- 1Department of Gastroenterology, Children's Hospital Affiliated to Zhengzhou University, Zhengzhou, Henan, China
- 2Zhengzhou University, Zhengzhou, Henan, China
- 3Department of Clinical Nutrition, Children's Hospital Affiliated to Zhengzhou University, Zhengzhou, Henan, China
- 4Zhengzhou Key Laboratory of Children's Digestive Diseases, Children's Hospital Affiliated to Zhengzhou University, Zhengzhou, Henan, China
Introduction: This study aimed to develop a dynamic nomogram model to predict the risk of Clostridioides difficile infection (CDI) in children with ulcerative colitis (UC).
Methods: This was a retrospective study that clinical data from pediatric diagnosis and treatment with UC at Zhengzhou University Children's Hospital between January 2018 and December 2024 were retrospectively reviewed. Patients were classified into CDI (n = 35) and non-CDI (n = 86) groups based on the presence or absence of CDI. Predictor variables were selected using least absolute shrinkage and selection operator (LASSO) regression and subsequently entered into a multivariate logistic regression model. Nomograms were then constructed based on the final logistic regression analysis. The model's performance and clinical utility were assessed using receiver operating characteristic (ROC) curves, calibration plots, and decision curve analysis (DCA). Internal validation was performed using 1,000 bootstrap resamples.
Results: A total of 121 children were included in the study. Based on LASSO and multivariate logistic regression analysis of 24 candidate variables, five independent risk factors for CDI in children with UC were identified: Pediatric Ulcerative Colitis Activity Index (PUCAI), erythrocyte sedimentation rate (ESR), vitamin D (Vit D), fecal calprotectin (FC), and antibiotic use exceeding seven days (all p < 0.05). The nomograms constructed with the above variables demonstrated excellent discriminative ability (C-index = 0.964, 95% CI: 0.932–0.997). The Hosmer-Lemeshow test (χ2 = 12.529, p = 0.129) and bootstrap validation revealed good concordance between the predicted probabilities and actual outcomes. Decision curve analysis (DCA) indicated significant net clinical benefit, and the model maintained robust consistency across relevant clinical subgroups.
Conclusions: PUCAI, ESR, Vit D, FC, and use of antibiotic use exceeding seven days were the five independent risk factors for CDI in children with UC. The resulting nomogram may support clinicians in early diagnosis and timely adjustment of therapeutic strategies.
Introduction
Ulcerative colitis (UC) is a chronic, relapsing-remitting inflammatory disease characterized by mucosal diseases confined to the colon, with approximately 15%–20% of cases presenting during childhood or adolescence (1). Its pathogenesis involves multifactorial mechanisms, including genetic susceptibility, environmental exposures (e.g., infections, diet, and lifestyle), gut microbiota alterations, and immune dysregulation. Compared to adult-onset UC, children with UC typically presents with abrupt onset, rapid progression, extensive mucosal involvement, and an unpredictable course marked by alternating remission and acute exacerbations. Clinical management is often complicated by frequent hospitalizations, a high rate of corticosteroid resistance, and a colectomy rate reaching 5%–6% within one year of diagnosis (2, 3). Despite the efficacy of long-term immunosuppressive therapies - such as 5-aminosalicylic acid (5-ASA), systemic and topical corticosteroids, immunomodulators, and biologics - in inducing and maintaining remission, these treatments increase susceptibility to opportunistic infections, suboptimal mucosal healing, and secondary loss of therapeutic response. Among opportunistic infections, Clostridioides difficile infection (CDI) and cytomegalovirus (CMV) represent significant contributors to disease exacerbation in children with UC.
CDI poses substantial public health challenges due to its high incidence, recurrence, and associated mortality. According to Centers for Disease Control and Prevention (CDC) surveillance data, community-associated CDI accounted for 75% of pediatric cases in 2019, with an incidence reaching 25.8 per 100,000 children (4). CDI is a significant health concern with consequences for pediatric's growth and overall morbidity. Individuals with inflammatory bowel disease (IBD), including both adults and children, exhibit increased susceptibility, with CDI prevalence rates of 13% and 6.9%, respectively (5, 6). Although Clostridioides difficile (CD) is a non-invasive, spore-forming, obligate anaerobic Gram-positive bacillus, it produces enterotoxins (toxins A and B) and tissue-degrading enzymes (e.g., collagenase, hyaluronidase, chondroitin sulfate lyase) that compromise epithelial integrity by disrupting tight junctions and the actin cytoskeleton. This cascade leads to fluid secretion, localized inflammation, and a spectrum of clinical manifestations ranging from mild diarrhea to life-threatening complications such as pseudomembranous colitis, toxic megacolon, intestinal perforation, systemic inflammatory response syndrome (SIRS), sepsis, and death (7).
The treatment strategy for CDI depend on the severity of the infection, the presence of recurrence, and individual patient factors. The main antibiotic options include metronidazole, vancomycin, or fidaxomicin. For initial CDI episodes, it is recommended to first discontinue the suspected inciting antibiotics. Vancomycin or metronidazole are typically used as first-line agents, with fidaxomicin considered in select cases (8). In cases of recurrent CDI, distinct therapeutic approaches are indicated: (1) For patients whose initial episode was treated with vancomycin or metronidazole, fidaxomicin is recommended for the first recurrence; (2) For those initially treated with fidaxomicin, adjunctive bezlotoxumab therapy should be combined with standard oral antibiotic regimens (vancomycin or fidaxomicin). Multiple recurrent CDI cases warrant fecal microbiota transplantation (FMT) or bezlotoxumab supplementation following standard antibiotic pretreatment (7). FMT can regulate the gut microbiota and has been approved by the FDA for the treatment of recurrent CDI. However, the results of FMT in the treatment of UC are inconsistent and its efficacy is questionable. Large-scale randomized controlled trials have not been conducted for verification and inclusion in the pediatric population (9). Therefore, this study excluded pediatric treated with FMT.
