detection
A total of 251 MedDRA preferred terms met predefined criteria for disproportionality signals across all QIVs. Notable vaccine-specific safety signals included syncope and seizures associated with Afluria, injection-site reactions linked primarily to egg-based vaccines, and respiratory symptoms observed with FluMist.
Among evaluated rare adverse events, Guillain-Barré syndrome (GBS) was the sole condition meeting both statistical thresholds, with 42 reported cases (ROR = 1.71, 95% CI: 1.25–2.35; IC025 = 0.28). The median time to symptom onset for GBS was 14.5 days (IQR: 4.5–32 days), with distribution across vaccines as follows: Fluzone (n = 16), Fluarix (n = 7), FluMist (n = 6), FluLaval (n = 4), Afluria (n = 3), and Flucelvax (n = 0). Table 2 presents the top five MedDRA preferred terms showing positive disproportionality signals, while Table 3 provides a comprehensive summary of rare adverse events.
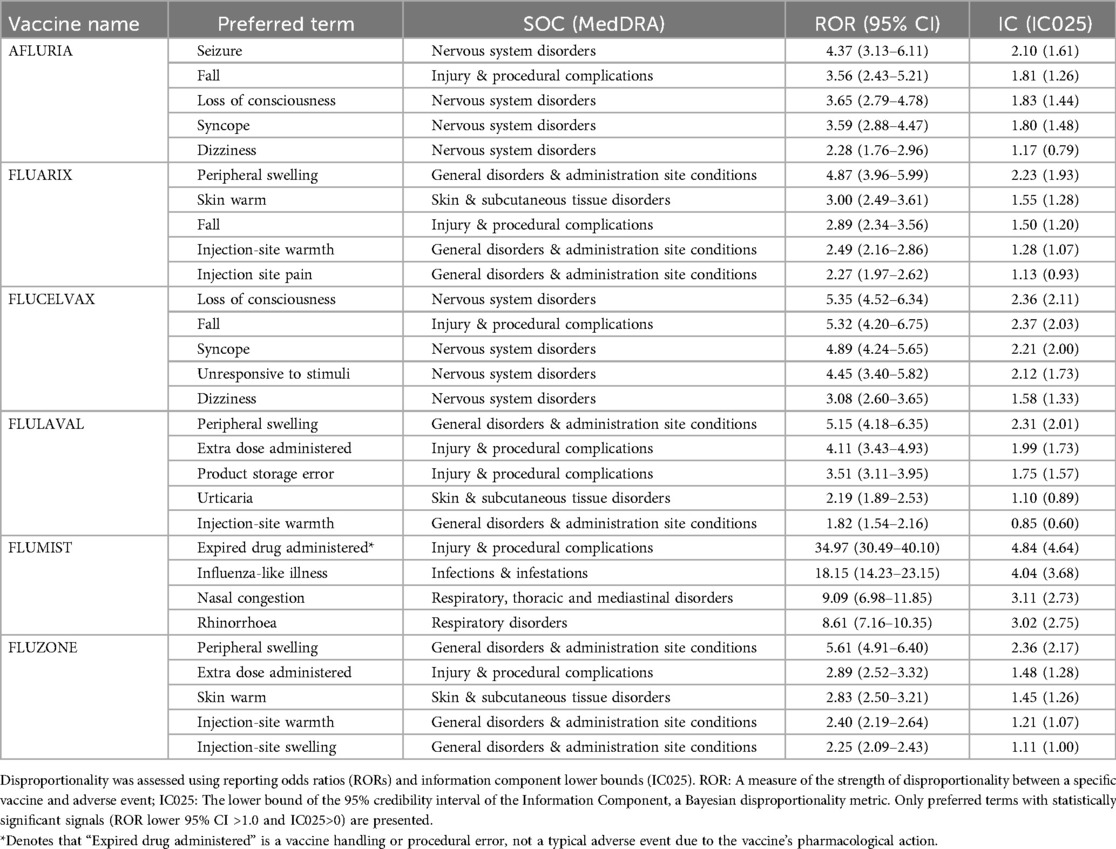
Table 2. Top five MedDRA preferred terms showing positive disproportionality signals associated with quadrivalent influenza vaccines in pediatric populations.
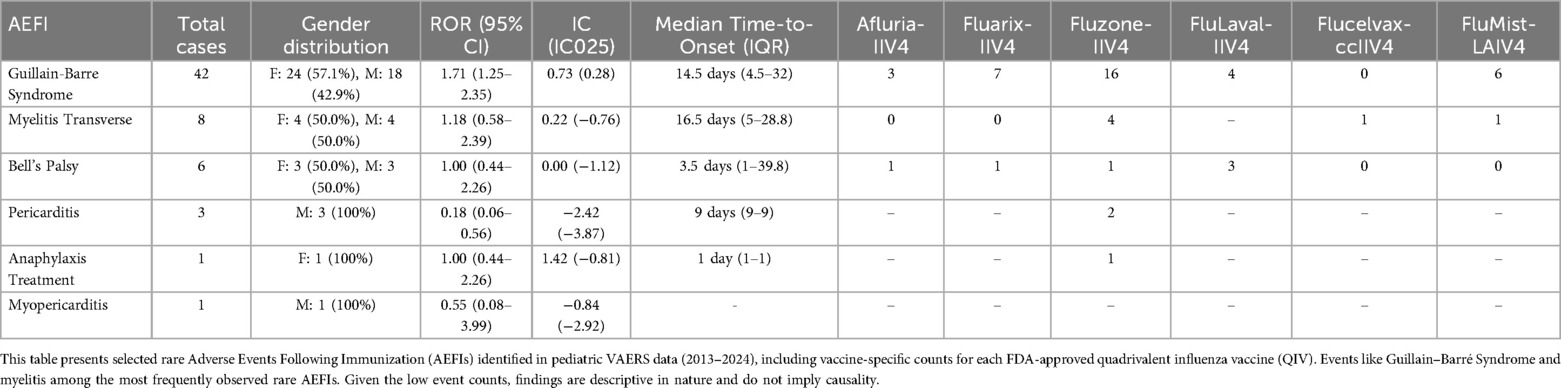
Table 3. Summary of rare adverse events following immunization (AEFIs) with quadrivalent influenza vaccines in pediatric populations.
