- 1Department of Gastroenterology, National Children’s Medical Center, Children’s Hospital of Fudan University, Shanghai, China
- 2Department of Rheumatology, National Children’s Medical Center, Children’s Hospital of Fudan University, Shanghai, China
Objectives: Tofacitinib is an oral Janus kinase (JAK) inhibitor initially used for the treatment of arthritis. It demonstrated to effectively induce and maintain remission in adults with inflammatory bowel disease (IBD). However, data on its safety and efficacy in children with ulcerative colitis (UC), particularly in children with comorbid arthropathy, remained limited. This study aimed to evaluate the safety and efficacy of tofacitinib in treating children with UC who also had comorbid arthropathy.
Methods: We conducted a retrospective cohort study enrolling children with UC and comorbid arthropathy who received tofacitinib treatment at the Gastroenterology Department of the Children's Hospital of Fudan University from January 2018 to December 2024. All enrolled UC patients underwent blood tests, stool tests, and colonoscopies, with the Pediatric Ulcerative Colitis Activity Index (PUCAI) used to assess clinical indicators, clinical response, and clinical remission.
Results: A total of 16 patients met the inclusion criteria, all of whom presented with comorbid arthropathy. The mean age at onset was 7.1 ± 3.7 years, with a mean body mass index (BMI) of 14.6 ± 2.0 kg/m2. All patients had previously failed biologic therapy with infliximab. The majority patients initiated tofacitinib treatment at a starting dose of 2.5 mg twice daily (bid) and adjusted based on clinical response, with a maximum dose of 5 mg bid. Fecal calprotectin and endoscopic scores decreased significantly by weeks 14, 21, and 30, while albumin and BMI levels increased (all p < 0.05). The mean PUCAI scores also demonstrated a significant decline. One patient (6.25%) achieved clinical response by week 7, nine (56.25%) by week 14, and five (31.25%) by week 21. Six patients (37.5%) achieved clinical remission by week 30.
Conclusions: Our study provided promising evidence for the safety and efficacy of tofacitinib as part of the treatment regimen for children with UC complicated with arthropathy. Further large-scale, prospective studies are needed to confirm these findings.
Introduction
Inflammatory bowel diseases (IBD), including Crohn disease (CD) and ulcerative colitis (UC), are chronic, relapsing inflammatory conditions of the gastrointestinal tract. The prevalence of IBD is increasing globally, particularly among children (1). Symptoms include weight loss, diarrhea, bloody stools, and abdominal pain. Currently, there is no cure for IBD, and the primary treatment goal is to maintain clinical remission without the use of glucocorticoids (2). Treatment options include conventional pharmacological therapies and surgical intervention (3). Beyond gastrointestinal symptoms, up to 40% of children with IBD experience extraintestinal manifestations (EIMs), which can involve the joints, skin, eyes, and hepatobiliary system. These EIMs contribute significantly to disease burden, with studies reporting that children with EIMs have poorer health-related quality of life (QoL) compared to those without, including increased pain, fatigue, and psychosocial distress. For instance, musculoskeletal EIMs—such as arthritis—affect 10%–20% of childhood IBD cases and are a leading cause of disability, while dermatologic and ocular manifestations further exacerbate morbidity. Given their profound impact on physical and emotional well-being, early recognition and multidisciplinary management of EIMs are essential to improving outcomes in children with IBD (4–6). In some cases, arthritis may precede gastrointestinal symptoms (7, 8).
Targeting the Janus kinase (JAK) pathway has emerged as a promising therapeutic strategy for IBD. Tofacitinib, an oral JAK inhibitor, selectively inhibits JAK1 and JAK3 in the JAK-STAT pathway, thereby reducing proinflammatory cytokines (4). Approved by the US Food and Drug Administration (FDA) in 2012 for severe active rheumatoid arthritis and in 2018 for UC, tofacitinib has been available in China for the past two years. However, data on its use in chilldhood IBD, particularly in patients with associated inflammatory arthropathy, remain limited (5). Therefore, we conducted a retrospective cohort study to evaluate the efficacy and safety of tofacitinib in children with UCcomplicated by arthropathy.
