- 1Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden
- 2Astrid Lindgren Children’s Hospital, Karolinska University Hospital, Stockholm, Sweden
- 3FUTURE, Center for Functional Tissue Reconstruction, University of Oslo, Oslo, Norway
- 4Hospital das Clínicas, Ribeirão Preto Medical School, University of São Paulo, Ribeirão Preto, Brazil
- 5Centre for Neonatal and Paediatric Infection & Vaccine Institute, Institute of Infection and Immunity, City St George’s University of London, London, United Kingdom
- 6Fetal Medicine Unit, St George’s University Hospitals NHS Foundation Trust, University of London, London, United Kingdom
- 7Vascular Biology Research Centre, Molecular and Clinical Sciences Research Institute, City St George’s University of London, London, United Kingdom
- 8Royal College of Obstetricians and Gynaecologists, London, United Kingdom
Emerging evidence suggests a potential link between maternal SARS-CoV-2 infection during early pregnancy and the development of congenital heart defects (CHD) in offspring. Although vertical transmission of SARS-CoV-2 is rare, the virus has been associated with placental complications and increased maternal morbidity. Recent studies from China report increased rates of CHD and anomalies such as situs inversus when infection occurs during gestational weeks 4–6, a critical window for cardiac development. Additional reports from different parts of the world also highlight post-pandemic increases in specific cardiac anomalies, including ventricular septal defects (VSDs). Multiple mechanisms may underlie these associations. SARS-CoV-2 can induce placental inflammation, compromise the cytotrophoblast barrier, and impair nutrient and gas exchange, potentially leading to fetal hypoxia and disrupted morphogenic signalling. Furthermore, maternal inflammation and elevated cytokines, along with viral effects on ACE2-expressing fetal cardiac progenitors, could further affect proliferation, differentiation, and apoptosis during cardiac development. Co-infections, hormone disruption, and maternal stress could also contribute. There is an urgent need for longitudinal studies with comprehensive maternal-fetal data, including infection timing, vaccine status, and biological sampling. These will be essential to delineate the multifactorial impacts of maternal infection on fetal cardiac development and long-term outcomes. Special focus should be placed on infections during early pregnancy (weeks 4–7), the period of cardiac septation and left-right asymmetry establishment, to determine causality and inform prevention strategies.
Is maternal SARS-CoV-2 infection in the first trimester associated with congenital heart defects?
Currently, there is limited evidence regarding an association between SARS-CoV-2 infection during pregnancy and the development of congenital anomalies (1). Although reports of vertical transmission to the fetus are rare (2, 3), SARS-CoV-2 is known to increase maternal mortality and morbidity and to cause placental complications, including stillbirth and pre-eclampsia (4–6). Recent data suggesting a link between congenital anomalies and SARS-CoV-2 exposure during early gestation highlight the need for further investigation to clarify the potential risks and mechanisms involved.
In November 2023, Wang et al. reported an increase in the incidence of situs inversus in a large birth cohort following the lifting of pandemic-associated restrictions in China (7). This was further supported by a Chinese case-control study that analysed 52 pregnancies with situs inversus and 208 matched controls between January and October 2023 (8). While no overall association was found between SARS-CoV-2 infection and situs inversus, a significant correlation emerged when infection occurred specifically during gestational weeks 4–6. Infections outside this critical window showed no association, even after adjusting for covariates. Another study also showed that SARS-CoV-2 infection during weeks 5–6 of the first trimester significantly increased the risk of fetal left-right (LR) asymmetry disorders (9). A separate study from China found that newborns of mothers infected with COVID-19 during pregnancy had a significantly higher prevalence of cardiac ultrasound abnormalities (10%) compared to the control group (4%) (10). Most abnormalities were observed in cases where maternal infection occurred before 8 weeks of pregnancy. In addition, the single-centre annual incidence of congenital heart defects (CHD) peaked at 5.5% in 2023, marking a notable increase (10). A summary of the findings of the studies from China is shown in Table 1.