For context, the background incidence of Guillain-Barré syndrome in children is estimated at approximately 0.3–1.3 per 100,000 person-years; thus, while GBS met both disproportionality thresholds (ROR and IC), this likely reflects reporting enrichment rather than a true increase in clinical incidence (21, 22, 28, 29).
3.5 Temporal trends
Annual reporting volume peaked in 2018 with 1,840 reports, followed by a significant reduction ranging from 24.8% to 66.5% during 2021–2024, coinciding with disruptions related to the COVID-19 pandemic. Figure 1 illustrates the annual pediatric VAERS reports by quadrivalent influenza vaccine formulation from 2013 to 2024, clearly demonstrating the impact of the pandemic on reporting patterns.
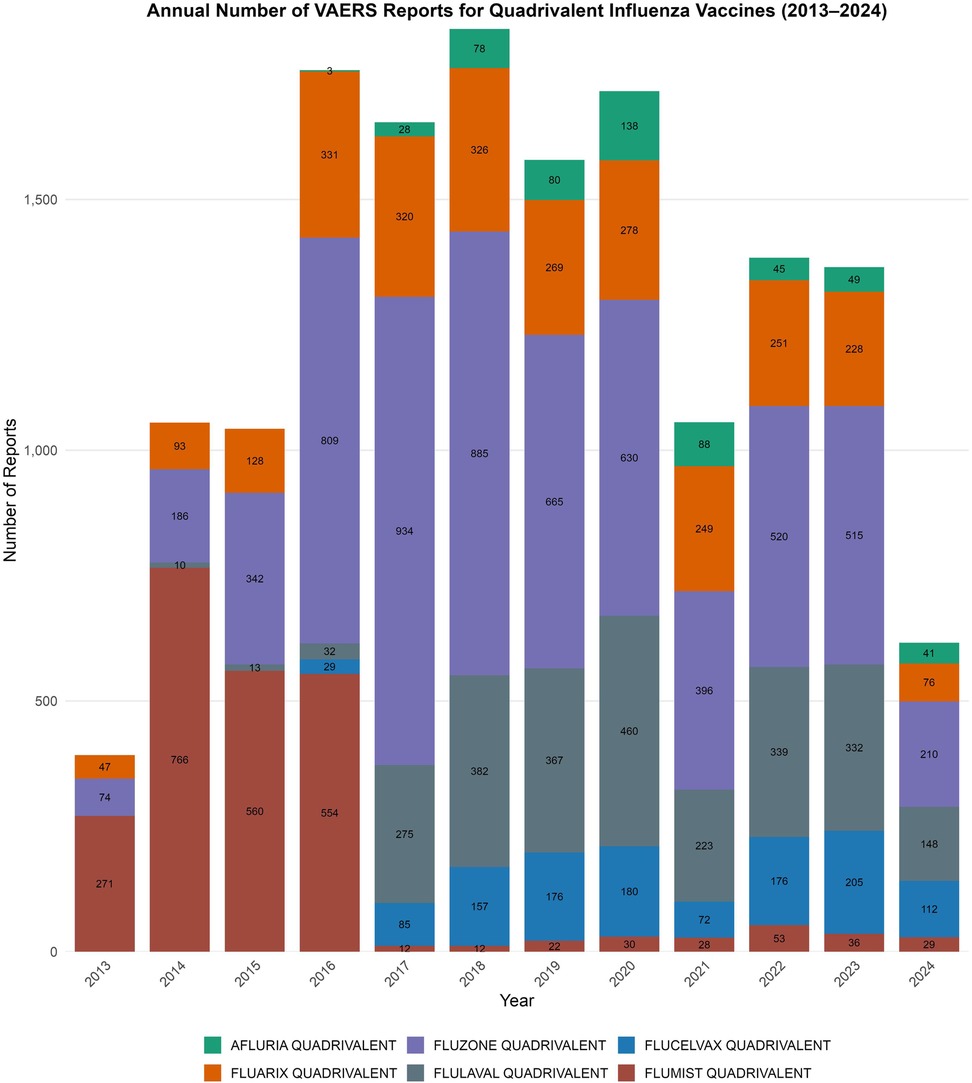
Figure 1. Annual pediatric VAERS reports by quadrivalent influenza vaccine formulation, 2013–2024. Stacked bar chart displaying the number of vaccine-specific adverse event reports submitted to VAERS each year for children under 18 years of age. Vaccines included Fluzone, Fluarix, FluLaval, FluMist, Flucelvax, and Afluria. A peak in overall reporting was observed in 2018, followed by a substantial decline during the COVID-19 pandemic period (2021–2024). Differences in volume across formulations and timeframes reflect variations in usage, market share, and potential reporting behavior.