Methods
Study cohort
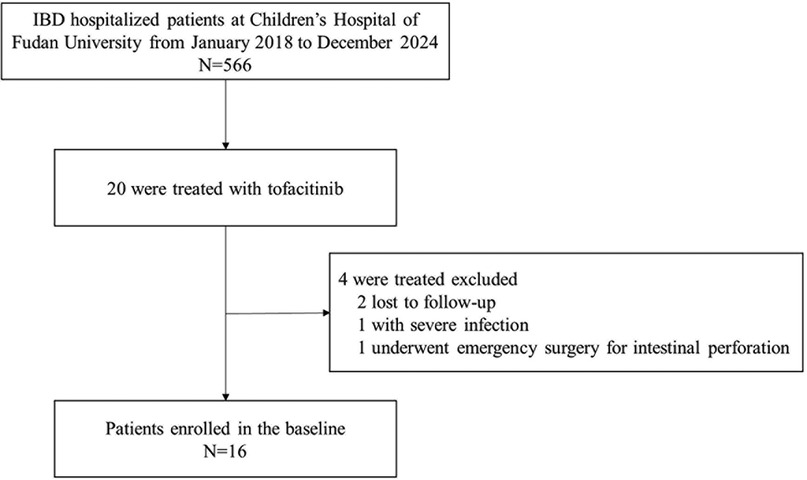
We conducted a perspective cohort study at the IBD Center of the Children's Hospital of Fudan University from January 2018 to December 2024 (Figure 1).
Inclusion criteria
1. Patients must meet the clinical, laboratory, and endoscopic diagnostic criteria outlined in Inflammatory Bowel Disease in Children and Adolescents: Recommendations for Diagnosis–The Porto Criteria (2005).
2. Patients were required to have arthritis manifestations (either monoarticular or polyarticular) confirmed by imaging studies.
3. Tofacitinib must be administered for the first time.
Exclusion criteria
1. Patients with incomplete clinical data.
2. Patients requiring urgent surgical intervention due to complications such as intestinal obstruction, perforation, or gastrointestinal bleeding.
3. Patients with severe underlying conditions, including malignancies, moderate-to-severe heart failure, or multiple organ dysfunction syndrome.
4. Patients with active infections such as tuberculosis or hepatitis B virus infection.
5. Patients with thrombotic disorders or coagulation dysfunction.
Data collection
We reviewed electronic medical records to collect demographic, clinical, laboratory, radiographic, and endoscopic data. Baseline characteristics included patient demographics (age, gender, BMI, medical history), disease features (symptoms, UC subtype), tofacitinib treatment details (induction and maintenance doses, duration, prior therapies), biochemical markers (serum C-reactive protein, albumin, hemoglobin), fecal calprotectin and endoscopic findings. Clinical response and adverse events were assessed at weeks 7, 14, 21, and 30.
Clinical outcomes
We evaluated PUCAI scores at weeks 7, 14, and 21. Disease activity was categorized as follows: 0–9 (no activity), 10–34 (mild activity), 35–64 (moderate activity), and 65–85 (severe activity) (6). Adverse events previously associated with tofacitinib were retrospectively assessed. Treatment failure was defined as the absence of significant symptom improvement, discontinuation of tofacitinib, or referral for surgery. Relapse was defined as therapeutic failure after an initial response or discontinuation of treatment.
Clinical remission was defined as a PUCAI score < 10 and corticosteroid-free treatment without the need for new medications or colectomy. Clinical response was defined as a decrease in PUCAI score by ≥20 or by one activity level from baseline.
Statistical analysis
Data were analyzed using SPSS 26.0. Continuous variables were expressed as mean ± standard deviation (SD) or median (interquartile range), while categorical data were expressed as percentages and absolute numbers. A P-value <0.05 was considered statistically significant.
