- 1Department of Pediatrics, Jinhua Maternal and Child Health Care Hospital, Jinhua, Zhejiang, China
- 2Cancer Institute (Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education), The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China
Objective: Current model or biomarkers are far from satisfactory to predict pediatric simple febrile seizures (SFS). This study aimed to explore novel serum biomarkers in children with SFS and analyze their clinical significance.
Methods: A total of 57 SFS children admitted to the pediatric ward of Jinhua Maternal and Child Health Hospital from June 2022 to October 2024 were enrolled as the observation group, 61 sepsis patients and 45 healthy children from the pediatric health clinic during the same period were included as the control groups. Serum levels of 25-hydroxyvitamin D and diverse cytokines were measured using enzyme-linked immunosorbent assay (ELISA). Univariate logistic regression was used to identify the predictive ability of risk factors for SFS.
Results: The SFS group exhibited significantly lower levels of IL-17 (SFS: 5 vs. Health: 12 and Sepsis: 16) compared to the control groups. Conversely, the SFS group showed significantly higher levels of pro-inflammatory cytokines (IFN-α: 22 vs. 4 and 5; and IL-10: 12 vs. 10 and 9) than the control groups. ROC curve revealed that during SFS episodes, IFN-α can powerfully in discriminating SFS and health cohorts.
Conclusion: Serum level of IFN-α hold significant predictive value for SFS occurrence.
Introduction
Febrile seizures (FS) are the most common convulsive disorder in childhood, usually associated with a fever of ≥38°C and an incidence of 2%–11% (1, 2). The highest incidence of FS is at 18 months and prevalence peaking in children aged 6 months to 5 years without central nervous system infection nervous system infections or other etiologies of seizures, and with no prior history of febrile seizures (3). According to the 2011 diagnostic criteria established by the American Academy of Pediatrics (AAP) (4), simple febrile seizures (SFS) are defined as generalized tonic-clonic convulsion that spontaneously resolve within 15 min, with return to an alert mental status, and do not recur within a 24 h period, in a patient aged 6–60 months, associated with documented fever higher than 38°C, in the absence of a pre-existing metabolic disorder, neurological abnormality or previous seizure.
Despite the majority of affected children have a good prognosis, they carry a risk of subsequent epilepsy, impacting children's cognition and increasing socioeconomic burdens (5). The exact causes of SFS remain unknown, although some studies indicate a possible association with environmental and genetic factors (6, 7). Therefore, understanding the risk factors for SFS is crucial for implementing early interventions to mitigate long-term complications (8). Previously much efforts have been made to predict FS but demonstrated limited predictive ability, with AUC values <0.8, including serum brain-derived neurotrophic factor (AUC = 0.723) (9), neurotrophin-3 (AUC = 0.678) (10), hypomagnesemia (AUC = 0.731) (11), neutrophil to lymphocyte ratio (NLR, AUC = 0.768) and mean platelet volume (MPV)/platelet count (PLT) ratio (MPR, AUC = 0.689) (12), neutrophil lymphocyte platelet ratio (NLPR, AUC = 0.774) (13). Therefore, our study aims to develop SFS predictive markers that can significantly improve predictive ability and holds practical value in clinic.
Research has demonstrated that FS and convulsions induce neuronal damage mediated by inflammatory and immune responses (14). However, limited studies report the relationship between serum cytokine profiles and clinical manifestations in SFS patients. It is thus tempting to believe that cytokines that play important roles in immune microenvironment may serve as promising biomarkers in SFS prediction.
In this study, we collected serum cytokines and demographic data in retrospective cohorts of children with SFS, sepsis patients and healthy controls. Next, we investigated sensitivity and specificity of individual variables, aiming to establish a robust risk prediction models and innovative strategies for clinical diagnosis and early prevention.