Due to overlapping clinical manifestations - such as fever, abdominal pain, and diarrhea - differentiating between a acute children with UC and concurrent CDI presents a diagnostic challenge. Several diagnostic modalities for CDI are currently employed, including enzyme immunoassays (EIA) for toxins A and B, glutamate dehydrogenase (GDH) detection, nucleic acid amplification tests (NAAT), toxin-producing culture, and next-generation sequencing (NGS) (10). However, these techniques differ in sensitivity and specificity. Given these limitations, the development of a simple, rapid, and noninvasive predictive tool is critical for children with UC at high risk of CDI. This study constructed nomograms model to predict CDI risk in children with UC. The model offers a clinically practical approach to facilitate early diagnosis and prompt initiation of standardized treatment, improving clinical outcomes in children with UC combined with CDI.
Methods
Study population
This retrospective, single-center clinical study was conducted at the Affiliated Children's Hospital of Zhengzhou University. Children admitted with UC between January 2018 and December 2024 were identified through the electronic medical records system using the search terms “ulcerative colitis” and/or “Clostridioides difficile infection” in accordance with the 2019 Expert Consensus on the Diagnosis and Treatment of Inflammatory Bowel Disease in Children (11). Inclusion criteria were as follows: (1) a confirmed UC diagnosis (for at least 3 months) (12); (2) age between 0 and 18 years; and (3) performed and documented CD testing (CDI or non-CDI). Exclusion criteria included: (1) severe cardiac, pulmonary, hepatic, or renal dysfunction; (2) prior use of metronidazole or vancomycin within one month before admission; (3) vitamin D (Vit D) supplementation within the previous six months (to avoid potential confounding effects of exogenous Vit D supplementation on disease progression in children with UC combined with CDI); (4) history of fecal microbiota transplantation; (5) and incomplete clinical records lacking any of the 24 potential predictor variables. Based on these criteria, 35 children were ultimately enrolled. The remaining patients did not meet the inclusion criterion of documented CDI.
The diagnosis of CDI was based on the 2017 Clinical Practice Guidelines for CDI in Adults and Children (13) from the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA), which define CDI as the presence of gastrointestinal symptoms (diarrhea, increased frequency of bowel movements, bloody stools, intestinal spasms, and/or urgency) combined with one of the following criteria: (1) positive for CD GDH and positive for CD toxin A or B by enzyme-linked immunosorbent assay; (2) positive for CD GDH, negative for CD toxin A and B by EIA, and positive for CD by real-time quantitative polymerase chain reaction (PCR); and/or (3) colonoscopy or histopathology showing pseudomembranous enteritis.
The study protocol was approved by the Ethics Committee of the Affiliated Children's Hospital of Zhengzhou University (approval number: 2024-079-002), and all data were anonymized prior to analysis.
Disease assessment standards
Disease activity was stratified into active and remission phases. Within the active phase, severity was further subcategorized as mild, moderate, or severe based on clinical criteria. The Pediatric Ulcerative Colitis Activity Index (PUCAI) was employed to assess disease activity (14): remission (PUCAI < 10), mild activity (10–34), moderate activity (35–64), and severe activity (>65). In this study, disease activity in UC is categorized into two main groups: primary relapse and chronic relapsing UC. Primary relapse refers to patients who experience a disease flare after initially achieving remission following diagnosis based on as sustained clinical remission (PUCAI < 10). Chronic relapsing UC refers to patients who experience ≥1 disease flare per year, which could be confirmed by endoscopy. These relapses are further classified based on frequency: infrequent (one episode or fewer per year), frequent (two or more episodes per year), or continuous (persistent UC symptoms without achieving remission). Additionally, an early relapse is defined as a recurrence of symptoms occurring within less than three months after remission was achieved through prior treatment (15).
Disease extent was classified according to the Paris classification system (16). Extraintestinal manifestations included ocular involvement (e.g., iritis, scleritis, uveitis), hepatobiliary disorders (e.g., fatty liver, primary sclerosing cholangitis, cholelithiasis), dermatologic and mucosal findings (e.g., oral ulcers, erythema nodosum, pyoderma gangrenosum), musculoskeletal complications (e.g., peripheral arthritis, spondyloarthritis), and thromboembolic events (17). Additional complications comprised intraepithelial neoplasia of the intestinal mucosa, bowel perforation, lower gastrointestinal hemorrhage, toxic megacolon, and malignancy.
Data collection
Clinical data were retrieved from the institution's electronic medical database in accordance with principles of data accessibility and clinical relevance. Extracted variables included: (1) demographic and clinical characteristics, including age (years), sex, body mass index (kg/m2), family history, presence of extraintestinal manifestations, upper gastrointestinal involvement, comorbidities, clinical relapse type, extent of colonic diseases, PUCAI, and antibiotic use exceeding seven days (days); (2) laboratory parameters, including white blood cell count (*109/L), hemoglobin (g/L), platelet count (mg/L), albumin (g/L), erythrocyte sedimentation rate (mm/h), procalcitonin (ng/ml), interleukin-6 (pg/ml), tumor necrosis factor-α (pg/ml), immunoglobulin A (g/L), Vitamin D (nmol/L), CD4+/CD8+ T-cell ratio (%), fecal calprotectin (ug/g), CMV IgM status, and CDI results (CD toxin genes were detected in children with UC fecal samples by real-time quantitative PCR). The PUCAI and laboratory parameters were selected for the period between admission and CDI testing (before the child developed symptoms that required CDI testing).
Statistical analysis
Statistical analyses were performed using SPSS version 26.0. For continuous variables with normal distribution, data were expressed as mean ± standard deviation (χ¯ ± s) and compared using the independent samples t-test. Non-normally distributed continuous variables were reported as median and interquartile range (P25, P75) and analyzed using the Mann–Whitney U-test. Categorical variables were presented as frequencies and percentages (%) and compared using the χ2 test.
To enhance the generalizability of the predictive model, the least absolute shrinkage and selection operator (LASSO) regression was applied to identify key features associated with children with UC combined with CDI. Variables selected by LASSO regression were subsequently entered into a multivariate logistic regression model. Statistically significant predictors were incorporated into the construction of a nomogram, with corresponding odds ratios (ORs), 95% confidence intervals (CIs), and P-values reported. Internal validation was conducted using a 1,000-sample bootstrap resampling technique. Model performance, including discrimination, calibration and clinical utility, was assessed through receiver operating characteristic (ROC) curves, calibration plots, and decision curve analysis (DCA). Model construction and validation were performed using R software (version 4.2.4). A two-tailed P < 0.05 was considered statistically significant.