Results
General and clinical characteristics
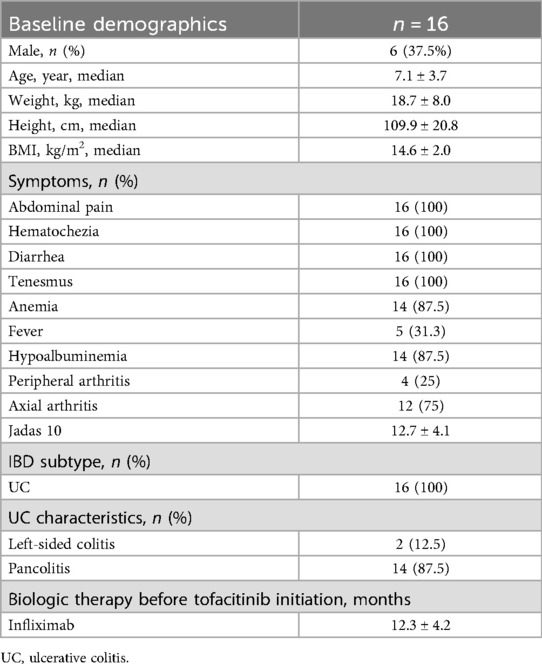
Sixteen patients were included in the study. The male-to-female ratio was approximately 3:5. The mean age at onset was 7.1 ± 3.7 years, with a mean weight of 18.7 ± 8.0 kg, height of 109.9 ± 20.8 cm, and BMI of 14.6 ± 2.0 kg/m2. All patients presented with abdominal pain, diarrhea, hematochezia, and tenesmus. Anemia and hypoalbuminemia were observed in 14 patients (87.5%), while fever was reported in five (31.3%). All 16 children presented with arthropathy, of which 4 cases were peripheral arthritis and 12 cases were axial arthritis. The Jadas 10 score was 12.7 ± 4.1 (7) [1] (Table 1).
Clinical response and remission
Clinical symptoms, including abdominal pain, hematochezia, diarrhea, tenesmus, and arthropathy, improved significantly by weeks 7, 14, 21, and 30. All patients had previously failed infliximab therapy, with a median washout period of 12.3 ± 4.2 months. Twelve patients (75.0%) were on steroids at the initiation of tofacitinib. By week 30, only 2 patients (87.5%) discontinued steroids (Figure 2).
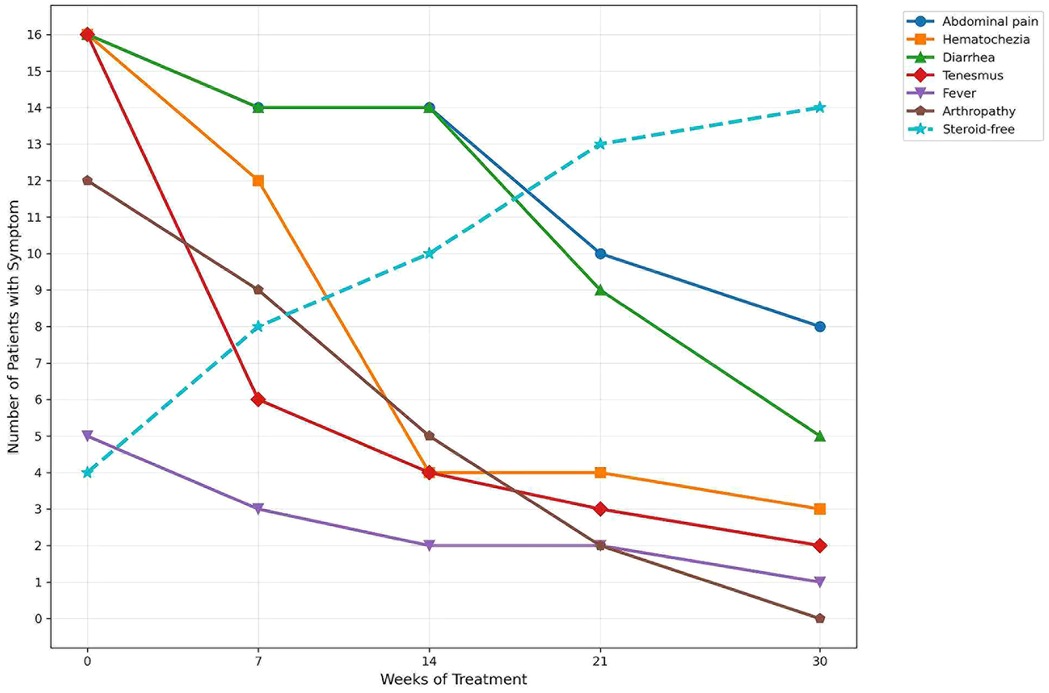
Figure 2. During JAK treatment, patients with IBD experience improvements in their digestive systems and systemic symptoms.