Materials and methods
Study population
This retrospective study was designed to analyze SFS cases admitted to the department of pediatrics at Jinhua maternal and child health care hospital between June 2022 and October 2024.Inclusion criteria encompassed: (1) Children meeting the diagnostic criteria for SFS; (2) Age at 2–5 years old; (3) No recent supplementation with vitamin D-containing medications; and (4) First episode of seizure. Exclusion criteria comprised: (1) central nervous system infections, fever-sensitive epilepsy, immune-mediated encephalitis, inherited metabolic disorders, or similar conditions; (2) Electrolyte imbalances, hypoglycemia, or related abnormalities; (3) Intracranial tumors; (4) Vitamin D deficiency cases secondary to malnutrition; and (5) There have been episodes of seizure in the past. Finally, 57 pediatric patients with SFS were admitted in this study, 61 sepsis patients (who experienced fever above 38°C but did not develop seizures) and 45 age-matched healthy children undergoing routine physical examinations at the pediatric healthcare outpatient clinic during the same period were included as the control groups. The research protocol was approved by the Medical Ethics Committee of Jinhua maternal and child health care hospital. Written informed consent forms were obtained from all legal guardians of the participants prior to enrollment.
Data collection
From the medical records, we gathered clinical details about children (including age, sex, and family history of FS or epilepsy), disease characteristics (including duration of the first episode, highest temperature before the first episode, and number of seizures episodes prior to the visit), and serum examination information (25-Hydroxyvitamin D (OHD25), interleukin 1β (IL-1β), IL-2, IL-4 IL-5, IL-6, IL-8, IL-10, IL-12, tumor necrosis factor α (TNF-α), interferon α (INF-α), and INF-γ). All blood tests were conducted on the consultation day. Briefly, 3 ml of venous blood from admitted SFS within one hour of admission, and serum OHD25 (Zhejiang Diseth Diagnostics Co., LTD., batch No. K2407023) and cytokine (cytokine detection kit, Qingdao Kaisirui Biotechnology Co., Ltd., Lot No. 240601) levels were tested by enzyme-linked immunosorbent assay (ELISA) in strict accordance with the kit instructions to complete the specimen test.
Statistical analysis
Data were presented as counts (percentages) for categorical variables such as sex, and group-wise comparisons were conducted using the chi-squared test. Data were described as mean and standard deviation (SD) for continuous variables and compared using the t test or rank sum test (Mann–Whitney test) for non-normally distributed measurement data. P < 0.05 was used as the criterion for statistically significant differences. Risk factors for occurrence of SFS analyze using a univariate logistic regression model. The area under the curve (AUC) was performed by pROC package in R. The optimal cutoff values for the biomarkers were determined based on the Youden index maximization principle. All data analysis was performed by R (V. 4.2.3) unless otherwise stated.
Results
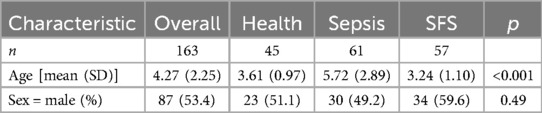
A total of 57 children (34 males and 23 females, 1.48:1) with SFS were included in this study, with a median age of 3 years, while the health and sepsis control groups included 45 cases (23 males and 22 females, 1.05:1) and 61 cases (30 males and 31 females, 0.97:1) with a median age of 4- and 5- years, respectively. All participants were aged 2–9 years. There was no statistically significant difference in sex between the three groups (P > 0.05, Table 1), while the sepsis group had an older age than the SFS and health groups.
As for the serum markers, we found that the SFS group had significantly lower cytokine IL-17 (5 vs. 12 and 16, P = 0.003) than the control groups (Table 2). In contrast, inflammatory cytokines such as IL-10 (12 vs. 10 and 9) and INF-α (22 vs. 4 and 5) were significantly up-regulated in the SFS group. To further understand the correlation between risk factors and SFS occurrence, we first performed univariate logistic regression based on the SFS and health groups. The results showed that OHD25 was associated with decreased risk of SFS (OR = 0.76, 95% CI: 0.68–0.84, P < 0.001), the higher OHD25 conferred a lower the risk of SFS occurrence. On the contrary, the high levels of IL-6 (OR = 1.97, 95% CI: 1.41–2.77, P < 0.001), IL-8 (OR = 1.09, 95% CI: 1.03–1.14, P < 0.001), IL-10 (OR = 1.09, 95% CI: 1.02–1.15, P = 0.01) and IFN-α (OR = 1.38, 95% CI: 1.17–1.62, P < 0.001) were associated with increased risk of SFS occurrence (Figure 1).