Among the inactivated vaccines, the median time to adverse event onset was 0 days (IQR: 0–1 day), with 75% of events occurring within 24 h post-vaccination. FluMist demonstrated a slightly broader onset range (IQR: 0–2 days).
To assess potential underreporting during the COVID-19 pandemic, we compared pediatric VAERS reporting volume with national influenza vaccination coverage estimates. Coverage among children aged 6 months-17 years declined from 63.7% (2019–20) to 58.6% (2020–21),and remained relatively stable at 57.8% (2021–22) and 57.4% (2022–23), with a further decline to 55.4% (2023–24) (30–33).By contrast, VAERS reports dropped by 42%–66.5%, a disproportionate decline relative to vaccine uptake, supporting the inference that pandemic-related factors-such as reduced healthcare access, shifting reporting priorities, and system burden-likely contributed to underreporting rather than a true reduction in adverse event incidence.
3.6 Comprehensive safety signal analysis
Figure 2 presents disproportionality analyses for the 30 most frequently reported adverse events following quadrivalent influenza vaccination in children. The forest plots demonstrate vaccine-specific reporting odds ratios (ROR) and Bayesian information components (IC) with 95% confidence intervals for each of the six FDA-approved QIVs. Significant signals were defined by ROR lower bound >1.0 and IC025>0, with notable variations observed across different vaccine formulations.

Figure 2. Disproportionality analysis of the 30 most frequently reported adverse events following quadrivalent influenza vaccination in children. Forest plot showing reporting odds ratios (ROR) and Bayesian Information Components (IC) with 95% confidence intervals for the 30 most frequently reported adverse events in VAERS (2013–2024), following administration of six FDA-approved quadrivalent influenza vaccines in children. ROR: A measure of the strength of disproportionality between a specific vaccine and adverse event. IC025: The lower bound of the 95% credibility interval of the Information Component, a Bayesian disproportionality metric. Significant signals were defined by ROR lower bound >1.0 and IC025>0. Blue squares represent point estimates; horizontal lines denote 95% confidence intervals. The red dashed line indicates the null value (ROR = 1.0). Arrowheads reflect intervals exceeding the plot range. Subfigures: (a) Afluria (b) Fluarix (c) Flucelvax (d) FluLaval (e) FluMist (f) Fluzone.
To further characterize the safety signal landscape, Figure 3 displays volcano plots of disproportionality signals for each QIV. These plots effectively visualize the relationship between statistical significance and effect size, with each point representing an individual MedDRA preferred term. The x-axis represents the log2-transformed reporting odds ratio (ROR), while the y-axis indicates the log10 of the false discovery rate (FDR)-adjusted p-value. Terms positioned further to the right and higher on the graph represent stronger disproportionality signals, with dot size indicating the number of reports.
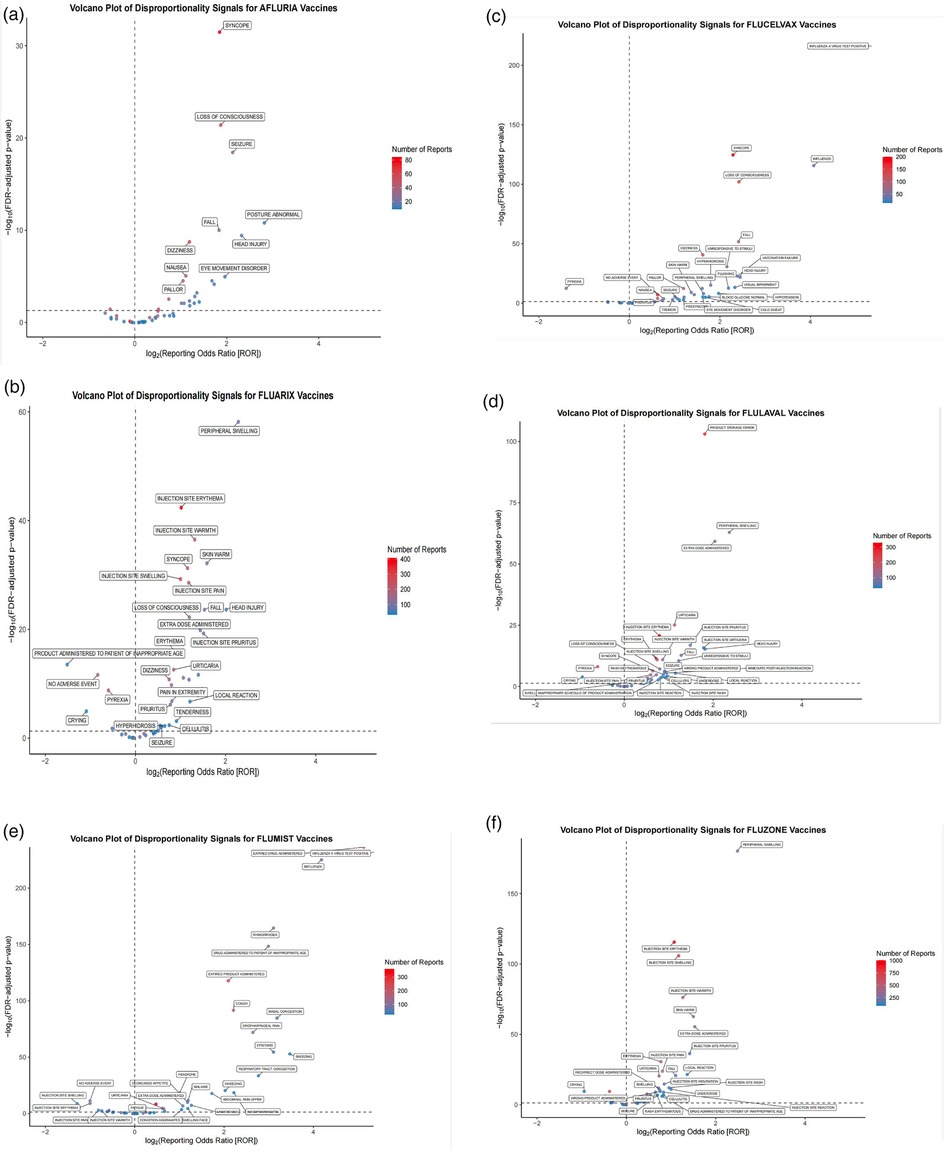