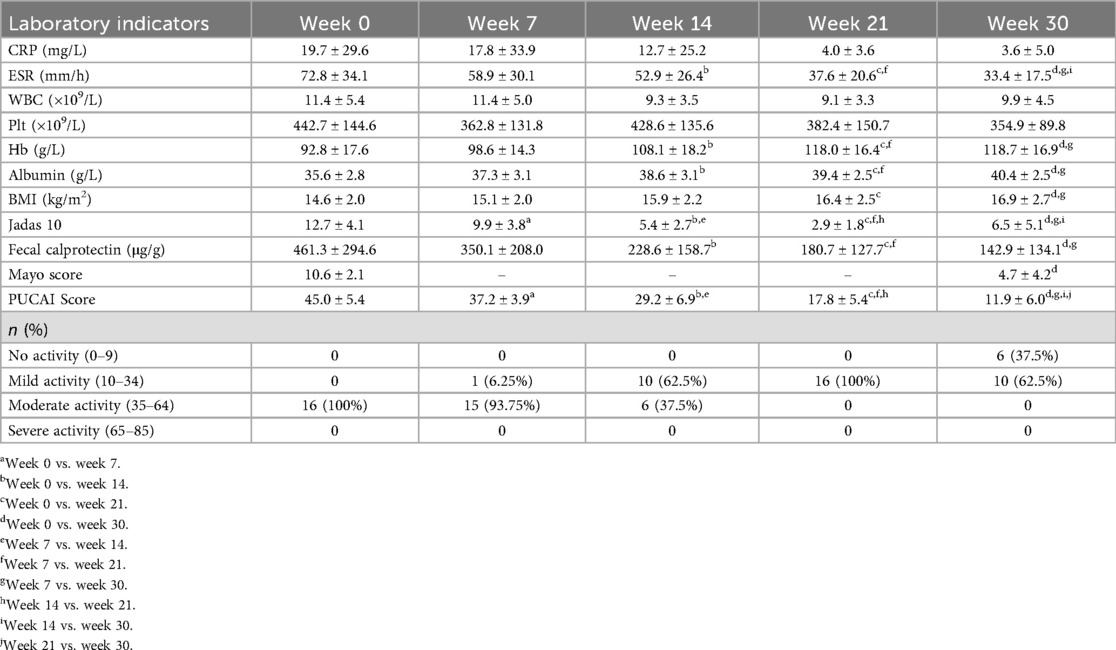
Fecal calprotectin decreased significantly by weeks 14, 21, and 30, while albumin and BMI levels increased (all P < 0.05). Endoscopic scores (Mayo score) showed a significant reduction from weeks 0 to 30. Jadas 10 score decreased from weeks 0 to 30. The mean PUCAI score decreased from 45.0 ± 5.4 to 11.9 ± 6.0. At the 0-week, all sixteen patients were in moderate disease activity. From week 7 to week 30, the number of patients with no active disease increased to six (37.5%), and the number of patients with mild activity increased to ten (62.5%) (Table 2). One patient (6.25%) achieved clinical response by week 7, and nine (56.25%) at week 14, five (31.25%) at week 21. Six patients (37.5%) achieved clinical remission by week 30.