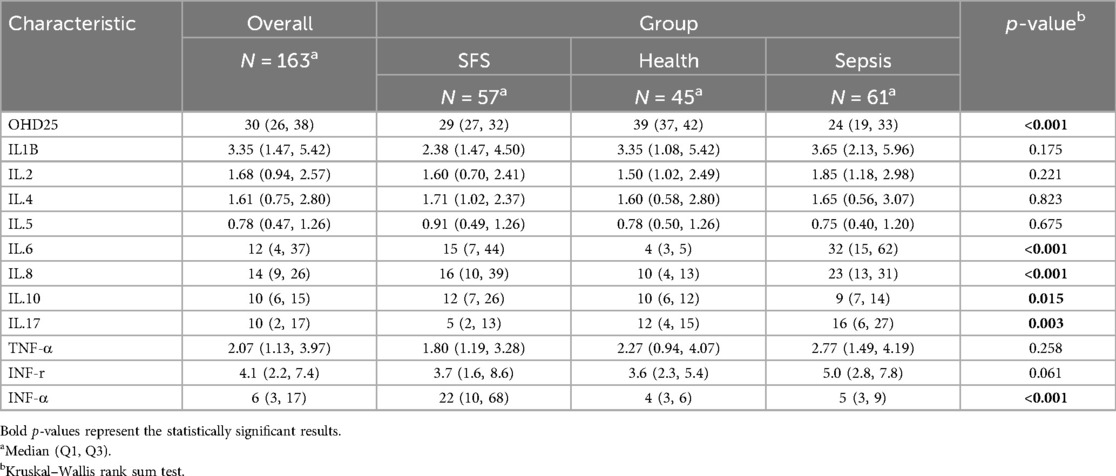
Table 2. Comparison of serum OHD25 (ng/ml) and cytokines (pg/ml) levels between SFS and two control groups.
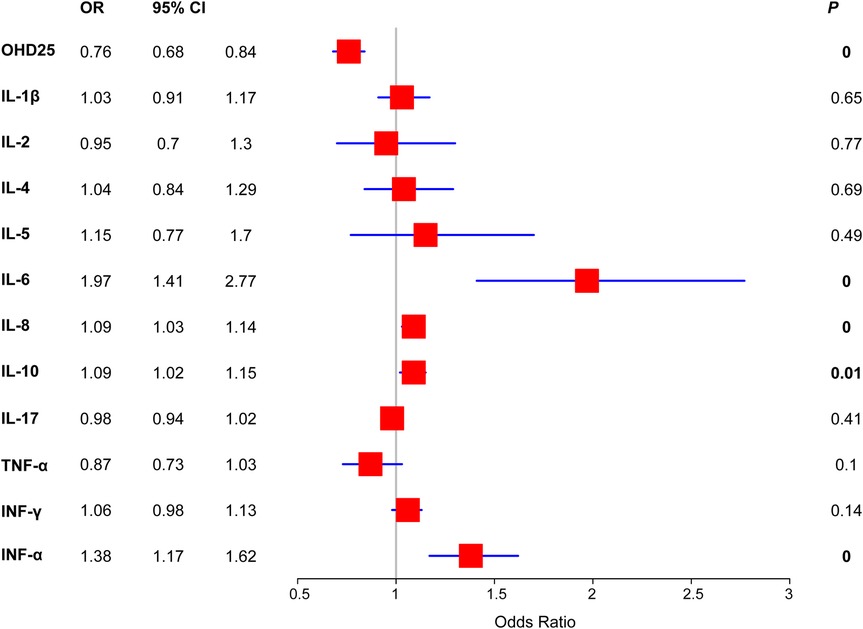
Figure 1. Forest plot of the SFS risk of serum markers detected in this study. Binary logistic regression was used to determine the risk of each serum markers.