Figure 3. Volcano plots of disproportionality signals for quadrivalent influenza vaccines in pediatric populations. Disproportionality analysis of adverse event reports submitted to VAERS (2013–2024) for six FDA-approved quadrivalent influenza vaccines administered to children. Each volcano plot displays individual MedDRA preferred terms, with the x-axis representing the log2-transformed reporting odds ratio (ROR) and the y-axis indicating the -log10 of the false discovery rate (FDR)-adjusted p-value. Terms plotted further to the right and higher on the graph represent stronger disproportionality signals. The number of reports is indicated by dot size. Subfigures: (a) Afluria, (b) Fluarix, (c) Flucelvax, (d) FluLaval, (e) FluMist, (f) Fluzone.
To further explore formulation-specific safety profiles, we conducted a sensitivity analysis stratified by vaccine type-inactivated influenza vaccines (IIV4) vs. live attenuated influenza vaccine (LAIV4). This analysis integrated findings from Tables 1, 2 and Figures 2–4. IIV4s (Fluzone, Fluarix, FluLaval, Afluria, and Flucelvax) collectively accounted for 84.6% of reports and were more frequently associated with neurological and injection-site events, such as syncope, seizures, and injection-site erythema. In contrast, LAIV4 (FluMist) was more commonly linked to upper respiratory symptoms including rhinorrhea and nasal congestion. Time-to-onset patterns were broadly similar, with most events occurring within the first day post-vaccination, though LAIV4 showed a slightly wider onset range (IQR: 0–2 days). These trends are consistent with known reactogenicity profiles and vaccine administration routes.
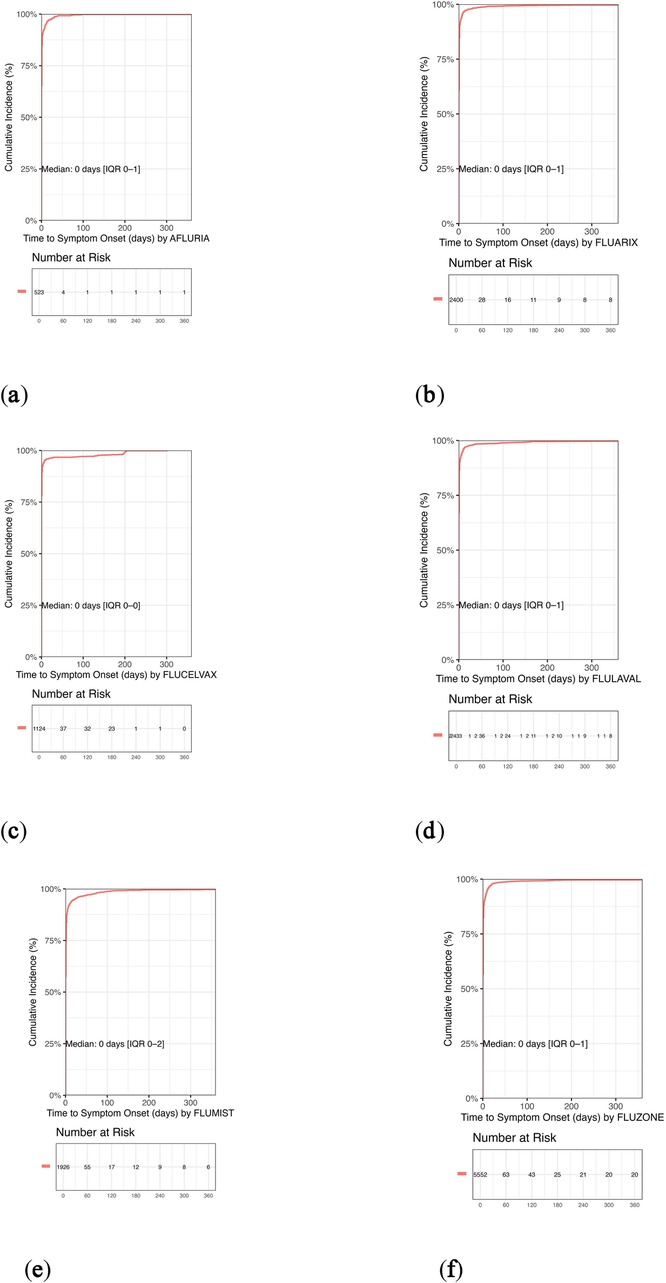
Figure 4. Kaplan–Meier curves of cumulative incidence of adverse events following quadrivalent influenza vaccination in pediatric populations. Kaplan–Meier curves depict the cumulative incidence of adverse events over time for each quadrivalent influenza vaccine formulation based on pediatric VAERS data (2013–2024). Across all vaccines, the median onset was 0 days, indicating an acute response profile. Over 95% of adverse events occurred within the first 7 days, with most reported on the day of vaccination. (a) Afluria: Median onset 0 days (IQR: 0–1); approximately 95% of events occurred on day 0. (b) Fluarix: Median onset 0 days (IQR: 0–1); similar to other inactivated formulations. (c) Flucelvax: Median onset 0 days (IQR: 0–0); exhibited the most rapid onset profile. (d) FluLaval: Median onset 0 days (IQR: 0–1); consistent with inactivated vaccine trends. (e) FluMist: Median onset 0 days (IQR: 0–2); slightly broader distribution typical of live attenuated vaccines. (f) Fluzone: Median onset 0 days (IQR: 0–1); rapid onset maintained despite higher report volume. The rapid onset patterns across all vaccines support the acute nature of most reported events and align with known immunization response timelines.
3.7 Time to symptom onset patterns
Figure 4 presents Kaplan–Meier curves depicting the cumulative incidence of adverse events over time for each quadrivalent influenza vaccine formulation. Across all vaccines, the median onset was 0 days, indicating an acute response profile. Over 95% of adverse events occurred within the first 7 days, with most reported on the day of vaccination. These rapid onset patterns support the acute nature of most reported events and align with known immunization response timelines.
4 Discussion
4.1 Principal findings