Dose
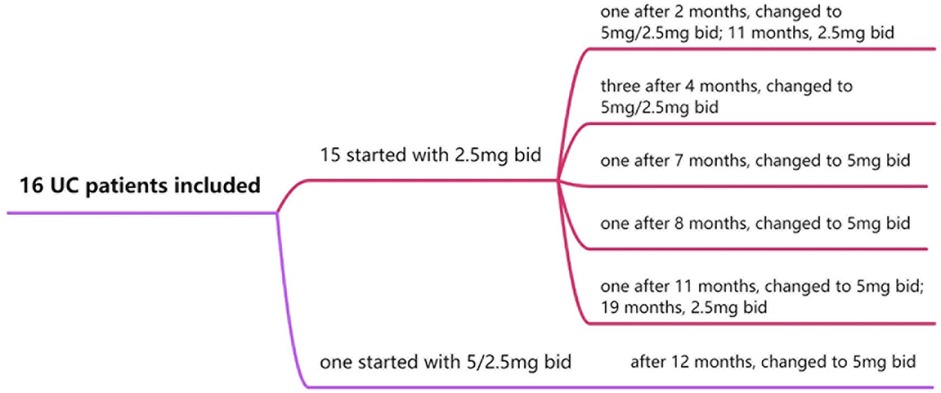
All children with UCcompleted the 30-week treatment course. Tofacitinib dosages were adjusted based on clinical symptoms, joint scores, changes in serum C-reactive protein (CRP) and fecal calprotectin level. Fifteen children started with an initial dose of 2.5 mg twice daily (bid). Among them: 1 case was adjusted to 5 mg in the morning and 2.5 mg in the evening at 2 months, then reduced to 2.5 mg bid at 11 months; 3 cases were adjusted to 5 mg in the morning and 2.5 mg in the evening at 4 months; 2 cases were escalated to 5 mg bid at 7 and 8 months, respectively; 1 case was increased to 5 mg bid at 11 months and later reduced to 2.5 mg bid at 19 months. The remaining 1 patient started at 5 mg in the morning and 2.5 mg in the evening, then adjusted to 5 mg bid at 12 months (Figure 3).
Adverse events
No serious drug-related adverse events were reported, and no thromboembolic events were observed. Eight mild infectious events were recorded in 16 patients, none of which required discontinuation of tofacitinib.
Discussion
Our study demonstrated the potential of tofacitinib as a salvage therapy for childhood IBD, particularly in patients with inflammatory arthritis. By week 30, 37.5% of patients achieved corticosteroid-free remission, with significant improvements in clinical symptoms and joint symptoms. Additionally, reductions were observed in fecal calprotectin and endoscopic scores, along with a notable decline in PUCAI score. Adverse events were limited to mild infections.
This study represented the first Asian report on the efficacy and safety of tofacitinib in childhood UC with associated arthropathy. Among the patients, one (6.25%) achieved clinical response by week 7, nine (56.25%) by week 14, and five (31.25%) by week 21. Overall, six patients (37.5%) achieved clinical remission by the 30-week mark. Tofacitinib demonstrated moderate efficacy in both inducing and maintaining remission in children with UC. In the OCTAVE Induction 1 and 2 trails–two Phase III clinical studies—the induction relief effect of tofacitinib (10 mg twice daily) was evaluated after 8 weeks of treatment. The results revealed that the tofacitinib group had significantly higher rates of clinical remission (18.5% vs. 8.2%, p < 0.001) and endoscopic improvement (31.3% vs. 15.6%, p < 0.001) compared to the placebo group (7). The OCTAVE Sustain study further assessed the efficacy of tofacitinib in maintenance therapy. Patients receiving tofacitinib (5 mg or 10 mg twice daily) exhibited significantly higher clinical remission rates at 52 weeks compared to the placebo group (34.3% vs. 40.6% vs. 11.1%, p < 0.001) (8). Recent real-word studies on tofacitinib in adults have reported higher remission rates than those observed in our study. For instance, a multicenter study in the United Kingdom by Honap et al. demonstrated a 74% response rate and a 44% corticosteroid-free remission rate at week 8. However, this cohort was less refractory to prior biologics, with 18% being biologic naïve and only 36% refractory to two biologics. Notably, in this adult study, patients with primary non-response were significantly younger than responders, suggesting that the younger age of our cohort may have influenced response rates (9). Similarly, in a Spanish real-life cohort study by Chaparro et al. (10), 60% of patients achieved a response, and 31% attained clinical remission by week 8.