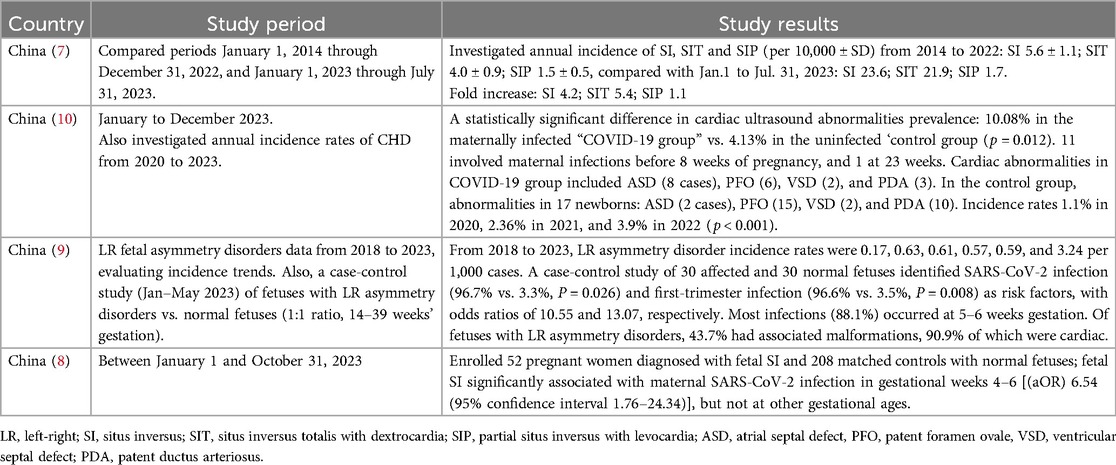
Table 1. Summary of the findings regarding maternal SARS-CoV-2 infection and congenital heart defects from studies conducted by researchers in China.
A single-centre US study investigated prenatal diagnosis rates of newborns with critical congenital heart defects (CCHDs) admitted for cardiac intervention during the COVID-19 pandemic (March 2020 to March 2021) and compared them with pre-pandemic periods (2009–2012 and 2013–2016). This study found that during the pandemic, patients had a 2.17 times higher odds of receiving a prenatal diagnosis of CCHD compared to the pre-pandemic period (11). Subsequent to this, a larger US-based study analysing data from the Centers for Disease Control and Prevention (CDC) demonstrated an increase in cyanotic congenital heart anomalies during the COVID-19 pandemic, though this study did not include analysis of CCHD subtypes, maternal SARS-CoV-2 infection, stillbirths, or pregnancy termination (12).
Unfortunately, analyses of the impact of COVID-19 maternal infections on CHD and other anomalies from other countries is limited, with further research pending. In Canada, pandemic-relevant congenital anomaly prevalence data is not available after 2020 (13). In Europe, the Joint Research Centre (JRC) technical report on European Surveillance of Congenital Anomalies (EUROCAT) presented the results of statistical monitoring for pan-European trends across 94 anomaly subgroups for 2012–2021 (14). This found that the incidence of patent ductus arteriosus (PDA) as an isolated CHD in term infants decreased significantly over the study period. However, this analysis did not include all European countries due to incomplete registry coverage. Two studies outside the registry have demonstrated an increase in CHD. In Turkey, a retrospective cohort study of 254 infants identified that preterm infants weighing up to 1.5 kg with maternal SARS-CoV-2 infection had a significantly higher rate of PDA (38% vs. 15%) (15). Similarly, a study in Ukraine found that 13.8% of fetuses of mothers infected with SARS-CoV-2 during pregnancy had congenital anomalies, with cardiovascular malformations second in prevalence only to facial anomalies (16).