To further identify the novel risk factors in predicting SFS occurrence, we performed ROC curve analysis and we found that three biomarkers showed excellent prediction power in determining SFS occurrence. The OHD25 [optimal cutoff value = 36.335 ng/ml, sensitivity = 94.7%, specificity = 75.6%; AUC = 0.888, 95% confidence interval (95% CI) = 0.821–0.954], IL-6 (optimal cutoff value = 6.425 pg/ml, sensitivity = 80.7%, specificity = 97.8%, AUC = 0.924, 95% CI = 0.869–0.979) and IFN-α (optimal cutoff value = 9.375 pg/ml, sensitivity = 75.4%, specificity = 95.6%, AUC = 0.895, 95% CI = 0.829–0.962) demonstrated high predictive value for SFS, with IL-6 exhibiting the highest discriminative power (AUC = 0.924, Figure 2A).
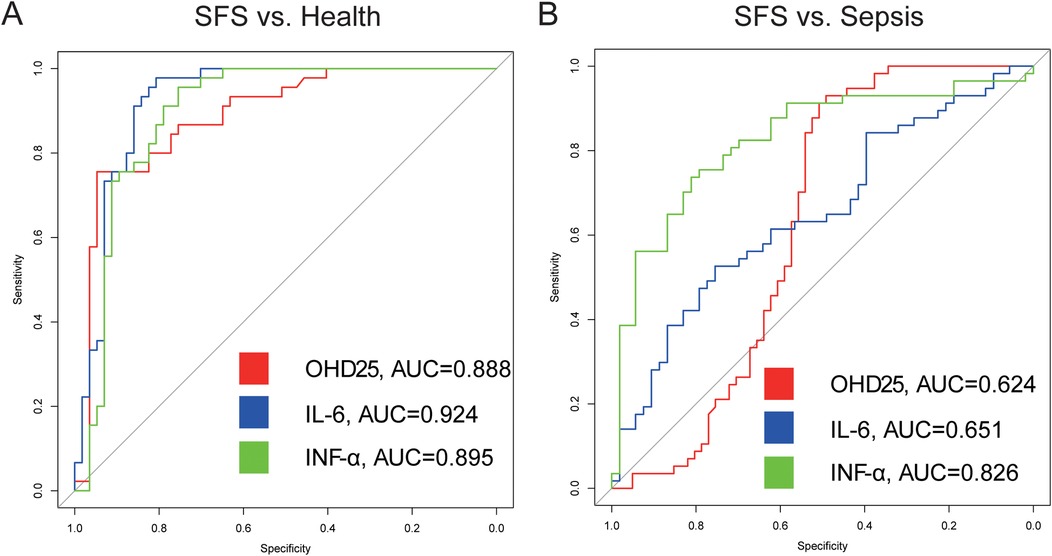
Figure 2. SFS ROC curve. (A) Receiver operating characteristics of OHD25, IL-6 and INF-α in discriminating SFS from health group. (B) Receiver operating characteristics of OHD25, IL-6 and INF-α in discriminating SFS from sepsis group.
Then, to rule out the possibility of nonspecific increased level of cytokines because of fever, we also compared the discriminative power between the SFS and the sepsis groups. Results showed that IFN-α (AUC = 0.826) also demonstrated high predictive value for SFS, which was consistent with the above comparison results (Figure 2B).
Discussion
SFS represent the most common form of seizures in children (15).The identification of novel biomarkers during SFS episodes holds significant potential for predicting SFS occurrence and enabling early preventive interventions to avoid abuse treatment and physical examination (16). Our study reveals elevated serum levels of pro-inflammatory cytokines (IL-6, IL-8, IL-10, and IFN-α) in SFS patients, indicating their contributory role in FS initiation. In addition, the robust performance of mono indicator IFN-α (AUC > 0.8) in predicting SFS compared to both sepsis and health groups is superior to other published models and markers. Excessive pro-inflammatory cytokine release may induce blood-brain barrier (BBB) dysfunction, neuronal injury, and precipitating seizures. Thus, dysregulation of the pro-inflammatory cytokine equilibrium may constitute a pivotal mechanism underlying FS development.