Results
Screening for optimal predictive markers of children with UC combined with CDI
All eligible patients were categorized into two groups: CDI (n = 35) and non-CDI (n = 86) based on the presence or absence of combined with CDI (Figure 1). The median time interval from children with UC diagnosis to the collection of disease activity scores and laboratory parameters was 1 (0.5–2) days. The median time interval from CDI diagnosis to completion of the aforementioned assessments was 3 (2.5–4) days. The baseline characteristics of the patients are shown in Table 1. Based on non-zero coefficients identified through LASSO logistic regression, seven candidate predictors were selected from the initial pool of 24 variables (Figures 2A,B), including PCUAI, Hb, ESR, Vit D, FC, and antibiotic use exceeding seven days. These variables were subsequently entered as independent predictors in a binary logistic regression model, with the presence of CDI in children with UC as the dependent variable. Multivariate logistic regression analysis identified PUCAI, ESR, Vit D, FC, and prolonged antibiotic use as independent risk factors for CDI in children with UC (Table 2).
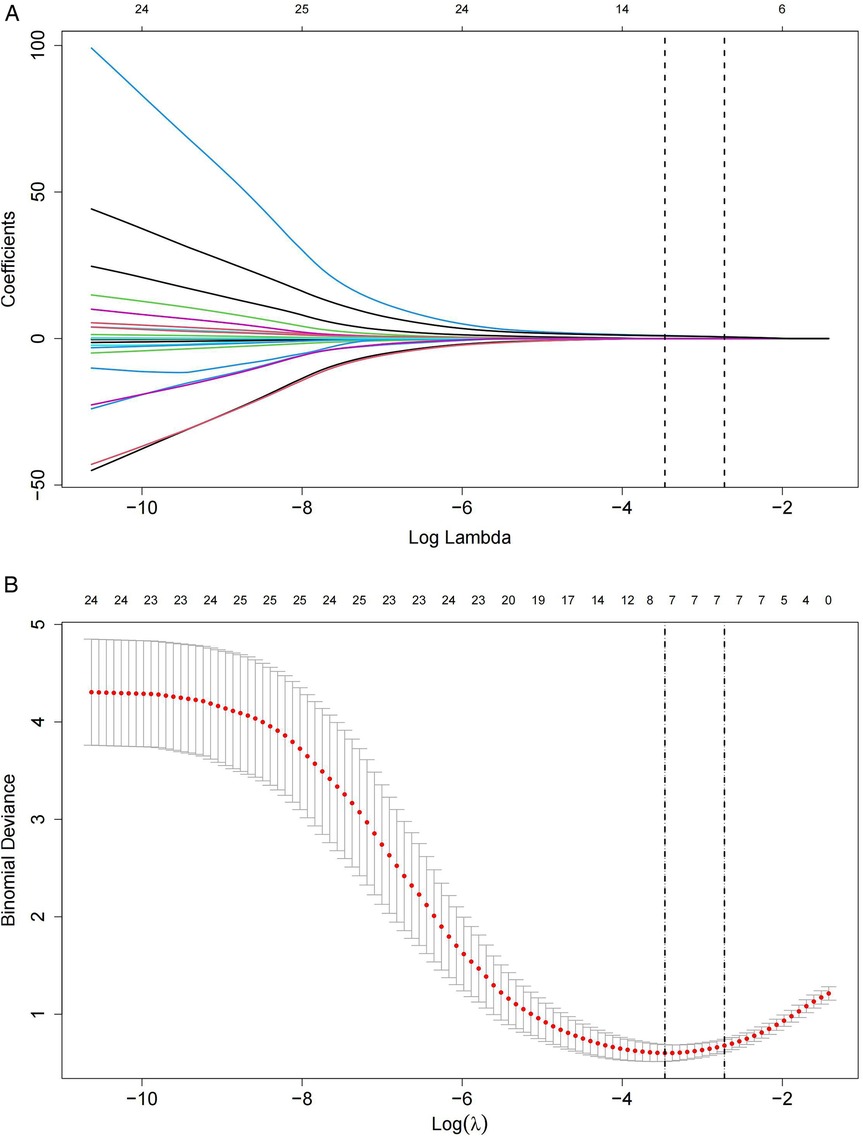
Figure 2. Lasso regression model of children with UC combined with CDI. (A) Lasso coefficient profile plot for 24 feature predictors, with variable values marked at the top of the plot. (B) The Lasso regression model's parameters were selected using five-fold cross-validation and the minimum criteria. Seven non-zero coefficients were identified by drawing two vertical lines at the optimal point of the minimum mean squared error (left) and one standard error above the minimum mean squared error (right).
Development of a prediction model
Two forms of nomograms was developed using five independent predictors that were statistically significant in the multivariate logistic regression analysis (Figure 3). In the static nomogram, each variable contributed to the calculation of its respective score, and the overall score was obtained by summing these individual scores. The risk probability of CDI in children with UC was determined by mapping the overall score to the corresponding value on the risk axis. In this model, higher total scores correlate with an increased likelihood of CDI occurrence in children with UC. The dynamic nomogram enables real-time predictive utility by displaying the risk of CDI and its 95% CI upon entering five variables per patient.