Tofacitinib has been explored as a treatment option for refractory IBD in several studies. For instance, one study demonstrated that among 19 patients with severe refractory CD, 58% were able to continue tofacitinib treatment, and 46% exhibited a positive response during endoscopic evaluation (11). In another study involving 21 children and adolescents aged 2–18 years, tofacitinib treatment led to a significant reduction in clinical activity index over 52 weeks, with some patients achieving clinical remission (12). Additionally, a retrospective study reported that 71% of children treated with a combination of tofacitinib and biologics achieved a glucocorticoid-free response at the 6-month mark (13).
Tofacitinib has been approved by the US FDA for the treatment of active polyarticular juvenile idiopathic arthritis (JIA) in children aged 2 years and older, particularly for patients who have shown inadequate response or intolerance to one or more TNF inhibitors (14). Clinical trials have demonstrated that tofacitinib significantly reduces the risk of disease flare-ups and improves disease activity levels. For example, in a 48-week Phase III clinical trial, the JIA-ACR70 and JIA-ACR90 response rates for the tofacitinib group were 60.0% and 33.6%, respectively, with efficacy progressively improving over time. Additionally, 26% of patients achieved disease-free status within 44 weeks of treatment (15). Arthropathy is a common complication in patients with IBD, particularly in those with CD and UC. It often presents as peripheral arthropathy, frequently affecting joints such as the hips and knees (16). While tofacitinib is primarily used to manage intestinal inflammation, its mechanism of action—targeting the JAK-STAT signaling pathway—may also help control systemic inflammation associated with IBD, including arthropathy (12). Tofacitinib has shown promise in treating IBD-related arthropathy, especially in cases where conventional therapies have failed (17). At our center, we observed that tofacitinib may indirectly alleviate joint symptoms by improving intestinal inflammation, particularly in patients who did not respond to traditional treatments. These findings align with other studies, highlighting the potential dual benefit of tofacitinib in managing both intestinal and extraintestinal manifestations of IBD. Additionally, tofacitinib is an oral small-molecule drug, which is more cost-effective compared to biologic agents. This makes it a more accessible and affordable treatment option for children in less developed regions.
The safety profile of tofacitinib is consistent with that of other JAK inhibitors, with common adverse events including infections (such as herpes zoster), upper respiratory tract infections, non-melanoma skin cancer, and cardiovascular events (18, 19). In the OCTAVE Sustain trial, the tofacitinib group exhibited higher rates of overall infections and herpes zoster compared to the placebo group, but no significant increase in serious adverse events was observed (18). However, tofacitinib has been associated with an elevated risk of cardiovascular events, such as thrombosis and myocardial infarction (20, 21). In our study, drug-related adverse events were limited to mild infections, with no reported cases of thromboembolic events.
Tofacitinib is typically administered twice daily, with a recommended dose of either 5 mg or 10 mg. Clinical trials have demonstrated that the 10 mg dose offers high efficacy and a favorable safety profile (22, 23). In some studies, tofacitinib dosages have ranged from 0.5 mg to 15 mg, with the 10 mg dose significantly improving clinical and endoscopic response rates by week 8 (24). For children, the dosage may need to be adjusted based on age and weight (25). At our center, treatment is initiated at a dose of 2.5 mg bid and adjusted according to clinical response, with a maximum dose of 5 mg bid.