Huang et al. (17) found that seizure episodes in rats are associated with significantly elevated levels of inflammatory cytokines IL-6 and TNF-α. Increased IL-6 expression may activate NMDA receptors, leading to impaired cerebral autoregulation and hippocampal neuronal damage. IL-8, a pro-inflammatory cytokine and neutrophil-activating peptide, is primarily secreted by monocyte-derived macrophages, astrocytes, and microglia. It plays a critical role in post-injury neuronal repair by promoting neurite outgrowth and stimulating neurotrophic factor production. Clinical evidence indicates that IL-8 exacerbates seizure activity, with its elevation correlating positively with seizure severity (18). By altering the pro-convulsive microenvironment, IL-8 may further potentiate FS development. Consistent with these findings, our study demonstrates marked upregulation of IL-6 and IL8 during SFS episodes, suggesting their promotive role in SFS development. Notably, early anti-inflammatory therapy could be considered for children with pronounced inflammatory responses to mitigate SFS progression to epilepsy.
Another interesting finding in this study is the markedly decreased OHD25 in the fever groups (SFS and sepsis) than the health control group. Bhat et al. (19) found that 43.5% of children with SFS exhibit insufficient serum OHD25 levels, while 30.85% demonstrates deficiency and 25.56% maintain normal levels, which was consistent with our observation in this study. Additionally, injected subcutaneously to NMRI mice with vitamin D supports the direct anticonvulsant role of vitamin D in the brain (20). Therefore, routine monitoring of serum OHD25 is recommended for children over 2 years old, not only to support growth but also to mitigate SFS risk, as vitamin D deficiency constitutes an independent risk factor for SFS.
Taken together, IFN-α emerge as a novel promising biomarker for SFS diagnosis. Early intervention in SFS patients could improve prognosis but warrant validation through large-scale multicentric trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
No animal studies are presented in this manuscript. The studies involving humans were approved by the Medical Ethics Committee of Jinhua Maternal and Child Health Care Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
JM: Conceptualization, Formal analysis, Data curation, Writing – original draft, Funding acquisition. CH: Investigation, Writing – original draft, Software. FS: Resources, Writing – original draft, Methodology. ZW: Formal analysis, Writing – original draft, Methodology. WH: Project administration, Investigation, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the project grant from the Jinhua Science and Technology Department (No. 2022-4-192).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kubota J, Higurashi N, Hirano D, Isono H, Numata H, Suzuki T, et al. Predictors of recurrent febrile seizures during the same febrile illness in children with febrile seizures. J Neurol Sci. (2020) 411:116682. doi: 10.1016/j.jns.2020.116682
2. Subcommittee on Febrile Seizures, American Academy of Pediatrics. Neurodiagnostic evaluation of the child with a simple febrile seizure. Pediatrics. (2011) 127(2):389–94. doi: 10.1542/peds.2010-3318
3. Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. (1994) 35(Suppl 2):S1–6. doi: 10.1111/j.1528-1157.1994.tb05932.x
4. Oluwabusi T, Sood SK. Update on the management of simple febrile seizures: emphasis on minimal intervention. Curr Opin Pediatr. (2012) 24(2):259–65. doi: 10.1097/MOP.0b013e3283506765