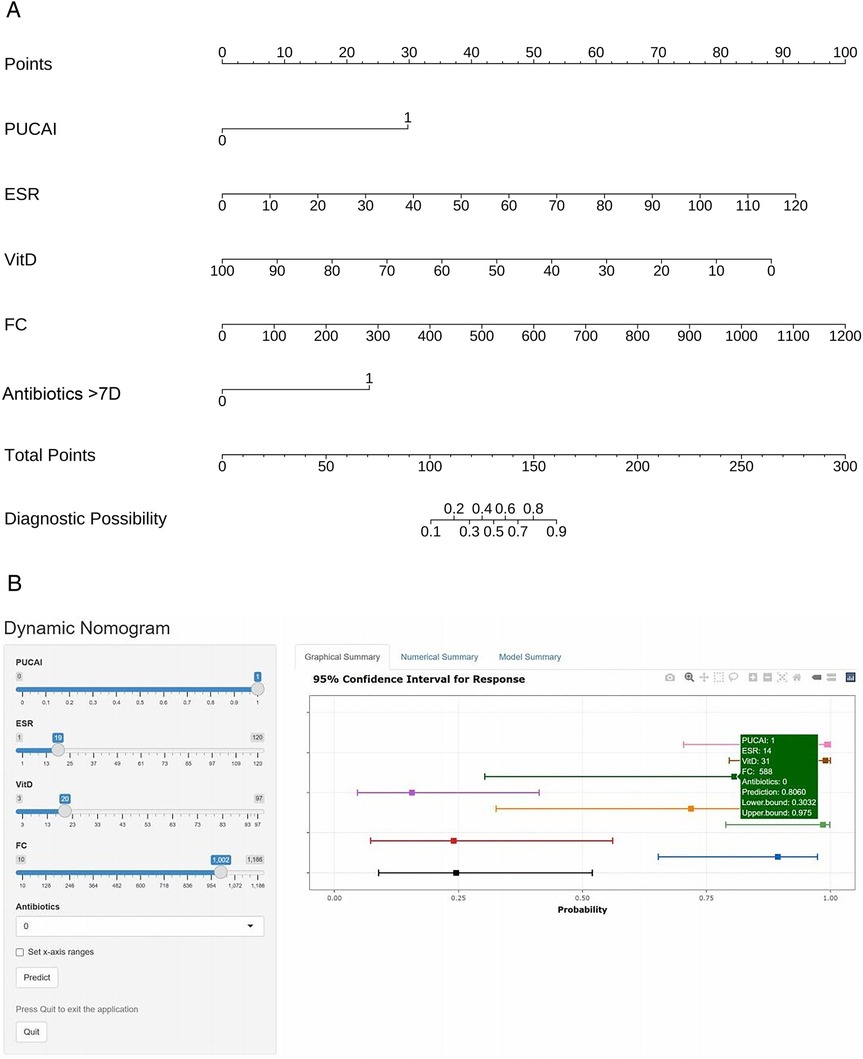
Figure 3. Construction of static-dynamic nomogram prediction models for children with UC combined with CDI. (A) Static nomogram for children with UC combined with CDI. (B) Online dynamic nomogram for children with UC combined with CDI. (https://plotsite.shinyapps.io/nomograme/).
To determine the score cut-off values for low, medium, and high wind directions, the maximum Youden's Index (YI) in the ROC curve was used to select the row with the largest YI from the data, and the corresponding threshold was found. Based on clinical practicability and data distribution, three-level stratification was carried out (Table 3). High risk: select the point with the largest YI; Medium risk: the lower limit is set at sensitivity = 100% and specificity = 50%, balancing the rate of missed diagnosis and false diagnosis; the upper limit is the YI maximum point; Low risk: the sensitivity is close to 100%, which can basically rule out the risk of CDI.
Predictive model performance and validation
The Hosmer-Lemeshow test was used to evaluate the model fit. The results showed that χ2 = 12.529, p = 0.129, indicating that the model does not have poor goodness of fit. The area under the ROC curve (AUC) reflects the model's ability to distinguish between different patient categories (Figure 4A). The C-index of this model was 0.964 (95% CI: 0.932–0.997), indicating that it can predict children with UC combined with CDI with a 96.4% confidence level. The sensitivity, specificity, PPV, and NPV were 80.4%, 90.6%, 80.4%, and 97.5%, respectively. The clinical applicability of the model was evaluated using DCA (Figure 4B). The results revealed that, across a broad range of threshold probabilities, the model's net benefit consistently exceeded that of the two extreme strategies (including all children with UC combined with CDI or none of them combining CDI), highlighting its potential clinical utility. Internal validation was completed using 1,000 Bootstrap analyses, and the results showed that the model has the ability to distinguish children with UC combined with CDI, with an average absolute error of only 0.022 (Figure 4C). Further evaluation of the model's performance in clinically relevant subgroups was conducted. The subgroup analysis of the forest plot revealed that the predictive efficacy of the model in terms of demographic and clinical characteristics was consistent (p > 0.05), indicating that the model can accurately and simply predict the risk of CDI in patients with UC (Figure 5).
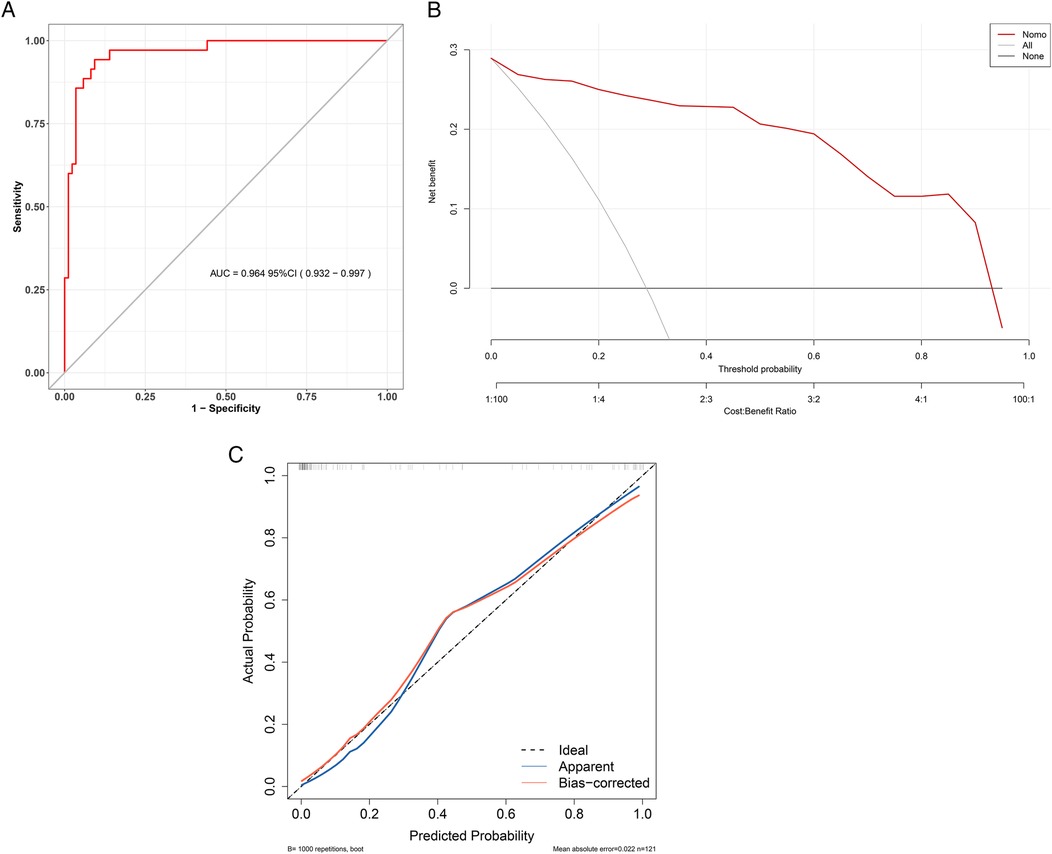
Figure 4. Performance evaluation of a children with UC combined with CDI prediction model. (A) ROC curve for children with UC combined with CDI. The y-axis represents the sensitivity, while the x-axis represents 1-specificity. The ROC curve would align with the gray diagonal if the model's performance were equivalent to random guessing. The red curve illustrates the model's sensitivity and 1-specificity at various thresholds, with greater proximity to the upper left corner indicating higher model accuracy. (B) DCA for children with UC combined with CDI. In the DCA, the red line represents the risk model for children with UC combined with CDI. The thin line corresponds to the strategy of assuming all children have children with UC combined with CDI, while the thick line represents the strategy of assuming none have children with UC combined with CDI. Model validation. (C) The x-axis represents the predicted risk of children with UC combined with CDI, while the y-axis represents the actual diagnosis of children with UC combined with CDI. The red solid line indicates the performance of the nomogram, and the black dotted line represents the ideal calibration line. A closer alignment of the red line to the black dotted line signifies better model calibration.