This large-scale post-marketing surveillance study provides robust evidence supporting the overall safety of quadrivalent influenza vaccines (QIVs) in pediatric populations. The observed 5.29% rate of serious adverse events (SAEs) reflects the inherent characteristics of passive surveillance systems. Large-scale active surveillance studies conducted through the Vaccine Safety Datalink demonstrate substantially lower rates of confirmed serious adverse events, with one study of over 590,000 live attenuated vaccine doses finding only rare confirmed cases of anaphylaxis (1.7 per million doses) and syncope (8.5 per million doses) (34), while another study of over 91,000 inactivated vaccine doses in young children found no evidence of serious medically attended events (35). However, interpretation must consider the inherent limitations of passive surveillance systems, which typically capture only 1%–10% of actual adverse events and are more likely to detect serious outcomes (36).
4.2 Guillain-Barré syndrome risk-benefit assessment
Among the rare adverse events assessed, Guillain-Barré syndrome (GBS) was the only one that met both signal detection thresholds (ROR=1.71), consistent with selected epidemiological studies (37). While some meta-analyses have found no confirmed risk of vaccine-associated GBS (38), CDC surveillance has reported modest relative risk increases within expected post-vaccination intervals (17). The median onset of 14.5 days is biologically plausible and supports a potential immune-mediated mechanism.
Importantly, the absolute risk of GBS following QIV administration remains exceedingly low. Even at the upper limit of the confidence interval, the estimated excess risk is 1–2 additional cases per million doses. This must be balanced against the substantially higher GBS incidence following natural influenza infection, which is estimated to be 4–7 times greater (37, 39). Recent pediatric surveillance data indicate a background incidence of Guillain-Barré syndrome of approximately 0.34–0.69 per 100,000 person-years in children under 16 years (22). Therefore, although GBS met both disproportionality thresholds (ROR and IC), this signal most likely reflects nhanced reporting rather than a clinically meaningful increase in incidence.
4.3 Age-dependent reactogenicity
The elevated SAE rates observed in children aged 6 months to 5 years are clinically noteworthy. This group exhibited 2- to 4-fold higher SAE rates compared to adolescents, which may reflect age-related differences in immune response and heightened parental or provider vigilance in reporting (40, 41). The immature immune system characteristics in early childhood, including differences in innate and adaptive immune responses, may contribute to varying reactogenicity profiles across pediatric age groups (40, 42).
4.4 Platform-specific safety profiles
Flucelvax demonstrated the lowest SAE rate (2.35%), suggesting potential safety advantages associated with cell culture-based platforms, including the absence of egg-adaptive mutations (43) and possibly lower innate immunogenicity due to production processes. Conversely, the elevated frequency of neurological adverse events associated with Afluria is noteworthy, especially given prior reports linking this formulation to febrile seizures (44, 45). These findings support the hypothesis that manufacturing methods and antigenic composition may influence vaccine reactogenicity.