In conclusion, our study supports the efficacy and safety of tofacitinib in the treating children with UC and associated arthritis. But our study had a relatively small sample size, which may limit the statistical power and generalizability of the results. However, multicenter and long-term studies are still needed to further evaluate its therapeutic effects and safety profile.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the institutional ethics committee of Children's Hospital of Fudan University with the approval No. 198 [2018]. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
SM: Methodology, Investigation, Visualization, Writing – original draft, Project administration, Conceptualization. SW: Conceptualization, Methodology, Project administration, Investigation, Visualization, Writing – review & editing. WH: Writing – review & editing, Visualization, Methodology, Investigation, Project administration, Conceptualization. YH: Investigation, Methodology, Writing – review & editing, Conceptualization, Project administration. YS: Formal analysis, Methodology, Visualization, Data curation, Resources, Conceptualization, Project administration, Writing – review & editing, Supervision, Investigation.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the patients for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gasparetto M, Guariso G. Highlights in IBD epidemiology and its natural history in the paediatric age. Gastroenterol Res Pract. (2013) 2013:829040. doi: 10.1155/2013/829040
2. Yu YR, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. (2017) 26(6):349–55. doi: 10.1053/j.sempedsurg.2017.10.003
3. Bruscoli S, Febo M, Riccardi C, Migliorati G. Glucocorticoid therapy in inflammatory bowel disease: mechanisms and clinical practice. Front Immunol. (2021) 12:691480. doi: 10.3389/fimmu.2021.691480
4. Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. (2017) 376(18):1723–36. doi: 10.1056/NEJMoa1606910
5. Dolinger MT, Rolfes P, Phan BL, Dubinsky MC. Letter: tofacitinib use for biologic-refractory paediatric inflammatory bowel disease. Aliment Pharmacol Ther. (2019) 50(8):966–7. doi: 10.1111/apt.15496
6. Turner D, Otley AR, Mack D, Hyams J, de Bruijne J, Uusoue K, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology. (2007) 133(2):423–32. doi: 10.1053/j.gastro.2007.05.029
7. Backström M, Tynjälä P, Ylijoki H, Aalto K, Kärki J, Pohjankoski H, et al. Finding specific 10-joint juvenile arthritis disease activity score (JADAS10) and clinical JADAS10 cut-off values for disease activity levels in non-systemic juvenile idiopathic arthritis: a Finnish multicentre study. Rheumatology (Oxford). (2016) 55(4):615–23. doi: 10.1093/rheumatology/kev353
8. Panés J, Sandborn WJ, Schreiber S, Sands BE, Vermeire S, D'Haens G, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. (2017) 66(6):1049–59. doi: 10.1136/gutjnl-2016-312735
9. Honap S, Chee D, Chapman TP, Patel M, Kent AJ, Ray S, et al. Real-world effectiveness of tofacitinib for moderate to severe ulcerative colitis: a multicentre UK experience. J Crohns Colitis. (2020) 14(10):1385–93. doi: 10.1093/ecco-jcc/jjaa075
10. Chaparro M, Garre A, Mesonero F, Rodríguez C, Barreiro-de Acosta M, Martínez-Cadilla J, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. (2021) 15(1):35–42. doi: 10.1093/ecco-jcc/jjaa145
11. Wiles CA, Shah NB, Bell J, Pabla BS, Scoville EA, Dalal RL, et al. Tofacitinib adherence and outcomes in refractory inflammatory bowel disease. Crohns Colitis 360. (2021) 3(4):otab075. doi: 10.1093/crocol/otab075
12. Moore H, Dubes L, Fusillo S, Baldassano R, Stein R. Tofacitinib therapy in children and young adults with pediatric-onset medically refractory inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2021) 73(3):e57–62. doi: 10.1097/MPG.0000000000003190