Discussion
CDI is a common opportunistic pathogen affecting both adults and pediatric patients, particularly in those with IBD. Adult studies have demonstrated that patients with UC are more susceptible to CDI (18). This predisposition may be attributed to the pathological characteristics of UC, which typically presents as continuous inflammatory lesions affecting the colorectum mucosa. Concurrent CDI in UC patients is associated with prolonged hospitalization, increased frequency of disease relapse, suboptimal therapeutic response to medications, and elevated risk of colectomy. Consequently, early clinical identification of such patients has emerged as a critical issue in IBD management. To date, there remains a scarcity of research on risk factors for CDI in children with UC populations, and no standardized diagnostic protocol for CDI detection has been established. To facilitate real-time clinical decision-making, this study developed an intuitive dynamic nomogram prediction tool utilizing readily accessible clinical parameters. This instrument enables risk stratification and prediction of CDI infection, thereby providing a valuable resource for proactive clinical intervention.
Based on fecal and blood biomarkers, feature selection was performed using Lasso regression, ultimately identifying five variables for inclusion in the risk prediction model for children with UC complicated by CDI. To guide clinical practice, the optimal cut-off value maximizing the YI was applied for risk stratification. For low-risk patients, regular follow-up is recommended; intermediate-risk patients should undergo CDI testing; and high-risk patients should receive empirical anti-CDI therapy concurrently with diagnostic testing. Internal validation demonstrated excellent performance with a mean absolute error of 0.022, indicating that the model provides stable and reliable prediction of CDI risk in children with UC patients.
To evaluate the predictive accuracy of the model, we conducted subgroup analyses across clinically relevant categories (gender, age, comorbidities, disease activity, and treatment history). Forest plot results demonstrated consistent predictive performance across demographic and clinical characteristics. A recent systematic review and meta-analysis on CDI in IBD patients identified age and male gender as significant risk factors for CDI (19). The discrepancy between our findings and previous reports may be attributed to population differences, as the present study exclusively enrolled pediatric patients under 18 years of age.
Diagnostic and therapeutic decisions for children with UC rely on disease activity, as measured by PUCAI, a scoring system used to assess disease severity in children with UC. Findings from this study reveal that the PUCAI demonstrates significant predictive utility for clinical outcomes in adult patients with acute severe colitis (20). Strong suspicion of CDI is warranted in children with elevated PUCAI. Most patients with IBD and CDI exhibit moderate to severe PUCAI (33% and 66%, respectively) (21). The results of the multivariate regression analysis in this study showed that moderate to severe children with UC, as indicated by PUCAI, was identified as an independent risk factor for concurrent CDI. Subgroup analysis conducted by Fang et al. similarly demonstrated a significant association between disease activity and CDI (22). Active disease stages, characterized by impaired mucus layer formation and compromised epithelial barrier function, may increase the susceptibility of children with UC to pathogenic colonization and invasion. Thus, PUCAI was incorporated into a nomogram model children with UC combined with CDI. A bidirectional relationship exists between CDI and changes in disease activity: while elevated IBD disease activity may increase susceptibility to CDI, the infection itself can conversely exacerbate IBD severity (23). Some studies suggest this observed association may be attributable to symptomatic overlap and disproportionate CDI testing in active disease states. Further investigation is required to elucidate this potential detection bias and establish causal mechanisms (22).