4.5 Impact of the COVID-19 pandemic
The marked decline in annual adverse event reporting (24.8%–66.5%) during 2021–2024 likely reflects the multifactorial consequences of the COVID-19 pandemic, including reduced pediatric immunization visits, shifts in healthcare delivery, and reporting fatigue among healthcare providers (24, 46, 47). Additionally, changes in public health messaging, vaccine formulation preferences, and seasonal uptake may have further confounded reporting trends.
4.6 Public health implications
These findings may inform tailored vaccine recommendations, particularly in pediatric subgroups. For instance, cell-based QIVs may offer preferable safety profiles for immunocompromised children or those with a history of seizures (48, 49). Continued surveillance and stratified safety assessments are essential for optimizing vaccine selection across diverse populations (50).
4.7 Study limitations
This study is subject to several limitations inherent to the VAERS system, including underreporting, reporting and selection bias, absence of denominator data, and the inability to determine causality without an unvaccinated comparator group. These constraints highlight the importance of corroborating findings with active surveillance systems and well-controlled epidemiological studies.
5 Conclusions
This 12-year analysis of 15,458 pediatric reports from the VAERS database provides compelling evidence affirming the overall safety of FDA-licensed quadrivalent influenza vaccines (QIVs) in children. The predominance of non-serious, self-limiting adverse events and the relatively low rate of serious outcomes underscore a favorable benefit-risk profile.
Although Guillain-Barré syndrome (GBS) met signal detection thresholds, its absolute incidence remained exceedingly low. These findings reinforce the safety of QIVs and support continued adherence to national influenza vaccination guidelines.
Importantly, formulation-specific differencessuch as the notably lower SAE rate observed with Flucelvax-suggest that cell-based QIVs may be preferable in younger or medically vulnerable subgroups, although further research is warranted. Age-related variation in reactogenicity also underscores the need for targeted risk communication and vigilant monitoring, particularly in children aged 6 months–5 years.
Given the observed drop in reporting during the COVID-19 pandemic, future efforts should integrate active surveillance systems such as the Vaccine Safety Datalink (VSD) to complement passive data sources and ensure timely signal detection. Pediatric healthcare providers can remain confident in recommending annual influenza vaccination, while supporting ongoing safety evaluation through systematic reporting and data sharing.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HF: Project administration, Conceptualization, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. YH: Visualization, Writing – original draft. XW: Data curation, Resources, Writing – original draft. YZ: Resources, Writing – review & editing. YL: Data curation, Investigation, Software, Writing – review & editing. YS: Project administration, Supervision, Validation, Visualization, Writing – original draft. LP: Conceptualization, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Grohskopf LA, Alyanak E, Broder KR, Walton EK, Clippard JR, Shay DK, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices—united States, 2019–20 influenza season. MMWR Recomm Rep. (2019) 68(3):1–21. doi: 10.15585/mmwr.rr6803a1
2. Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. (2012) 8(1):81–8. doi: 10.4161/hv.8.1.17623
3. Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. (2013) 12(9):1085–94. doi: 10.1586/14760584.2013.824709
4. U.S. Food and Drug Administration. Vaccines Licensed for Use in the United States [Internet]. Silver Spring (MD): FDA (2024). Available online at: https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
5. Chen RT, Shimabukuro TT, Martin DB, Zuber PL, Weibel D, Sturkenboom M. Enhancing vaccine safety capacity globally: a lifecycle perspective. Vaccine. (2015) 33(Suppl 4):D46–54. doi: 10.1016/j.vaccine.2015.06.073
6. Shimabukuro TT, Nguyen M, Martin D, DeStefano F. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine. (2015) 33(36):4398–405. doi: 10.1016/j.vaccine.2015.07.035
7. Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the vaccine adverse event reporting system, United States, October 1, 2009–January 31, 2010. Vaccine. (2010) 28(45):7248–55. doi: 10.1016/j.vaccine.2010.09.021
8. McMahon AW, Iskander J, Haber P, Brabazon T, Ball R, Goodman MJ, et al. Adverse events after inactivated influenza vaccination among children less than 2 years of age: analysis of reports from the vaccine adverse event reporting system, 1990–2003. Pediatrics. (2005) 115(2):453–60. doi: 10.1542/peds.2004-1519
9. Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM, VSD Rapid Cycle Analysis Influenza Working Group. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the vaccine safety datalink project, 2010–2011. Vaccine. (2012) 30(11):2024–31. doi: 10.1016/j.vaccine.2012.01.027