13. Dolinger MT, Spencer EA, Lai J, Dunkin D, Dubinsky MC. Dual biologic and small molecule therapy for the treatment of refractory pediatric inflammatory bowel disease. Inflamm Bowel Dis. (2021) 27(8):1210–4. doi: 10.1093/ibd/izaa277
14. Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. (2017) 77:521–46. doi: 10.1007/s40265-017-0701-9
15. Brunner HI, Akikusa JD, Al-Abadi E, Bohnsack JF, Boteanu AL, Chedeville G, et al. Safety and efficacy of tofacitinib for the treatment of patients with juvenile idiopathic arthritis: preliminary results of an open-label, long-term extension study. Ann Rheum Dis. (2024) 83(11):1561–71. doi: 10.1136/ard-2023-225094
16. Horton DB, Sherry DD, Baldassano RN, Weiss PF. Enthesitis is an extraintestinal manifestation of pediatric inflammatory bowel disease. Ann Paediatr Rheumatol. (2012) 1(4):10.5455/apr.102920121510. doi: 10.5455/apr.102920121510
17. Wang Y, Wan Z, Jin R, Xu T, Ouyang Y, Wang B, et al. Tofacitinib for extraintestinal manifestations of inflammatory bowel disease: a literature review. Int Immunopharmacol. (2022) 105:108517. doi: 10.1016/j.intimp.2022.108517
18. Moran K, Null K, Huang Z, Lissoos T, Kane S. Retrospective claims analysis indirectly comparing medication adherence and persistence between intravenous biologics and oral small-molecule therapies in inflammatory bowel diseases. Adv Ther. (2019) 36(9):2260–72. doi: 10.1007/s12325-019-01037-x
19. Chang S, Murphy M, Malter L. A review of available medical therapies to treat moderate-to-severe inflammatory bowel disease. Am J Gastroenterol. (2024) 119(1):55–80. doi: 10.14309/ajg.0000000000002485
20. Charles-Schoeman C, Wicker P, Gonzalez-Gay MA, Boy M, Zuckerman A, Soma K, et al. Cardiovascular safety findings in patients with rheumatoid arthritis treated with tofacitinib, an oral Janus kinase inhibitor. Semin Arthritis Rheum. (2016) 46(3):261–71. doi: 10.1016/j.semarthrit.2016.05.014
21. Sinh P, Cross R. Cardiovascular risk assessment and impact of medications on cardiovascular disease in inflammatory bowel disease. Inflamm Bowel Dis. (2021) 27(7):1107–15. doi: 10.1093/ibd/izaa258
22. Lichtenstein GR, Bressler B, Francisconi C, Vermeire S, Lawendy N, Salese L, et al. Assessment of safety and efficacy of tofacitinib, stratified by age, in patients from the ulcerative colitis clinical program. Inflamm Bowel Dis. (2023) 29(1):27–41. doi: 10.1093/ibd/izac084
23. Nash P, Kerschbaumer A, Dörner T, Dougados M, Fleischmann RM, Geissler K, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. (2021) 80(1):71–87. doi: 10.1136/annrheumdis-2020-218398
24. Pathmakanthan S, Stack WA. Novel treatments in inflammatory bowel disease. Hosp Med. (1999) 60(1):19–23. doi: 10.12968/hosp.1999.60.1.1019
Keywords: arthropathy, tofacitinib, ulcerative colitis, children, safety and efficacy
Citation: Miao S, Wang S, Hu W, Huang Y and Shi Y (2025) Safety and efficacy of tofacitinib in children with ulcerative colitis complicated with arthropathy: a single-center study. Front. Pediatr. 13:1643668. doi: 10.3389/fped.2025.1643668
Received: 9 June 2025; Accepted: 31 October 2025;
Published: 14 November 2025.
Edited by:
Jan De Laffolie, University of Giessen, GermanyReviewed by:
Dragana Lazarevic, University Clinical Center Nis, SerbiaKenneth Ernest-Suarez, University of Costa Rica, Costa Rica
Copyright: © 2025 Miao, Wang, Hu, Huang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Shi, c2hpeXVfODIxMDA4QDE2My5jb20=; Ying Huang, eWh1YW5nODE1QDE2My5jb20=
 Shijian Miao
Shijian Miao Shengnan Wang
Shengnan Wang Wenhui Hu1
Wenhui Hu1 Ying Huang
Ying Huang Yu Shi
Yu Shi