ESR and FC are both indicators reflecting the inflammatory activity of children with UC. In this study, children with UC combined with CDI exhibited elevated ESR and FC levels. At the same time, there are also research results showing that FC levels correlate positively with PUCAI, ESR, PLT, and CRP in children with UC (24). ESR, cost-effective and clinically practical biomarker for assessing inflammatory activity, reflects the rate of erythrocyte sedimentation in plasma, which is accelerated by elevated concentrations of globulin, fibrinogen, and complement. In IBD diagnosis, ESR demonstrates a sensitivity of 66% and specificity of 84% (25). It correlates with endoscopic inflammatory activity in children with UC and serves as a laboratory marker to predict disease severity. Changes in the gut microbiota and metabolites can trigger immune responses in epithelial cells, resulting in systemic inflammation. Wan et al. reported that certain metabolites elevated in UC patients with CDI, such as putrescine, maltose, 4-hydroxybenzoic acid, 4-hydroxybutyrate, and aminomalonic acid, were positively correlated with ESR (26). Our study demonstrated that higher ESR levels in UC patients combined with CDI compared to those without. FC, a non-invasive inflammatory biomarker, are calcium-zinc binding protein complexes belonging to the S100 family, consisting of two hydrophobic, non-covalently linked S100 A8/A9 regions. These proteins account for approximately 60% of the total cytoplasmic protein content in neutrophils, with smaller amounts found in monocytes, macrophages, and epithelial cells. FC is useful for the differential diagnosis of diseases [e.g., UC vs. Crohn's disease (CD), IBD vs. irritable bowel syndrome], assessing inflammatory activity, and predicting short-term relapse. Although FC demonstrates good sensitivity and specificity for diagnosing IBD, its optimal cut-off value can vary depending on factors such as age, diet, lifestyle, disease type, medications, defecation timing, sample storage, and detection methods. The AGA Clinical Practice Guidelines recommend that elevated FC levels may indicate an inflammatory source, and fecal testing for CDI is essential in UC patients with symptoms consistent with the disease and elevated biomarkers (e.g., FC, CRP, fecal lactoferrin) (27). Certainly, FC can also be elevated due to other infections (e.g., Salmonella, Campylobacter, or certain pathogenic strains of Escherichia coli). Children with UC combined with CDI exhibited markedly increased FC levels in our study. Therefore, when there is aggravation of clinical manifestations and elevation of inflammatory markers, clinicians should be alert to opportunistic infections.
The active form of Vit D, 1,25-dihydroxyvitamin D (1,25-(OH)₂-D), plays a critical role in bone formation, calcium and phosphorus metabolism, and cellular growth and differentiation. Increasing evidence suggests that the Vit D/VDR signaling pathway is crucial for maintaining the integrity of the intestinal barrier, modulating immune responses, and regulating the microbiome (28). Tight junctions between intestinal epithelial cells are essential for the intestinal mucosal barrier, controlling permeability. When these tight junctions are impaired (through downregulation and disruption of tight junction proteins), intestinal permeability increases, allowing pathogens to invade and activating a sustained immune response (29). Therefore, the Vit D/VDR system may significantly influence UC development. For instance, Bakke et al. (30) observed reduced VDR expression and impaired Vit D/VDR signaling in UC patients, while a meta-analysis of 987 UC patients and 1,247 controls found significantly lower serum Vit D levels in UC patients (OR = 1.90, 95% CI: 1.38–2.62) (31). Multivariate regression analysis in our study identified hypovitaminosis D as an independent risk factor for CDI in children with UC. β2-defensin, an antimicrobial peptide that inhibits the action of CD toxins and alleviates colitis, is stimulated by plasma 1,25-(OH)₂-D. Based on these mechanisms, Ananthakrishnan et al. investigated the relationship between plasma 1,25-(OH)₂-D and CDI in 188 IBD patients, including 35 combined with CDI. Patients combined with CDI had significantly lower mean plasma 1,25-(OH)₂-D levels compared to those with IBD (20.4 ng/ml vs. 27.1 ng/ml, p = 0.002). The risk of CDI decreased by 4% for each 1 ng/ml increase in Vit D levels (OR = 0.96, 95% CI: 0.93–0.99, p = 0.046). Furthermore, the risk of CDI was higher in patients with Vit D levels below 20 ng/ml compared to those with levels above 20 ng/ml (OR = 3.1, 95% CI: 1.2–8.3, p = 0.02) (32). Our study similarly demonstrated a negative correlation between Vit D levels and the occurrence of children with UC combined with CDI. Consequently, serum Vit D levels were incorporated as a predictive factor in the construction of the children with UC nomogram model. Children with UC, often referred to as the “green tumor”, is associated with reduced Vit D levels due to both non-disease-related factors (e.g., limited sunlight exposure and reduced physical activity) and disease-related factors (e.g., decreased dietary intake, malabsorption, and increased basal metabolism). Children with UC combined with CDI triggers inflammatory response and affects Vit D level.
Environmental risk factors for CDI include antibiotic and proton pump inhibitor (PPI) use, escalation of IBD therapy, prolonged hospitalization, immune dysfunction, and a history of gastrointestinal surgery. The primary defense against CDI is the normal intestinal flora. Antibiotic therapy disrupts the intestinal microbiota, facilitating CDI colonization and toxin production, which damages the cytoskeletal structure of epithelial cells and induces inflammation (33). The risk of CDI is influenced by the type, quantity, and duration of antibiotic use. Antibiotics most frequently associated with CDI include fluoroquinolones, clindamycin, and broad-spectrum penicillins and cephalosporins. A meta-analysis of 14 studies found prior antibiotic exposure was linked to a higher incidence of CDI in children (OR: 2.14; 95% CI: 1.31–3.52) (34). Balram et al. (35) conducted a meta-analysis of 22 studies, showing that antibiotic use within 30 days prior to diagnosis was associated with CDI occurrence in IBD patients (OR: 1.85, 95% CI: 1.36–2.52). Despite being a cornerstone of treatment for inflammatory diseases, frequent antibiotic use has been linked to an increased incidence of CDI in UC patients. In this study, antibiotic use exceeding seven days was found to be a risk factor for CDI combined with children with UC, suggesting that early use of metronidazole or vancomycin, in accordance with guidelines, may reduce the risk of CDI.
The dynamic nomogram prediction model constructed by five routine indicators in this study provides an intuitive and individualized warning tool for clinical practice. To help clinicians risk stratification of low, moderate, and high risk patients, and take active and effective countermeasures, so as to shorten the length of hospital stay, reduce medical costs and improve the efficiency of antibiotic management. Although this study provides a new scheme for the prediction of risk factors for children with UC combined with CDI, it still has some limitations. First, this study is a single-center, small-sample retrospective analytical study, which may suffer from data collection and selection bias. Therefore, it is necessary to carry out a prospective study with multiple centers and large samples in the future, and carry out external validation to further explore other predictive factors and improve the accuracy and stability of the model. Second, the relationship between IBD therapeutic regimens and CDI risk remains incompletely characterized. During clinical data collection, we observed that the pediatric cohort demonstrated low prevalence of conventional risk factors with no statistically significant differences, consequently these variables were excluded from risk factor analysis. Future large-scale clinical trials will incorporate dedicated investigations to elucidate potential associations between treatment modalities and CDI pathogenesis. Third, the genome of CD is mutable, so more virulent strains may emerge. These strains vary in geographic distribution, genetic composition, virulence factors and antibiotic susceptibility. Therefore, this study can only determine whether one is infected with CD or not, and is not applicable to predict specific strain types. Therefore, it is to be explored in further studies in the future. Fourth, substantial heterogeneity exists in reported colectomy rates among IBD patients with concurrent CDI. Future investigations should focus on delineating clinical outcomes in this pediatric subpopulation to optimize therapeutic management strategies.