The EUROCAT data from 2019 to 2023 has identified a rise in the incidence of situs inversus (Figure 1a); while there was a decline in live births and stillbirths with the condition in 2021, there was an increase in pregnancy terminations for situs inversus during the same period and an increase in the incidence of situs inversus between 2021 and 2023. Prevalence data analysed by EUROCAT demonstrated a slight decline in the incidence of CHD from 65.75 cases per 10,000 births in 2020 to 63.01 in 2021, followed by an increase to 71.63 cases per 10,000 births in 2022 and 71.47 in 2023. This trend reflects a rebound after the initial COVID-19 pandemic period, with overall prevalence remaining higher than in 2020 and 2021. (Figure 1b) (17). The EUROCAT data also show an increase in the prevalence of VSDs from 32.42 in 2019 to 37.39 in 2022, further increased to 39.5 in 2023, while the prevalence of tetralogy and pentalogy of Fallot cases decreased from 2019 to 2022, accompanied by an increase in 2023.
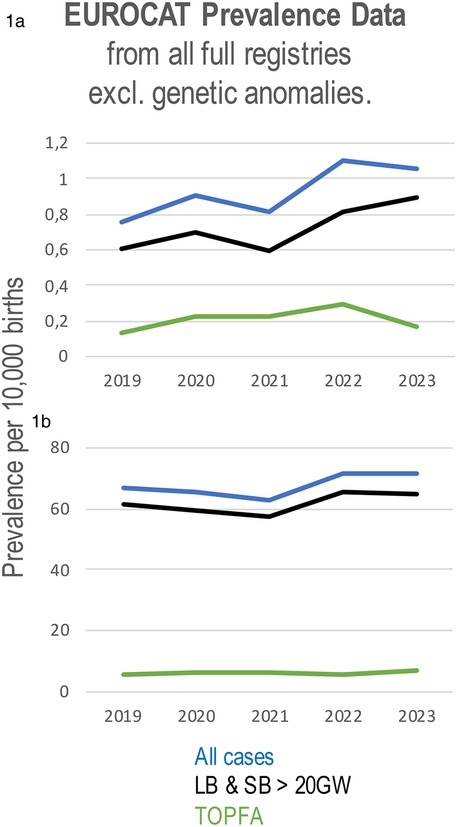
Figure 1. Prevalence of situs inversus (a) and all congenital heart defects (b) per 10,000 births using data from all full registries of EUROCAT from 2019 to 2023. CHD prevalence includes live births and stillbirths from 20 weeks’ gestation (LB & SB >20GW), cases of termination of pregnancy due to fetal anomaly (TOPFA), and all cases combined.
Additionally, surveillance data from the Argentinian National Registry of Congenital Anomalies (Registro Nacional de Anomalías Congénitas, RENAC), report an upward trend in cases of tetralogy and pentalogy of Fallot, with rates increasing from 2.12 (95% CI: 1.60–2.76) in 2019 to 2.41 (1.83–3.11) in 2020, 2.70 (2.08–3.46) in 2021, 2.16 (1.61–2.84) in 2022, and 2.84 (2.17–3.66) in 2023 (18).
We have examined Brazil's publicly available data from 2019 to 2023 to calculate the annual incidence of selected congenital anomalies per 10,000 live births (19). Starting from the pandemic years, there has been an increase in reported cases of congenital malformations of the circulatory system (Q20–Q28) (20), including defects in cardiac septa (Q21), the great arteries (Q25), cardiac chambers and connections (Q20), pulmonary and tricuspid valves (Q22), the peripheral vascular system (Q27), and other heart malformations (Figure 2). Of note, the incidence of malformations in cardiac septa steadily increased from 3.36 in 2019 to 5.17 in 2023 (19). Similarly, the data revealed a rising incidence in ASDs, which continues to increase through 2023 (19).