10. Duffy J, Weintraub E, Hambidge SJ, Jackson LA, Kharbanda EO, Klein NP, et al. Febrile seizure risk after vaccination in children 6–23 months. Pediatrics. (2016) 138(1):e20160320. doi: 10.1542/peds.2016-0320
11. Centers for Disease Control and Prevention. Flu Vaccines and People with Egg Allergies [Internet]. Atlanta (GA): CDC (2023). Available online at: https://www.cdc.gov/flu/vaccines/egg-allergies.html
12. Grohskopf LA, Sokolow LZ, Broder KR, Olsen SJ, Karron RA, Jernigan DB, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the advisory committee on immunization practices-United States, 2016–17 influenza season. MMWR Recomm Rep. (2016) 65(5):1–54. doi: 10.15585/mmwr.rr6505a1
13. Centers for Disease Control and Prevention. Cell-Based Flu Vaccines [Internet]. Atlanta (GA): CDC (2023). Available online at: https://www.cdc.gov/flu/vaccine-types/cell-based.html
14. Rajaram S, Boikos C, Gelone DK, Gandhi A. Influenza vaccines: the potential benefits of cell-culture isolation and manufacturing. Ther Adv Vaccines Immunother. (2020) 8:2515135520908121. doi: 10.1177/2515135520908121
15. Block SL, Yogev R, Hayden FG, Ambrose CS, Walker RE, Hultquist M. Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age. Vaccine. (2008) 26(38):4940–6. doi: 10.1016/j.vaccine.2008.07.013
16. Centers for Disease Control and Prevention. Live Attenuated Influenza Vaccine [LAIV] (the Nasal Spray Flu Vaccine) [Internet]. Atlanta (GA): CDC (2023). Available online at: https://www.cdc.gov/flu/vaccine-types/nasalspray.html
17. Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. (2014) 58(8):1149–55. doi: 10.1093/cid/ciu005
18. Martín Arias LH, Sanz R, Sáinz M, Treceño C, Carvajal A. Guillain-Barré syndrome and influenza vaccines: a meta-analysis. Vaccine. (2015) 33(31):3773–8. doi: 10.1016/j.vaccine.2015.05.013
19. Institute for Vaccine Safety, Johns Hopkins Bloomberg School of Public Health. Do Vaccines Cause Guillain-Barré Syndrome? [Internet]. Baltimore (MD): Johns Hopkins Bloomberg School of Public Health (2018). Available online at: https://www.vaccinesafety.edu/do-vaccines-cause-guillain-barre-syndrome/
20. Children’s Hospital of Philadelphia Vaccine Education Center. Vaccines and Guillain-Barré Syndrome [Internet]. Philadelphia (PA): CHOP (2021). Available online at: https://www.chop.edu/vaccine-education-center/vaccine-safety/vaccines-and-other-conditions/guillain-barre-syndrome
21. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. (2011) 36(2):123–33. doi: 10.1159/000324710
22. Levison LS, Thomsen RW, Markvardsen LK, Christensen DH, Sindrup SH, Andersen H. Pediatric Guillain-Barré syndrome in a 30-year nationwide cohort. Pediatr Neurol. (2020) 107:57–63. doi: 10.1016/j.pediatrneurol.2020.01.017
23. Bramer CA, Kimmins LM, Swanson R, Kuo J, Vranesich P, Jacques-Carroll LA, et al. Decline in child vaccination coverage during the COVID-19 pandemic—Michigan care improvement registry, may 2016–May 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(20):630–1. doi: 10.15585/mmwr.mm6920e1
24. Santoli JM, Lindley MC, DeSilva MB, Kharbanda EO, Daley MF, Galloway L, et al. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb Mortal Wkly Rep. (2020) 69(19):591–3. doi: 10.15585/mmwr.mm6919e2
25. Food and Drug Administration. Guidance for Industry: Good Pharmacovigilance Practices and Pharmacoepidemiologic Assessment [Internet]. Silver Spring (MD): FDA (2005). Available online at: https://www.fda.gov/media/71546/download
26. Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. (2014) 67(8):850–7. doi: 10.1016/j.jclinepi.2014.03.012
27. Moro PL, Haber P, McNeil MM. Challenges in evaluating post-licensure vaccine safety: observations from the centers for disease control and prevention. Expert Rev Vaccines. (2019) 18(11):1045–51. doi: 10.1080/14760584.2019.1676154
28. Tam CC, O'Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barré syndrome and preceding infection with Campylobacter, influenza and Epstein–Barr virus in the general practice research database. PLoS One. (2007) 2(4):e344. doi: 10.1371/journal.pone.0000344
29. Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barré syndrome with influenza vaccine and influenza-like illness using the United Kingdom general practice research database. Am J Epidemiol. (2009) 169(3):382–8. doi: 10.1093/aje/kwn310
30. Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2020–2021 Influenza Season [Internet]. Atlanta (GA): CDC (2022). Available online at: https://www.cdc.gov/fluvaxview/coverage-by-season/2020-2021.html
31. Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2021–22 Influenza Season [Internet]. Atlanta (GA): CDC (2022). Available online at: https://www.cdc.gov/fluvaxview/coverage-by-season/2021-2022.html
32. Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2022–23 Influenza Season [Internet]. Atlanta (GA): CDC (2023). Available online at: https://www.cdc.gov/fluvaxview/coverage-by-season/2022-2023.html
33. Centers for Disease Control and Prevention. Flu Vaccination Coverage, United States, 2023–24 Influenza Season [Internet]. Atlanta (GA): CDC (2024). Available online at: https://www.cdc.gov/fluvaxview/coverage-by-season/2023-2024.html