Conclusion
The clinical data of children with UC, with or without CDI, were used to develop a dynamic nomogram prediction model by Lasso regression and multifactor logistic regression analyses. Five predictors (PUCAI, ESR, Vit D, FC, and antibiotic use exceeding seven days) were identified. PUCAI, ESR, VitD, FC and antibiotic use exceeding seven days serve as indicators of inflammatory response intensity and immune function status, and may also signal the presence of an inflammatory storm during infection. According to the specific score cut-off value of the model, risk stratification was performed to guide clinical decision-making. The model not only has good discrimination, calibration, clinical utility, but also has good consistency in clinically relevant subgroups (demographic and clinical characteristics). We hope to conduct prospective studies to identify potential predictors, and conduct multi-center external validation to strengthen the extrapolation of the model and improve the efficiency of clinical diagnosis and treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Henan Children's Hospital, Zhengzhou Children's Hospital Medical Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZL: Writing – original draft, Methodology. FX: Methodology, Writing – original draft. JZ: Writing – original draft, Data curation, Software. ZY: Writing – review & editing, Investigation, Formal analysis. XL: Writing – review & editing. YW: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the [Henan Provincial Science and Technology Research Project] [Grant numbers 222102310320].
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bethlehem L, Estevinho MM, Grinspan A, Magro F, Faith FF, Colombel J-F. Microbiota therapeutics for inflammatory bowel disease: the way forward. Lancet Gastroenterol Hepatol. (2024) 9(5):476–86. doi: 10.1016/s2468-1253(23)00441-7
2. Hoeve KV, Hoffman I, D’hoore A, Ferrante M, Vermeire M. Long-term outcome of immunomodulator use in pediatric patients with inflammatory bowel disease. Digest Liver Dis. (2020) 52(2):164–72. doi: 10.1016/j.dld.2019.09.008
3. Hyams JS, Thomas SD, Gotman N, Haberman Y, Karns R, Schirmer M, et al. Clinical and biological predictors of response to standardised paediatric colitis therapy (PROTECT): a multicentre inception cohort study. Lancet. (2019) 393(10182):1708–20. doi: 10.1016/s0140-6736(18)32592-3
4. Shirley D-A, Tornel W, Warren CA, Moonah S. Clostridioides difficile infection in children: recent updates on epidemiology, diagnosis, therapy. Pediatrics. (2023) 152(3):e2023062307. doi: 10.1542/peds.2023-062307
5. Kelly CR, Fischer M, Allegretti JR, LaPlante K, Stewart DB, Limketkai BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. (2021) 116(6):1124–47. doi: 10.14309/ajg.0000000000001278
6. Chandrakumar A, Zohni H, El-Matary W. Clostridioides difficile infection in children with inflammatory bowel disease. Inflamm Bowel Dis. (2020) 26(11):1700–6. doi: 10.1093/ibd/izz285
7. Prehn JV, Reigadas E, Vogelzang EH, Bouza E, Hristea S, Guery B, et al. European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. (2021) 27 Suppl 2:S1–21. doi: 10.1016/j.cmi.2021.09.038
8. Davidovics ZH, Michail S, Nicholson MR, Kociolek LK, Pai N, Hansen R, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection and other conditions in children: a joint position paper from the north American society for pediatric gastroenterology, hepatology, and nutrition and the European society for pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. (2019) 68(1):130–43. doi: 10.1097/mpg.0000000000002205
9. Lauwers E, Sabino J, Hoffman I, Hoeve KV. Faecal microbiota transplantation in children: a systematic review. Acta Paediatr. (2024) 113(9):1991–2002. doi: 10.1111/apa.17167
10. Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA institute. Clin Gastroenterol Hepatol. (2017) 15(2):166–74. doi: 10.1016/j.cgh.2016.10.024
11. Subspecialty Group of Gastroenterology T S O P, Chinese Medical Association, Subspecialty Group of Clinical Nutrition T S O P, Chinese Medical Association. Expert consensus on the diagnosis and management of pediatric inflammatory bowel disease. Chin J Pediatr. (2019) 57(7):501–7. doi: 10.3760/cma.j.issn.0578-1310.2019.07.002
12. Ajbar A, Cross E, Matoi S, Hay CA, Baines LM, Saunders B, et al. Diagnostic delay in pediatric inflammatory bowel disease: a systematic review. Dig Dis Sci. (2022) 67(12):5444–54. doi: 10.1007/s10620-022-07452-5
13. Mcdonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and society for healthcare epidemiology of America (SHEA). Clin Infect Dis. (2018) 66(7):e1–48. doi: 10.1093/cid/cix1085
14. Souza HSPD, Fiocchi C, Iliopoulos D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat Rev Gastroenterol Hepatol. (2017) 14(12):739–49. doi: 10.1038/nrgastro.2017.110
15. Chinese Society of Gastroenterology, Inflammatory Bowel Disease Group, Chinese Quality Control and Evaluation Center for the Diagnosis and Treatment of Inflammatory Bowel Disease, Kaichun W, Minhui C, Jiaming Q. Chinese guidelines for the diagnosis and treatment of ulcerative colitis (2023, Xi'an). Chin J Gastroenterol. (2024) 29(3):145–73. doi: 10.3969/j.issn.1008-7125.2024.03.003
16. Ghione S, Sarter H, Fumery M, Armengol-Debeir L, Savoye G, Ley D, et al. Dramatic increase in incidence of ulcerative colitis and Crohn’s disease (1988–2011): a population-based study of French adolescents. Am J Gastroenterol. (2018) 113(2):265–72. doi: 10.1038/ajg.2017.228