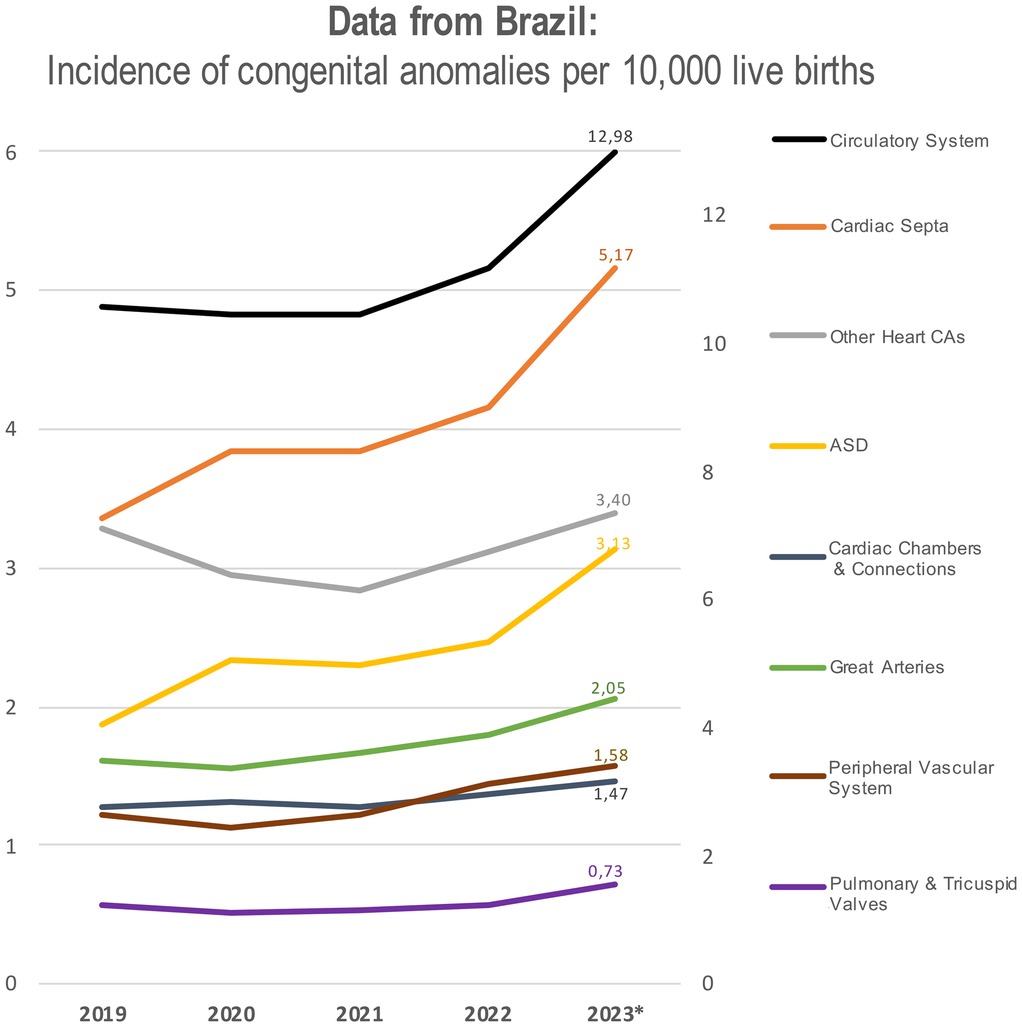
Figure 2. Annual incidence of congenital malformations of the circulatory system per 10,000 live births in Brazil from 2019 to 2023. Categories include specific CHD types: defects of cardiac septa, chambers and connections, great arteries, pulmonary/tricuspid valves, and peripheral vascular system, classified according to ICD-10 Q20–Q28. The graph has two axes: the right axis specifically refers to circulatory system malformation, while the left axis represents the incidence of all other congenital malformations.
As reflected in the findings discussed above, the impact of SARS-CoV-2 infection in early pregnancy may be associated with a statistically significant increase in CHD. It should be emphasised that COVID-19 vaccination in early pregnancy does not appear to be associated with a risk of congenital anomalies (21).