34. Daley MF, Clarke CL, Glanz JM, Xu S, Hambidge SJ, Donahue JG, et al. The safety of live attenuated influenza vaccine in children and adolescents 2 through 17 years of age: a vaccine safety datalink study. Pharmacoepidemiol Drug Saf. (2018) 27(1):59–68. doi: 10.1002/pds.4349
35. Glanz JM, Newcomer SR, Hambidge SJ, Daley MF, Narwaney KJ, Xu S, et al. Safety of trivalent inactivated influenza vaccine in children aged 24–59 months in the vaccine safety datalink. Arch Pediatr Adolesc Med. (2011) 165(8):749–55. doi: 10.1001/archpediatrics.2011.112
36. Lazarus R, Klompas M. Electronic Support for Public Health—Vaccine Adverse Event Reporting System (ESP:VAERS). Final Report [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (2010). Available online at: https://digital.ahrq.gov/ahrq-funded-projects/electronic-support-public-health-vaccine-adverse-event-reporting-system
37. Kwong JC, Vasa PP, Campitelli MA, Hawken S, Wilson K, Rosella LC, et al. Risk of Guillain-Barré syndrome after seasonal influenza vaccination and influenza health-care encounters: a self-controlled study. Lancet Infect Dis. (2013) 13(9):769–76. doi: 10.1016/S1473-3099(13)70104-X
38. Petráš M, Králová Lesná I, Dáňová J, Čelko AM. Is an increased risk of developing Guillain-Barré syndrome associated with seasonal influenza vaccination? A systematic review and meta-analysis. Vaccines (Basel). (2020) 8(2):150. doi: 10.3390/vaccines8020150
39. Ghaderi S, Gunnes N, Bakken IJ, Magnus P, Trogstad L, Håberg SE. Risk of Guillain-Barré syndrome after exposure to pandemic influenza A(H1N1)pdm09 vaccination or infection: a Norwegian population-based cohort study. Eur J Epidemiol. (2016) 31(1):67–72. doi: 10.1007/s10654-015-0047-0
40. Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. (2015) 282(1821):20143085. doi: 10.1098/rspb.2014.3085
41. Parrella A, Gold M, Marshall H, Braunack-Mayer A, Baghurst P. Parental perspectives of vaccine safety and experience of adverse events following immunisation. Vaccine. (2013) 31(16):2067–74. doi: 10.1016/j.vaccine.2013.02.011
42. Gidengil C, Goetz MB, Newberry S, Maglione M, Hall O, Larkin J, et al. Safety of vaccines used for routine immunization in the United States: an updated systematic review and meta-analysis. Vaccine. (2021) 39(28):3696–716. doi: 10.1016/j.vaccine.2021.03.079
43. Tree JA, Richardson C, Fooks AR, Clegg JC, Looby D. Comparison of large-scale mammalian cell culture systems with egg culture for the production of influenza virus A vaccine strains. Vaccine. (2001) 19(25–26):3444–50. doi: 10.1016/S0264-410X(01)00053-6
44. Li-Kim-Moy J, Yin JK, Rashid H, Khandaker G, King C, Wood N, et al. Systematic review of fever, febrile convulsions and serious adverse events following administration of inactivated trivalent influenza vaccines in children. Euro Surveill. (2015) 20(24):21159. doi: 10.2807/1560-7917.ES2015.20.24.21159
45. Centers for Disease Control and Prevention (CDC). Update: recommendations of the advisory committee on immunization practices (ACIP) regarding use of CSL seasonal influenza vaccine (afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep. (2010) 59(31):989–92.20703207
46. Li Y, Li J, Dang Y, Chen Y, Tao C. Adverse events of COVID-19 vaccines in the United States: temporal and spatial analysis. JMIR Public Health Surveill. (2024) 10:e51007. doi: 10.2196/51007
47. Block J. Is the US’s vaccine adverse event reporting system broken? Br Med J. (2023) 383:2582. doi: 10.1136/bmj.p2582
48. Essink BJ, Heeringa M, Jeanfreau RJ, Finn D, Matassa V, Edelman J, et al. Safety and immunogenicity of cell-based quadrivalent influenza vaccine: a randomized trial. Pediatrics. (2022) 150(5):e2022057509. doi: 10.1542/peds.2022-057509
49. Centers for Disease Control and Prevention. Febrile Seizures and Vaccines [Internet]. Atlanta (GA): CDC (2024). Available online at: https://www.cdc.gov/vaccine-safety/about/febrile-seizures.html
50. Centers for Disease Control and Prevention. About CDC’s Vaccine Safety Monitoring Program [Internet]. Atlanta (GA): CDC (2024). Available online at: https://www.cdc.gov/vaccine-safety-systems/about/cdc-monitoring-program.html
Keywords: quadrivalent influenza vaccine, pediatric safety, adverse events, VAERS, Guillain–Barré syndrome, post-marketing surveillance
Citation: Fu H, Huang Y, Wang X, Zhang Y, Liu Y, Su Y and Pan L (2025) Pharmacovigilance-based 12-year post-marketing safety analysis of quadrivalent influenza vaccines in children: a VAERS surveillance study. Front. Pediatr. 13:1640498. doi: 10.3389/fped.2025.1640498
Received: 3 June 2025; Accepted: 18 August 2025;
Published: 2 September 2025.
Edited by:
Dechuan Kong, Shanghai Municipal Center for Disease Control and Prevention (SCDC), ChinaReviewed by:
Mercedes Macías Parra, National Institute of Pediatrics, MexicoMustafa Kursat Sahin, Ondokuz Mayıs University, Türkiye
Copyright: © 2025 Fu, Huang, Wang, Zhang, Liu, Su and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonglong Su, eW9uZ2xvbmdzdTRAZ21haWwuY29t; Lili Pan, MzYyNDA1MDc5QHFxLmNvbQ==
 Honghong Fu
Honghong Fu Yirong Huang2
Yirong Huang2 Xinjin Wang
Xinjin Wang