5. Wang Q, Sun W, Zhao J, Tong L, Li B. Development and validation of a nomogram for the estimation of the prognosis of patients presenting with a febrile seizure. BMC Pediatr. (2024) 24(1):655. doi: 10.1186/s12887-024-05132-z
6. Laino D, Mencaroni E, Esposito S. Management of pediatric febrile seizures. Int J Environ Res Public Health. (2018) 15(10):2232. doi: 10.3390/ijerph15102232
8. Shen F, Lu L, Wu Y, Suo G, Zheng Y, Zhong X, et al. Risk factors and predictors of recurrence of febrile seizures in children in nantong, China: a retrospective cohort study. BMC Pediatr. (2024) 24(1):420. doi: 10.1186/s12887-024-04895-9
9. Cokyaman T, Kasap T, Sehitoglu H. Serum brain-derived neurotrophic factor in the diagnosis of febrile seizure. Pediatr Int. (2021) 63(9):1082–6. doi: 10.1111/ped.14567
10. Bakri AH, Hassan MH, Ahmed AE, Halim PR, El-Sawy SA, Mohamed MM, et al. Biochemical assessments of neurotrophin-3 and zinc involvement in the pathophysiology of pediatric febrile seizures: biochemical markers in febrile seizures. Biol Trace Elem Res. (2022) 200(6):2614–9. doi: 10.1007/s12011-021-02886-w
11. Baek SJ, Byeon JH, Eun SH, Eun BL, Kim GH. Risk of low serum levels of ionized magnesium in children with febrile seizure. BMC Pediatr. (2018) 18(1):297. doi: 10.1186/s12887-018-1271-z
12. Liu Z, Li X, Zhang M, Huang X, Bai J, Pan Z, et al. The role of mean platelet volume/platelet count ratio and neutrophil to lymphocyte ratio on the risk of febrile seizure. Sci Rep. (2018) 8(1):15123. doi: 10.1038/s41598-018-33373-3
13. Sogutlu Y, Altas U. Predictive value of neutrophil-lymphocyte ratio and other inflammation indices in febrile seizures in children. J Clin Med. (2024) 13(17):5330. doi: 10.3390/jcm13175330
14. Kim K, Kwak BO, Kwon A, Ha J, Kim SJ, Bae SW, et al. Analysis of plasma multiplex cytokines and increased level of IL-10 and IL-1Ra cytokines in febrile seizures. J Neuroinflammation. (2017) 14(1):200. doi: 10.1186/s12974-017-0974-7
15. Kwon A, Kwak BO, Kim K, Ha J, Kim SJ, Bae SH, et al. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. (2018) 59:5–10. doi: 10.1016/j.seizure.2018.04.023
16. Papez J, Labounek R, Jabandziev P, Ceska K, Slaba K, Oslejskova H, et al. Multivariate linear mixture models for the prediction of febrile seizure risk and recurrence: a prospective case-control study. Sci Rep. (2023) 13(1):17372. doi: 10.1038/s41598-023-43599-5
17. Huang WS, Zhu L. MiR-134 expression and changes in inflammatory cytokines of rats with epileptic seizures. Eur Rev Med Pharmacol Sci. (2018) 22(11):3479–84. doi: 10.26355/eurrev_201806_15174
18. Kocaturk M, Kirmit A. Evaluation of IL-10, IFN-gamma, and thiol-disulfide homeostasis in patients with drug-resistant epilepsy. Neurol Sci. (2022) 43(1):485–92. doi: 10.1007/s10072-021-05331-x
19. Bhat JA, Bhat TA, Sheikh SA, Wani ZA, Ara R. Status of 25-hydroxy vitamin D level in simple febrile seizures and its correlation with recurrence of seizures. Avicenna J Med. (2020) 10(1):6–9. doi: 10.4103/ajm.ajm_57_19
Keywords: febrile seizures, IL-6, IFN-α, vitamin D, ROC curve
Citation: Mei J, Hu C, Sheng F, Wang Z and Hu W (2025) Novel serum biomarker for powerfully predicting pediatric simple febrile seizures. Front. Pediatr. 13:1646261. doi: 10.3389/fped.2025.1646261
Received: 16 June 2025; Accepted: 30 July 2025;
Published: 14 August 2025.
Edited by:
Pasquale Striano, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Elisabetta Amadori, Giannina Gaslini Institute (IRCCS), ItalyKamil Dzwilewski, Medical University of Gdansk, Poland
Copyright: © 2025 Mei, Hu, Sheng, Wang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinzhi Mei, bWp6Xzh4aW5AMTI2LmNvbQ==; Wangxiong Hu, d3hodUB6anUuZWR1LmNu
 Jinzhi Mei1*
Jinzhi Mei1* Wangxiong Hu
Wangxiong Hu