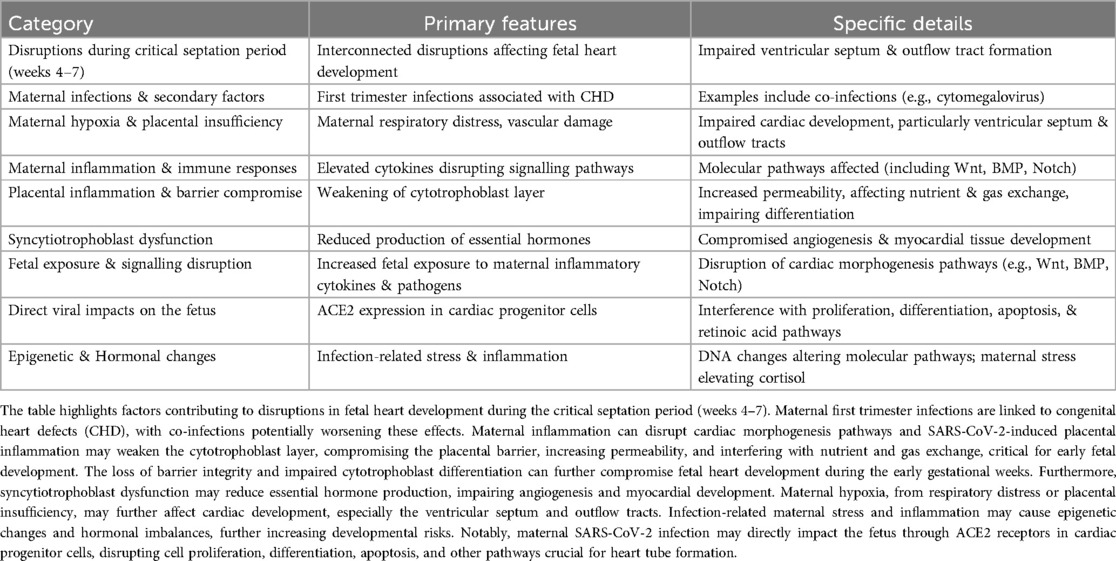
All these reports from China (7–10), Europe (14, 17), the US (11, 12), Brazil (19), Argentina (18), Turkey (15) and Ukraine (16), indicate a post-pandemic rise in specific CHDs, highlighting a critical gap in our understanding of the effects of early maternal SARS-CoV-2 infection on fetal development. These effects may be explained by interconnected disruptions affecting fetal heart development, particularly during the critical septation period between weeks 4 and 7 of gestation (22), with maternal first trimester infections being linked to CHD (23). Secondary factors like co-infections [e.g., cytomegalovirus (CMV)] (23, 24) and maternal inflammation, including elevated cytokines, can also disrupt cardiac morphogenesis pathways (25, 26). Furthermore, SARS-CoV-2 induced placental inflammation may weaken the cytotrophoblast layer, compromising the placental barrier, nutrient, and gas exchange (27, 28). This disruption, combined with maternal hypoxia from respiratory distress or vascular damage, may affect cardiac development, especially the ventricular septum and outflow tracts (29–31). The compromised cytotrophoblast barrier may also allow increased fetal exposure to maternal inflammatory cytokines (28) and pathogens, disrupting signalling pathways crucial for cardiac morphogenesis, such as Wnt, BMP, and Notch (29, 32).
Infection-induced loss of placental barrier integrity also affects hormone production (e.g., hCG) (33), impacting angiogenesis and myocardial development. Additionally, the virus may directly affect fetal cells via ACE2 expression in cardiac progenitors, disrupting proliferation, differentiation, and apoptosis (28). Maternal stress, elevated cortisol, and epigenetic changes (e.g., DNA methylation) could further complicate heart development. While direct animal studies linking COVID-19 to CHD are currently unavailable, related data demonstrate placental dysfunction, fetal growth restriction, and neurodevelopmental delays (34). This evidence supports a plausible mechanistic pathway worth exploring in animal models focused on cardiac outcomes. These complex factors (summarized in Table 2) highlight the need for further research into the impacts of maternal SARS-CoV-2 infection on fetal heart development.
To understand the effects of early maternal SARS-CoV-2 infection on fetal development, comprehensive and detailed data collection is now essential. This should include demographic information such as age, ethnicity, socioeconomic status, and pre-existing health conditions, enhanced by environmental and lifestyle factors like stress, diet, and exercise should be considered, as these can also impact outcomes, as these factors may influence maternal and fetal health outcomes.