17. Jakobsen C, Bartek J, Wewer V, Vind I, Munkholm P, Groen R, et al. Differences in phenotype and disease course in adult and paediatric inflammatory bowel disease–a population-based study. Aliment Pharmacol Ther. (2011) 34(10):1217–24. doi: 10.1111/j.1365-2036.2011.04857.x
18. Rodemann JF, Dubberke ER, Reske KA, Seo DH, Stone CD. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol. (2007) 5(3):339–44. doi: 10.1016/j.cgh.2006.12.027
19. Amakye D, Ssentongo P, Patel S, Dalessio S, Kochhar S, Momin A, et al. Global patterns of Clostridioides difficile infection in patients with inflammatory bowel disease: a systematic review and meta-analysis of prevalence, epidemiology, and risk factors. Crohns Colitis 360. (2025) 7(2):otaf024. doi: 10.1093/crocol/otaf024
20. Hyams JS, Brimacombe M, Haberman Y, Walters T, Gibson G, Mo A, et al. Clinical and host biological factors predict colectomy risk in children newly diagnosed with ulcerative colitis. Inflamm Bowel Dis. (2022) 28(2):151–60. doi: 10.1093/ibd/izab061
21. Kochhar R, Ayyagari A, Goenka MK, Dhali GK, Aggarwal R, Mehta GK. Role of infectious agents in exacerbations of ulcerative colitis in India. A study of Clostridium difficile. J Clin Gastroenterol. (1993) 16(1):26–30. doi: 10.1097/00004836-199301000-00008
22. Fang S-B, Song Y-Q, Zhang C-Y, Wang L-B. Risk factors for Clostridioides difficile infection in children and adolescents with inflammatory bowel disease: a systematic review and meta-analysis. World J Pediatr. (2022) 18(1):27–36. doi: 10.1007/s12519-021-00486-1
23. Banaszkiewicz A, Kowalska-Duplaga K, Pytrus T, Pituch H, Radzikowski A. Clostridium difficile infection in newly diagnosed pediatric patients with inflammatory bowel disease: prevalence and risk factors. Inflamm Bowel Dis. (2012) 18(5):844–8. doi: 10.1002/ibd.21837
24. Plume JL, De A, Mutalib M. Assessing the correlation between fecal calprotectin, blood markers and disease activity in pediatric inflammatory bowel disease. Ann Gastroenterol. (2024) 37(4):436–41. doi: 10.20524/aog.2024.0892
25. Holtman GA, Leeuwen YL-V, Reitsma JB, Berger MY. Noninvasive tests for inflammatory bowel disease: a meta-analysis. Pediatrics. (2016) 137(1):e20152126. doi: 10.1542/peds.2015-2126
26. Wan J, Zhang Y, He W, Tian Z, Lin J, Liu Z, et al. Gut microbiota and metabolite changes in patients with ulcerative colitis and Clostridioides difficile infection. Front Microbiol. (2022) 13:802823. doi: 10.3389/fmicb.2022.802823
27. Singh S, Ananthakrishnan AN, Nguyen NH, Cohen BL, Velayos FS, Weiss JM, et al. AGA clinical practice guideline on the role of biomarkers for the management of ulcerative colitis. Gastroenterology. (2023) 164(3):344–72. doi: 10.1053/j.gastro.2022.12.007
28. Dalal RS, Mitri J, Goodrick H, Allegretti JR. Risk of gastrointestinal infections after initiating vedolizumab and anti-TNFα agents for ulcerative colitis. J Clin Gastroenterol. (2023) 57(7):714–20. doi: 10.1097/mcg.0000000000001733
29. Bella SD, Sanson G, Monticelli J, Zerbato V, Principe L, Giuffrè M. Clostridioides difficile infection: history, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin Microbiol Rev. (2024) 37(2):e0013523. doi: 10.1128/cmr.00135-23
30. Bakke D, Sun J. Ancient nuclear receptor VDR with new functions: microbiome and inflammation. Inflamm Bowel Dis. (2018) 24(6):1149–54. doi: 10.1093/ibd/izy092
31. Liu C, Liu X, Shi H, Chen F, Sun L, Gao X, et al. The correlation between serum 25-hydroxyvitamin D level and ulcerative colitis: a systematic review and meta-analysis. Eur J Gastroentero Hepatol. (2023) 35(12):1375–81. doi: 10.1097/meg.0000000000002670
32. Ananthakrishnan AN, Cagan A, Gainer VS, Cheng S-C, Cai T, Szolovits P, et al. Higher plasma vitamin D is associated with reduced risk of Clostridium difficile infection in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. (2014) 39(10):1136–42. doi: 10.1111/apt.12706
33. Shore BM, Weaver KN, Allegretti JR, Herfarth HH, Barnes EL. Prevalence of Clostridioides difficile infection after ileal pouch-anal anastomosis in patients with chronic antibiotic-dependent pouchitis and Crohn’s-like disease of the pouch. Inflamm Bowel Dis. (2023) 29(6):932–7. doi: 10.1093/ibd/izac165
34. Dang R, Alabaster A, Miranda-Katz M, Parmar D, Greenhow TL. Impact of antecedent antibiotic usage on community-associated Clostridioides difficile infection in pediatrics. Pediatr Infect Dis J. (2021) 40(5):426–8. doi: 10.1097/inf.0000000000002991
Keywords: ulcerative colitis, Clostridioides difficile, children, nomograms, risk factors
Citation: Li Z, Xue F, Zhang J, Yu Z, Li X and Wang Y (2025) A nomogram for predicting the risk of Clostridioides difficile infection in children with ulcerative colitis: development and validation. Front. Pediatr. 13:1641220. doi: 10.3389/fped.2025.1641220
Received: 4 June 2025; Accepted: 22 September 2025;
Published: 9 October 2025.
Edited by:
Jan De Laffolie, University of Giessen, GermanyReviewed by:
Duolong Zhu, Baylor College of Medicine, United StatesKaren Van Hoeve, University Hospitals Leuven, Belgium
Copyright: © 2025 Li, Xue, Zhang, Yu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhidan Yu, emhpZGFueXUyMDEzQDEyNi5jb20=; Xiaoqin Li, bGl4aWFvcWlueXNAMTI2LmNvbQ==; Yuesheng Wang, d2FuZ3l1ZXNoZW5neXNAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Ziying Li
Ziying Li Fumin Xue
Fumin Xue Jing Zhang3
Jing Zhang3 Xiaoqin Li
Xiaoqin Li