Clinical data should track the severity, timing and SARS-CoV-2 strain of maternal infection, including comorbidities, hospitalisation, and medication use, alongside fetal health indicators like growth measurements, ultrasounds, and any congenital abnormalities. Additionally, information on maternal vaccination status, type of vaccine, timing relative to pregnancy, and booster doses, are also important. Insight into the maternal immune response, such as viral load, circulating antibody and cytokine levels, can elaborate on the biological effects of the infection or vaccination.
To better understand the effects of early maternal SARS-CoV-2 infection on fetal development, several types of samples should be collected across multiple time points during pregnancy. Maternal blood samples are essential to explore the DNA methylation patterns and mRNA abundance, providing insight into how maternal infection may alter gene expression, focusing on the immune response and fetal development genes. Amniotic fluid or cord blood samples can be analysed for fetal DNA, to explore biomarkers and epigenetic changes linked to the maternal condition, in addition to inflammatory cytokines and viral load, helping to understand the direct impact of the infection to the fetus. Placental tissue, collected at birth, would allow for the assessment of epigenetic landscape modifications that affect fetal development. Imaging data, such as regular ultrasounds, should also be collected to monitor fetal growth, development, and any abnormalities. By collecting these samples, we can track how maternal infection and vaccination, along with other factors like stress and medication, could influence fetal congenital heart defect formation. We can also explore genetic and environmental interactions that may contribute to long-term effects. In addition, future studies stratifying risk should also explore fetal genetic conditions, such as trisomy 21 (Down syndrome), in which approximately 40%–50% of individuals present with CHD (35).
We must continue to collect and analyse data on the development of CHD following maternal SARS-CoV-2 infection and conduct detailed studies to determine how maternal infection may influence the development of CHD and other anomalies. Areas of particular importance for future research are infection during the critical 4–7-week period in early pregnancy, and whether SARS-CoV-2 contributes to left-right asymmetry, septal development and lateral visceralisation disruption, all of which have significant implications for maternal and fetal health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AS: Conceptualization, Data curation, Investigation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. CMC: Data curation, Investigation, Formal analysis, Validation, Writing – review & editing. PTH: Investigation, Formal analysis, Validation, Writing – review & editing. AK: Conceptualization, Data curation, Formal analysis, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Samara A, Souter V, Coutinho CM, Khalil A. In need of robust evidence of non-association of pregestational and early pregnancy SARS-CoV-2 infections with congenital anomalies. EClinicalMedicine. (2024) 74:102729. doi: 10.1016/j.eclinm.2024.102729
2. Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. (2021) 224(1):35–53.e3. doi: 10.1016/j.ajog.2020.07.049
3. Allotey J, Chatterjee S, Kew T, Gaetano A, Stallings E, Fernández-García S, et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. Br Med J. (2022) 376:e067696. doi: 10.1136/bmj-2021-067696
4. Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. Br Med J. (2020) 370:m3320. Update in: BMJ. 2022 377:o1205. doi: 10.1136/bmj.o1205. doi: 10.1136/bmj.m3320
5. Metz TD, Clifton RG, Hughes BL, Sandoval GJ, Grobman WA, Saade GR, et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. (2022) 327(8):748–59. doi: 10.1001/jama.2022.1190
6. Palaska E, Golia E, Zacharogianni E, Bothou A, Tziriridou-Chatzopoulou M, Dagla M, et al. Risk of transmission of COVID-19 from the mother to the foetus: a systematic review. J Mother Child. (2024) 28(1):94–101. doi: 10.34763/jmotherandchild.20242801.d-24-00032
7. Wang Y, Guo Z, Ye B, Liu L, Mao X, Luo Y, et al. Association of SARS-CoV-2 infection during early weeks of gestation with situs inversus. N Engl J Med. (2023) 389(18):1722–4. doi: 10.1056/NEJMc2309215
8. Guo Z, Luo Y, Bi Y, Liu L, Qi Y, Yan J, et al. Association between situs inversus and maternal SARS-CoV-2 infection at gestational age 4–6 weeks. Med. (2024) 5(11):1433–1441.e3. doi: 10.1016/j.medj.2024.07.009
9. Li Y, Wang Y, Wu H, Li Q, Li S, Qiu C, et al. Increased risk of fetal left-right asymmetry disorders associated with maternal SARS-CoV-2 infection during the first trimester. Sci Rep. (2024) 14(1):11422. Erratum in: Sci Rep. 2024 14(1):16602. doi: 10.1038/s41598-024-67864-3. doi: 10.1038/s41598-024-61778-w
10. Ren H, Zhang X, Zhang S, Pan J, Wang W. The association of increased incidence of congenital heart disease in newborns with maternal COVID-19 infection during pregnancy. Sci Rep. (2024) 14(1):24866. doi: 10.1038/s41598-024-76690-6
11. Gupta D, Vuong T, Wang S, Korst LM, Pruetz JD. Update on prenatal detection rate of critical congenital heart disease before and during the COVID-19 pandemic. Pediatr Cardiol. (2024) 45(5):1015–22. doi: 10.1007/s00246-024-03487-9
12. Khalil A, Painter I, Souter V. Congenital heart defects during COVID-19 pandemic. Ultrasound Obstet Gynecol. (2025) 65(5):546–51. doi: 10.1002/uog.29126
13. Public Health Agency of Canada. Congenital Anomalies in Canada: 2022. Ottawa: Public Health Infobase (2022). Available online at: https://health-infobase.canada.ca/congenital-anomalies/data-tool/ (Accessed November 16, 2024).
14. Kinsner-Ovaskainen A, Perraud A, Garne E, Morris J. JRC-EUROCAT report on statistical monitoring of congenital anomalies (2012-2021) (2024). Available online at: https://eu-rd-platform.jrc.ec.europa.eu/system/files/public/eurocat/2024_EUROCAT_Statistical_Monitoring_Report.pdf (Accessed November 17, 2024).
15. Çıplak G, Becerir C, Sarı FN, Alyamaç Dizdar E. Effect of maternal coronavirus disease on preterm morbidities. Am J Perinatol. (2024) 41(S 01):e1835–40. doi: 10.1055/s-0043-1769471
16. Korinets Y, Prokopchuk N. Congenital and hereditary pathology in women contracted COVID-19 during pregnancy. Sci J Polonia Univ. (2024) 61:158–67. doi: 10.23856/6120
17. EUROCAT data: prevalence. EU RD Platform. (N/A) Available online at: https://eu-rd-platform.jrc.ec.europa.eu/eurocat/eurocat-data/prevalence_en (Accessed July 20, 2025).
18. La Red Nacional de Anomalías Congénitas de Argentina (RENAC). 2019-2023 Reports. The National Registry of Congenital Anomalies of Argentina. (2024). Available online at: https://ine.gov.ar/index.php/otrasareas/renac (Accessed December 25, 2024).
19. Ministério da Saúde. Painel de monitoramento: Anomalias congênitas. Secretaria de Vigilância em Saúde (SVS), Brasil (n.d.). Available online at: https://svs.aids.gov.br/daent/centrais-de-conteudos/paineis-de-monitoramento/natalidade/anomalias-congenitas/ (Accessed November 25, 2024).
20. World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10). Geneva: WHO (2019). Q20–Q28. Congenital malformations of circulatory system. Available online at: https://icd.who.int/browse10/2019/en (Accessed July 14, 2025).
21. Kharbanda EO, DeSilva MB, Lipkind HS, Romitti PA, Zhu J, Vesco KK, et al. COVID-19 Vaccination in the first trimester and major structural birth defects among live births. JAMA Pediatr. (2024) 178(8):823–9. doi: 10.1001/jamapediatrics.2024.1917
22. Lamers WH, Moorman AF. Cardiac septation: a late contribution of the embryonic primary myocardium to heart morphogenesis. Circ Res. (2002) 91(2):93–103. doi: 10.1161/01.res.0000027135.63141.89
23. Su H, Guo E, Woodward M, He JR, Dwyer T, Rahimi K, et al. First trimester maternal infections and offspring congenital heart defects: a systematic review and meta-analysis. Eur Heart J. (2024) 45(Supplement_1):ehae666.3637. doi: 10.1093/eurheartj/ehae666.3637
24. Wang T, Li Q, Chen L, Ni B, Sheng X, Huang P, et al. Maternal viral infection in early pregnancy and risk of congenital heart disease in offspring: a prospective cohort study in central China. Clin Epidemiol. (2022) 14:71–82. doi: 10.2147/CLEP.S338870
25. Mitchell T, MacDonald JW, Srinouanpranchanh S, Bammler TK, Merillat S, Boldenow E, et al. Evidence of cardiac involvement in the fetal inflammatory response syndrome: disruption of gene networks programming cardiac development in nonhuman primates. Am J Obstet Gynecol. (2018) 218(4):438.e1–438.e16. doi: 10.1016/j.ajog.2018.01.009
26. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. (2018) 49(3):397–412. doi: 10.1016/j.immuni.2018.07.017
27. Leon RL, Sharma K, Mir IN, Herrera CL, Brown SL, Spong CY, et al. Placental vascular malperfusion lesions in fetal congenital heart disease. Am J Obstet Gynecol. (2022) 227(4):620.e1–e8. doi: 10.1016/j.ajog.2022.05.038
28. Khalil A, Blakeway H, Samara A, O'Brien P. COVID-19 and stillbirth: direct vs indirect effect of the pandemic. Ultrasound Obstet Gynecol. (2022) 59(3):288–95. doi: 10.1002/uog.24846
29. Ward EJ, Bert S, Fanti S, Malone KM, Maughan RT, Gkantsinikoudi C, et al. Placental inflammation leads to abnormal embryonic heart development. Circulation. (2023) 147(12):956–72. doi: 10.1161/CIRCULATIONAHA.122.061934
30. O'Hare CB, Mangin-Heimos KS, Gu H, Edmunds M, Bebbington M, Lee CK, et al. Placental delayed villous maturation is associated with fetal congenital heart disease. Am J Obstet Gynecol. (2023) 228(2):231.e1–231.e11. doi: 10.1016/j.ajog.2022.08.013
31. Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. (2016) 96(4):1509–65. doi: 10.1152/physrev.00029.2015
32. Li Y, Du J, Deng S, Liu B, Jing X, Yan Y, et al. The molecular mechanisms of cardiac development and related diseases. Sig Transduct Target Ther. (2024) 9(1):368. doi: 10.1038/s41392-024-02069-8
33. Matthiesen NB, Henriksen TB, Agergaard P, Gaynor JW, Bach CC, Hjortdal VE, et al. Congenital heart defects and indices of placental and fetal growth in a nationwide study of 924,422 liveborn infants. Circulation. (2016) 134(20):1546–56. doi: 10.1161/CIRCULATIONAHA.116.021793
34. Creisher PS, Perry JL, Zhong W, Lei J, Mulka KR, Ryan WH, et al. Adverse outcomes in SARS-CoV-2-infected pregnant mice are gestational age-dependent and resolve with antiviral treatment. J Clin Invest. (2023) 133(20):e170687. doi: 10.1172/JCI170687
Keywords: SARS-CoV2, congenital heart defects, maternal infection, COVID-19, CHD
Citation: Samara A, Coutinho CM, Heath PT and Khalil A (2025) Is maternal SARS-CoV-2 infection in the first trimester associated with congenital heart defects? Front. Pediatr. 13:1643423. doi: 10.3389/fped.2025.1643423
Received: 8 June 2025; Accepted: 30 July 2025;
Published: 18 August 2025.
Edited by:
Cecile Tissot, Clinique des Grangettes, SwitzerlandReviewed by:
Dijana Majstorović, Juraj Dobrila University of Pula, CroatiaCopyright: © 2025 Samara, Coutinho, Heath and Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Asma Khalil, YWtoYWxpbEBzZ3VsLmFjLnVr
 Athina Samara
Athina Samara Conrado Milani Coutinho4
Conrado Milani Coutinho4 Paul T. Heath
Paul T. Heath Asma Khalil
Asma Khalil